Abstract
AdΔΔ is an oncolytic adenoviral mutant that has been engineered to selectively target tumors with deregulated cell cycle and apoptosis pathways. AdΔΔ potentiates apoptotic cell death induced by drugs, including mitoxantrone and docetaxel, which are commonly used to treat prostate cancer. Here, we demonstrate that AdΔΔ can also interact synergistically with dietary phytochemicals known to have anti-cancer activities, without incurring the toxic side effects of chemodrugs. Curcumin, genistein, epigallocatechin-gallate, equol, and resveratrol efficiently killed both androgen-receptor positive (22Rv1) and negative cell lines (PC-3, DU145) in combination with adenoviral mutants. Synergistic cell killing was demonstrated with wild-type virus (Ad5) and AdΔΔ in combination with equol and resveratrol. EC50 values for both phytochemicals and viruses were reduced three- to eightfold in all three combination-treated cell lines. The most potent efficacy was achieved in the cytotoxic drug- and virus-insensitive PC-3 cells, both in vitro and in vivo, while cell killing in normal bronchial epithelial cells was not enhanced. Although equol and resveratrol induced only low levels of apoptosis when administered alone, in combination with wild-type virus or AdΔΔ, the level of apoptotic cell death was significantly increased in PC-3 and DU145 cells. In vivo studies using suboptimal doses of AdΔΔ and equol or resveratrol, showed reduced tumor growth without toxicity to normal tissue. These findings identify novel functions for AdΔΔ and phytochemicals in promoting cancer cell killing and apoptosis, suggesting the use of these natural nontoxic compounds might be a feasible and currently unexploited anti-cancer strategy.
Adam and colleagues demonstrate that AdΔΔ, an oncolytic adenoviral mutant that potentiates drug-induced apoptotic cell death, can also interact synergistically with dietary phytochemicals such as equol and resveratrol. Treatment of human prostate cancer cell lines with combinations of virus and phytochemicals led to increased cell killing. In mice, suboptimal doses of phytochemicals showed reduced tumor growth without incurring toxicity.
Introduction
Adenoviruses can be readily engineered to specifically replicate in and lyse tumor cells, leaving normal tissue unharmed. This approach (virotherapy) has been applied to numerous viral mutants with promising results in various cancers including prostate (Parato et al., 2005; Small et al., 2006; Liu et al., 2007; Ekblad and Hallden, 2010). Prostate cancer is the third leading cause of cancer-related death in Western men, due to frequent progression to metastatic disease with resistance to all currently available therapies (Jemal et al., 2011). The development of novel therapeutics with different mechanisms of action to overcome treatment resistance, such as virus-drug combinations, is therefore of high priority.
The clinical safety of virotherapy has been demonstrated in thousands of patients (Parato et al., 2005; Liu et al., 2007). The majority of trials evaluated mutants deleted in the E1B55K-gene (dl1520, also known as ONYX-015 and H101), which is complemented in tumors by nonfunctional p53 and mRNA transport pathways (O'Shea et al., 2004; Parato et al., 2005). Clinical efficacy when administered as single agents was poor, with greatly improved responses in combination with conventional cancer therapeutics (Garber, 2006; Liu et al., 2007). For example, the Ad5-CD/TKrep mutant (a dl1520 variant) significantly lengthened prostate-specific antigen doubling times in prostate cancer patients up to 5 years after treatment when combined with 5-fluorocytosine, ganciclovir, and radiotherapy (Freytag et al., 2007). Chemotherapy in combination with prostate-selective adenoviral mutants with replication regulated by androgen-response elements also resulted in enhanced antitumor efficacy (Dilley et al., 2005; Small et al., 2006).
We recently demonstrated that the E1ACR2-deleted dl922-947 mutant, defective in pRb-binding, had superior efficacy in prostate cancer cells compared to the prototype dl1520 mutants (Radhakrishnan et al., 2010). In combination with cytotoxic drugs, oncolytic potency could be greatly enhanced by deletion of the viral anti-apoptotic E1B19K gene, a functional Bcl-2 homologue (Leitner et al., 2009). To optimize viral efficacy we generated the AdΔΔ mutant with both the E1B19K- and E1ACR2-gene deletions that selectively and synergistically enhance cytotoxic drug-induced apoptosis (Öberg et al., 2010).
To further decrease unwanted toxicity to normal tissue, we explored whether nontoxic natural dietary compounds with proven anticancer activities could interact synergistically with oncolytic mutants, in a manner analogous to the synergistically enhanced cell killing observed with virus and chemotherapeutic drugs. Phytochemicals include dietary isoflavone compounds that inhibit the growth and development of several malignancies, including prostate cancer (Surh, 2003; de Souza et al., 2010). For example, dietary intake of the soybean-derived phytochemicals genistein and daidzein was found to reduce prostate cancer progression in phase II trials (Kwan et al., 2010). It was also demonstrated that the intestinal metabolism of daidzein to the more active compound equol correlates with a lower risk of prostate cancer (Akaza et al., 2004; Yuan et al., 2007). Recently, equol entered Phase II trials for benign prostatic hyperplasia (Ausio Pharmaceuticals; http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00962390). Epigallocatechin-gallate (EGCG) from green tea and curcumin from turmeric are potent antioxidants, currently undergoing evaluation in several trials for cancer prevention and treatment (Thomasset et al., 2007; Goel and Aggarwal, 2010). Resveratrol, produced by grapes and berries in response to fungal infections, is also being evaluated in numerous cancer trials (Jang et al., 1997; de la Lastra and Villegas, 2005) (e.g., http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00256334). Resveratrol has also been reported to have additive anticancer effects in pancreatic cell lines in combination with the oncolytic parvovirus H-1PV (Raykov et al., 2009).
