Abstract
We assessed immunoglobulin G (IgG) isotype responses with specificity for the variant surface antigens (VSA) of heterologous Plasmodium falciparum isolates by using flow cytometry and plasma from healthy Gabonese adults and from children during and after two consecutive malaria episodes. The individual isolate-specific antibody profiles differed markedly in terms of their isotype content but were similar for healthy adults and healthy uninfected children. In healthy adults, IgG3 and IgG2 responses were the highest, while in healthy children, IgG3 and IgG4 predominated. A transiently elevated IgG1 response was observed during the second of two successive malaria episodes in children, signaling P. falciparum infection-induced cross-reactive anti-VSA responses. Our findings highlight the prominence of IgG3 in the overall profile of these responses but also indicate a marked age-related increase in the prevalence of anti-VSA antibodies of the classically noncytophilic IgG2 isotype, possibly reflecting the high frequency of the histidine-131 variant of FcγRIIA in the Gabonese population.
The existence of clonally variant surface antigens (VSA) of Plasmodium falciparum that are inserted into the membranes of infected erythrocytes was first demonstrated 2 decades ago (25). A number of subsequent studies have shown that VSA comprise targets of antibody responses that are enhanced with age and are associated with protection from malaria (9, 11, 16, 20, 27, 38, 47, 55). Such associations have been reported in the context of responses to VSA expressed by both autologous and heterologous parasite isolates, which may contribute to the putatively rapid acquisition of immunity to malaria (24). P. falciparum erythrocyte membrane protein-1 (PfEMP-1) is considered to be the principal target of anti-VSA antibodies, although rifin proteins, a second, polymorphic, parasite-derived family of antigens that are inserted into the infected erythrocyte membrane, also induce antibody responses in exposed populations (2, 7, 31, 32, 42). Studies incorporating longitudinal components have shown that, in most but not all cases, antibodies with specificity for the VSA expressed by the autologous parasites causing a given malaria attack are infrequent or absent prior to the attack but are enhanced and sustained posttreatment (10, 13, 21, 26, 37, 46). In contrast, the profile of antibody responses to the VSA expressed by heterologous parasite isolates shows no such consistent pattern during and after a malaria attack, although the responses have been shown to be elevated in a proportion of individuals in all longitudinal studies reported to date (9, 13, 21, 26, 46). Subclinical pediatric P. falciparum infections are associated with raised levels of antibodies that interact with the VSA of heterologous isolates (9). Such findings, along with the known antibody-mediated recognition of VSA expressed by parasites from distant geographical regions, perhaps point to a predominance of variant specificity over cross-reactivity in these responses that is rather less marked than has been conjectured (3, 6, 43).
The cytophilic immunoglobulin G (IgG) isotypes, IgG1 and IgG3, are known to mediate the in vitro phagocytosis of P. falciparum-infected erythrocytes (23, 54), but studies incorporating measurements of IgG isotype-mediated responses directed to P. falciparum VSA are scarce. Piper and colleagues (50), by using flow cytometric techniques, reported a predominance of IgG1 antibodies with specificity for VSA of heterologous isolates in the sera of Papua New Guinean (semi-immune) adults, with smaller amounts of IgG3 and negligible amounts of IgG2 and IgG4. Kenyan children with uncomplicated malaria, on the other hand, display a predominantly IgG3-mediated antibody response to VSA of autologous isolates in parallel with the expected IgM response (27). In the study presented here, we investigated and compared the profiles of IgG isotype antibodies with specificity for the VSA expressed by a panel of locally collected heterologous parasite isolates from Gabonese adults and children. The latter comprised participants in an extended longitudinal study, thus allowing comparisons within and between groups that had differing initial clinical presentations as well as subsequent infection histories (33, 34).
MATERIALS AND METHODS
Study site.
A study, with the reference code 1/95-C, was initiated in 1995 at the Albert Schweitzer Hospital in Lambaréné, Gabon, a site in equatorial central Africa where malaria is hyperendemic on account of the perennial transmission of P. falciparum (59). The estimated annual entomologic inoculation rate for Lambaréné is about 50 infective bites/person/yeast (52).
Ethical clearance.
Ethical clearance for the study was given by the ethics committee of the International Foundation for the Albert Schweitzer Hospital in Lambaréné. Children were included in the study after informed consent was obtained from the parent or guardian.
Study design.
Details of the study design, patient enrollment, care, and treatment given have been described elsewhere (28, 29, 34). Briefly, 100 children presenting with severe malaria were admitted to the hospital, and an equal number presenting with mild malaria were included. The latter were pair matched to children with severe malaria by gender, age, and area of residence. Children were included in the severe-malaria group if they had a P. falciparum parasitemia of >1,000/μl, were older than 6 months, were not homozygous for hemoglobin S, had severe anemia (<5 g of hemoglobin/dl) and/or hyperparasitemia (>250,000 parasites/μl), and had or did not have other signs of severe malaria, for example, loss of consciousness, hypoglycemia, lactic acidosis, or respiratory distress (58). The level of consciousness was determined by using the Blantyre coma score (58). Mild malaria was defined as a parasitemia of between 1,000 and 50,000 parasites/μl on admission, no schizontemia, <50 circulating leukocytes containing malarial pigment/μl, >8 g of hemoglobin/dl, >50 platelets/nl, <12 leukocytes/nl, <3 mM lactate, and >50 mg of glucose/dl of blood. Exclusion criteria for the mild-malaria controls were signs of severe malaria, concomitant acute infection, prior hospitalization for any reason, and intake of antimalarial agents within the preceding week.
Plasma samples.
The plasma samples that were used were selected from those collected during the 1/95-C study from the children presenting with severe and mild malaria whose mean age (± standard error) was 47 (±3) months.
We focused on samples from four different time points: (i) the point of acute infection at admission (immediately prior to treatment), (ii) the first posttreatment reinfection, (iii) the healthy phase at least 6 months after admission (healthy I) when the child was aparasitemic for the preceding 6 weeks as confirmed by the examination of Giemsa-stained thick blood smears prepared during the routine fortnightly follow-up home visits undertaken as part of the study, and (iv) the healthy phase at least 24 months after inclusion (healthy II) when children were again known to be asymptomatic and aparasitemic through home visits and the examination of thick blood smears.
