Abstract
Members of the peroxidase-cyclooxygenase superfamily catalyze biochemical reactions essential to a broad spectrum of biological processes, including host defense, thyroid hormone biosynthesis, and modification of extracellular matrix, as well as contributing to the pathogenesis of chronic inflammatory diseases. We recently identified a novel member of this family, vascular peroxidase-1 (VPO1) that is highly expressed in the human cardiovascular system. Its biosynthesis and enzymatic properties are largely unknown. Here, we report that VPO1 was rapidly and efficiently secreted into the extracellular space when the gene was stably expressed in human embryonic kidney cells (HEK). Secreted VPO1 was a monomer with complex N-linked oligosaccharides and exhibited peroxidase activity. Biosynthesis of endogenous VPO1 by cultured human umbilical vein endothelial cells (HUVECs) share features exhibited by heterologous expression of recombinant VPO1 (rVPO1) in HEK. Pro-inflammatory agents, lipopolysaccharide and tumor necrosis factor-α, induce expression of VPO1 mRNA and protein in HUVECs. Furthermore, murine and bovine sera, and human plasma contain enzymatically active VPO1. rVPO1 exhibits spectral and enzymatic properties characteristic of the peroxidase-cyclooxygenase family, except with regard to its heat stability. rVPO1 catalyzes tyrosyl radical formation and promotes dityrosine cross-linking. Taken together, these data demonstrate that VPO1 is a glycosylated heme peroxidase that is actively secreted into circulating plasma by vascular endothelial cells and shares several features with other members of the peroxidase-cyclooxygenase family, including the catalysis of dityrosine formation.
Keywords: peroxidase-cyclooxygenase, VPO1, glycosylated protein, plasma concentration, biosynthesis, dityrosine formation
Peroxidases figure prominently in biochemistry, catalyzing reactions critical to the integrity and viability of cells throughout the plant and animal kingdoms [1]. Members of the peroxidase-cyclooxygenase superfamily, formerly known as the animal peroxidase family, include myeloperoxidase (MPO), eosinophil peroxidase (EPO), lactoperoxidase (LPO), and thyroid peroxidase (TPO) [2]. These peroxidases catalyze a wide variety of biochemical reactions, including those essential for host defense, biosynthesis of thyroid hormone, and modification of the extracellular matrix. In addition, MPO, EPO, LPO, and TPO have been implicated in the pathogenesis or progression of chronic inflammatory diseases, including atherosclerosis, systemic vasculitis and autoimmune thyroid disease [3, 4].
We have recently reported the identification and characterization of vascular peroxidase-1 (VPO1), a peroxidase that exhibits high levels of expression in the heart and vascular system [5]. Structural features, spectral properties, and enzymatic activity of VPO1 suggest that it is a member of the peroxidase-cyclooxygenase family [2]. Given the roles of MPO in vascular diseases such as atherosclerosis [6–11] and antinuclear cytoplasmic antigen (ANCA)-associated vasculitis [12–14], and the expression of VPO1 in vascular tissue, we studied the biosynthesis of VPO1, both in an immortalized cell line stably expressing normal VPO1 and in primary endothelial cells expressing endogenous VPO1. We also characterized the ability of VPO1 to catalyze dityrosine formation and compared its activity to that of MPO and LPO. Collectively, our data demonstrate that VPO1 is a glycosylated monomeric heme protein, constitutively secreted by vascular endothelial cells and present in circulating human plasma. Furthermore, VPO1 expression and secretion were stimulated by pro-inflammatory factors, TNF-α and lipopolysaccharide (LPS) and, like other members of the peroxidase-cyclooxygenase family, VPO1 catalyzed dityrosine formation. Thus, VPO1 has the tissue distribution and biochemical properties to contribute to inflammatory pathology in the vascular system.
Materials and Methods
Reagents
Hematin, sodium butyrate (NaBu), luminol, bovine LPO, 3,3’,5,5’-tetramethylbezidine (TMB) and TMB liquid system, tyrosine, tyrosine ethyl ester, 3-chloro-L-tyrosine, and 3-nitro-L-tyrosine were purchased from Sigma-Aldrich (St. Louis, MO); EasyTagExpress Protein labeling mix [35S]-methionine (37 TBq/mmol) from Perkin Elmer; chemiluminescent substrate for immunoblots from Pierce Biotechnology (Rockford, IL). pcDNA3.1/His was obtained from Invitrogen (Carlsbad, CA), and Matchmaker Mammalian Two-Hybrid System was purchased from Clontech (Mountain View, CA). A monospecific rabbit polyclonal antibody against human MPO was as previously described [15].
Cell culture
Both wild-type and VPO1-stably expressing HEK293 cells were maintained in DMEM media with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. Suspension culture was performed in 293 Serum Free Medium II (Invitrogen, Carlsbad, CA) as described in below. Stably transfected HEK293 cells expressing normal MPO were employed for studies of MPO biosynthesis, as previously described [16].
Subcloning
A tandem (His)6-myc-(His)6 tag was fused to the C- terminus of full-length human VPO1 in pcDNA3.1/His and to the C- terminus of truncated VPO1 including N-terminal 666 amino acids, respectively. For mammalian hybrid assay, human VPO1 (30–1479 aa) was subcloned into DNA-binding vector (pM) or activation vector (pVP16), respectively, to produce pM-VPO1 (30–1479 aa) or pVP16-VPO1 (30–1479 aa).
Construction of VPO1-stably expressing cell lines
Two cell lines stably expressing full length VPO1 and one cell line stably expressing a truncated form of VPO1 (1–666 aa) were created for these studies. GJ-4 is untagged full-length VPO1-stably expressing cells derived from HEK293 cells as previously described [5]. In addition, two tagged constructs, full-length VPO1 [pDNA3.1/VPO1-(His)6-myc-(His)6] and a truncated form of VPO1 including N-terminal 666 amino acids [pDNA3.1/VPO1 1–666 aa-(His)6], were transfected into HEK 293 cells, respectively, using methods previously described [5], to establish VPO1-expressing stable cell lines.
Suspension culture of VPO1-stably expressing cells and induction of VPO1 expression
For expression and purification of recombinant VPO1 (rVPO1), the previously described method [5] was scaled up to increase the amount of protein available for the studies here. In brief, 0.5 × 106 cells/ml were grown in 100 ml spinner bottles with 40 ml of 293 Serum-free Medium II (Invitrogen) at 37°C and 8% CO2 with stirring at 100 rpm. After three days, 60 ml of fresh serum-free medium was added and the culture was continued for four more days. Subsequently, 200 ml fresh media was added and the total volume transferred to 500 ml spinner bottles to culture for an additional four days under the same conditions. When cell density reached 3 × 106/ml, VPO1 expression was induced by addition of 200 ml fresh media containing NaBu and hematin. The final concentrations of NaBu and hematin were 2.5 mM and 0.5 µg/ml, respectively. The cells were cultured under these conditions for 3–4 days before harvest. Cells were pelleted at 1000 × g at 4°C for 10 min and the supernatant recovered for subsequent VPO1 purification or for use in immunoblots.
