Abstract
Previous studies demonstrated that interleukin-12 (IL-12)-dependent gamma interferon (IFN-γ) responses have a major role in restricting in vivo bacterial growth during infection of mice with group B streptococci (GBS), important human pathogens. Like IL-12, IL-18 is a potent IFN-γ inducer. The role of IL-18 in experimental GBS infection was investigated here. Significant elevations of IL-18 levels over baseline values were detected in plasma samples from neonatal mice rendered septic with GBS. Neutralization of IL-18 significantly increased mortality and bacterial burden (P < 0.05). In contrast, administration of recombinant IL-18 (rIL-18) before or after GBS challenge remarkably improved survival and decreased blood colony counts, in association with increased IFN-γ production by spleen cells. The beneficial effects of rIL-18 were counteracted by administration of neutralizing anti-IFN-γ monoclonal antibodies, indicating that the effects of IL-18 were mediated by IFN-γ. Finally, low rIL-18 doses that had no effect of their own on bacterial burden could act in synergy with rIL-12 to protect neonatal mice during GBS infection. Collectively, our data indicate that IL-18 responses have an important role in host defenses against GBS and that rIL-18 may be useful in alternative strategies to treat neonatal GBS disease.
Group B streptococci (GBS) have been recognized as major agents of septicemia and meningitis in neonates and in adults with chronic underlying conditions (2). In spite of a significant reduction of early-onset neonatal disease by use of intrapartum antibiotic prophylaxis, mortality and permanent disability rates from GBS disease continue to be high. Cytokines appear to have an important role in host defenses against GBS (5, 8, 9, 25, 26), although an excessive release of tumor necrosis factor alpha (TNF-α) has been associated with pathophysiologic phenomena (11, 15, 23, 33). Gamma interferon (IFN-γ) may have a crucial role in stimulating anti-GBS defenses. IFN-γ production is severely impaired during early life, a fact which may explain at least partially the increased susceptibility of neonates to infections by GBS and other pathogens (6, 8, 22, 36). Administration of recombinant IFN-γ was effective in protecting neonatal mice from GBS-induced lethality and in limiting in vivo GBS growth (7). Recombinant interleukin-12 (rIL-12) also improved survival and impaired in vivo bacterial growth (24). These effects were mediated at least partially by increased IFN-γ production (10, 24).
Interleukin-18 (IL-18) is a potent IFN-γ inducer, especially in combination with IL-12. IL-18 is a member of the IL-1 family of ligands and is produced mainly as a precursor protein (24 kDa) that requires proteolytic activation by an IL-1β-converting enzyme to liberate the 18-kDa mature active protein (14, 16). IL-18 mRNA is expressed in a wide range of cells, including Kupffer cells, macrophages, T cells, B cells, dendritic cells, osteoblasts, keratinocytes, astrocytes, and microglia (28). The IL-18 receptor system and its signal transduction pathway are analogous to those of IL-1 (34). In addition to IFN-γ, IL-18 can induce the synthesis of IL-1β, TNF-α, IL-8, and various chemokines (21, 30, 31), probably through the activation of nuclear factor κB (NF-κB) (28). Recent studies investigated the role of IL-18 in the host response to infection. In its capacity as a IFN-γ-inducing factor, IL-18 has an important role in natural immunity and acquired immunity (1, 21). Accordingly, IL-18 is protective against infection caused by a number of intracellular and extracellular pathogens (3, 4, 17, 20, 32, 35). However, during experimental endotoxemia in mice, neutralization of IL-18 protected against lipopolysaccharide-induced liver injury and lethality (12, 29), suggesting a pathogenic role of IL-18 in lethal endotoxemia.
In view of the pleiotropic effects of IL-18, it was of interest to explore its role in experimental GBS disease. Our data indicate that endogenous IL-18 contributes significantly to host defenses against GBS. Moreover, prophylactic or therapeutic administration of rIL-18 results in decreased bacterial burden and increased survival.
MATERIALS AND METHODS
Bacteria.