In the current study, we selected these five phytochemicals for their anticancer properties and for their reported effects on intracellular signaling pathways. In particular, our choice focused on the effects of the phytochemicals on the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-OH kinase (PI3K) cascades, which are known to regulate the expression of the major viral uptake and internalization receptors, coxsackie and adenovirus receptor (CAR) and the αVβ3 and αVβ5 integrins (Li et al., 1998; Bewley et al., 1999; Anders et al., 2003). We demonstrate that prostate cancer cell killing was synergistic and selective for cancer cells with the AdΔΔ mutant in combination with equol and resveratrol, even in treatment-insensitive PC-3 cells and in tumor xenografts in vivo. Phytochemical-induced viral uptake was part of the underlying mechanism for the response, together with further increases in equol- and resveratrol-induced caspase-dependent apoptosis and cell killing in combination with AdΔΔ. These findings suggest that combining oncolytic adenoviruses with nontoxic dietary phytochemicals is a promising approach for the development into novel prostate cancer therapies.
Materials and Methods
Cancer cell lines, viruses, and reagents
The human metastatic prostate cancer cell lines 22Rv1, DU145 (ATCC, USA), PC-3 (ECACC, UK), A549 lung carcinoma, and embryonic kidney HEK293 cell lines (ATCC) were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 mg/L streptomycin, and 584 mg/L L-glutamine. All cell lines were authenticated by STR-profiling (Cancer Research UK and LGC Standards, UK) and verified to be identical to the profiles reported by ATCC at the end of the studies. Wild-type adenovirus type 5 (Ad5), the AdΔΔ mutant (AdE1ACR2- and AdE1B19K-deleted), the nonreplicating Ad5-GFP mutant (CMV-GFP cassette replacing E1-genes), and dl312 (E1A- and E3B-deleted) were produced and characterized as previously described (Öberg et al., 2010; Wang et al., 2003). The viral particle (vp) to infectious units (pfu) was 10–40 vp/pfu for all viruses. Curcumin, EGCG (Merck Biosciences, UK), equol, genistein (Apin Chemicals, UK), resveratrol (Sigma, USA), and trichostatin-A (Calbiochem, USA) were reconstituted in dimethyl sulfoxide (DMSO).
Cell viability and synergy assays
Cell viability was evaluated 3–6 days after treatment with phytochemicals or inhibitors or infection with virus by the MTS assay (Promega). Dose–response curves were generated, and EC50 values (effective concentration killing 50% of cells) were calculated as previously described (Cheong et al., 2008; Radhakrishnan et al., 2010). In combination assays, fixed concentrations of phytochemicals were selected that killed 10%–30% of cells alone. Synergistic interactions were evaluated by combining serially diluted phytochemicals (0.001–500 μM) and viral mutants (0.004–100,000 particles per cell [ppc]) at constant ratios to determine combination indexes (CIs) (Chou, 2006; Cheong et al., 2008). The combination ratios (ppc:nM) were 1:125, 1:625, 1:3125, and 1:15,625 (DU145 and PC3) or 1:250, 1:1250, 1:6250, and 1:31,250 (22Rv1). CI values≤0.9 were considered synergistic; 0.9<CI<1.1, additive; and CI values≥1.1, antagonistic (Chou, 2006). Each data point was generated from triplicate samples, n=3–5.
Immunoblotting
Cells were lysed in 5xRIPA buffer (0.75 M NaCl, 5% NP40, 2.5% deoxycholic acid, 0.5% sodium dodecyl sulfate [SDS], and 0.25 M Tris, pH 8.0) containing a protease inhibitor cocktail (Roche). Total protein (10–20 μg) was analyzed on 10%–15% SDS reducing polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes (Invitrogen) and detected by the following antibodies: hexon (1:2000; Autogen Bioclear), E1A (1:1000; Santa Cruz), β-tubulin (1:20,000; Sigma), actin (1:1000, Santa Cruz), poly ADP ribose polymerase (PARP) (1:200; Santa Cruz Biotechnology), and secondary antibodies conjugated to horseradish peroxidase (Dako). Visualization was by ECL Western Blot Detection Reagent (GE Healthcare, UK).
Quantitative PCR
DNA was extracted 4, 24, 48, and 72 hr after treatment using the DNA Blood Mini Kit (Qiagen) and viral genomes quantified in 10 ng of total DNA with specific primers and SYBR Green Master mix as described (Leitner et al., 2009). Results are expressed as the ratio of viral genome copies at each time point relative to that at 4 hr after infection to normalize for cellular uptake. Data are presented from representative studies, n≥3.
Reverse-transcription quantitative PCR
Total RNA was extracted using the RNeasy kit (Qiagen) 3–48 hr after treatment, first-strand cDNA was synthesized from 1 μg of total RNA using MMLV-reverse transcriptase and random hexamer primers followed by specific primers for E1A and 18S RNA as described (Leitner et al., 2009). Results are expressed as the ratio of E1A to 18S cDNA in each sample, n=3.
Viral replication assays (TCID50)
Cells and media were collected 24–72 hr post-infection, freeze-thawed, and analyzed by the limiting dilution assay (TCID50) on HEK293 cells (Wang et al., 2003). Each sample was determined in triplicate, data from three independent studies were averaged and expressed as pfu/cell±SD.
Flow cytometry
For uptake of virus, cells (1×105) were pretreated for 24 hr with equol (100 μM), resveratrol (10 μM), or media alone; infected with Ad5GFP (22Rv1: 25 ppc; DU145: 50 ppc, PC3: 300 ppc); and 48 hr later stained with 2.5 μg/ml propidium iodide (PI; Invitrogen) and analyzed on a FACSCalibur cytometer (Becton Dickinson Immunocytometry Systems). Cell surface receptor expression was determined using mouse monoclonal antibodies targeting: CAR (1:500; ATCC), αVβ3-integrin (1:400; Chemicon), αVβ5-integrin (1:520; CRUK) and a secondary goat anti-mouse F(ab′)2 fluorescein isothiocyanate–conjugated antibody (1:30; Dako). For cell cycle analysis, cells were treated as above, washed in phosphate-buffered saline, fixed in 70% ethanol, treated with 5 μg RNase A (Sigma) and 10 μg PI, and analyzed on the FACSCalibur. Cell death markers were identified by detection of caspase-3 activation (Caspase-3 antibody Apoptosis Kit; BD Pharmingen), Annexin V staining (Alexa Fluor 488 conjugate; Molecular Probes/Invitrogen) and changes in mitochondrial membrane potential (Δψm) with tetramethylrhodamine ethyl ester perchlorate (Molecular Probes/Invitrogen) as previously described (Leitner et al., 2009).