Purification of His-tagged human VPO1
Culture supernatant (1 L) containing His-tagged VPO1 was mixed with 200 ml of 100 mM potassium phosphate, pH 8.0, and loaded onto a 0.8 × 5.0 cm column of DEAE-Sepharose Fast Flow at 4°C. The column was washed with 50 ml of 20 mM potassium phosphate, pH 8.0, containing 100 mM NaCl. Sequentially, the column was eluted by 25 ml of 20 mM potassium phosphate, pH 8.0, containing 0.5 M NaCl. The NaCl concentration of the concentrated eluent containing VPO1 was adjusted to 0.3 M and imidazole was added to final concentration of 2.5 mM in order to reduce the non-specific binding. The salt-adjusted eluent was loaded onto a column with 0.5 ml of HisPur™ Cobalt Resin (Thermo Fisher Scientific (Rockford, IL) and the column was washed with 10 ml of wash buffer (20 mM potassium phosphate, pH 8.0, 0.3 M NaCl and 2.5 mM imidazole). Elution was achieved using 2 ml of wash buffer with 0.5 M imidazole.
The rVPO1-enriched eluent was then loaded onto 1 × 110 cm column of Sephacryl S-300 (GE Healthcare, Piscataway, NJ) and eluted by 20 mM potassium phosphate at a rate of 0.25 ml/min. The eluent was collected at a rate of 4 ml/tube. The protein concentration (A280), Soret absorbance (A412), and peroxidase activity (TMB oxidation) in eluent fractions were monitored to identify fractions with the most enzymatically active rVPO1. Peak fractions were pooled and further concentrated using Centricon Centrifugal Filter Devices (Cutoff 100 kDa) into a volume of 0.5 ml. Protein concentrations were determined using the Bio-Rad Protein Assay based on the Bradford dye-binding procedure. Bovine serum albumin served as protein standard. The spectrum of oxidized rVPO1 was recorded from 250 nm to 700 nm using a Beckman DU-640 Spectrophotometer.
VPO1 enzymatic activity
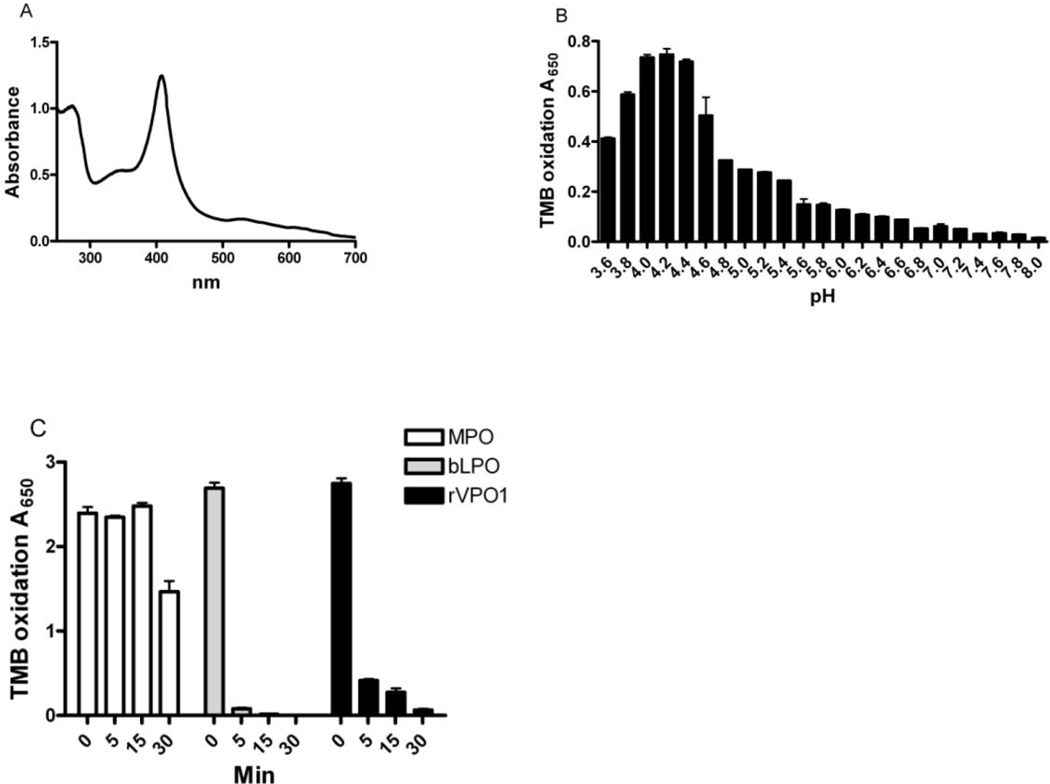
TMB was used as the substrate to measure peroxidase activity. VPO1 was added into 100 µl of TMB liquid system. After 30 min, the absorbance at 650 nm was recorded. To characterize thermal stability of VPO1, purified rVPO1 as well as MPO and LPO were heated at 92 °C for 5 min, 15 min and 30 min, respectively. Heat-treated VPO1 (final concentration, 1 µM), MPO (50 nM) and LPO (50 nM) were assayed as described above and absorbance recorded at 650 nm after 30 min. Optimal pH of VPO1-mediated TMB oxidation was determined in a 100 µl reaction containing 25 mM buffer, pH from 3.6 to 8.0 with 0.2 intervals (pH 3.6 to 5.6, potassium acetate buffer; pH 5.8 to 8.0, potassium phosphate buffer), 0.5 mM TMB, 50 µM H2O2, 250 nM rVPO1. The absorbance at 650 nm was monitored after 30 min incubation.
Anti-VPO1 antibody
A region of VPO1 that was predicted to be antigenic was identified using DNAStar software (Madison, WI, USA) and the corresponding peptide (residues 49–63 of VPO1) was synthesized, purified by reverse-phase high-performance liquid chromatography, and conjugated with keyhole limpet hemocyanin from Sigma-Genosys (The Woodlands, TX, USA). Anti-VPO1 antibody was raised against the conjugated peptide (Sigma-Genosys) in rabbits. The antiserum was further purified by using highly cross-linked agarose beads conjugated with the immunogen, residues 49–63 of VPO1. The peptide immunogen used to raise the VPO1 antibody is from the N-terminal region of VPO1 that is not conserved in MPO, LPO, EPO and TPO. Consequently, the anti-VPO1 antibody selectively recognizes VPO1.
Quantitation of VPO1
Truncated VPO1 (1–666) protein was used as a standard for quantitation of VPO1 in biological samples. His-tagged VPO1(1–666)-stably expressing HEK293 cells were established using the similar procedures to those described in [5], and the His-tagged VPO1 (1–666) was affinity-purified from the supernatant of centrifuged suspension cultures using HisPur™ Cobalt resin (Thermo Fisher Scientific, Inc. Rockford, IL). VPO1(1–666) migrated as single ~82 kDa band on SDS-PAGE stained with Coomassie Brilliant Blue, and its concentration was determined by using the Bio-Rad Protein Assay.
VPO1 in human plasma or murine sera was quantified by performing densitometry of immunoblots. Purified His tagged VPO1(1–666) (1.2 pmol, 2.4 pmol and 3.6 pmol) as standards and 1 µl of plasma sample (diluted in 10 µl of 1× loading buffer, run in duplicate) were separated by SDS-PAGE and quantitated by densitometry. Plasma samples from 21 unrelated healthy adult human donors and 12 serum samples from BALB/c mice were evaluated. The density of immunoreactive bands recognized by affinity-purified anti-VPO1 antibody was measured using ImageJ software (The National Institute of Health) and the plasma or serum VPO1 (170 kDa) concentration calculated from the standard curve derived using the truncated VPO1 (1–666) protein.
