Abstract
n-3 Polyunsaturated fatty acids (PUFA) are increasingly consumed as food additives and supplements; however, the side effects of these fatty acids, especially at high doses, remain unclear. We previously discovered a high fat n-3 PUFA diet made of fish/flaxseed oils promoted significant weight gain in C57BL/6 mice, relative to a control, without changes in food consumption. Therefore, here we tested the effects of feeding mice high fat (HF) and low fat (LF) n-3 PUFA diets, relative to a purified control diet (CD), on locomotor activity using metabolic cages. Relative to CD, the HF n-3 PUFA diet, but not the LF n-3 PUFA diet, dramatically reduced ambulatory, rearing, and running wheel activities. Furthermore, the HF n-3 PUFA diet lowered the respiratory exchange ratio. The data suggest mixed fish/flaxseed oil diets at high doses could exert some negative side effects and likely have limited therapeutic applications.
Keywords: Fish oil, Flaxseed oil, Body weight
1. Introduction
n-3 Polyunsaturated fatty acids (PUFAs), the bioactive components of fish and flaxseed oil, are routinely consumed by the public as food additives or supplements. They are also recognized to have utility for treating metabolic and inflammatory diseases [1–5]. Generally, dietary studies evaluating n-3 PUFA efficacy in differing model systems have relied on low doses of n-3 PUFAs. With animal studies, investigators have commonly used ~5% (weight/weight) fish or flaxseed oil as intervention, corresponding to approximately 2–6% of total energy as n-3 PUFAs [6]. This dose, especially of fish oil, is often selected to model n-3 PUFA intake of Greenland Eskimos that consume n-3 PUFAs in the range of 1–6% of total energy [7,8]. A few studies have tested the effects of fish or flaxseed oils at higher doses with mixed results on functional endpoints [9–11]. Overall, very little is known about the effects of high doses of n-3 PUFAs, which could have a unique therapeutic niche or could exert negative side effects, raising potential safety issues for the general public.
We previously reported long-term administration of high levels of a mixed fish/flaxseed oil diet to C57BL/6 mice promoted significant body weight gain [12]. These findings suggested high doses of n-3 PUFAs increased body weight by lowering activity since we ruled out changes in food consumption [12]. Therefore, the objective of this study was to determine if short-term dietary consumption of a high dose of n-3 PUFAs could lower energy expenditure prior to any large differences in body weight. Studies were conducted in comparison to a low fat (LF) purified mouse control diet (CD) and a LF n-3 PUFA diet.
2. Experimental methods
2.1. Mice and diets
All experiments with mice fulfilled guidelines established by the East Carolina University Brody School of Medicine for euthanasia and humane treatment. Male C57BL/6 mice (~4–6 weeks old) were placed for 3 weeks on two experimental diets, developed in collaboration with Harlan-Laboratories (Madison, WI). Mice were administered either a purified control diet (CD), 5% fat by weight, a LF n-3 PUFA diet, or a HF n-3 PUFA diet, 20% fat by weight, as previously described [13]. The composition of the diets is listed in Table 1.
Table 1.
Composition of control, low fat, and high fat n-3 PUFA diets.