A panel of 21 positive-control plasma samples from clinically healthy Gabonese adults was tested in parallel. These donors were all over 18 years of age, were residents of Lambaréné, and consequently had had life-long continuous exposure to P. falciparum infection. They are thus considered representative of the semi-immune population in the study area. P. falciparum parasitemia in these individuals was not assessed but, if present, could be assumed to be minimal.
Screening for P. falciparum infection by the PfHRPII ELISA.
In order to verify the parasitological status of individuals at the healthy time points, the levels in plasma of the P. falciparum histidine-rich protein II (PfHRPII) antigen, present during or for a short period immediately following the termination of an active P. falciparum infection, were determined with a commercially available monoclonal antibody-based sandwich enzyme-linked immunosorbent assay (ELISA), which was used according to the manufacturer's instructions (Malaria AG Celisa; Cellabs, Brookevale, Australia).
Parasite isolates and culture.
A panel of six P. falciparum isolates was used. These were obtained from patients enrolled in a separate outpatient study conducted in 1997 at the Albert Schweitzer Hospital. Isolates with reference codes Cys002, Cys007, Cys030, and Cys035 were obtained from children presenting with severe malarial anemia, while isolates Cym030 and Cym033 were obtained from children presenting with mild malaria. All cases were confirmed monoinfections with P. falciparum, and all were shown by routine standardized merozoite surface antigen-based PCR genotyping techniques to be polyclonal, each with at least three different strains (C. Yone, unpublished observations). Details of the methods used for the collection and culture of parasites have been described elsewhere (55). Briefly, peripheral venous blood was centrifuged and the erythrocytes obtained were spin washed twice. Pellets containing infected erythrocytes were then cryopreserved in liquid nitrogen for subsequent in vitro adaptation. Primary isolates were adapted to in vitro culture according to the method of Trager and Jensen (56). Briefly, cells were resuspended in complete medium supplemented with 10% heat-treated, prescreened nonimmune AB+ serum (from the blood bank of the University Hospital, Tübingen, Germany) and were then incubated in an atmosphere of 5% CO2, 5% O2, and 90% N2. Fresh O+ erythrocytes depleted of lymphocytes (University Hospital, Tübingen, Germany) were periodically added. Isolates were initially expanded over a short period of 8 to 10 multiplication cycles (48 h), after which identical stabilates of cultures containing mostly asexual ring forms were cryopreserved for subsequent culture and use in cytometric assays (see below).
Flow cytometric measurement of IgG antibody isotype responses with specificity for P. falciparum-infected erythrocyte surfaces.
Details of the methods, including the flow cytometric (fluorescence-activated cell sorting) assay employed, have been described elsewhere (50, 55). The following additional procedures were used here: trophozoite-infected erythrocytes (T-IE) were washed in RPMI 1640 and resuspended in phosphate-buffered saline (PBS)-1% bovine serum albumin (BSA) at 10 to 15% parasitemia. Fifty microliters of the T-IE suspension was then transferred to round-bottomed 96-well tissue culture plates (Costar, Corning, N.Y.), and 50 μl of plasma samples previously diluted 1:50 in PBS-1% BSA were added to each well. Negative- and positive-control plasma samples were included in each assay, comprising, respectively, plasma samples from 31 European donors resident in Germany with no history of contact with Plasmodium species and plasma samples from 21 healthy African adults resident in Lambaréné. After 30 min of incubation at room temperature, the plates were spin washed three times with PBS-1% BSA at 1,000 rpm for 2 min. Fifty microliters of mouse anti-human IgG at a 1/100 dilution or mouse anti-human IgG1, IgG2, IgG3, or IgG4 (SkyBio, Wyboston, Bedford, United Kingdom) at a 1/50 dilution was added to the wells, and the plates were again incubated for 30 min at room temperature, followed by spin washing as described above. Finally, a fluorescein isothiocyanate-coupled goat anti-mouse IgG antibody (Southern Biotech, Birmingham, Ala.), containing 50 μg of ethidium bromide/ml, was added at a 1/100 dilution. Plates were incubated for a further 30 min and washed as described above. Samples were assayed by fluorescence-activated cell sorting immediately after the double staining with a FACScan (Becton Dickinson, Heidelberg, Germany) using CellQuest 3.3 software. An event was defined as the passage of one cell through the cytometer optical system. We counted 10,000 events per sample, and the geometric mean of the fluorescence intensities was calculated. The mean fluorescence intensity (MFI) was defined as the difference between the geometric mean of the fluorescence emitted by the T-IE and the geometric mean of the fluorescence emitted by the noninfected erythrocytes (background).
Statistical analyses.
Analyses were performed by using StatView for Windows 5.0.1 (SAS Institute Inc., Cary, N.C.) running on Windows XP (Microsoft Corp., Redmond, Wash.). Pairwise comparisons of continuous variables were performed with the nonparametric Wilcoxon sign rank test, and for unpaired comparisons the Mann-Whitney U test was applied. The level of significance was set at a two-tailed P value of <0.05.
RESULTS
Sample sizes.
Plasma samples from 21 healthy semi-immune Gabonese adults were used. From the cohort of Gabonese children, we analyzed a total of 57 plasma samples from children in the acute phase, 60 samples from children at the first reinfection, 58 samples from children at the first healthy phase, and 82 samples from children at the second healthy phase. The smaller numbers of individuals available for pairwise comparisons at the different time points are explained at the appropriate points in the following subsections.
Anti-VSA IgG antibody isotype responses of healthy Gabonese adults and children.
The results of the PfHRPII ELISA confirmed the results of microscopy, namely, that none of the children had active P. falciparum infections at either of the healthy-phase time points (data not shown). A typical example of the patterns of isotype-specific anti-VSA activity that we detected, expressed as MFIs, is given in Table 1, showing that the background level of binding to nonparasitized erythrocytes was frequently higher in samples from healthy children than in samples from adults.
TABLE 1.
Typical isotype-specific fluorescence intensity values obtained with plasma samples from healthy Gabonese children and adults and used for calculation of MFI values
| Cell type (dot plot quadrant)b | Fluorescence intensity
|
|||
|---|---|---|---|---|
| Healthy childa
|
Healthy adult
|
|||
| IgG1 | IgG3 | IgG1 | IgG3 | |
| Double-stained cells (upper right quadrant) | 54.25 | 57.05 | 23.14 | 55.53 |
| Nonparasitized stained cells (lower right quadrant) | 36.80 | 45.87 | 11.45 | 16.20 |
| Meanb | 17.45 | 11.18 | 11.69 | 39.33 |
Plasma samples were from the healthy II time point.