Mammalian two-hybrid assay
Human VPO1 (30–1479 aa) was subcloned into DNA-binding vector (pM) or activation vector (pVP16), respectively. Two constructs, pM-VPO1 (30–1479 aa) and pVP16-VPO1 (30–1479 aa), were cotransfected into HEK293 cells. Mammalian two-hybrid assay was performed as previously described [17], with slight modifications. The enzymatic activity of transcriptional product of chloramphenicol acetyltransferase (CAT) was evaluated by using FAST CAT® chloramphenicol acetyltransferase Assay Kit (Molecular Probes, Inc.).
PAGE and immunoblotting
Samples or plasma (diluted by 1:10 by PBS) for SDS-PAGE analysis were mixed with SDS-sample buffer [50 mM Tris/HCl (pH 6.7), 2% (w/v) SDS, 200 mM dithiothreitol, 10% (w/v) glycerol, and 0.05% bromophenol blue] and heated 100°C for 3 min. Proteins were separated by electrophoresis in 12.5% (w/v) polyacrylamide gel and stained with Coomassie Brilliant Blue or electroblotted to PVDF membrane for probing with antibodies. Immunoblots for VPO1 were probed and processed as previously described [5]. Immunoblots for MPO-related proteins were performed as previously described [16].
For 4–15% native PAGE, purified His-tagged VPO1 was mixed with equal volume of 2 X sample buffer (50 mM Tris-Cl, pH6.7, 20% glycerol) and loaded onto 4–15% Tris/glycine gel (Bio-Rad). Proteins were electrophoresed at 10 V/cm until the bromophenol blue reached the bottom of the gel. The resulting gel was stained with Coomassie Brilliant Blue.
Biosynthesis of VPO1 and MPO
Biosynthesis of VPO1 and of MPO was assessed using the same procedures as previously described [16]. Briefly, HEK293 cells, either wild-type or stable transfectants expressing VPO1 or MPO, were grown at low density in media supplemented with hemin (2 µg/ml) for 24 hrs and then in methionine-free RPMI media containing 5% dialyzed fetal calf serum and antibiotics for 1 hr prior to radiolabeling. Cells were biosynthetically pulse-labeled for the indicated time with [35S]-methionine and then collected for analysis or chased by the addition of 1000-fold excess of unlabeled methionine. Cells were recovered and solubilized; and the solubilized cells and culture media immunoprecipitated with the appropriate antibody, as previously described [5, 16].
Biosynthesis of VPO1 endogenously expressed by human umbilical vein endothelial cells (HUVECs) was examined using a method modified from that described above. A nearly confluent growth of HUVECs was harvested from a T-75 flask using brief trypsinization, followed by washing, resuspension in supplemented endothelial basal medium 2 (EBM2 medium, Clonetics, Walkerville, MD), and culture in a fresh T-75 flask for an additional day, until the cells were 80–90% confluent. Media was removed by pipetting, and adherent cells were washed twice with PBS. Cells were starved for 1 hr in 5 ml of EMB media (Fisher Scientific, Pittsburgh, PA) without supplements prior to labeling for 2 hrs with [35S]-methionine, followed by a 20 hr chase with 1 mM unlabeled methionine. Cells and culture supernatant were immunoprecipitated as described previously [16].
Induction of endogenous VPO1 expression by pro-inflammatory factors
HUVECs were cultured to 60–70% confluence in M199 media with supplements and 10% FBS, LPS (100 ng/ml) or TNF-α (2 ng/ml) was added to the media at the indicated time prior to harvest. Samples were used for preparation of total RNA and proteins, respectively. Total RNA was extracted by using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcription was performed using SuperScript™ Reverse Transcriptase (Invitrogen, Carlsbad, CA). mRNA expression of VPO1 was analyzed by StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA) with primers: 5’-ATCGCAAACCTGTCGGGCTGTACC-3’ (forward) and 5’-AGTTGCTCTAGAGAGGCAAGTCCC-3’ (backward). Expression of β–actin (forward primer: 5’- ACG TTG CTA TCC AGG CTG TGC TAT-3’; backward primer: 5’- GCG ACG TAG CAC AGC TTC TCC TTA-3’) served as internal control. VPO1 protein expression after LPS or TNF-α treatment was detected by PAGE and immunoblotting.
The effects of LPS and TNF-α on VPO1 secretion by HUVECs were assessed using biosynthetic radiolabelling, as described above. LPS (100 and 500 ng/ml) or TNF-α (2 ng/ml) was added to experimental cultures. Cells were cultured for 0, 6, 14.5, and 22 hours before starving for 1 hr in methionine-free medium and labeling for 2 hrs, as described above. Throughout the starvation and pulse-labeling LPS and TNF-α concentrations were maintained. Pulse-labeled cells were chased for 19 hrs and VPO1 immunoprecipitated from culture media as described above.
Dityrosine formation
Peroxidase-mediated oxidation of tyrosine was assessed by measuring the generation of dityrosine in 96-well black microplates using a fluorescence-based assay; 100 µl reaction containing 50 mM phosphate buffer, pH 7.2, 0.25 mM tyrosine or its derivatives, 50 µM H2O2, and 4 nM MPO, 4 nM LPO or 50 nM rVPO1 was performed at 22°C for 30 min. The fluorescence was recorded as relative fluorescence units (RFU) using a fluorometer (BioTek, Winooski, VT) at excitation/emission (320 nm/405 nm). The effects of pH and tyrosine concentration on dityrosine formation were examined using varying pH of the phosphate buffer and varying concentrations of tyrosine, respectively. Assays with varying tyrosine concentrations were performed at pH 7.2.
Results
Heterologous expression of VPO1 and MPO in HEK293 cells
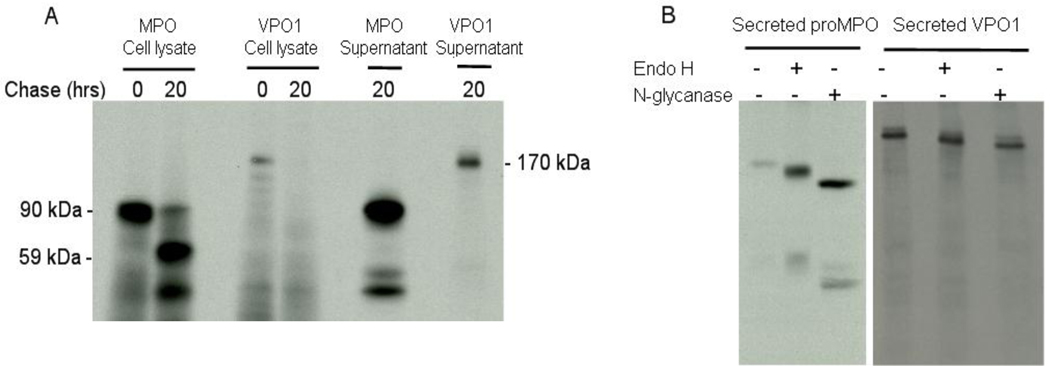
Heterologous expression of MPO in HEK293 cells recapitulates features of the normal biosynthesis of endogenous MPO in myeloid progenitors; the exogenously expressed protein undergoes co-translational glycosylation, interacts with molecular chaperones in the endoplasmic reticulum, acquires heme, and undergoes proper proteolytic processing and subsequent subcellular compartmentalization [16]. As occuring during MPO biosynthesis by cultured myeloid cell lines or normal bone marrow [18–21], a fraction of proMPO enters the secretory pathway and exits cells constitutively [21]. HEK293 cells stably transfected with normal MPO or with VPO1 (GJ-4) were biosynthetically radiolabeled with [35S]-methionine; cell lysates and conditioned culture media were then subjected to immunoprecipitation with antibodies against MPO or VPO1. The majority of the 90-kDa proMPO synthesized by HEK-MPO was proteolytically processed during the 20 hr chase into the normal heavy and light subunits of mature MPO and packaged into a membrane-bound intracellular compartment, with a fraction of the proMPO secreted into the culture medium (Fig. 1A). Similarly, GJ-4 cells produced VPO1 during the pulse period, but the protein was rapidly secreted into the culture medium with undetectable radiolabeled protein recovered from cell lysates after a 20 hr chase (Fig. 1A). All of the VPO1 synthesized during the pulse period was recovered in the extracellular medium.