GBS strain COH1, a highly virulent strain originally isolated from a septic neonate, was kindly provided by Craig Rubens, University of Washington, Seattle. Bacteria were grown to the mid-log phase in Todd-Hewitt broth (Oxoid, Milan, Italy) and diluted to the appropriate concentration in phosphate-buffered saline (PBS; 0.01 M phosphate, 0.15 M NaCl [pH 7.2]) prior to the inoculation of animals.
Neonatal mice.
Neonatal (<24-h-old) BALB/c mice were used. Parental mice were obtained from Charles River Italia (Calco, Italy). Pups from each litter were randomly assigned to control or experimental groups, marked, and kept with their mothers. Mice used in this study were housed under specific-pathogen-free conditions in the animal facilities of the Department of Pathology and Experimental Microbiology at the University of Messina. The mice were fed clean food and water ad libitum. All of the procedures were in agreement with the guidelines of the National Institutes of Health for the handling of laboratory animals, and the studies presented here were approved by the relevant national and institutional committees.
Neonatal sepsis model.
Mouse pups were infected subcutaneously with various GBS doses. The actual numbers of injected bacteria were confirmed by colony counts. Mice were observed daily for 8 days after inoculation. Deaths were never observed after 5 days. In addition, some of the mice were killed at certain intervals in order to measure bacterial burden and plasma cytokine levels.
Reagents and antibodies.
Murine rIL-18 was purchased from Euroclone (distributed by Celbio, Milan, Italy). The endotoxin content in this preparation was less than 1 endotoxin unit/μg, as determined by the manufacturer. Murine rIL-12, goat polyclonal anti-mouse IL-18, anti-mouse IFN-γ immunoglobulin G (IgG), and normal goat IgG were purchased from PeproTech EC Ltd. (London, United Kingdom).
Cytokine determinations.
In order to measure cytokine production during the course of infection, mouse pups were killed by decapitation at various times after GBS challenge. Mixed venous-arterial blood was collected in heparinized containers and centrifuged after 10 μl was saved for colony counts. Pooled plasma from five animals was stored at −70°C until measured for IL-18 and TNF-α by using, respectively, a mouse IL-18 enzyme-linked immunosorbent assay kit (MBL; R&D Systems, Minneapolis, Minn.) and a mouse TNF-α module set (BenderMedSystems, Vienna, Austria). The lower limits of detection for IL-18 and TNF-α were 25.6 and 16 pg/ml, respectively. To measure ongoing IFN-γ production in spleen cells, the organs from five animals were pooled and a single-cell suspension was prepared as described previously (7, 13). Cells were washed and resuspended in RPMI 1640 containing 10% heat-inactivated fetal calf serum, HEPES (10 mM), l-glutamine (2 mM), penicillin (50 IU/ml), and streptomycin (50 μg/ml) (all obtained from Invitrogen Life Technologies, San Giuliano Milanese, Italy) to a density of 2 × 106 cells per ml. One-milliliter aliquots were cultured in 24-well plates for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Spleen cells obtained from uninfected animals and cultured under the same conditions served as controls. After culturing, supernatants were removed and stored at −70°C until assayed by using a mouse IFN-γ enzyme-linked immunosorbent assay kit (Euroclone; sensitivity of 15 pg/ml).
Expression of data and statistical analysis.
IL-18, TNF-α, IFN-γ, and log CFU levels were expressed as means and standard deviations of three independent observations, each conducted on a different animal (IL-18, TNF-α, and log CFU) or on a different pool from five animals (IFN-γ). Differences in cytokine and CFU levels were assessed by a one-way analysis of variance and the Student-Newman-Keuls test. Survival data were analyzed with Kaplan-Meier survival plots followed by the Wilcoxon test. With both tests, differences were considered significant when P values were <0.05.
RESULTS
IL-18 levels during infection.