| Formula (g/Kg) | CD | LF n-3 PUFA | HF n-3 PUFA |
|---|---|---|---|
| Casein | 185 | 185 | 220 |
| L-Cystine | 2.5 | 2.5 | 3.0 |
| Corn Starch | 370 | 370 | 174 |
| Mineral Mix, AIN-93M-MX (94049) | 35 | 35 | 42 |
| Vitamin Mix, AIN-93-VX (94047) | 15 | 15 | 18 |
| Choline Bitartrate | 2.5 | 2.5 | 3.0 |
| TBHQ, antioxidant | 0.02 | 0.02 | 0.06 |
| Maltodextrin | 140 | 140 | 140 |
| Sucrose | 150 | 150 | 150 |
| Cellulose | 50 | 50 | 50 |
| Soybean Oil | 50 | 3.8 | 15 |
| Flaxseed Oil | - | 23.1 | 92.5 |
| Fish Oil | - | 23.1 | 92.5 |
2.2. Metabolic cage studies
Mice were placed in fully automated metabolic cages (TSE Systems) for 4 days to monitor activity during week 2. Mice were placed one per cage and were acclimated for 48 h followed by data collection for 48 h. Airflow through the cages was held constant at 0.5 L/min. 12 h light and dark cycles were maintained with ad libitum access to food and water. Locomotor activity in the metabolic cages was measured by the breaking of 32 infrared laser beams that span each cage in the xy and yz planes. TSE LabMaster software recorded in 20 min intervals each time a series of laser beams were broken by ambulatory, rearing, and running wheel activity. Metabolic activity was measured via indirect calorimetry recording maximal O2 consumption (VO2) and CO2 production (VCO2). VO2 and VCO2 values were normalized by the software to body weight in kilograms and are reported as ml/h/kg [14]. Respiratory exchange ratio (RER) was calculated as VCO2/VO2. The data were analyzed for average light and dark activity [14]. Significance was established using a two-way ANOVA followed by a Bonferroni Multiple Comparison t test using GraphPad Prism [14]. P values less than 0.05 were considered significant.
3. Results
3.1. Body weight and food consumption
Mice weighing 16–19 g were placed on diets. Final body weight gain, confirmed with measurements of adipose mass with Echo-MRI, was identical between the CD and the experimental diets (data not shown). Mice on the HF n-3 PUFA diet consumed less food relative to CD; however, there was no difference in the total kcal consumed between the differing diets relative to CD (data not shown).
3.2. Metabolic cage studies
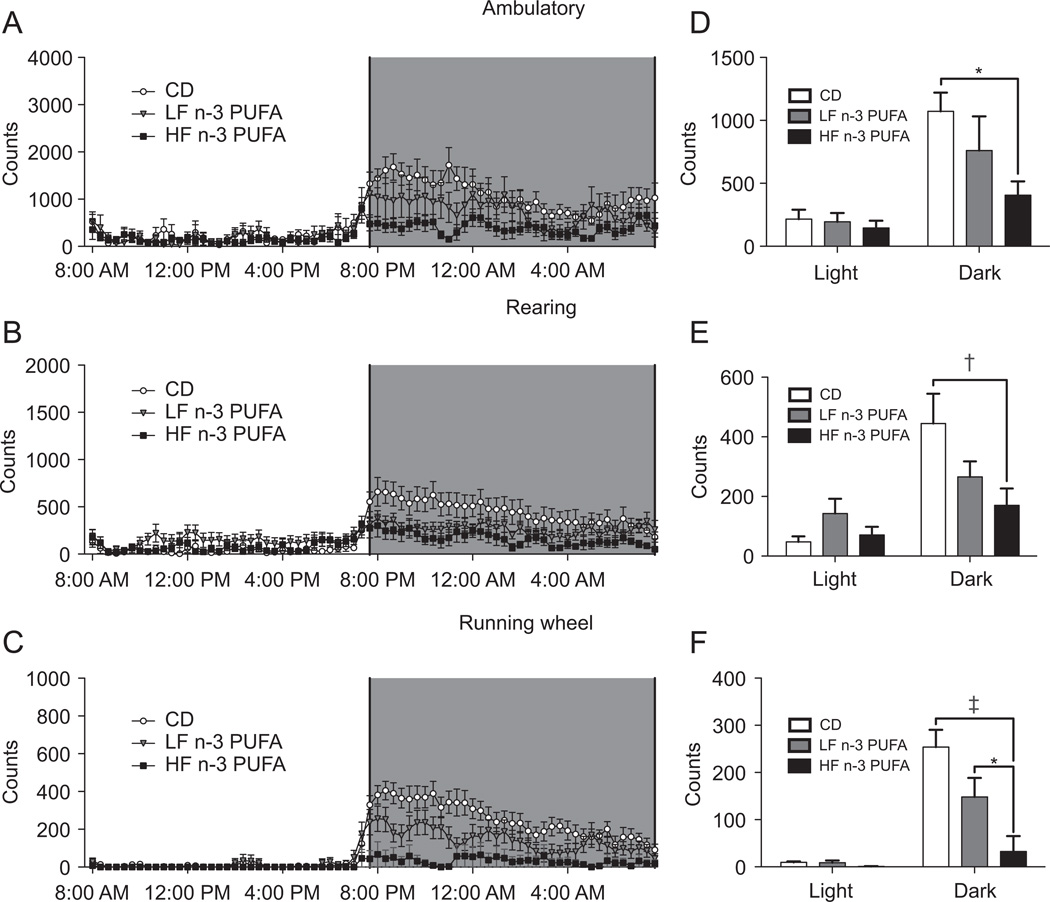
The time course of ambulatory, rearing, and running wheel activities are shown in Fig. 1A–C. These data were used to calculate the average activity for the entire light and dark cycles (Fig. 1D–F). There was a significant increase in activity during the dark cycle with all of the diets relative to the light cycle for all measurements (Fig. 1D–F).