Cells were obtained from the upper and lower right quadrants of a dot plot obtained from flow cytometric determinations. Means are as defined in Materials and Methods.
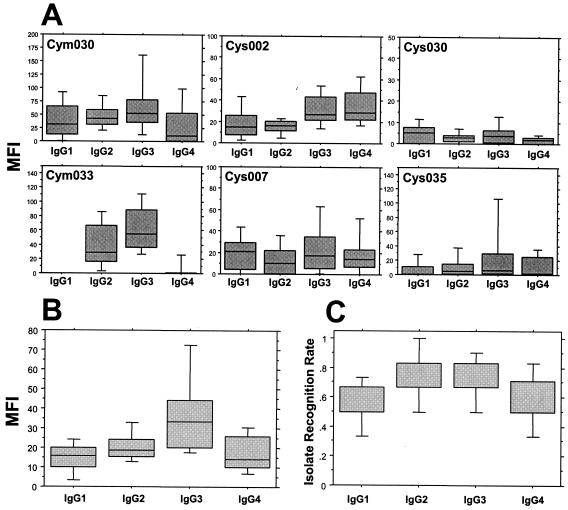
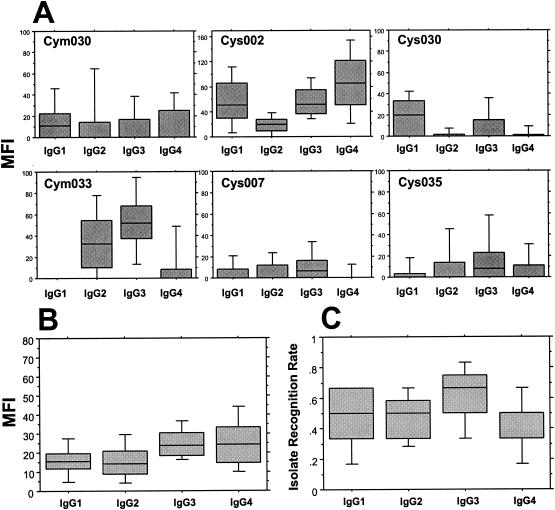
The IgG isotype profiles of anti-VSA antibody responses detected in plasma samples from healthy Gabonese adults and children (in this case, samples from healthy phase II individuals) are illustrated, respectively, in Fig. 1 and 2. The isolate-specific profiles showed no discernible pattern: IgG isotype responses to individual isolates varied both in the magnitude of anti-VSA antibodies of each isotype and in the relative predominance of any given isotype. For three of the parasite isolates tested (Cym030, Cym033, and Cys035), the highest level in adults was seen with IgG3 antibodies (Fig. 1A). In contrast, IgG1 antibodies were marginally the highest in adults' responses to two of the other parasite isolates (Cys007 and Cys030) but were undetectable in the response to isolate Cym033. The profiles seen with healthy children's samples were broadly similar to the adults', except for the relative predominance of IgG1 in the children's response to isolate Cym030 and a similarly predominant IgG4 response to isolate Cys002 (Fig. 2A).
FIG. 1.
Healthy semi-immune Gabonese adults' IgG isotype antibody responses to the VSA expressed by a panel of six heterologous P. falciparum isolates. (A) MFIs of individual isolate-specific profiles; (B) cumulated MFIs of isotype-specific responses; (C) cumulated isotype-specific IRRs. Box plots illustrate the medians with the 25th and 75th percentiles, and I bars indicate the 10th and 90th percentiles.
FIG. 2.
Healthy Gabonese childrens' IgG isotype antibody responses to the VSA expressed by a panel of six heterologous P. falciparum isolates. (A) MFIs of individual isolate-specific profiles; (B) cumulated MFIs of isotype-specific responses; (C) cumulated isotype-specific IRRs. Box plots illustrate medians with the 25th and 75th percentiles, and I bars indicate the 10th and 90th percentiles. Data are pooled from all children at the healthy II time point and without segregation according to malaria history, and the MFIs of nonresponders were considered to be 0.
Of particular interest in the adults' profile, the classically noncytophilic IgG2 and IgG4 anti-VSA responses were frequent and were notable for the fact that (i) the levels of IgG2 detected were equivalent to those of, for example, IgG1 and (ii) although IgG4 responses were for the most part the weakest, in the profile of responses to isolate Cys002, they were detected at a level equivalent to that of IgG3 and were markedly higher in this case than in that of either IgG1 or IgG2 (Fig. 1A).
The cumulated anti-VSA IgG isotype responses (medians of arithmetic means derived from pooled data) of healthy adults and children to all six isolates are shown in Fig. 1B and 2B. These pooled data show that, in healthy adults, antibodies of the IgG3 isotype predominate at a level significantly higher than those of the other isotypes (versus values for IgG1 or IgG4, P was <0.001, and versus values for IgG2, P was <0.005, as determined by the Wilcoxon rank test), with an overall hierarchy of the magnitude of anti-VSA responses as follows: IgG3 > IgG2 > IgG1 = IgG4 (Fig. 1B). In healthy children, there was no clear IgG isotype predominance: the levels of IgG3 and IgG4 were similar, and both were higher than the levels of either IgG1 or IgG2 (Fig. 2B).
In order to make qualitative comparisons of anti-VSA antibody responses, an isolate recognition rate (IRR) was determined. The IRR was based on an initial segregation into responder or nonresponder subgroups according to the thresholds determined for each isotype with each isolate (Table 2). The IRR was then defined as the proportion of isolates from the panel for which antibodies from a given individual showed specific binding above the threshold, as illustrated in Fig. 1C and 2C. The IRRs for IgG2 and IgG3 were similar in adults and were significantly higher than those for either IgG1 (P < 0.01) or IgG4 (P < 0.03) (Fig. 1C). In healthy children, the highest IRR (∼70%) was recorded for IgG3, with lower-level recognition (∼50%) for IgG1, IgG2, and IgG4 (Fig. 2C). Overall, the range of IRRs seen with adults' samples (60 to 80%) was higher than that seen with children's (50 to 70%).