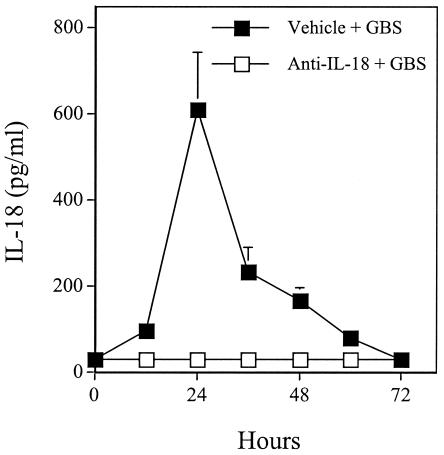
Plasma IL-18 values were below the limit of detection of the assay in uninfected control pups (data not shown). Significant IL-18 elevations over baseline values could be detected in plasma at 12 to 60 h after challenge with 60 CFU of GBS, peaking at 24 h (Fig. 1).
FIG. 1.
Plasma IL-18 levels in neonatal mice at various times after infection with GBS. Groups of pups were treated with goat polyclonal anti-IL-18 IgG (0.5 μg/pup) or vehicle (PBS) at 6 h before GBS challenge (60 CFU/mouse). Data represent means and standard deviations of three independent observations, each conducted on a different animal.
Effects of anti-IL-18 and rIL-18.
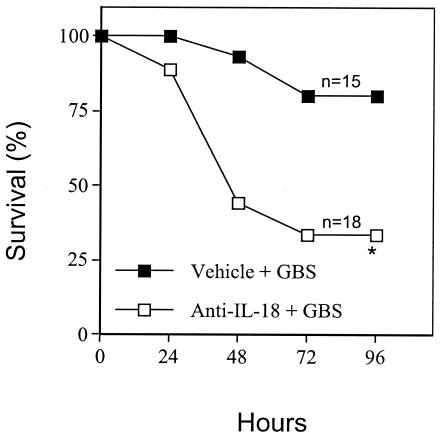
In further experiments, we explored the role of the IL-18 endogenously produced during GBS infection. Administration of goat polyclonal anti-IL-18 IgG (0.5 μg/pup) at 6 h before challenge totally prevented the elevations in immunoreactive IL-18 (Fig. 1). This treatment also markedly decreased survival in GBS-infected pups relative to controls injected with PBS before bacterial challenge (Fig. 2). In further experiments, similar detrimental effects of anti-IL-18 IgG were observed when controls consisted of normal goat IgG-treated pups instead of PBS-treated ones.
FIG. 2.
Effects of IL-18 blockade on GBS-induced lethality in neonatal mice. Pups were treated with goat polyclonal anti-IL-18 IgG (0.5 μg/pup) or vehicle (PBS) at 6 h before GBS challenge (15 CFU/pup). An asterisk indicates a P value of <0.05 for a comparison of the treated mice and the control mice.
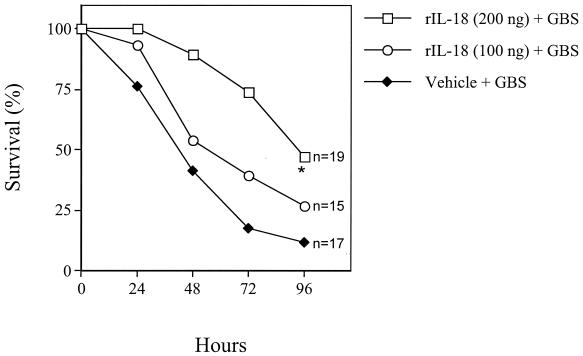
Since IL-18 blockade decreased survival in this model, we investigated whether treatment with rIL-18 would be beneficial. Mouse pups were injected with rIL-18 at 6 h before GBS challenge. Prophylactic treatment with 200 ng of rIL-18 per pup increased survival, as evidenced by significantly (P < 0.05) decreased lethality (Fig. 3). This effect was dose dependent, since no effects were observed with a lower IL-18 dose (100 ng) (Fig. 3). Doses of 300 and 400 ng per mouse did not result in further protection over that observed with 200 ng (data not shown). Treatment at the time of or 16 h after GBS challenge also was able to increase survival (data not shown).
FIG. 3.