Fig. 1.
HF n-3 PUFA diet lowered activity. (A) Ambulatory, (B) rearing, and (C) running wheel activities as a function of time for mice fed differing diets. Corresponding average light and dark cycle (D) ambulatory, (E) rearing and (F) running wheel activities. Counts represent the number of laser beams broken due to movement in the cage. Data are mean ± SEM, n = 7 mice per diet. Significance during the dark cycle compared to CD is indicated by *P < 0.05, †P < 0.01, ‡P < 0.001.
There were no statistical differences between the diets during the light cycle. The HF n-3 PUFA diet lowered average dark cycle ambulatory activity by 62% relative to CD (Fig. 1D). The LF n-3 PUFA diet had no significant effect compared to CD. Rearing activity was significantly lowered with the HF n-3 PUFA diet by 63% compared to CD (Fig. 1E). The LF n-3 PUFA diets had no effect on rearing activity relative to CD. For the ambulatory and rearing measurements of activity, there were no significant differences in activity between the LF n-3 PUFA and HF n-3 PUFA diets. Running wheel activity was lowered with the HF n-3 PUFA diet relative to CD by 87%, and 78% relative to the LF n-3 PUFA (Fig. 1F). The LF n-3 PUFA diet had no effect on running wheel activity relative to CD.
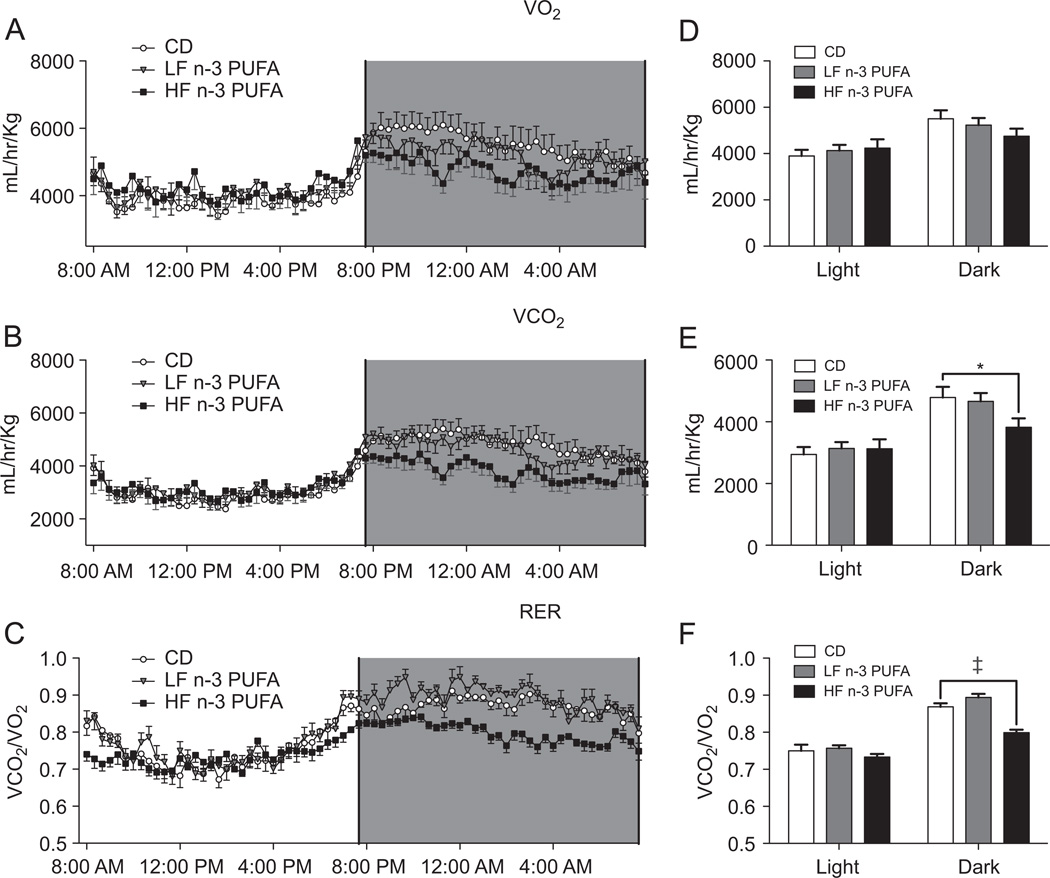
The time course of VO2, VCO2, and RER values is shown in Fig. 2A–C. These data were then used to calculate the average VO2, VCO2, and RER for the entire light and dark cycles (Fig. 2D–F). The average dark cycle VO2 (Fig. 2D) and VCO2 (Fig. 2E) were significantly higher for all of the diets relative to the light cycle. There were no differences between the diets on VO2. The HF n-3 PUFA diet lowered dark cycle VCO2 by 20% relative to CD and LF n-3 PUFA. RER values were significantly different for all of the diets between the light and dark cycles (Fig. 2F). During the dark cycle, the HF n-3 PUFA had lower RER values relative to CD and LF n-3 PUFA.