Figure 1. VPO1 as a secreted and glycosylated protein.
A. HEK293 cells stably expressing transfected MPO or VPO1 were biosynthetically radiolabelled with [32S]-Met for 1 hr and then harvested (0 hr chase) or chased with excess unlabelled Met and harvested at 20 hr. Cell lysates after pulse and chase and culture supernatants after chase were immunoprecipitated with the anti-MPO or anti-VPO1 antibody, the resulting immunoprecipitates were separated by SDS-PAGE, and the dried gels subjected to autoradiography. The data shown are representative of three independent experiments. B. proMPO and VPO1 that were biosynthetically radiolabelled and secreted by HEK293 stable transfectants were immunoprecipitated with antibodies against MPO and VPO1, respectively. Immunoprecipitates were incubated in digestion buffer alone or digested with endoglycosidase H (Endo H) or N-glycanase (N-glycanase), as described in Materials and Methods. Digests were separated by SDS-PAGE and the dried gels subjected to autoradiography. The data shown are representative of three independent experiments.
The N-linked oligosaccharide side chains of secreted proMPO differ from that of intracellular proMPO or of mature MPO; the carbohydrate side chains on secreted proMPO are partially endoglycosidase H-resistant, whereas those on intracellular proMPO or mature MPO are readily digested by endoglycosidase H [22]. These observations indicate that the oligosaccharides on the proMPO destined for constitutive extracellular release undergo processing during passage through the secretory pathway, resulting in conversion of some carbohydrates from high mannose to complex side chains, whereas those on mature MPO remain as high mannose residues. Secreted VPO1 was glycosylated, as indicated by susceptibility to digestion with endoglycosidase F (Fig. 1B). Similar to secreted proMPO, VPO1 demonstrated partial resistance to digestion with endoglycosidase H. Thus, VPO1 possessed endoglycosidase H-susceptible and complex (i.e. Endo H-resistant, N-glycanase-susceptible) carbohydrates, implying VPO1 was modified during transport through the Golgi.
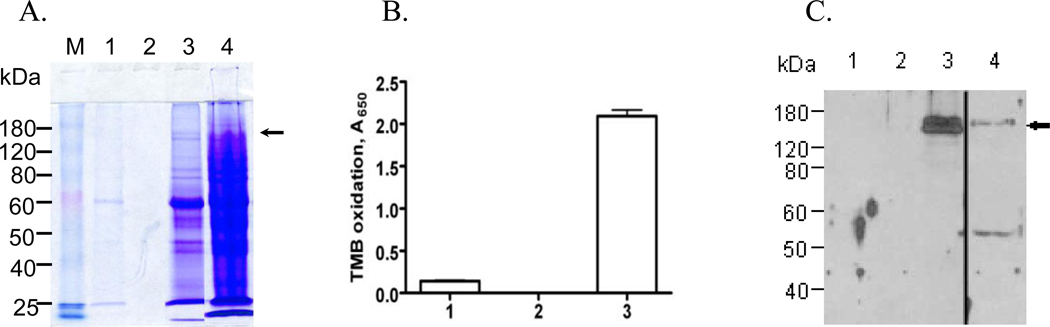
To determine if secreted VPO1 was functional, serum-free culture medium conditioned by GJ-4 cells treated with NaBu and hematin was used as a source for rVPO1. Secreted VPO1 was recovered from culture media of GJ-4 cells (Fig. 2A), concentrated, and assayed for capacity to oxidize TMB (Fig. 2B). The medium contained VPO1, as demonstrated by immunoblotting (Fig. 2C), exhibited significant peroxidase activity. Thus, GJ-4 cells constitutively secrete enzymatically active rVPO1.
Figure 2. VPO1 in cell culture media.
A. SDS-PAGE of samples from HEK293 transfectants stably expressing VPO1 grown in serum-free media and supplemented with NaBu and hematin, as described in Materials and Methods. M represents the protein mass marker. Culture media (lane 1) was harvested by centrifugation and concentrated ~150-fold by volume (Centricon, 100 kDa cutoff). The filtrate from the Centricon and the concentrated culture media were separated in lanes 2 and 3, respectively, whereas total cell lysate was analyzed in lane 4. B. The enzymatic activity of samples from lanes 1–3 in panel A was assessed as the oxidation of TMB, as detailed in Materials and Methods. Error bars show the range of three determinants and the results shown are representative of three independent experiments. C. Fractions 1–4 from panel A were subjected to SDS-PAGE and immunoblotting with anti-VPO1 antibody. Lane 4 came from the same blot as Lane 1–3 by removing the blank space between Lane 3 and 4. The arrows in panels A and C indicate VPO1. The data are representative of three independent experiments.