TABLE 2.
Threshold values of MFIs of isolate-specific IgG isotype anti-VSA responses
| Isolatea | Threshold MFIa for:
|
|||
|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | |
| Cym030 | 5.5 | 8.8 | 9.1 | 8.0 |
| Cym033 | 4.4 | 1.9 | 6.2 | 2.6 |
| Cys002 | 0.0 | 3.9 | 7.9 | 8.8 |
| Cys007 | 10.5 | 10.9 | 5.1 | 6.4 |
| Cys030 | 4.6 | 0.0 | 6.1 | 0.4 |
| Cys035 | 0.0 | 9.5 | 6.5 | 3.2 |
Thresholds were defined as the geometric mean plus 2 standard deviations of the MFIs of responses of a panel of samples from nonexposed Germans.
Relative amounts of IgG isotype antibodies in healthy adults and children with specificity for VSA of heterologous P. falciparum isolates.
Since IgG3 was the predominant isotype detected in the adults' anti-VSA antibody profile, we used the magnitude of the IgG3 response as a reference value to determine ratios with respect to the other isotypes in order to make comparisons of their relative amounts in healthy adults and children, as illustrated in Table 3. These analyses showed that IgG3 predominated over the other anti-VSA isotypes in both adults and children but that the amounts of IgG3 relative to both IgG1 and IgG4 were significantly greater in adults than in children regardless of the latter's history of malaria. The IgG3/IgG2 ratios in adults and children were similar. The most marked difference observed concerned the IgG3/IgG4 ratio in children with a history of severe malaria, in whom the ratio was close to unity compared with the almost double ratio of IgG3 to IgG4 in adults. The different ratios observed in children did not differ significantly after segregation and comparison according to their history of malaria.
TABLE 3.
Comparison of the ratios of IgG isotype antibodies of healthy Gabonese adults and children with specificities for VSA of a panel of six heterologous P. falciparum isolates
| Isotypes compared | Populationb | Clinical group | Anti-VSA isotype ratio
|
P valuea | ||
|---|---|---|---|---|---|---|
| Median | 25th percentile | 75th percentile | ||||
| IgG3/IgG1 | Adults | 2.650 | 1.833 | 3.315 | ||
| Children | All | 1.605 | 1.018 | 2.228 | 0.002 | |
| Mild malaria | 1.427 | 1.001 | 2.228 | 0.002 | ||
| Severe malaria | 1.537 | 1.075 | 2.213 | 0.007 | ||
| IgG3/IgG2 | Adults | 1.630 | 0.968 | 2.553 | ||
| Children | All | 1.726 | 1.194 | 3.035 | NS | |
| Mild malaria | 1.684 | 1.325 | 2.978 | NS | ||
| Severe malaria | 1.911 | 1.096 | 3.116 | NS | ||
| IgG3/IgG4 | Adults | 1.890 | 1.345 | 4.012 | ||
| Children | All | 1.388 | 0.762 | 1.967 | 0.002 | |
| Mild malaria | 1.392 | 0.823 | 2.504 | 0.031 | ||
| Severe malaria | 1.160 | 0.732 | 1.676 | <0.001 | ||
Determined by the Mann-Whitney U test for differences between adults and children. NS, not significant.
Children's plasma samples were taken at the healthy II time point.
Infection-related and temporally related changes in the profile of anti-VSA IgG isotype responses in Gabonese children.
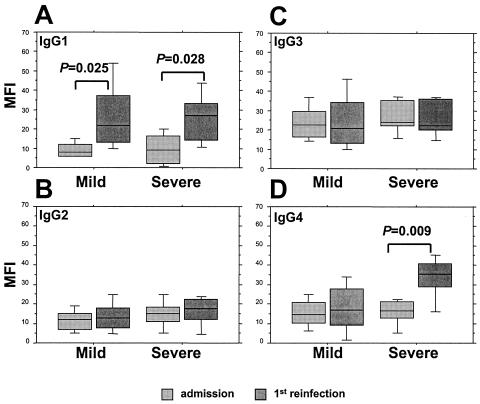
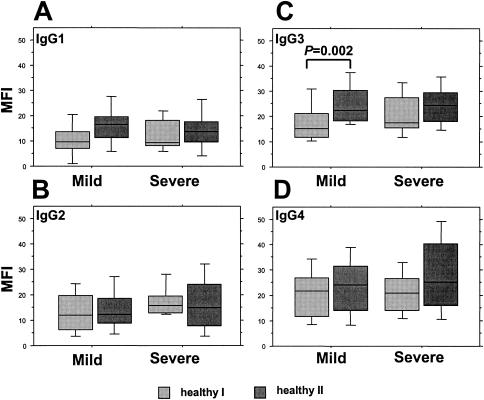
We measured anti-VSA IgG isotype responses in paired samples from a total of 18 individuals (8 in the mild- and 10 in the severe-malaria groups) at the admission and first reinfection time points and from a total of 33 individuals (18 in the mild- and 15 in the severe-malaria groups) at the healthy I and healthy II time points. The age at admission and the gender distribution of these subgroups did not differ from those of the entire group of children (data not shown). The median times to the first reinfection in those individuals with a history of mild and severe malaria were, respectively, 35 and 15 weeks (ranges, 4 to 53 weeks and 6 to 37 weeks). Their cumulated responses to all six heterologous isolates at the different infection and healthy time points are illustrated, respectively, in Fig. 3 and 4. At admission, the levels of all four isotypes in the two groups were similar, with IgG3 responses predominant (Fig. 3). At the first reinfection, the levels of IgG2 and IgG3 remained unchanged compared to their levels at admission (Fig. 3B and C), but the level of IgG1 showed a consistent and significant increase in both groups (Fig. 3A) and the level of IgG4 increased significantly at the first reinfection only in the severe-malaria group (Fig. 3D). A comparison of healthy-phase samples revealed a trend towards increased cytophilic-isotype (IgG1 and IgG3) responses in both groups over time but a significant increase only in IgG3 responses in the mild-malaria group (Fig. 4A and C). The noncytophilic IgG2 and IgG4 responses remained unchanged over time but with a trend towards enhanced IgG4 responses among those with a history of severe malaria (P was 0.069 in a comparison of values for healthy I- and healthy II-phase samples as determined by a Wilcoxon signed-rank test) (Fig. 4B and D). Of further interest, in both groups, the levels of IgG4 responses in healthy-phase samples were equivalent to (IgG3) or higher than (IgG1) those of the cytophilic isotypes at the same time points (Fig. 4A, B, and D). IgG4, furthermore, was the only isotype for which healthy-phase levels were noticeably higher than those recorded during acute infection (Fig. 3D and 4D).