Fig. 2.
HF n-3 PUFA diet lowered VCO2 and RER. (A) VO2, (B) VCO2, and (C) RER as a function of time for mice fed differing diets for 3 weeks. Corresponding average light and dark cycle (D) VO2, (E) VCO2, and (F) RER. VO2 and VCO2 are ml of gas consumed or expelled per hour normalized to body weight. Data are mean ± SEM, n = 7 mice per diet. Significance during the dark cycle compared to CD is indicated by *P < 0.05, ‡P < 0.001.
4. Discussion
We present evidence for the first time that a high dose of n-3 PUFAs lowered mouse activity. The reduction in activity with the HF n-3 PUFA diet was consistent with our previous study demonstrating this diet promoted significant body weight gain in mice [12]. The LF n-3 PUFA diet also had a tendency to reduce energy expenditure in some of the measurements. This raises the question of whether mixing fish and flaxseed oils has some potential effect that does not promote body weight loss and perhaps even increase body weight gain after long-term feeding due to a reduction in activity. More studies are needed to address this in addition to determining the effects of other fat sources (i.e. hydrogenated oils, coconut oil) on activity. Overall, the reduction in all three measures of activity (ambulatory, rearing, and running wheel) appeared dose dependent with the n-3 PUFA diets.
It is difficult to directly compare our data to other studies since there are very few studies in this area, which are not in complete agreement. For instance, an EPA enriched oral food supplement provided to advanced pancreatic cancer patients, who are prone to losing weight, enhanced energy expenditure, and provided some improvement in their metabolism [15]. However, in another set of studies, supplementing the diet of healthy males with fish or flaxseed oil had no effect on energy expenditure [16,17]. Clearly, more studies are needed in this area with n-3 PUFAs in both animals and humans.