Molecular organization of VPO1
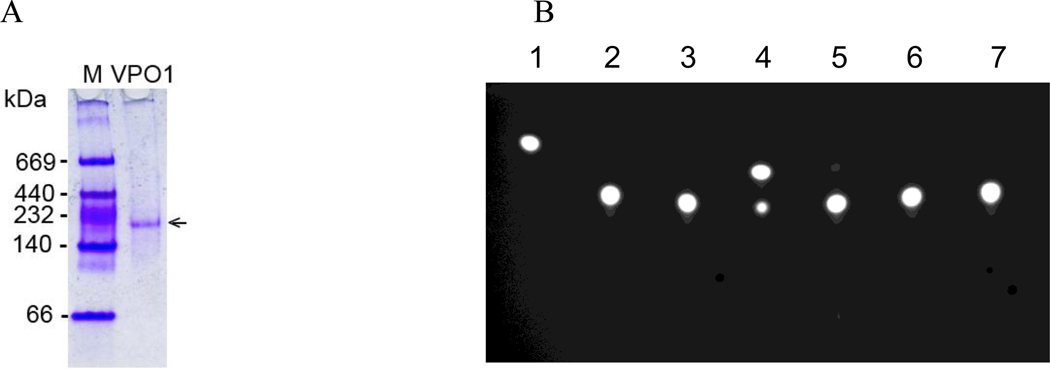
Although the mature form of MPO stored in the azurophilic granules of neutrophils is a dimer, with each half composed of a heavy and a light chain covalently bound to a heme group [23], secreted proMPO is monomeric [18–21]. VPO1 is the human homolog of peroxidasin, a peroxidase that participates in modifying the extracellular matrix during development in Drosophila and has been reported to form a homotrimer due to the coiled-coil structure of α-helices at its far C-terminal region [24]. To examine the molecular organization of human VPO1, we first purified His- and myc-tagged full-length VPO1 from serum-free media conditioned by stably transfected cells. When separated by native gel electrophoresis, the purified human VPO1 construct migrated as single species of ~170-kDa, consistent with it being in a monomeric form (Fig. 3A). To assess the potential for the existence of higher order structures in VPO1 using a different analytical approach, we performed a mammalian two-hybrid analysis. We subcloned the full length VPO1 (30–1479 aa) into DNA-binding vector and activation vector, respectively, and cotransfected the two constructs into HEK293 cells. In these constructs, the signal peptide of VPO1 was deleted to facilitate the translocation of the fusion proteins to the nucleus where they could interact. In the nucleus, interaction of the fusion proteins via DNA-binding domain and DNA-activation domain triggers the transcription of CAT, with subsequent acetylation of the boron-dipyrromethene fluorescent substrate as evidence for the presence of interacting species. Because the acetylated product migrates faster than the unmodified substrate, CAT activity can be indirectly assessed by thin layer chromatography. We were unable to detect fluorescent product (Fig. 3B), indicating the absence of inter-molecular interactions between domains within VPO1. Thus, in contrast to peroxidasin, VPO1 behaves as a monomeric polypeptide, similar to proMPO.
Figure 3. VPO1 as monomer.
A. Purified full-length VPO1-His-Myc was separated in a 4–15% native gel, as described in Materials and Methods. Native protein marker (M) was separated in lane 1 and an arrow indicates VPO1. B. The mammalian two-hybrid system was utilized in HEK293 cells to assess the presence of interactions between VPO1 molecules. The presence of the most rapidly migrating spot on the TLC plate represents product formation and indicates the presence of interactions between components. Lane 1 is the positive reference standard; 2. Non-transfected HEK293 cells; 3. Bait and prey vectors: pM/pVP16; 4. Positive control vector: pM3V16; 5. pM/pVP16-VPO1(30–1479); 6. pM-VPO1(30–1479)/pVP16; 7. pM-VPO1(30–1479)/pVP16-VPO1(30–1479). Data are representative of three independent experiments.
Endogenous VPO1 as a plasma protein and secreted by endothelial cells
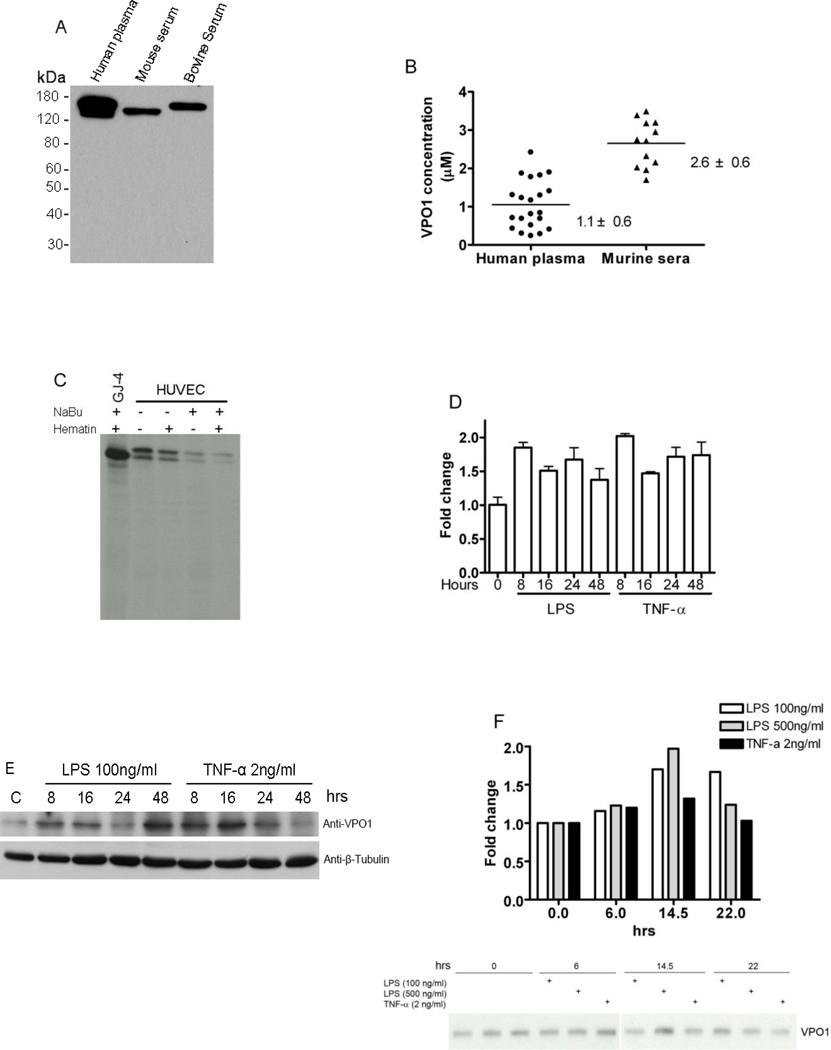
Given the presence of a 26-amino acid signal peptide in the amino acid sequence of VPO1 [5], together with our data demonstrating constitutive secretion of VPO1 from stably transfected heterologous cells (Fig. 1 and 2), we anticipated that endogenous VPO1 secreted by vascular endothelial cells in vivo will allow for the detection of this protein in the circulation. Analyses of human plasma, as well as murine and bovine sera, detected immunoreactive VPO1 as a single monomeric band (Fig. 4A). Quantitative analysis determined VPO1 at 1.1 ± 0.6 µM (range 0.2 – 2.4 µM) in normal human plasma (21 donors) and 2.6 ± 0.6 µM (range 1.7–3.5 µM) in serum from 12 BALB/c mice (Figure 4B).
Figure 4. Endogenous expression of VPO1.