FIG. 3.
Comparisons of the IgG isotype (IgG1[A], IgG2 [B], IgG3 [C], and IgG4 [D])-specific anti-VSA antibody profiles of matched groups of Gabonese children during two successive malaria episodes, segregated according to the severity of the admission episode. Box plots illustrate medians with the 25th and 75th percentiles, and I bars indicate the 10th and 90th percentiles, of cumulated MFIs of responses to the panel of six heterologous P. falciparum isolates.
FIG. 4.
Comparisons of the IgG isotype (IgG1 [A], IgG2 [B], IgG3 [C], and IgG4 [D])-specific anti-VSA antibody profiles of matched groups of Gabonese children at successive healthy-phase time points, segregated according to the severity of the admission episode. Box plots illustrate medians with the 25th and 75th percentiles, and I bars indicate the 10th and 90th percentiles, of cumulated MFIs of responses to the panel of six heterologous P. falciparum isolates.
Isolate recognition rates in Gabonese children.
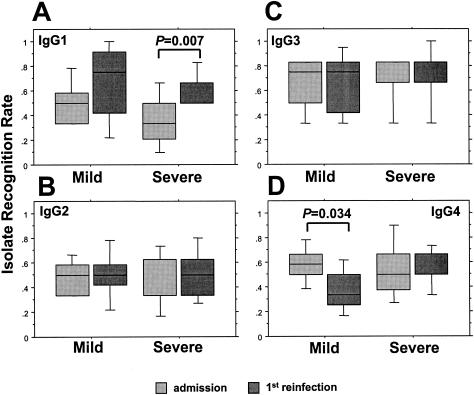
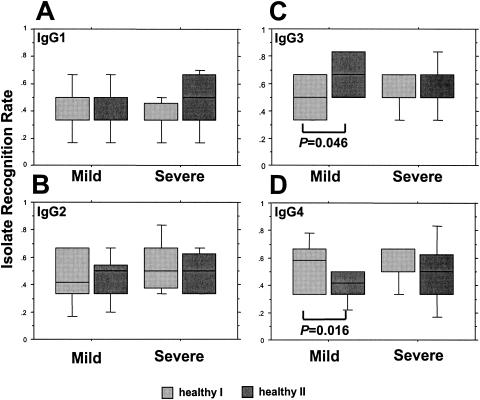
The IRRs at the different time points for children segregated according to clinical presentation are illustrated in Fig. 5 and 6. The IRRs mediated by IgG1 increased from admission to the first reinfection in both groups, significantly so in those with severe malaria (Fig. 5A). The levels of recognition mediated by either IgG2 or IgG3 were stable in both groups, but at the first reinfection assessment, IgG4-mediated recognition had declined significantly in the mild-malaria group from the level seen at admission (Fig. 5B-D). In healthy-phase samples, the only significant changes in IRRs concerned children with a history of mild malaria in whom IgG3-mediated recognition increased over the period of the study; a corresponding decline in IgG4-mediated recognition was observed (Fig. 6C and D).
FIG. 5.
The IgG isotype (IgG1 [A], IgG2 [B], IgG3 [C], and IgG4 [D])-specific IRRs of matched groups of Gabonese children during two successive malaria episodes, segregated according to the severity of the admission episode. Box plots illustrate medians with the 25th and 75th percentiles, and I bars indicate the 10th and 90th percentiles, of proportions of the panel of the six heterologous P. falciparum isolates that were recognized.
FIG. 6.
The IgG isotype (IgG1 [A], IgG2 [B], IgG3 [C], and IgG4 [D])-specific IRRs of matched groups of Gabonese children at successive healthy-phase time points, segregated according to the severity of the admission episode. Box plots illustrate medians with the 25th and 75th percentiles, and I bars indicate the 10th and 90th percentiles, of proportions of the panel of six heterologous P. falciparum isolates that were recognized.
DISCUSSION
This is the first study to report a detailed investigation, using large groups of African adults and children, of IgG antibody isotype responses with specificity for the polymorphic parasite VSA inserted into the membranes of infected erythrocytes. We assessed the activities directed to the VSA expressed by a panel of six locally collected heterologous parasite isolates, each known to be composed of several antigenically distinct parasite strains. These assessments were thus designed to address questions concerning changes in individuals' overall anti-VSA antibody isotype responses. They were also designed to serve as a measure of the ability of these individuals to mount such responses in order to enable comparisons, in the case of children, between groups of individuals who presented with malaria of differing levels of severity. These questions are somewhat distinct from those addressed by studies of individuals' responses to VSA expressed by homologous parasite isolates, i.e., by the parasites causing a given malaria episode.
In samples from both healthy adults and healthy children, the profiles of anti-VSA IgG responses to the panel of heterologous isolates that we used lacked any discernible isotype-specific pattern. We conclude that this lack of a pattern reflects the intrinsically polymorphic nature of VSA and hence the diversity of the potential B-cell epitopes that they may contain. The current consensus view is that PfEMP-1 is the principal target of the antibodies detected by the cytometric methods employed here, but the epitopic specificities of these antibodies can only be a matter of speculation, especially given that the isolates used comprise multiple strains, each of which could be assumed to be expressing distinct VSA. Another aspect of particular note concerns the detection of the anti-VSA antibodies of all four IgG isotypes, in the case of some isolates at very similar levels, which is consistent with an earlier report (23). The antibody response to crude parasite antigen preparations commonly comprises a mixture of all four IgG isotypes, but the response to defined P. falciparum asexual-stage antigens is usually more restricted and is dominated by cytophilic isotypes (4, 18, 41, 57). In the profile that we observed here, IgG3 antibodies did predominate, although they were equivalent to IgG4 in children in terms of magnitude and equivalent to IgG2 in adults in terms of the IRR. These findings contrast with the reported predominance of IgG1 in the profile observed in healthy Papua New Guinean adults (50). As a note of caution, that study reported results as percentages of positive cells rather than the MFIs that we used here, making direct comparisons invalid. The preferential induction of IgG3 responses to various P. falciparum asexual-stage antigens, including merozoite surface protein 1 (MSP-1), -2, and -3 and parasite glycosylphosphatidylinositols, is a well-described phenomenon (8, 12, 17, 45). This preference seems to be associated with the degree of polymorphism associated with the target antigen or epitope thereof, an observation with which in vitro antibody induction assays are largely concordant (19).