Effects of rIL-18 on GBS-induced lethality in neonatal mice. Pups were treated with two doses of rIL-18 (200 and 100 ng/pup) or vehicle (PBS) at 6 h before GBS challenge (60 CFU/pup). An asterisk indicates a P value of <0.05 for a comparison of the treated mice and the control mice.
Effects of rIL-18 on blood colony counts.
Colony counts were determined with blood samples obtained from animals treated with rIL-18, anti-IL-18 IgG, or PBS at 6 h before challenge. At 48 or 72 h after challenge, log CFU levels were significantly lower and higher in the blood of pups treated with rIL-18 and anti-IL-18 IgG, respectively, than in vehicle-treated controls (P < 0.05) (Table 1). These data indicated that blockade of endogenous IL-18 increased the severity of sepsis. Conversely, rIL-18 administration had the opposite effect and decreased bacterial burden (Table 1).
TABLE 1.
Effects of anti-IL-18 IgG or rIL-18 administration on blood counts in GBS-infected neonatal micea
| Treatment | Blood colony counts (mean ± SD log CFU/ml) at h:
|
|
|---|---|---|
| 48 | 72 | |
| PBS | 4.56 ± 1.27 | 5.9 ± 1.19 |
| Anti-IL-18 IgG | 7.16 ± 1.34b | 7.40 ± 1.56b |
| rIL-18 | 2.31 ± 1.08b | 4.01 ± 0.96b |
Neonatal mice were treated with 0.5 μg of goat polyclonal anti-IL-18 IgG or 200 ng of rIL-18 at 6 h before challenge with GBS (60 CFU/pup). Data represent three observations each conducted on a different animal.
The P value in a comparison with the respective PBS control was <0.05, as determined by analysis of variance and the Student-Newman-Keuls test.
TNF-α and IFN-γ levels.
Since it is known that IL-18 can increase the production of TNF-α (31), a cytokine previously shown to have a pathophysiologic role in this sepsis model (23), TNF-α levels in neonatal mice treated with rIL-18 or anti-IL-18 at doses previously shown to modify lethality were determined. Pups were treated with rIL-18 (200 ng/pup), anti-IL-18 (0.5 μg/pup), or vehicle at 6 h before challenge with 60 CFU, and plasma samples were collected after 24 or 48 h. Plasma TNF-α values did not differ in the three groups of mice (data not shown), indicating that neither rIL-18 nor anti-IL-18 treatment significantly affected TNF-α production in this model.
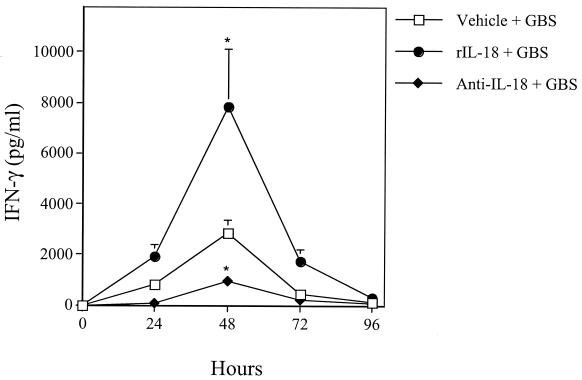
Because IL-18 is also a potent inducer of IFN-γ, a mediator that was previously shown to improve anti-GBS defenses, the effects of rIL-18 and anti-IL-18 on IFN-γ production also were investigated. To this end, neonatal spleen cells were explanted at various times after infection, and IFN-γ responses in supernatants of splenocyte cultures were measured (13). IFN-γ production was significantly enhanced by rIL-18 administration in GBS-infected pups relative to vehicle-treated controls (Fig. 4). Conversely, the IFN-γ response was impaired but not totally abolished by anti-IL-18 treatment (P < 0.05).
FIG. 4.