A. VPO1 expression in normal human plasma, and murine and bovine sera. Samples of human plasma, and murine and bovine sera were separated by SDS-PAGE and immunoblotted with anti-VPO1 antibody. B. Plasma and serum VPO1 concentration. VPO1 concentration was determined in 21 plasma samples from human healthy donors and 12 serum samples from BALB/c mice using Western blotting and densitometry analysis as described in Materials and Methods. The average of each sample is plotted. The average concentration + SD (standard deviation) of VPO1 in human plasma and murine serum is also indicated. C. VPO1 expression in endothelial cells. HUVECs were biosynthetically radiolabelled with [35S]-methionine, as detailed in Materials and Methods, in the absence (−) or presence (+) of NaBu, hematin, or both. Culture supernatants were immunoprecipitated with VPO1 antibody, and immunoprecipitates separated by SDS-PAGE and visualized by autoradiography of the dried gels. For comparison, media conditioned by HEK293 cells stably expressing VPO1 (GJ-4) was analyzed in parallel as a positive control. The data are the representative of five independent experiments. D. Detection of VPO1 mRNA expression induced by LPS and TNF-α. HUVECs were stimulated by LPS (100 ng/ml) or TNF-α (2 ng/ml) at indicated hours. VPO1 expression was determined by quantitative PCR as detailed in Materials and Methods (n=3). E. Detection of VPO1 protein expression induced by LPS and TNF-α. HUVECs were stimulated exactly as in D. Cell lysates were subjected to immunoblotting by using anti-VPO1 antibody and anti-β-Tubulin antibody (as loading control), respectively. C is control without addition of LPS or TNF-α. F. Detection of secreted VPO1 induced by LPS and TNF-α. HUVECs were stimulated by LPS (100 ng/ml and 500 ng/ml) or TNF-α (2 ng/ml) and radiolabelled as in C. Culture supernatants were harvested at indicated hours and immunoprecipitated with VPO1 antibody. The immunoprecipitates were separated by SDS-PAGE and visualized by autoradiography of the dried gels. Upper panel indicates densitometry of the secreted VPO1 related to 0-hr control based on autoradiography at lower panel.
Although we previously identified VPO1 mRNA in multiple tissues, including heart, lung, liver, pancreas, spleen, testis, thyroid and mouse embryonic tissues, we detected VPO1 protein only in mouse heart and cultured rat neonatal cardiomyocytes (H9c2 cells) as well as vessel walls [5]. Given the constitutive secretion of rVPO1 from HEK293 cells and the detection of VPO1 in plasma, the apparent contradiction in the distributions of mRNA and protein is likely related to the rapid secretion of the bulk of newly generated VPO1 from vascular sites.
Because of the relatively high expression of VPO1 in the cardiovascular system [5], we reasoned that VPO1 might be secreted normally into human plasma by vascular lining cells. To directly test this hypothesis, we investigated the endogenous expression of VPO1 in HUVECs using biosynthetic radioisotope labeling. Similar to GJ-4 cells, HUVECs synthesized and rapidly secreted VPO1 into the culture medium (Fig. 4C). However, in contrast to the VPO1 produced in the heterologous expression system, two isoforms of endogenous VPO1 were detected from endothelial cells. Although it is not known if the two isoforms reflect differential glycosylation or alternative N-terminal start sites for translation, normal human plasma also appears to contain two different sized proteins recognized by VPO1 antibody (Fig. 4A). It is also noteworthy that the presence of NaBu, the histone deacetylase inhibitor, hematin, or both agents did not influence expression of endogenous VPO1 by HUVECs (Fig. 4C), a behavior that contrasted with VPO1 expression in the HEK293 transfectants, where the construct is controlled by a CMV promoter and thus subject to modulation by hematin and NaBu.
To investigate if pro-inflammatory mediators regulate VPO1 expression in vascular endothelial cells, HUVECs were incubated with LPS (100 ng/ml) and TNF-α (2 ng/ml). Quantitative PCR demonstrated increased VPO1 mRNA 8–48 hrs post-treatment, peaking (~ 2 fold) at 8 hrs (Fig. 4D). Consistent with transcriptional upregulation, cell-associated VPO1 protein reached its peak at 16 hr following stimulation (Fig. 4E). Similar effects of LPS and TNF-α on secretion of newly synthesized VPO1 were seen and maximum effects were noted at 14.5 hr (Fig. 4F). In addition, higher amounts of LPS (500 ng/ml) did not further increased VPO1 expression or secretion beyond that seen with 100 ng/ml LPS (Fig. 4F). Thus, our data demonstrate that the pro-inflammatory stimuli, LPS and TNF-α, increase constitutive secretion of VPO1 by HUVECs.
Enzymatic properties of rVPO1
The native ferriheme UV-visible absorbance spectrum of purified His-tagged rVPO1, migrating as a single protein on SDS-PAGE, exhibited a sharp Soret maximum at 408 nm (Fig. 5A), similar to that reported for unmodified rVPO1 (~410 nm) and the Reinheit Zahl value (i.e. the ratio of absorbance at 408 nm to 280 nm) was 1.29. Peak peroxidase activity, as judged by oxidation of TMB, was in the pH range of 4.0–4.4 (Fig. 5B), a pH optimum slightly lower than that for TMB oxidation by MPO and EPO (pH 5.0 – 5.4) [25]. In comparing the thermal stability of MPO, LPO, and rVPO1, MPO was the most stable, showing no loss of activity at 92°C for 15 min (Fig. 5C) and consistent with previous studies that reported no loss of activity at 60°C for 1 hr [26] or only partial inactivation at 100°C for 30 min [27, 28]. Interestingly, whereas rVPO1 and bovine LPO (bLPO) lost nearly all activity at 92°C for 30 min, MPO remained ~60% of its original activity under the same experimental conditions. rVPO1 was relatively more heat stable than bLPO, maintaining >10% of activity up to 15 min. Taken together, these data demonstrate that the thermal stability of rVPO1 was intermediate between that of MPO and LPO.
Figure 5. Enzymatic properties of rVPO1.
A. UV-visible absorbance spectrum of human rVPO1. rVPO1 was purified as described in Materials and Methods. The oxidized spectrum of rVPO1 was recorded. B. Optimal pH of rVPO1 with TMB as substrate. C. Thermal stability of rVPO1, comparing with MPO and bLPO. The procedures are described in Materials and Methods. Data are the representative of three independent experiments.
Tyrosine cross-linking by VPO1
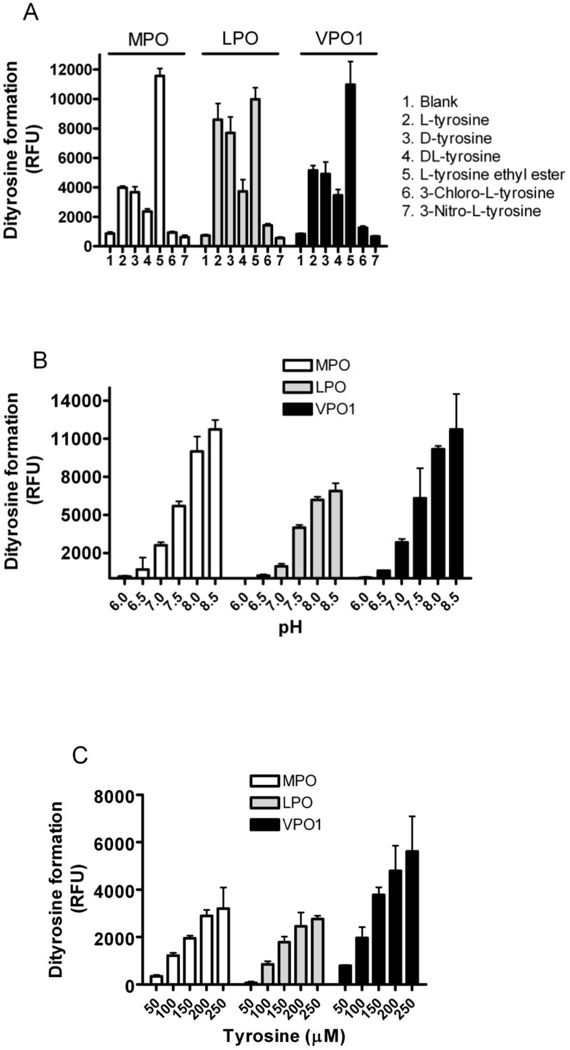
Since heme peroxidases catalyze the single electron oxidation of tyrosine to form tyrosyl radicals that in turn can cross-link to form dityrosine, we compared the capacity of rVPO1 to promote dityrosine formation with that of MPO and bLPO. Whereas MPO and bLPO were equally potent in catalyzing dityrosine formation from tyrosine ethyl ester, the specific activity of rVPO1 was less, requiring ~12.5 fold more enzyme on a molar basis (Fig. 6A). rVPO1 most readily oxidized tyrosine ethyl ester, with D- and L-tyrosine being much less susceptible to oxidation (Fig. 6A). Interestingly, the mixture of D- and L-tyrosine yielded ~65% dityrosine when compared to D- or L-isomer of tyrosine alone, suggesting that tyrosine with the same polar side group (either D-isomer or L-isomer) facilitated dityrosine catalysis by these heme peroxidases. Among the three peroxidases, bLPO demonstrated the most potent capacity on a molar basis to generate dityrosine from D- and L-isomer of tyrosine (Fig. 6A). Neither 3-chloro-L-tyrosine nor 3-nitro-L-tyrosine formed dityrosine cross-links (Fig. 6A), consistent with notion that tyrosyl bond formation occurs at the 3-position of tyrosine. Similar to other heme-containing peroxidases, rVPO1-mediated dityrosine formation was optimal under basic conditions; VPO1 produced approximately 150% of dityrosine at pH 8.5 in comparison to that at pH 7.5 (Fig. 6B). Dityrosine formation was dose-dependent at concentrations of tyrosine from 50–250 µM (Fig. 6C). Thus, our data demonstrate that rVPO1 catalyzed dityrosine formation and consequently may promote protein cross-linking in a variety of physiological and pathophysiological conditions.