In vivo tumor growth
PC-3 cells (1×107 cells) were grown subcutaneously in either one or both flanks of C57Bl/6 nu/nu or CD1 nu/nu mice as previously described (Öberg et al., 2010). Suboptimal doses of AdΔΔ (1×108–1×109 vp/injection) were administered intratumorally on day 2, 4, and 6 and 20–40 μg of equol or resveratrol (1 mg/ml in DMSO) administered intraperitoneally on day 1, 3, and 5. Control animals (mock) were treated with dl312 (50 μl) intratumorally and DMSO (20–40 μl) intraperitoneally at the same time points. Tumor growth was monitored by measurements twice weekly: volume=(length×width2×p)/6. Treatments were initiated when tumors were 100±20 μl and tumor growth and progression followed until tumors reached 1.44 cm2 (according to animal welfare regulations; UK Home Office). Measurements were double-blinded and analyzed by two-way ANOVA with Bonferroni multiple comparison post-tests; n=5–8/group. Tumor samples were collected and processed for immunohistochemistry when treatment groups were terminated. An anti-hexon antibody was used as previously described (Cheong et al., 2008; Radhakrishnan et al., 2010), representative images are presented after analyzing 10 high-powered fields of each section (three sections per tumor, three tumors per group).
Ethics statement
Animal studies were carried out in strict accordance with the UK Home Office Guidelines for Animals (Scientific Procedures) and the UKCCCR Guidelines for the Welfare of Animals in Experimental Neoplasia. All protocols were approved by the Committee on the Ethics of Animal Experiments of Queen Marys University London under the Home Office project license PPL 70/6393.
Statistical analysis
Statistical comparisons were carried out by one-way or two-way ANOVA with Bonferroni multiple comparison post-tests or Tukey's multiple comparison tests, or unpaired or paired two-tailed Student's t-test, as indicated in the figure legends. All data were prepared using the GraphPad Prism software version 4, and p values were considered significant if <0.05, very significant if <0.01, and extremely significant if <0.001.
Results
Phytochemicals enhance adenovirus-induced cell killing in prostate cancer cells
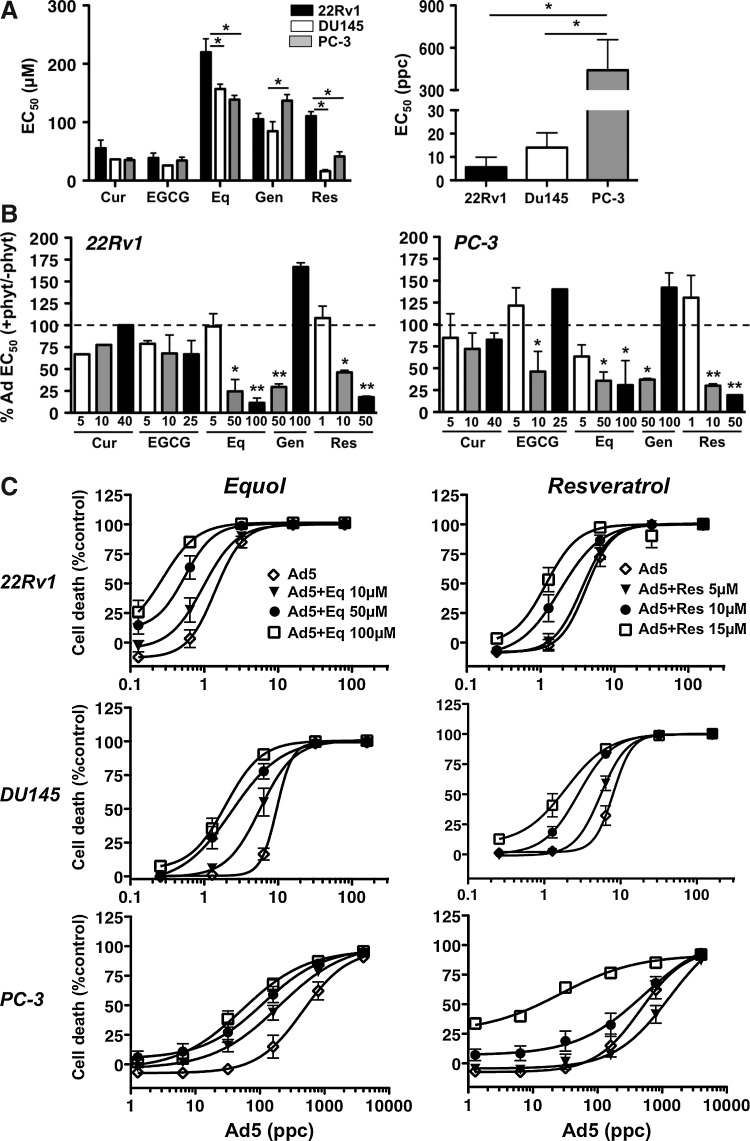
Cytotoxicity of curcumin, EGCG, equol, genistein, and resveratrol was assessed in one androgen receptor (AR)-positive (22Rv1) and two AR-negative (DU145 and PC-3) cell lines (Fig. 1A). Equol and genistein were the least cytotoxic, while curcumin and EGCG had more potent effects. The 22Rv1 cells were less sensitive to equol and resveratrol compared to DU145 and PC-3 cells (p<0.05). The cytotoxicity of wild type virus (Ad5) was highest in 22Rv1 and DU145 cells, with EC50 values of 5.6±4.3 ppc and 14.1±6.3 ppc, respectively, while PC-3 cells were highly insensitive at 430±182 ppc (Fig. 1A). In combination with phytochemicals at fixed nontoxic doses (killing <10%), Ad5 efficacy was enhanced, both in the virus-sensitive 22Rv1 and the virus-insensitive PC-3 cells. Some sensitization was observed with all phytochemicals, with significant decreases in EC50 values in 5–6 out of 14 combinations in each cell line (Fig. 1B). Similar effects were observed in DU145 cells (data not shown). Equol and resveratrol caused the greatest sensitization to Ad5 that was also dose dependent, in comparison to other phytochemicals that did not show dose-dependent sensitization (Fig. 1B, C). The cytotoxicity of equol and resveratrol was also increased in the presence of low doses of Ad5 (Fig. 1D). Importantly, in normal primary epithelial (NHBE) cells, sensitization was not detected when the same doses of equol or resveratrol were combined with Ad5 (Table 1). These results indicate that cancer-specific cell killing is greatly enhanced by combining adenovirus with selected dietary phytochemicals.