IFN-γ levels in culture supernatants of spleen cells explanted from neonatal mice at various times after GBS challenge. Pups were treated with rIL-18 (200 ng/pup), goat polyclonal anti-IL-18 (0.5 μg/pup), or vehicle (PBS) at 6 h before GBS challenge (60 CFU/pup). Data represent means and standard deviations of three independent observations, each conducted in duplicate on culture supernatants of spleen cells pooled from five animals. An asterisk indicates a value that was significantly (P < 0.05) different from the value for the vehicle-treated control, as determined by one-way analysis of variance and the Student-Newman-Keuls test.
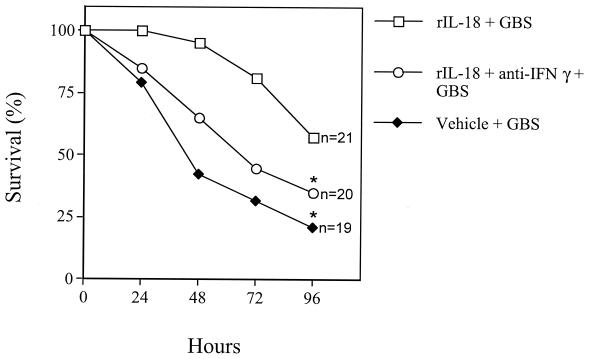
Next, to ascertain whether the beneficial activities of IL-18 were mediated by increased IFN-γ bioactivity, pups were treated with an anti-IFN-γ monoclonal antibody (5 μg per pup) plus rIL-18 (200 ng/pup) at 6 h before challenge with a 90% lethal dose. Infected pups treated with rIL-18 alone served as controls. Figure 5 shows that the effects of rIL-18 were counteracted by IFN-γ blockade. These data indicate that the effects of rIL-18 were mediated at least partially by IFN-γ.
FIG. 5.
Effects of combined treatment with rIL-18 and anti-IFN-γ on GBS-induced lethality in neonatal mice. Pups were treated with rIL-18 (200 ng/pup) alone, with rIL-18 (200 ng/pup) and goat polyclonal anti-IFN-γ (5 μg/pup), or with vehicle (PBS) at 6 h before GBS challenge (60 CFU/pup). An asterisk indicates a P value of <0.05 for a comparison with rIL-18-treated mice.
Effects of combined treatment with rIL-18 and rIL-12.
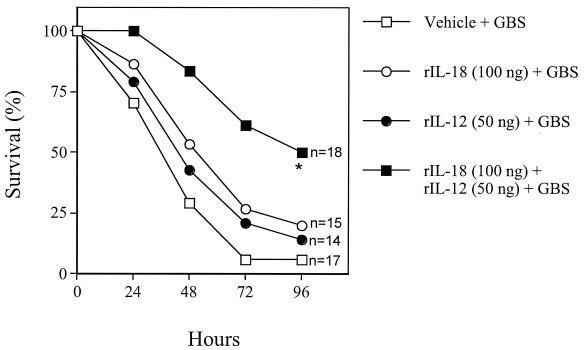
To determine whether IL-18 acts in synergy with IL-12, pups were inoculated with mixtures of rIL-18 and rIL-12 at 6 h before challenge with a 90% lethal dose. Treatment with a low dose of rIL-18 (100 ng) or rIL-12 (50 ng) was not effective when given alone. However, combined administration of the same low doses increased the survival of pups (Fig. 6). Combined treatment with optimal rIL-12 (100 ng) and rIL-18 (200 ng) doses did not result in increased protection over that observed with single doses (data not shown).
FIG. 6.
Effects of combined treatment with rIL-12 and rIL-18 on GBS-induced lethality in neonatal mice. Pups were treated with rIL-18 (100 ng/pup) and rIL-12 (50 ng/pup), alone or in combination, or with vehicle (PBS) at 6 h before GBS challenge (60 CFU/pup). An asterisk indicates a P value of <0.05 for a comparison of the treated mice and the control mice.