The prominence of IgG2 that we observed in the adults' anti-VSA antibody profile deserves further comment, since the role of IgG2 antibodies with specificity for plasmodial antigens remains a subject of some controversy. A recent study conducted in Burkina Faso showed an association between levels of parasite antigen-specific IgG2 and resistance to P. falciparum, which the authors attributed to the high prevalence in the study population of a polymorphism in the monocytes' FcγRIIA that results in an abnormally high affinity for Fcγ2 (5). This finding implies an influence on IgG isotype switching that allows for the preferential induction of protective IgG2 antibody responses to plasmodial antigens in these individuals. Protective effects have also been attributed to maternally transmitted parasite antigen-specific IgG2 responses in Cameroonian infants, in whom they were associated with a reduced risk of P. falciparum infection (14). Conversely, antiparasitic IgG2 responses were associated with a higher risk of developing severe malaria in Kenyan children, and purified IgG2 antibodies have been shown to block the ability of purified cytophilic antibodies to inhibit parasite growth in vitro (23, 41). The prevalence of the above-mentioned FcγRIIA mutation in the Gabonese population is very high (∼75% allele frequency; F. Ntoumi, personal communication), so an influence on IgG2 responses could be envisaged. We have found evidence of an association between anti-VSA IgG2 responses and protection from malaria (C. Yone et al., unpublished observations). On the other hand, the level of IgG2 (and of IgG4) in the profile of the IgG isotype responses of our child study cohort is negligible compared to levels of other defined parasite asexual-stage antigens (A. J. F. Luty, unpublished observations), and the level of semi-immune serum-mediated phagocytosis of P. falciparum-infected erythrocytes is apparently unaffected by the amount of anti-parasite IgG2 in samples from Gabonese adults (54). Defining a clear role for anti-VSA IgG2 antibodies therefore awaits further study.
We can offer no clear explanation for the prominence of IgG4 in the anti-VSA responses of both adults and children to one of the heterologous isolates we used, which was itself obtained from a child with severe malaria. Repeated exposure to immunogens can skew responses towards IgG4, raising the possibility that a cross-reactive epitope expressed on the “common” VSA associated with severe malaria may be responsible for the pattern that we observed here (1, 10, 44). At the molecular level, IgG4 responses are commonly, but not exclusively, directed to carbohydrate epitopes, but the well-described P. falciparum VSA (PfEMP-1 and rifins) are not known to be glycosylated. Peptide-induced IgG4 responses have nevertheless been reported for other nonplasmodial parasite infections, and antiplasmodial IgG4 antibodies are functionally important because of their ability to block the activities of cytophilic isotypes (5, 23, 51). In this context, the relatively enhanced IgG4 anti-VSA responses observed here in children, both during and after a malaria episode and especially in children with a history of severe malaria, merit a more detailed investigation. These findings reiterate the need, already evoked by others, for comprehensive analyses of antibody activities with defined specificities in different epidemiological settings (L. Hviid, T. Staalsoe, M. A. Nielsen, and T. G. Theander, Letter, Infect. Immun. 71:2296, 2003.).
The broad similarity in the isolate-specific profiles of the responses seen with samples from healthy children and adults suggests that antibody responses to a particular isolate, and thus, by implication, to any given VSA type, are rather stable over time. The most notable age-related changes in the overall profile of the anti-VSA IgG isotype responses that we observed here concern the decline in the relative prominence of IgG4 in children, with a corresponding increase in IgG2-mediated isolate recognition in adults, whereas IgG3 responses, as noted earlier, were sustained at similar levels. A multitude of factors can enhance or suppress Ig secretion, including monocyte- and T-cell-derived cytokines, such as tumor necrosis factor alpha, gamma interferon, interleukin-10 (IL-10), and IL-12, which are known to be associated with the acquisition of antimalarial immunity and/or with pathogenesis during P. falciparum infection (15, 22, 30, 35, 39, 40, 49). In this context, the profound effect that IL-10, for example, has on the pattern of isotype switching in naïve B cells may have far-reaching implications for the development of antiplasmodial antibody responses, given the dramatically increased levels of this cytokine associated with acute malaria episodes in African children (30, 35, 36, 48, 53).
An association between P. falciparum infection and enhanced IgG antibody responses to VSA expressed by heterologous isolates has been reported in several different studies (9, 13, 21, 26, 46). In the study presented here, IgG1 responses to VSA expressed by heterologous isolates displayed a clear, albeit transient, infection-related enhancement. Interestingly, this increase was seen only in samples taken at the time of the first posttreatment malaria attack, not in those taken during the admission episode. We speculate that this pattern may be a reflection of the chronology of IgG isotype switching events during a malaria episode, since IgG1 is, ontologically, the first isotype to be produced in response to any given protein antigen; in vitro experiments have shown that the switching of human B cells from IgG1 to IgG2 or IgG3 production requires several rounds of division (53). The follow-up surveillance that formed an integral part of this study inevitably resulted in the detection and treatment of reinfections at a stage in their evolution earlier than the admission episode at inclusion, which might therefore favor the detection of putatively earlier IgG1 anti-VSA responses in the reinfection samples. Whatever the reasons for these particular findings, the clear implication is that cross-reactive antibodies can be induced by P. falciparum VSA during malaria attacks in African children who have perennial exposure to infection. Such cross-reactivity may be more common than was previously thought to be the case (13, 43; Hviid et al., letter).
Acknowledgments
We are grateful to the children and their families for their participation in this study and to the staff of the Albert Schweitzer Hospital in Lambaréné, Gabon. We also thank Anselme Ndzengué and Marcel Nkeyi for technical assistance. The 1/95-C study was initiated in 1995 and inclusion into the study was completed in 1996. Follow-up surveillance continued until February 2002. We acknowledge the following members of the 1/95-C Study Team for their important contribution to the data included in the manuscript: Ruprecht Schmidt-Ott, Leopold G. Lehman, Doris Luckner, Bernhard Greve, Peter Matousek, Klaus Herbich, Daniela Schmid, Milena Sovric, Birgit Bojowald, Hanna Rudloff, Andreas Schindler, and Michel A. Missinou.