FIG. 1.
Adenovirus-induced cell killing is significantly increased in combination with phytochemicals in prostate cancer cells. (A) EC50 values for curcumin (Cur), epigallocatechin gallate (EGCG), equol (Eq), genistein (Gen), resveratrol (Res), and adenovirus wild type (right panel; Ad5) in 22Rv1, PC-3, and DU145 cells 6 days after treatment, averages±SEM, n≥2, *p<0.05; two-way ANOVA with Bonferroni post-test (left panel); one-way ANOVA with Tukey's multiple comparison test (right panel). (B) Changes in Ad5 EC50 values when combined with increasing doses (1–100 μM) of each phytochemical in 22Rv1 and PC-3 cells. Data are percentages of the EC50 for Ad5 alone, averages±SEM, n≥2, one-way ANOVA with Tukey's multiple comparison test on EC50 values; bars below the dashed lines illustrate sensitization compared with Ad5 alone. (C) Ad5 dose–response curves in 22Rv1, DU145, and PC-3 cells with and without equol or resveratrol at three doses. Cell death expressed as percentages of uninfected controls, averages±SD, n=3, dose-dependent sensitization for all conditions compared with virus infection alone, except for 5 μM resveratrol. The EC50 values for Ad5 and each phytochemical+Ad5 were analyzed for statistical significance; one-way ANOVA with Bonferroni multiple comparison tests, p<0.05 for the two highest doses of phytochemicals in all cell lines except in PC-3 cells with resveratrol, for which only the highest dose was significant. (D) Dose–response to equol (E) or resveratrol (R) alone or in combination with Ad5 (22Rv1: 1 particle per cell (ppc), PC-3: 100 ppc) in 22Rv1 and PC-3 cells. Data are shown as percentages of the EC50 for phytochemicals alone, averages±SEM of two experiments. EC50 values were analyzed for statistical differences for each phytochemical in each cell line, unpaired two-tailed Student's t-test.
Table 1.
Normal Human Bronchial Epithelial Cells Are Not Sensitized to Ad5 or AdΔΔ by Equol or Resveratrol
| Treatment | EC50 (ppc) |
|---|---|
| Ad5 | 6.6±0.4 |
| Ad5+equol 50 μM | 11.7±3.7 |
| Ad5+resveratrol 10 μM | 8.5±1.3 |
| Ad5+resveratrol 15 μM | 13.6±4.1 |
| AdΔΔ | 1.8±0.5 |
| AdΔΔ+equol 50 μM | 2.2±0.9 |
| AdΔΔ+resveratrol 10 μM | 2.4±0.7 |
| AdΔΔ+resveratrol 15 μM | 3.6±1.1 |
EC50 values determined for each virus with and without fixed doses of equol or resveratrol, n=2.
Equol and resveratrol increase viral uptake
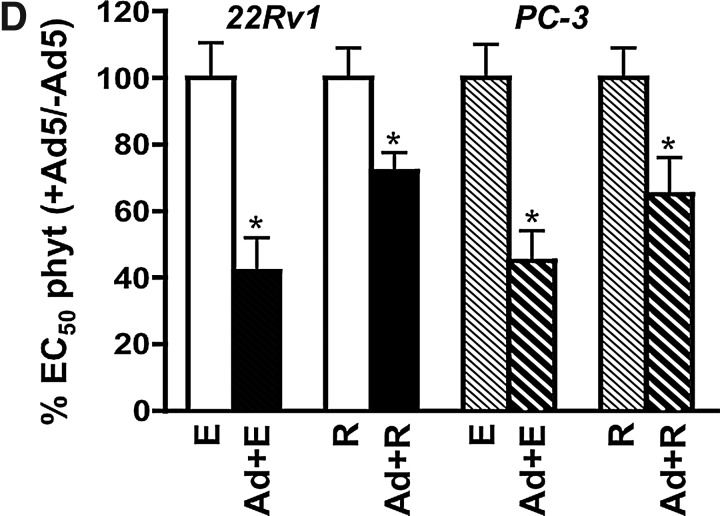
To investigate whether cellular permissiveness to virus was improved by equol or resveratrol, we first quantified viral uptake. In the presence of low nontoxic doses of equol and resveratrol, viral infectivity was significantly increased (0.001<p<0.05) in all three cell lines, as determined by AdGFP infection (Fig. 2A) and by quantification of viral genome copies (Fig. 2B). The greatest increases were observed with resveratrol in DU145 and with equol in PC-3 cells. The small but statistically significant 5%–40% (p<0.05) increases in AdGFP expression in 22Rv1 cells was comparable to that observed in cells treated with trichostatin A (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum), an established inducer of adenoviral uptake (Hemminki et al., 2003). Viral uptake was lowest in PC-3 and highest in 22Rv1 cells, correlating with the relative sensitivity to virus-induced cell death (Fig. 1A). PC-3 cells had low levels of all three major viral receptors, explaining the lower infectability; 2.9±0.2% (CAR), 4.8±0.1% (αVβ3) and 6.5±0.2% (αVβ5) (Fig. 2C). However, both equol and resveratrol significantly increased the population of cells expressing integrins (p<0.05), while CAR expression was unaffected (Fig. 2C). DU145 cells expressed the highest levels of all receptors although only the integrins were significantly increased (p<0.05) from 44±2% to 58±4% with equol and to 67±6% with resveratrol for αVβ3, and from 11±2% to 13.7±1% and 29.3±7% for αVβ5, respectively (Fig. 2C). The 22Rv1 cells did not express detectable levels of either class of integrins (<3% cells) and expression was not induced by equol or resveratrol. However, CAR levels increased from 66±1% to 71±1% by equol and to 73±2% by resveratrol (p<0.05). Interestingly, resveratrol and, to a lesser degree, equol stimulated cell surface expression of at least one receptor type in each cell line, suggesting that increased binding of virus and/or internalization contributed to the improved infectability (Fig. 2A, B). However, the increases in both viral uptake and receptor expression were too modest to fully explain the potent enhancement of cell killing with the combination treatments.