DISCUSSION
Sepsis still is frequently a fatal disease that involves the expression of a complex network of proinflammatory and anti-inflammatory cytokines. Previous studies demonstrated that IL-18 is produced during clinical infection caused by different organisms and in various animal models of infection (3, 4, 17, 20). The main finding of the present study is that IL-18 is produced during GBS sepsis and contributes significantly to host defenses. This finding was evidenced by the ability of GBS challenge to induce circulating IL-18 and by the ability of treatment with neutralizing anti-IL-18 to produce marked increases in both lethality and blood CFU levels. Conversely, administration of rIL-18 to mice before or after challenge improved their survival rates and decreased bacterial burden. These results were reminiscent of the effects of rIFN-γ administration previously observed with the same animal model (7). Moreover, in the present study, administration of rIL-18 was followed by significant elevations of IFN-γ production by spleen cells. Although IL-18 exerts some of its activities through induction of IFN-γ, recent data suggest that IL-18 has direct properties, including NF-κB activation, stimulation of proliferation and cytotoxicity of T and NK cells, induction of Fas ligand expression, and cytokine production (12, 27, 31). Therefore, we studied whether mechanisms different from the hypothesized IL-18-promoted IFN-γ production were involved in protection of neonatal mice against GBS sepsis. Our data showed that the effects of rIL-18 could be abolished almost completely by concomitant IFN-γ blockade, indicating an important role of IFN-γ in the observed beneficial effects of rIL-18.
Marked neonatal age-associated defects in IFN-γ production have been well documented (18, 22). Our results demonstrated that rIL-18 administration may restore at least partially the ability of neonates to produce IFN-γ. A reduced IL-18 response may contribute to neonatal age-associated defects in IFN-γ production. Interestingly, a recent study showed that cord blood mixed mononuclear cells produced significantly less IL-18, IL-12, and IFN-γ in response to GBS than did mixed mononuclear cells from adults (19). Moreover, IFN-γ production was enhanced by added rIL-18 and rIL-12 (19). These data, as well as those of the present study, suggest that exogenous rIL-18 may be useful for correcting neonatal deficiences in IL-18 and IFN-γ production and for decreasing neonatal susceptibility to GBS infections. Collectively, the results presented here and those of previous studies (7, 24) suggest that IL-18- and IL-12-induced IFN-γ is a crucial host response factor that acts mainly through increasing phagocyte-mediated killing of GBS. Since phagocyte functions are depressed in neonates, administration of exogenous IFN-γ, IL-12, IL-18, or combinations thereof may help to correct neonatal age-associated defects in innate immunity.
In the present study, low doses of rIL-18, which had no protective effects of their own, could act in synergy with rIL-12 to improve survival. Finally, rIL-18 was effective both prophylactically and therapeutically. Both of these observations may be clinically relevant.
In conclusion, the present study indicates that (i) endogenous IL-18 may have an important role as a host defense factor in GBS disesase and (ii) the administration of rIL-18 may be useful alone or in combination with rIL-12 for correcting neonatal age-associated defects in IFN-γ responses. These observations may be useful for the development of alternative strategies for treating neonatal GBS disease. Moreover, our data are in general agreement with the notion that IL-18 is an important innate immunity factor during bacterial infections.
Acknowledgments
This work was supported in part by the European Commission (HOSPATH contract no. QLK2-CT-2000-00336) and by grants from MIUR and the University of Messina.