Figure 6. Dityrosine formation by heme-containing peroxidases.
A. Formation of dityrosine from tyrosine and its derivates by MPO, bLPO and rVPO1. 100 µl reaction containing 50 mM phosphate buffer, pH 7.2, 0.25 mM tyrosine or its derivatives, 50 µM H2O2, and 4 nM MPO, 4 nM LPO or 50 nM rVPO1 was performed at 22 °C for 30 min. Relative fluorescence unit (RFU) was recorded as described in Materials and Methods. B. Effects of pH on dityrosine formation. The method is the same as in A except the pH as indicated. C. Dose-dependent formation of dityrosine. The method is the same as in A with the tyrosine concentration as indicated. Data are representative of three independent experiments and standard deviation is shown.
Discussion
We previously identified VPO1, the human homolog of the insect protein peroxidasin, as a member of the peroxidase-cyclooxygenase superfamily of proteins [5]. All members of the peroxidase-cyclooxygenase superfamily have their essential heme covalently linked to the protein backbone by ester bonds [2], a posttranslational modification due to autooxidation occurring during their biosynthesis [29, 30]. Although we identified VPO1 mRNA expression by RT-PCR in cardiovascular tissue, lung, liver, pancreas, spleen, testis and thyroid, we were unable to detect significant amounts of VPO1 protein in many of the tissues examined and noted only weak immunofluorescence in endothelial cells, smooth muscle cells, and H9c2 cells. In this report, we demonstrate that VPO1 is an abundant peroxidase that circulates in normal human plasma due to its constitutive production and rapid secretion from vascular endothelial cells.
Analysis of the predicted amino acid sequence of VPO1 suggests the presence of a 26 amino acid signal peptide, indicating that VPO1 enters the secretory pathway during its biosynthesis. Our data now demonstrate that VPO1, either as a recombinant protein expressed heterologously in HEK293 cells or as the endogenous product of vascular endothelial cells was synthesized and constitutively secreted into the culture medium. Consistent with this behavior in ex vivo cell culture systems, both normal human plasma and murine serum contain micromolar amounts of VPO1 (Fig. 4B). Immunochemical evidence suggesting the presence of MPO-related proteins in normal plasma has been reported previously [31], although the individual species responsible for immunoreactivity have not been well defined. For example, in a recent prospective study of more than 25,000 individuals in a community in the United Kingdom [32], serum levels of MPO-related protein, measured by ELISA, ranged from 454 to 951 pM. We estimate that the amount of VPO1 in human plasma may be ~ four orders of magnitude higher than that of MPO. This suggests that the relative contributions of the peroxidative actions of other non-MPO peroxidases may be underestimated and further studies of the roles of these proteins in normal physiology and disease states are needed. Interestingly, transcription, biosynthesis, and secretion of VPO1 from vascular endothelial cells was induced by the pro-inflammatory mediators, LPS and TNF-α (Fig. 4), providing a potential role for VPO1 in inflammatory diseases of the vascular system.
Whereas most of the newly synthesized proMPO undergoes proteolytic processing and targeting to the azurophil granule to be held in reserve for potential release later in response to secretogogues, our studies indicate that all of VPO1 synthesized by vascular cells was constitutively secreted. The glycosylation profile of VPO1 resembled that of secreted proMPO [22], in that both possessed complex N-linked oligosaccharides, as demonstrated by partial resistance to digestion with endoglycosidase H. The molecular organization of VPO1 and secreted proMPO was similar as well, as both were monomers. The VPO1 homologue in Drosophila, peroxidasin, exists as a homotrimer; thus, the monomeric nature of VPO1 represents a feature of human, but not insect homolog, although the functional consequences of the two distinct macromolecular species have not been explored.
The biologic functions that are served by circulating VPO1 in human plasma are not known. In general, peroxidases catalyze the oxidation of H2O2 to form Compound I, which in turn catalyzes oxidation of many biological substrates with the concomitant reduction of H2O2 and generation of water [1]. In the case of MPO, its best characterized biological activity occurs in the phagosome of neutrophils, where H2O2 formed from the phagocytic NADPH oxidase readily provides the substrate for generation of HOCl and other reactive species that are toxic to bacteria within the phagosome [3]. Despite the oxidant flux in activated phagocytes that can generate superoxide anion concentrations of ~25 µM, the steady state level of H2O2 is only ~2 µM in the phagosome, likely due to diffusion out of the phagosome or consumption by its rapid and efficient reaction with millimolar amounts of MPO [33, 34]. Indeed, in the absence of MPO, phagosomal concentrations of H2O2 can reach ~30 µM, as in the neutrophils of MPO-deficient subjects [34]. Unexpectedly, the ambient levels of H2O2 in normal plasma are on the same order of magnitude as that in normal phagosomes. For example, a study of 236 family members related to individuals with hypertension [35], the plasma levels of H2O2 measured electrochemically ranged from 1 to 5.6 µM. It is likely that plasma concentrations of H2O2 are influenced by peroxidative actions of circulating peroxidases, including VPO1.
VPO1 possesses structural features not present in MPO that may contribute to its capacity to localize in specific vascular sites and support targeted biological modifications of substrates. The N-terminus of VPO1 includes five leucine-rich repeats (LRRs) and four immunoglobulin C-2 type (Ig C-2) domains, both motifs that have been implicated as supporting protein-protein interactions [36]. Peroxidasin, a VPO1 homolog in Drosophila, is highly expressed in hemocyte, gastric cecae, fat body and oocyte, and is secreted into extracellular spaces [24], where it has been associated with dityrosine cross-linking of extracellular matrix proteins, basement membrane and chorionic membranes during development [24]. Whether mammalian heme peroxidases contribute to dityrosine cross-linking in the biosynthesis of extracellular matrices of the vascular wall is largely unknown, although our data demonstrate the capacity of VPO1 to promote such modifications.