FIG. 2.
Equol and resveratrol enhance viral uptake but attenuate viral replication. (A) Cells were infected with the nonreplicating Ad5GFP mutant alone (22Rv1, 25 ppc; DU145, 50 ppc; PC-3, 300 ppc) and in combination with equol (100 μM) or resveratrol (10 μM) and analyzed by flow cytometry 48 hr later. Results are percent GFP-positive cells in combination-treated versus AdGFP infected alone; averages±SD, n=3. (B) Cells were infected with wild-type virus (Ad5) (22Rv1, 25 ppc; DU145, 50 ppc; PC3, 300 ppc) and treated with equol or resveratrol as above. Viral E2A DNA was quantified by quantitative PCR (qPCR) 4 hr after infection. Data are normalized to cellular GAPDH DNA in each sample and to viral E2A DNA in cells infected with Ad5 alone, averages±SD, n=3. (C) Equol (100 μM) and resveratrol (10 μM) increase expression of cell surface receptors essential for adenoviral entry. The proportion of receptor-positive cells was determined by flow cytometry 24 hr after treatment, averages±SD, n=3. (A–C) Data analyzed in each cell line by one-way ANOVA with Bonferroni multiple comparison test, compared to untreated cells. (D) Viral DNA amplification over time (3–72 hr) was determined by qPCR analysis with primers to hexon DNA in 22Rv1, DU145, and PC-3 cells infected with Ad5 (100 ppc) with and without the addition of equol (E, 100 μM) or resveratrol (R, 10 μM). Results are normalized to cellular actin DNA in each sample and to viral DNA present 3 hr after infection with Ad5 alone, averages±SD, n=3. (E) Viral replication over time (3–72 hr) was determined by TCID50 assays in samples treated as described above for 22Rv1, DU145, and PC-3 cells. Results are averages±SD, n=3. (D–E) Two-way ANOVA with Bonferroni post-tests comparing each result over the time period to Ad-infected untreated cells for each time point. (F) Cells infected with Ad5 (22Rv1 and DU145, 100 ppc; PC-3, 300 ppc) with and without simultaneous additions of equol (E, 100 μM) or resveratrol (R, 10 μM) analyzed for viral E1A mRNA and protein expression 24 and 48 hr later, averages±SD, n=2–3, representative immunoblots.
Viral replication but not early viral gene expression is attenuated in the presence of equol or resveratrol
Although viral uptake was increased in response to equol and resveratrol, viral genome amplification was significantly reduced (p<0.05) in PC-3 and DU145 cells (48–72 h; Fig. 2D). Reduced levels were also observed in 22Rv1 cells but were only significantly lower with resveratrol 72 hr after treatment. The reduced amplification of DNA was accompanied by attenuated viral replication, with the highest virion production in DU145 cells (1.6×104±5.3×103 pfu/cell), with lower levels in PC-3 (1.1×103±4.1×102 pfu/cell) and 22Rv1 cells (1.9×103±7.1×102 pfu/cell) (Fig. 2E). In DU145 and PC-3 cells equol or resveratrol significantly reduced replication up to 10-fold at 72 hr (DU145, p<0.01; PC-3, p<0.001) and a smaller decrease was observed in 22Rv1 cells, as expected from the genome data (Fig. 2D). Interestingly, E1A mRNA and protein levels were expressed at high levels, similar to those in untreated cells (Fig. 2F), although the expression of late proteins was not increased, in agreement with the replication data (data not shown). These findings demonstrate that equol and resveratrol attenuate viral DNA amplification but not early gene expression, indicating a role for E1A and/or other early viral genes in the enhancement of cell killing.
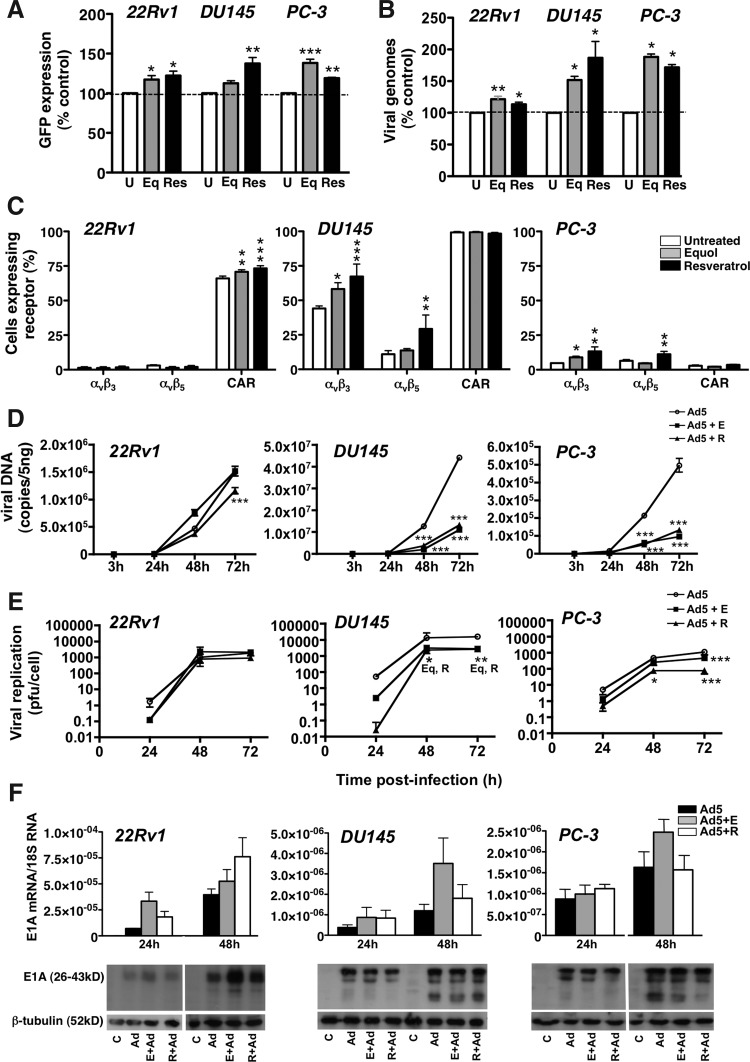
Adenoviruses enhance phytochemical-induced apoptosis in PC-3 and DU145 but not in 22Rv1 cells
Initial studies revealed that both phytochemicals increase the proportion of cells in sub-G1, indicating that apoptotic pathways might be induced (Supplementary Fig. S2). Further studies showed that equol and resveratrol increased mitochondrial depolarization (Δψm) in all three cell lines, while Ad5 did not cause apoptotic death, as expected (Fig. 3A) (Abou El Hassan et al., 2004; Leitner et al., 2009). Depolarization was further increased over time in combination with Ad5 in PC-3 and DU145, but not in 22Rv1 cells. The proportion of PC-3 cells with intact Δψm significantly (p<0.001) decreased from 89% (virus), 57% (equol), and 51% (resveratrol) to 34% (equol/virus) and 29% (resveratrol/virus). A smaller decrease was observed in combination-treated DU145 cells. In contrast, in 22Rv1 cells, Ad5 attenuated the equol- and resveratrol-induced reductions in Δψm (Fig. 3A). These findings were verified at the molecular level, with parallel increases in active caspase 3 in PC-3 and DU145 cells in combination with Ad5, while virus prevented further caspase activation in 22Rv1 cells (Fig. 3B). The induction of apoptotic pathways was confirmed in PC-3 and DU145 cells which showed increased membrane exposure of phosphatidylserine over time in combination-treated cells (Fig. 3C). When the pan-caspase inhibitor zVAD-fmk was included in cell viability assays, sensitization was completely blocked in DU145 cells, and in the PC-3 cells viral EC50 values were significantly (p<0.01) decreased (Fig. 3D and Table 2). The inhibitor did not prevent sensitization in 22Rv1 cells, although the inhibitor did reduce equol- and resveratrol-induced caspase 3 activation in the absence of virus (Fig. 4F). These data demonstrate that equol and resveratrol caused caspase-dependent apoptotic death in all three prostate cancer cell lines, but in combination with virus, apoptosis was further induced only in DU145 and PC-3 cells. Furthermore, the sensitization to cell killing could be fully blocked by a pan-caspase inhibitor in DU145 cells and partly in PC-3 cells.
FIG. 3.
Resveratrol and equol induce apoptosis. (A) Cells infected with Ad5 (22Rv1 and DU145, 100 ppc; PC-3, 300 ppc) (open diamond dashed line) and equol at 100 μM (open square dashed line) or resveratrol at 10 μM (open circle dashed line) were added alone or in combination with Ad5 (equol; black square solid line or resveratrol black circle solid line). Cells were stained with tetramethylrhodamine ethyl ester perchlorate (TMRE) and 4′,6-diamidino-2-phenylindole (DAPI) and analyzed by flow cytometry after 24–96 hr. Cells that retained TMRE staining (intact, active mitochondria) and remained negative for DAPI (intact cellular membrane) were considered live, means of duplicates±SD, n=3, expressed as percent of live cells (Δψm). Two-way ANOVA with Bonferroni post-tests, significant differences indicated at 96 hr: 22Rv1; °°combination-treated or ***single agent treated compared to untreated cells, DU145; °°°combination-treated or ***single-agent treated compared to untreated or virus infected cells, PC-3: °°°combination-treated compared to each single agent treatment, and ***single agent treatments compared to untreated cells. B) Cells infected with Ad5 and treated with phytochemicals as described in A, analyzed by flow cytometry for active caspase 3, averages±SD of one experiment representative of three independent studies. Significant results at 96 hr, two-way ANOVA with Bonferroni post-tests: 22Rv1; *single agents compared to untreated cells, PC-3 and DU145; °°°combination-treated compared to each single agent treated; and ***single agents compared to untreated cells. (C) Cells were infected with Ad5 as described in A and treated with equol at 50 or 100 μM or resveratrol at 5 or 10 μM. After 24–96 hr, cells were stained with Alexa fluor 488–conjugated annexin V and propidium iodide (PI). Cells that were negative for annexin V and PI cells were plotted as live cells under each condition. Results are means of duplicates from two to four experiments±SD. Significant results at 96–120 hr, two-way ANOVA with Bonferroni post-tests: DU145; *treatments compared to single agent or to °untreated, PC-3; Eq combinations compared to single agent treated, Res combinations compared to virus-infected cells. (D) Cells infected with Ad5 (0.256–10,000 ppc), with or without the pan-caspase inhibitor zVAD-fmk at 25 μM, and with or without equol 100 μM or resveratrol 10 μM. Cell viability was assessed 6 days post-infection, one representative study out of two to three. Two-way ANOVA with Bonferroni post-tests on the corresponding average EC50 values; ***Ad5 EC50 versus both phytochemicals in all three cell lines, sensitization was significantly reduced with zVADfmk in DU145 (***) and PC-3 cells (**).
Table 2.
Changes in Ad5 EC50 Values in 22Rv1, PC-3, and DU145 Cells Treated With and Without Equol (Eq) or Resveratrol (Res) in Combination With the Pan-Caspase Inhibitor zVAD-fmk
| |
% of Ad5 EC50 |
|
|
|---|---|---|---|
| 22Rv1 | PC-3 | DU145 | |
| Ad5 | 100±14 | 100±23 | 100±9 |
| + 25 μM zVAD | 71±11 | 59±13 | 76±12 |
| Ad5+50 μM Eq | 19±3 | 29±11 | 26±2 |
| + 25 μM zVAD | 5±4 | 47±7 | 104±6 |
| Ad5+10 μM Res | 71±9 | 33±8 | 88±3 |
| + 25 μM zVAD | 66±10 | 57±12 | 128±18 |
Data expressed as % of Ad5 EC50-values, averages±SD, n=2–4.
FIG. 4.