Editor: J. T. Barbieri
REFERENCES
- 1.Akira, S. 2000. The role of IL-18 in innate immunity. Curr. Opin. Immunol. 12:59-63. [DOI] [PubMed] [Google Scholar]
- 2.Baker, C. J. 1986. Group B streptococcal infections in newborns. N. Engl. J. Med. 314:1702-1708. [DOI] [PubMed] [Google Scholar]
- 3.Bohn, E., A. Sing, R. Zumbil, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-γ-inducing factor) regulates early cytokine production and promotes resolution of bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 4.Brieland, J. K., C. Jackson, S. Hurst, D. Loebenberg, T. Muchamuel, R. Debets, R. Kastelein, T. Churakova, J. Abrams, R. Hare, and A. O'Garra. 2000. Immunomodulatory role of endogenous interleukin-18 in gamma interferon-mediated resolution of replicative Legionella pneumophila lung infection. Infect. Immun. 68:6567-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusumano, V., F. Genovese, G. Mancuso, M. Carbone, M. T. Fera, and G. Teti. 1996. Interleukin-10 protects neonatal mice from lethal group B streptococcal infection. Infect. Immun. 64:2850-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cusumano, V., G. Mancuso, F. Genovese, M. Cuzzola, M. Carbone, J. A. Cook, J. B. Cochran, and G. Teti. 1997. Neonatal hypersusceptibility to endotoxin correlates with increased TNF production in mice. J. Infect. Dis. 176:168-176. [DOI] [PubMed] [Google Scholar]
- 7.Cusumano, V., G. Mancuso, F. Genovese, D. Delfino, C. Beninati, E. Losi, and G. Teti. 1996. Role of gamma interferon in neonatal mouse model of group B streptococcal disease. Infect. Immun. 64:2941-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuzzola, M., G. Mancuso, C. Beninati, C. Biondo, F. Genovese, F. Tomasello, T. H. Flo, T. Espevik, and G. Teti. 2000. β2 Integrins are involved in cytokine responses to whole gram-positive bacteria. J. Immunol. 164:5871-5876. [DOI] [PubMed] [Google Scholar]
- 9.Cuzzola, M., G. Mancuso, C. Beninati, C. Biondo, C. von Hunolstein, G. Orefici, T. Espevik, T. H. Flo, and G. Teti. 2000. Human monocyte receptors involved in tumor necrosis factor responses to group B streptococcal products. Infect. Immun. 68:994-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derrico, C. A., and K. J. Goodrum. 1996. Interleukin-12 and tumor necrosis factor alpha mediate innate production of gamma interferon by group B Streptococcus-treated splenocytes of severe combined immunodeficiency mice. Infect. Immun. 64:1314-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello, C. A. 1991. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J. Infect. Dis. 163:1177-1184. [DOI] [PubMed] [Google Scholar]
- 12.Faggioni, R., R. C. Cattley, J. Guo, S. Flores, H. Brown, M. Qi, S. Yn, D. Hill, S. Scully, C. Chen, D. Brankow, J. Lewis, C. Baikalov, H. Yamane, T. Meng, F. Martin, S. Hu, T. Boone, and G. Senaldi. 2001. IL-18-binding protein protects against lipopolysaccharide-induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver-disease in mice. J. Immunol. 167:5913-5920. [DOI] [PubMed] [Google Scholar]
- 13.Freudenberg, M. A., Y. Kumazawa, S. Meding, J. Langhorne, and G. Galanos. 1991. Gamma interferon production in endotoxin-responder and nonresponder mice during infection. Infect. Immun. 59:3484-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, et al. 1997. Caspase-1 processes IFN γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386:619-623. [DOI] [PubMed] [Google Scholar]
- 15.Givner, L. B., L. Gray, and T. M. O'Shea. 1995. Antibodies to tumor necrosis factor-alpha: use as adjunctive therapy in established group B streptococcal disease in newborn rats. Pediatr. Res. 38:551-554. [DOI] [PubMed] [Google Scholar]
- 16.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, et al. 1997. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 17.Guifang, C., R. Kastelein, and C. A. Hunter. 2000. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect. Immun. 68:6932-6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill, H. R. 1993. Modulation of host defenses with interferon-γ in pediatrics. J. Infect. Dis. 167:523-528. [DOI] [PubMed] [Google Scholar]