Overall, the potential therapeutic value of high n-3 PUFA doses appears very limited. It is interesting that despite lowering activity, the HF n-3 PUFA diet reduced serum triglycerides (data not shown). Therefore, the data suggest high doses of n-3 PUFAs are effective in their accepted role of lowering triglycerides (TG) even when they lower activity. There are a few cases where an increase in body fat mass due to lower activity could have some value. For instance, HIV positive patients can have elevated TGs and high energy expenditure, yet low body weight, which are in part driven by aggressive anti-viral therapy [18,19]. The elevated TGs are a risk factor for cardiovascular disease, which is prevalent in HIV positive patients [20]. Perhaps short-term intervention with a high dose of fish/flaxseed oils would assist in lowering TGs and lowering energy expenditure. Of course, extensive functional and mechanistic studies in animals and eventually humans would be required to address at what specific dose, composition of n-3 PUFAs, and duration could these fatty acids be utilized for these cohorts.
Acknowledgments
We thank Barbara Mickelson who assisted in designing the diets.
The research was supported by a grant from the NIH (R15AT006152) to S.R.S.
Footnotes
Conflict of interest
Author disclosures: B.D. Rockett, M. Harris, and S.R. Shaikh, no conflicts of interest.
Author contributions
B.D.R. designed the study, collected and analyzed data, wrote the manuscript, M.H. participated in data collection and analysis and S.R.S. assisted in writing and was responsible for the entire project.
References
- 1.Fetterman JW, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 2009;66:1169–1179. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 2.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 3.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann. Nutr. Metab. 2009;55:123–139. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- 4.Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hyper triglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Hoy SM, Keating GM. Omega-3 ethylester concentrate: a review of its use in secondary prevention post-myocardial infarction and the treatment of hyper triglyceridaemia. Drugs. 2009;69:1077–1105. doi: 10.2165/00003495-200969080-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kim W, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids—physiological relevance of dose. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:155–158. doi: 10.1016/j.plefa.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang HO, Dyerberg J, Sinclair HM. The composition of the eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 8.Dyerberg J, Bang HO, Hjorne N. Fatty acid composition of the plasma lipids in Greenland eskimos. Am. J. Clin. Nutr. 1975;28:958–966. doi: 10.1093/ajcn/28.9.958. [DOI] [PubMed] [Google Scholar]
- 9.Cunnane SC, Ganguli S, Menard C, et al. High alpha-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. British J. Nutr. 1993;69:443–453. doi: 10.1079/bjn19930046. [DOI] [PubMed] [Google Scholar]
- 10.Burns CP, Halabi S, Clamon G, et al. Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia. Cancer. 2004;101:370–378. doi: 10.1002/cncr.20362. [DOI] [PubMed] [Google Scholar]
- 11.Kremer JM, Lawrence DA, Petrillo GF, et al. Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal anti-inflammatory drugs. Clinical and immune correlates. Arthritis Rheum. 1995;38:1107–1114. doi: 10.1002/art.1780380813. [DOI] [PubMed] [Google Scholar]
- 12.Rockett BD, Salameh M, Carraway K, et al. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J. Lipid Res. 2010;51:1284–1297. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockett BD, Franklin A, Harris M, et al. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonraft organization in EL4 and B cells. J. Nutr. 2011;141:1041–1048. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J-Y, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber MD, McMillan DC, Preston T, et al. Metabolic response to feeding in weight-losing pancreatic cancer patients and its modulation by a fish-oil-enriched nutritional supplement. Clin. Sci. 2000;98:389–399. [PubMed] [Google Scholar]
- 16.Bortolotti M, Tappy L, Schneiter P. Fish oil supplementation does not alter energy efficiency in healthy males. Clin. Nutr. 2007;26:225–230. doi: 10.1016/j.clnu.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Jones PJH, Jew S, AbuMweis S. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metab. Clin. Exp. 2008;57:1198–1203. doi: 10.1016/j.metabol.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Macallan DC, Noble C, Baldwin C, et al. Energy expenditure and wasting in human immunodeficiency virus infection. N. Engl. J. Med. 1995;333:83–88. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]
- 19.Kosmiski LA, Kuritzkes DR, Lichtenstein KA, et al. Fat distribution and metabolic changes are strongly correlated and energy expenditure is increased in the HIV lipodystrophy syndrome. AIDS. 2001;15:1993–2000. doi: 10.1097/00002030-200110190-00012. [DOI] [PubMed] [Google Scholar]
- 20.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]