This study was supported in part by the fortune programme of the Medical Faculty, University of Tübingen, by the European Union INCO Programme (contract number INCO-DC IC18 CT98 0359), and by the Deutsche Forschungsgemeinschaft (project reference Ku775/12-1).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aalberse, R. C., R. van der Gaag, and J. van Leeuwen. 1983. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J. Immunol. 130:722-726. [PubMed] [Google Scholar]
- 2.Abdel-Latif, M. S., A. Khattab, C. Lindenthal, P. G. Kremsner, and M.-Q. Klinkert. 2002. Recognition of variant rifin antigens by human antibodies induced during natural Plasmodium falciparum infections. Infect. Immun. 70:7013-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguiar, J. C., G. R. Albrecht, P. Cegielski, B. M. Greenwood, J. B. Jensen, G. Lallinger, A. Martinez, I. A. McGregor, J. N. Minjas, J. Neequaye, et al. 1992. Agglutination of Plasmodium falciparum-infected erythrocytes from east and west African isolates by human sera from distant geographic regions. Am. J. Trop. Med. Hyg. 47:621-632. [DOI] [PubMed] [Google Scholar]
- 4.Aribot, G., C. Rogier, J. L. Sarthou, J. F. Trape, A. T. Balde, P. Druilhe, and C. Roussilhon. 1996. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa). Am. J. Trop. Med. Hyg. 54:449-457. [DOI] [PubMed] [Google Scholar]
- 5.Aucan, C., Y. Traoré, F. Tall, B. Nacro, T. Traoré-Leroux, F. Fumoux, and P. Rihet. 2000. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 68:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barragan, A., P. G. Kremsner, W. Weiss, M. Wahlgren, and J. Carlson. 1998. Age-related buildup of humoral immunity against epitopes for rosette formation and agglutination in African areas of malaria endemicity. Infect. Immun. 66:4783-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 8.Boutlis, C. S., P. K. Fagan, D. C. Gowda, M. Lagog, C. S. Mgone, M. J. Bockarie, and N. M. Anstey. 2003. Immunoglobulin G (IgG) responses to Plasmodium falciparum glycosylphosphatidylinositols are short-lived and predominantly of the IgG3 subclass. J. Infect. Dis. 187: 862-865. [DOI] [PubMed] [Google Scholar]
- 9.Bull, P. C., B. S. Lowe, N. Kaleli, F. Njuga, M. Kortok, A. Ross, F. Ndungu, R. W. Snow, and K. Marsh. 2002. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J. Infect. Dis. 185: 1688-1691. [DOI] [PubMed] [Google Scholar]
- 10.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanagh, D. R., C. Dobaño, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. T. G. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 69:1207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyay, R., A. Sharma, V. K. Srivastava, S. S. Pati, S. K. Sharma, B. S. Das, and C. E. Chitnis. 2003. Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens. Infect. Immun. 71:597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deloron, P., B. Dubois, J. Y. Le Hesran, D. Riche, N. Fievet, M. Cornet, P. Ringwald, and M. Cot. 1997. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin. Exp. Immunol. 110:212-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodoo, D., F. M. Omer, J. Todd, B. D. Akanmori, K. A. Koram, and E. M. Riley. 2002. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 185: 971-979. [DOI] [PubMed] [Google Scholar]
- 16.Dodoo, D., T. Staalsoe, H. Giha, J. A. L. Kurtzhals, B. D. Akanmori, K. Koram, S. Dunyo, F. K. Nkrumah, L. Hviid, and T. G. Theander. 2001. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect. Immun. 69:3713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrante, A., and C. M. Rzepczyk. 1997. Atypical IgG subclass antibody responses to Plasmodium falciparum asexual stage antigens. Parasitol. Today 13:145-148. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira, M. U., E. A. Kimura, A. M. Katzin, L. L. Santos-Neto, J. O. Ferrari, J. M. Villalobos, and M. E. de Carvalho. 1998. The IgG-subclass distribution of naturally acquired antibodies to Plasmodium falciparum, in relation to malaria exposure and severity. Ann. Trop. Med. Parasitol. 92:245-256. [DOI] [PubMed] [Google Scholar]
- 19.Garraud, O., R. Perraut, A. Diouf, W. S. Nambei, A. Tall, A. Spiegel, S. Longacre, D. C. Kaslow, H. Jouin, D. Mattei, G. M. Engler, T. B. Nutman, E. M. Riley, and O. Mercereau-Puijalon. 2002. Regulation of antigen-specific immunoglobulin G subclasses in response to conserved and polymorphic Plasmodium falciparum antigens in an in vitro model. Infect. Immun. 70:2820-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giha, H. A., T. Staalsoe, D. Dodoo, C. Roper, G. M. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 2000. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol. Lett. 71:117-126. [DOI] [PubMed] [Google Scholar]
- 21.Giha, H. A., T. Staalsoe, D. Dodoo, I. M. Elhassan, C. Roper, G. M. H. Satti, D. E. Arnot, T. G. Theander, and L. Hviid. 1999. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect. Immun. 67:4092-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grau, G. E., T. E. Taylor, M. E. Molyneux, J. J. Wirima, P. Vassalli, M. Hommel, and P. H. Lambert. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 320:1586-1591. [DOI] [PubMed] [Google Scholar]
- 23.Groux, H., and J. Gysin. 1990. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res. Immunol. 141:529-542. [DOI] [PubMed] [Google Scholar]
- 24.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 25.Hommel, M., P. H. David, and L. D. Oligino. 1983. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J. Exp. Med. 157:1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal, J., P. Perlmann, and K. Berzins. 1993. Serological diversity of antigens expressed on the surface of erythrocytes infected with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 87:583-588. [DOI] [PubMed] [Google Scholar]
- 27.Kinyanjui, S. M., P. Bull, C. I. Newbold, and K. Marsh. 2003. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J. Infect. Dis. 187: 667-674. [DOI] [PubMed] [Google Scholar]
- 28.Kun, J. F., B. Mordmuller, B. Lell, L. G. Lehman, D. Luckner, and P. G. Kremsner. 1998. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet 351:265-266. [DOI] [PubMed] [Google Scholar]