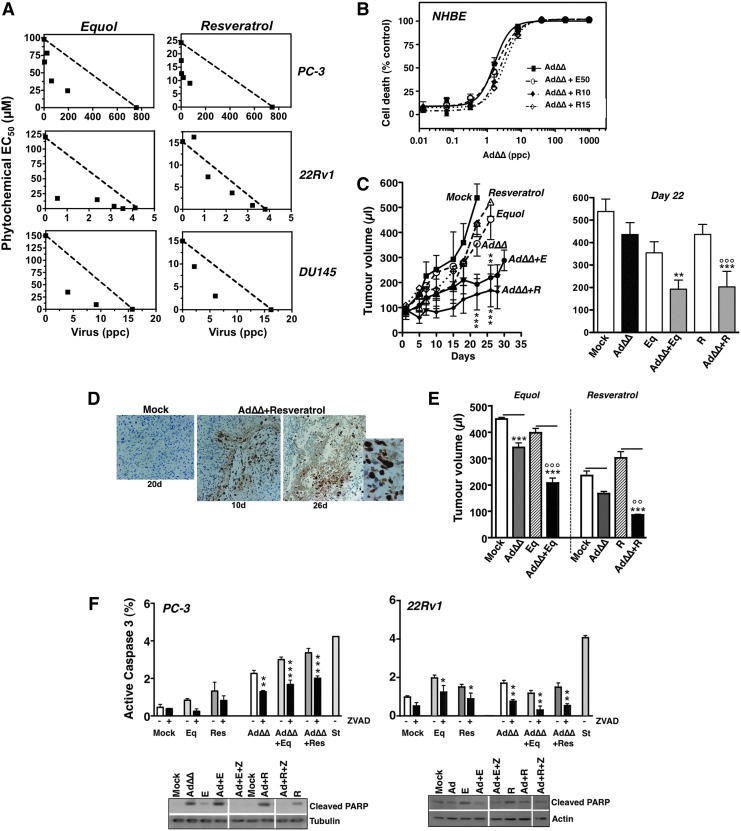
AdΔΔ interacts synergistically with equol and resveratrol and inhibits tumor growth in vivo. (A) Isobolographs generated from EC50 values at two to four constant ratios of equol or resveratrol in combination with the replication-selective AdΔΔ in PC-3, 22Rv1, and DU145 cells. The straight lines represent the theoretical additive values with synergistic (CI ≤0.9) and antagonistic (CI ≥1.1) interactions illustrated by data points under and above the lines, respectively. Representative data, n=3. (B) Dose–response to AdΔΔ with and without equol (50 μM) or resveratrol (10 and 15 μM) in normal primary epithelial (NHBE) cells. Representative data, n=2. (C) PC-3 tumor xenografts were grown subcutaneously in one flank in C57Bl6 athymic mice and treated with suboptimal doses of AdΔΔ (3×108 vp per injection) on day 2, 4, and 6, or equol (40 mg/kg) or resveratrol (20 mg/kg) on day 1, 3, and 5, or a combination of AdΔΔ and each phytochemical, 5–8 animals/group, repeated twice. Two-way ANOVA with Bonferroni multiple comparisons post-tests, *** resveratrol and virus at 22 and 26 days compared to resveratrol alone; **equol and virus treated compared to equol alone. Right panel: At day 22 after treatment combination-treated tumors were smaller than AdΔΔ-treated and mock-treated tumors (***). AdΔΔ+resveratrol–treated tumors were significantly different from resveratrol-only tumors (°°°). (D) Immunohistochemistry (IHC) staining for the late viral hexon protein in tumors treated with AdΔΔ and resveratrol, 10 days (10× magnification) and 26 days (right two images; 10× and 40× magnification) after treatment. Mock-treated (nonreplicating dl312 mutant) tumors 20 days after treatment (left image; 20× magnification). (E) Tumor growth in animals with two tumors each; one tumor injected with AdΔΔ or dl312 and both tumors subjected to resveratrol, equol, or vehicle administered intraperitoneally. Tumor measurements on day 16 after treatment, n=8. Simultaneous infection with AdΔΔ significantly reduced tumor growth compared to corresponding noninfected cells (***), and compared to AdΔΔ alone (°° and °°°). The horizontal lines indicate the paired tumors in each animal group. Two-way ANOVA with Bonferroni multiple comparisons post-tests. (F) Activation of caspase 3 in PC-3 and 22Rv1 cells infected with AdΔΔ (PC-3, 300 ppc; 22Rv1, 10 ppc) and treated with equol (E, 100 μM), resveratrol (R, 10 μM), or zVAD-fmk (25 μM) for 72 hr at the indicated combinations. Activation of caspase 3 was determined by flow cytometry, averages±SD, n=3; two-way ANOVA with Bonferroni multiple comparisons post-tests comparing cells treated with and without zVADfmk. Lower panels: Immunoblotting of cells treated as described for the caspase 3 analysis and cleaved PARP was identified. E, equol; R, resveratrol; Z, zVADfmk.
The tumor-selective AdΔΔ mutant synergistically enhances cell killing with equol and resveratrol
Next we explored whether our potent replication-selective AdΔΔ mutant also enhanced cell killing in combination with equol and resveratrol. In PC-3 cells the phytochemicals caused strong synergy with both Ad5 and AdΔΔ, with CI values<0.9 in 14 out of 16 conditions at four different ratios (Fig. 4A and Table 3). In 22Rv1 and DU145 cells, at least one combination ratio resulted in strong synergistic effects on cell killing. No sensitization was observed in normal NHBE cells, either with AdΔΔ or Ad5 when combined with phytochemicals at the same low doses (Fig. 4B and Table 1). These results demonstrate that the oncolytic AdΔΔ mutant can selectively enhance equol- and resveratrol-induced cancer cell killing to similar or even higher levels than with wild-type virus. In contrast, the nonreplicating E1A-deleted mutant dl312 did not synergize with any of the phytochemicals; the EC50 value for dl312 was >1×105 ppc and was unaffected by the combination with equol or resveratrol (not shown), suggesting that the synergistic effects are specific and involve early viral gene expression and viral replication.
Table 3.