Acknowledgements
This work was supported by the National Heart, Blood and Lung Institute at the National Institutes of Health grant R01 HL086836 and the American Heart Association (AHA) grant 0635122N (to G.C). The Nauseef lab is supported by National Institutes of Health grant AI70958 and AI044642 (WMN), a Merit Review Grant from the Veterans Administration, and with resources and use of facilities at the Iowa City Department of Veterans Affairs Medical Center, Iowa City, IA 52246. V.J.T was supported by the National Heart, Blood and Lung Institute at the National Institutes of Health grant grants HL067967 and HL094230.
Abbreviations
- VPO1
vascular peroxidase 1
- MPO
myeloperoxidase
- EPO
eosinophil peroxidase
- LPO
lactoperoxidase
- TPO
thyroid peroxidase
- HEK293
human embryonic kidney 293 cell line
- TMB
3,5,5’-tetramethylbenzodine
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PBS
phosphate buffered saline
- RT-PCR
reverse transcription polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none declared
References
- 1.Battistuzzi G, Bellei M, Bortolotti CA, Sola M. Redox properties of heme peroxidases. Arch Biochem Biophys. 2010;500:21–36. doi: 10.1016/j.abb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Zamocky M, Jakopitsch C, Furtmuller PG, Dunand C, Obinger C. The peroxidase-cyclooxygenase superfamily: Reconstructed evolution of critical enzymes of the innate immune system. Proteins. 2008;72:589–605. doi: 10.1002/prot.21950. [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 5.Cheng G, Salerno JC, Cao Z, Pagano PJ, Lambeth JD. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic Biol Med. 2008;45:1682–1694. doi: 10.1016/j.freeradbiomed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353–359. doi: 10.1097/00041433-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 7.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR-/- mice. Arterioscler Thromb Vasc Biol. 2008;28:84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 8.Nicholls SJ, Hazen SL. Myeloperoxidase, modified lipoproteins, and atherogenesis. J Lipid Res. 2009;50(Suppl):S346–S351. doi: 10.1194/jlr.R800086-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 10.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr Opin Cardiol. 2006;21:322–328. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 11.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 12.Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368:404–418. doi: 10.1016/S0140-6736(06)69114-9. [DOI] [PubMed] [Google Scholar]
- 13.Erdbrugger U, Hellmark T, Bunch DO, Alcorta DA, Jennette JC, Falk RJ, Nachman PH. Mapping of myeloperoxidase epitopes recognized by MPO-ANCA using human-mouse MPO chimers. Kidney Int. 2006;69:1799–1805. doi: 10.1038/sj.ki.5000354. [DOI] [PubMed] [Google Scholar]
- 14.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 15.Nauseef WM, Root RK, Malech HL. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest. 1983;71:1297–1307. doi: 10.1172/JCI110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goedken M, McCormick S, Leidal KG, Suzuki K, Kameoka Y, Astern JM, Huang M, Cherkasov A, Nauseef WM. Impact of two novel mutations on the structure and function of human myeloperoxidase. J Biol Chem. 2007;282:27994–28003. doi: 10.1074/jbc.M701984200. [DOI] [PubMed] [Google Scholar]
- 17.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 18.Yamada M, Hur SJ, Toda H. Isolation and characterization of extracellular myeloperoxidase precursor in HL-60 cell cultures. Biochem Biophys Res Commun. 1990;166:852–859. doi: 10.1016/0006-291x(90)90888-t. [DOI] [PubMed] [Google Scholar]
- 19.Nauseef WM. Myeloperoxidase biosynthesis by a human promyelocytic leukemia cell line: insight into myeloperoxidase deficiency. Blood. 1986;67:865–872. [PubMed] [Google Scholar]
- 20.Olsson I, Persson AM, Stromberg K. Biosynthesis, transport and processing of myeloperoxidase in the human leukaemic promyelocytic cell line HL-60 and normal marrow cells. Biochem J. 1984;223:911–920. doi: 10.1042/bj2230911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnljots K, Olsson I. Myeloperoxidase precursors incorporate heme. J Biol Chem. 1987;262:10430–10433. [PubMed] [Google Scholar]
- 22.Andersson E, Hellman L, Gullberg U, Olsson I. The role of the propeptide for processing and sorting of human myeloperoxidase. J Biol Chem. 1998;273:4747–4753. doi: 10.1074/jbc.273.8.4747. [DOI] [PubMed] [Google Scholar]
- 23.Fiedler TJ, Davey CA, Fenna RE. X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8 A resolution. J Biol Chem. 2000;275:11964–11971. doi: 10.1074/jbc.275.16.11964. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. Embo J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozeman PM, Learn DB, Thomas EL. Assay of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. J Immunol Methods. 1990;126:125–133. doi: 10.1016/0022-1759(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 26.Moguilevsky N, Garcia-Quintana L, Jacquet A, Tournay C, Fabry L, Pierard L, Bollen A. Structural and biological properties of human recombinant myeloperoxidase produced by Chinese hamster ovary cell lines. Eur J Biochem. 1991;197:605–614. doi: 10.1111/j.1432-1033.1991.tb15950.x. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch JG. Further studies on preparation and properties of phagocytin. J Exp Med. 1960;111:323–337. doi: 10.1084/jem.111.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee S, Stampler J, Furtmuller PG, Obinger C. Conformational and thermal stability of mature dimeric human myeloperoxidase and a recombinant monomeric form from CHO cells. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbapap.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 29.DePillis GD, Ozaki S, Kuo JM, Maltby DA, Ortiz de Montellano PR. Autocatalytic processing of heme by lactoperoxidase produces the native protein-bound prosthetic group. J Biol Chem. 1997;272:8857–8860. doi: 10.1074/jbc.272.14.8857. [DOI] [PubMed] [Google Scholar]
- 30.Colas C, Ortiz de Montellano PR. Autocatalytic radical reactions in physiological prosthetic heme modification. Chem Rev. 2003;103:2305–2332. doi: 10.1021/cr0204303. [DOI] [PubMed] [Google Scholar]
- 31.Olsen RL, Steigen TK, Holm T, Little C. Molecular forms of myeloperoxidase in human plasma. Biochem J. 1986;237:559–565. doi: 10.1042/bj2370559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50:159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Winterbourn CC, Garcia RC, Segal AW. Production of the superoxide adduct of myeloperoxidase (compound III) by stimulated human neutrophils and its reactivity with hydrogen peroxide and chloride. Biochem J. 1985;228:583–592. doi: 10.1042/bj2280583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 35.Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension. 2000;36:878–884. doi: 10.1161/01.hyp.36.5.878. [DOI] [PubMed] [Google Scholar]
- 36.Enkhbayar P, Kamiya M, Osaki M, Matsumoto T, Matsushima N. Structural principles of leucine-rich repeat (LRR) proteins. Proteins. 2004;54:394–403. doi: 10.1002/prot.10605. [DOI] [PubMed] [Google Scholar]