- 29.Kun, J. F., R. J. Schmidt-Ott, L. G. Lehman, B. Lell, D. Luckner, B. Greve, P. Matousek, and P. G. Kremsner. 1998. Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambarene, Gabon. Trans. R. Soc. Trop. Med. Hyg. 92:110-114. [DOI] [PubMed] [Google Scholar]
- 30.Kurtzhals, J. A., V. Adabayeri, B. Q. Goka, B. D. Akanmori, J. O. Oliver-Commey, F. K. Nkrumah, C. Behr, and L. Hviid. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768-1772. [DOI] [PubMed] [Google Scholar]
- 31.Kyes, S. A., J. A. Rowe, N. Kriek, and C. I. Newbold. 1999. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 96:9333-9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leech, J. H., J. W. Barnwell, M. Aikawa, L. H. Miller, and R. J. Howard. 1984. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J. Cell Biol. 98:1256-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lell, B., J. May, R. J. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, D. Schmid, K. Herbich, F. P. Mockenhaupt, C. G. Meyer, U. Bienzle, and P. G. Kremsner. 1999. The role of red blood cell polymorphisms in resistance and susceptibility to malaria. Clin. Infect. Dis. 28: 794-799. [DOI] [PubMed] [Google Scholar]
- 34.Luty, A. J., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, F. Migot-Nabias, P. Deloron, R. S. Nussenzweig, and P. G. Kremsner. 1999. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 179: 980-988. [DOI] [PubMed] [Google Scholar]
- 35.Luty, A. J. F., D. J. Perkins, B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, J. B. Weinberg, and P. G. Kremsner. 2000. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68:3909-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malisan, F., F. Briere, J. M. Bridon, N. Harindranath, F. C. Mills, E. E. Max, J. Banchereau, and H. Martinez-Valdez. 1996. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J. Exp. Med. 183:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 38.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 39.May, J., B. Lell, A. J. Luty, C. G. Meyer, and P. G. Kremsner. 2000. Plasma interleukin-10:tumor necrosis factor (TNF)-alpha ratio is associated with TNF promoter variants and predicts malarial complications. J. Infect. Dis. 182: 1570-1573. [DOI] [PubMed] [Google Scholar]
- 40.Mordmuller, B. G., W. G. Metzger, P. Juillard, B. M. Brinkman, C. L. Verweij, G. E. Grau, and P. G. Kremsner. 1997. Tumor necrosis factor in Plasmodium falciparum malaria: high plasma level is associated with fever, but high production capacity is associated with rapid fever clearance. Eur. Cytokine Netw. 8:29-35. [PubMed] [Google Scholar]
- 41.Ndungu, F. M., P. C. Bull, A. Ross, B. S. Lowe, E. Kabiru, and K. Marsh. 2002. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 24:77-82. [DOI] [PubMed] [Google Scholar]
- 42.Newbold, C., A. Craig, S. Kyes, A. Rowe, D. Fernandez-Reyes, and T. Fagan. 1999. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int. J. Parasitol. 29:927-937. [DOI] [PubMed] [Google Scholar]
- 43.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75:281-292. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen, M. A., T. Staalsoe, J. A. Kurtzhals, B. Q. Goka, D. Dodoo, M. Alifrangis, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J. Immunol. 168:3444-3450. [DOI] [PubMed] [Google Scholar]
- 45.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 46.Ofori, M. F., D. Dodoo, T. Staalsoe, J. A. L. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oguariri, R. M., S. Borrmann, M.-Q. Klinkert, P. G. Kremsner, and J. F. J. Kun. 2001. High prevalence of human antibodies to recombinant Duffy binding-like α domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect. Immun. 69:7603-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Othoro, C., A. A. Lal, B. Nahlen, D. Koech, A. S. Orago, and V. Udhayakumar. 1999. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179: 279-282. [DOI] [PubMed] [Google Scholar]
- 49.Perkins, D. J., J. B. Weinberg, and P. G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182: 988-992. [DOI] [PubMed] [Google Scholar]
- 50.Piper, K. P., D. J. Roberts, and K. P. Day. 1999. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp. Parasitol. 91:161-169. [DOI] [PubMed] [Google Scholar]
- 51.Sterla, S., H. Sato, and A. Nieto. 1999. Echinococcus granulosus human infection stimulates low avidity anticarbohydrate IgG2 and high avidity antipeptide IgG4 antibodies. Parasite Immunol. 21:27-34. [DOI] [PubMed] [Google Scholar]
- 52.Sylla, E. H., J. F. Kun, and P. G. Kremsner. 2000. Mosquito distribution and entomological inoculation rates in three malaria-endemic areas in Gabon. Trans. R. Soc. Trop. Med. Hyg. 94:652-656. [DOI] [PubMed] [Google Scholar]
- 53.Tangye, S. G., A. Ferguson, D. T. Avery, C. S. Ma, and P. D. Hodgkin. 2002. Isotype switching by human B cells is division-associated and regulated by cytokines. J. Immunol. 169:4298-4306. [DOI] [PubMed] [Google Scholar]
- 54.Tebo, A. E., P. G. Kremsner, and A. J. Luty. 2002. Fcgamma receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin. Exp. Immunol. 130:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tebo, A. E., P. G. Kremsner, K. P. Piper, and A. J. Luty. 2002. Low antibody responses to variant surface antigens of Plasmodium falciparum are associated with severe malaria and increased susceptibility to malaria attacks in Gabonese children. Am. J. Trop. Med. Hyg. 67:597-603. [DOI] [PubMed] [Google Scholar]
- 56.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 57.Wahlgren, M., K. Berzins, P. Perlmann, and M. Persson. 1983. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin. Exp. Immunol. 54:135-142. [PMC free article] [PubMed] [Google Scholar]
- 58.Warrell, D., M. Molyneux, and P. Beales. 1990. Severe and complicated malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):1-65. [Google Scholar]
- 59.Wildling, E., S. Winkler, P. Kremsner, C. Brandts, L. Jenne, and W. Wernsdorfer. 1995. Malaria epidemiology in the province of Moyen Ogoov, Gabon. Trop. Med. Parasitol. 46:77-82. [PubMed] [Google Scholar]