- 19.La Pine, T. R., J. L. Joyner, N. H. Augustine, S. D. Kwak, and H. R. Hill. 2003. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B streptococci. Pediatr. Res. 54:276-281. [DOI] [PubMed] [Google Scholar]
- 20.Lauw, F. N., J. Branger, S. Florquin, P. Speelman, S. J. H. van Deventer, S. Akira, and T. van der Poll. 2002. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 168:372-378. [DOI] [PubMed] [Google Scholar]
- 21.Leung, B. P., S. Culshaw, J. Alaster Gracie, D. Hunter, C. A. Canetti, C. Campbell, F. Cunha, F. Y. Liew, and I. B. McInnes. 2001. A role for IL-18 in neutrophil activation. J. Immunol. 167:2879-2886. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, D. B., A. Larsen, and C. B. Wilson. 1986. Reduced interferon-γ mRNA levels in human neonates. J. Exp. Med. 163:1018-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancuso, G., V. Cusumano, J. A. Cook, E. Smith, F. Squadrito, G. Blandino, and G. Teti. 1994. Efficacy of tumor necrosis factor alpha and eicosanoid inhibitors in experimental models of neonatal sepsis. FEMS Immunol. Med. Microbiol. 9:49-54. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso, G., V. Cusumano, F. Genovese, N. Gambuzza, C. Beninati, and G. Teti. 1997. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect. Immun. 65:3731-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancuso, G., A. Midiri, C. Beninati, G. Piraino, A. Valenti, G. Nicocia, D. Teti, J. A. Cook, and G. Teti. 2002. Mitogen-activated protein kinases and NF-kappa B are involved in TNF-alpha responses to group B streptococci. J. Immunol. 169:1401-1409. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso, G., F. Tomasello, M. Migliardo, D. Delfino, J. Cochran, J. A. Cook, and G. Teti. 1994. Beneficial effects of interleukin-6 in neonatal models of group B streptococcal disease. Infect. Immun. 62:4992-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto, S., K. Tsuji-Takayama, Y. Aizawa, K. Koide, M. Takeuchi, T. Ohta, and M. Kurimoto. 1997. Interleukin-18 activates NF-κB in murine T helper type 1 cells. Biochem. Biophys. Res. Commun. 234:454-457. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 29.Netea, M. G., G. Fantuzzi, B. G. Kullberg, R. J. L. Stuuyt, E. J. Pulido, R. C. McIntyre, L. A. B. Joosten, J. W. M. Van der Meer, and C. A. Dinarello. 2000. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J. Immunol. 164:2644-2649. [DOI] [PubMed] [Google Scholar]
- 30.Netea, M. G., B. J. Kullberg, I. Verschueren, and J. W. M. Van der Meer. 2000. Interleukin-18 induces production of proinflammatory cytokines in mice: no intermediate role for the cytokines of the tumor necrosis factor family and interleukin-1β. Eur. J. Immunol. 30:3057-3060. [DOI] [PubMed] [Google Scholar]
- 31.Puren, A. J., G. Fantuzzi, Y. Gu, M. S. Su, and C. A. Dinarello. 1998. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNF-α production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 101:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakao, Y., K. Takeda, H. Tsutsui, T. Kaissho, F. Nomura, H. Okamura, K. Nakanishi, and S. Akira. 1999. IL-18-deficient mice are resistent to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int. Immunol. 11:471-480. [DOI] [PubMed] [Google Scholar]
- 33.Teti, G., G. Mancuso, and F. Tomasello. 1993. Cytokine appearance and effects of anti-tumor necrosis factor antibodies in a neonatal rat model of group B streptococcal infection. Infect. Immun. 61:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torigoe, K., S. Ushio, T. Okura, S. Kobayashi, M. Taniai, T. Kunikata, T. Murakami, O. Sanou, H. Kojima, M. Fujii, T. Ohta, M. Ikeda, H. Ikegami, and M. Kurimoto. 1997. Purification and characterization of the human interleukin-18 receptor. J. Biol. Chem. 272:25737-25742. [DOI] [PubMed] [Google Scholar]
- 35.Wei, X., B. P. Leung, W. Niedbala, D. Piedrafita, G. Feng, M. Sweet, L. Dobbie, A. J. Smith, and F. L. Liew. 1999. Altered immune responses and susceptibility to Leishmania major and Staphlylococcus aureus infection in IL-18-deficient mice. J. Immunol. 163:2821-2828. [PubMed] [Google Scholar]
- 36.Wilson, C. B. 1986. Immunologic basis for increased susceptibility of the neonate to infection. J. Pediatr. 108:111-116. [DOI] [PubMed] [Google Scholar]