Average Combination Indexes (CI) of Adenoviral Mutants in Combination with Equol or Resveratrol in Prostate Cancer Cell Lines
| |
|
Ad5 |
AdΔΔ |
||
|---|---|---|---|---|---|
| Ratio (ppc/nM) | Equol | Resveratrol | Equol | Resveratrol | |
| PC-3 | 1:125 | 0.35±0.09 | 0.40±0.31 | 0.46±0.06 | 0.61±0.12 |
| 1:625 | 0.27±0.10 | 0.37±0.42 | 0.52±0.07 | 0.69±0.18 | |
| 1:3125 | 0.38±0.27 | 0.46±0.40 | 0.72±0.16 | 0.80±0.28 | |
| 1:15,625 | 0.92±0.17 | 0.99±0.09 | 0.72±0.07 | 0.89±0.16 | |
| DU145 | 1:15,625 | 0.65±0.17 | 0.94±0.10 | 0.34±0.21 | 0.42±0.07 |
| 22Rv1 | 1:6250 | 0.81±0.09 | 0.78±0.10 | 0.79±0.10 | 0.80±0.07 |
| 1:31,250 | 0.83±0.05 | 0.60±0.33 | 0.44±0.12 | 1.02±0.12 | |
Data are presented as CI averages±SD, n≥3.
CI ≤0.9=synergistic effects; CI ≥1.1=antagonistic; 0.9<CI<1.1=additive effects.
Combination treatments with AdΔΔ and phytochemicals cause potent tumor regression in PC-3 xenograft models
We previously demonstrated that AdΔΔ inhibited growth of both DU145 and PC-3 tumor xenografts in athymic mice in a dose-dependent manner (Öberg et al., 2010). In this study, animals with PC-3 xenografts were treated with low doses of AdΔΔ (3×108 vp), equol (40 mg/kg), or resveratrol (20 mg/kg) (Fig. 4C; left panel). No agent alone was efficacious at these low doses, while in combination with AdΔΔ tumor growth rates were reduced. Tumors were significantly smaller (0.01<p<0.001) 22 days after treatment in combination-treated animals, compared to each single agent treatment groups (Fig. 4C; right panel). Animals with AdΔΔ and mock-treated tumors were discontinued after 22 days due to tumor burden, while after 26 days, combination-treated tumors were still significantly (0.01<p<0.001) smaller than phytochemical-treated tumors. Expression of the late viral hexon gene was detected in all tumors infected with AdΔΔ but not in mock-infected (dl312) control tumors at these time points (Fig. 4D), indicating that replication and spread can proceed over time, even in the presence of phytochemicals that might attenuate initial replication. In areas with high levels of hexon staining, the appearance of dead/necrotic tissue was also noted at the later time points, indicating virus and/or phytochemical induced cell killing (26 days after treatment; Fig. 4D; right panels). In a second study, animals with tumors in both flanks were given phytochemicals intraperitoneally and AdΔΔ or dl312 in one tumor (Fig. 4E). Tumors treated with both resveratrol and AdΔΔ were significantly smaller (91.0±6.3 μl; p<0.01) than tumors treated only with resveratrol (307.9±64.5 μl) or AdΔΔ (172.4±22.3 μl). Similar growth reductions were observed for tumors treated with equol and AdΔΔ (212.7±51.82; p<0.001) compared to those treated only with equol (403.5±45.19) or AddΔΔ (347.3±47.01). In conclusion, all tested combination treatments had higher antitumor efficacy compared with single-agent treatments.
The synergistic effects of AdΔΔ in combination with equol and resveratrol are caused by increased caspase 3 activation in PC-3 but not in 22Rv1 cells
Similar to the findings with wild-type Ad5, the AdΔΔ mutant potentiated equol- and resveratrol-induced caspase 3 activation (Fig. 4F). Addition of the pan-caspase inhibitor zVAD attenuated the activation of caspase 3 in cells treated with single agents (equol, resveratrol, and AdΔΔ), as well as in the combination-treated cells. However, complete inhibition of activation could not be achieved under these conditions. Under identical conditions, PARP cleavage was detected at high levels in the combination treated PC-3 cells and was subsequently blocked by addition of zVAD (Fig. 4F; lower left panel), suggesting a decrease in synergistic cell killing, in agreement with the Ad5 sensitization data (Fig. 3D). In DU145 cells we noted almost identical effects on caspase 3 activation as in the PC-3 cells (not shown). In contrast, in 22Rv1 cells, AdΔΔ in combination with equol or resveratrol caused no further increase in caspase 3 or PARP cleavage (Fig. 4F; right panels). Interestingly, all combinations with AdΔΔ or Ad5 in 22Rv1 cells seemed to attenuate phytochemical-mediated induction of these markers, despite potent enhancement of cell killing (Fig. 1C, D, 4A and Table 3). These findings demonstrate that caspase-regulated, apoptotic death plays a significant role in the synergistic cell killing in PC-3 cells but not in 22Rv1 cells.
Discussion
Here we demonstrate that oncolytic virotherapy can be greatly improved by combining the recently developed tumor-selective AdΔΔ mutant with dietary cancer-preventive phytochemicals, both in treatment-sensitive (22Rv1, DU145) and treatment-insensitive (PC-3) prostate cancer models. We previously reported that combining AdΔΔ and the current standard of care for treatment-resistant prostate cancers, the chemotherapeutics docetaxel and mitoxantrone, was highly efficacious (Öberg et al., 2010). Neither cytotoxic drugs nor phytochemicals had detectable effects in normal cells, nor did the treatments sensitize normal cells to AdΔΔ or wild-type virus. We propose that AdΔΔ and phytochemicals may cause fewer unwanted clinical side effects than similar combinations with mitoxantrone or docetaxel, even though the administered dose levels might be reduced for both types of compounds. Furthermore, the sensitization to virus by low doses of phytochemicals, in particular by resveratrol, greatly reduced tumor growth in vivo. Optimization of the dose and mode of delivery is likely to further improve the antitumor efficacy of this combination. Oral administration at higher doses might be preferable, which might allow the generation of active metabolites, as has previously been reported for curcumin and equol (Yuan et al., 2007; Goel and Aggarwal, 2010).
All tested phytochemicals, including curcumin, EGCG, genistein, equol, and resveratrol, improved cell killing in combination with Ad5, in agreement with previous reports on the anticancer efficacy for these compounds (Surh, 2003; de Souza et al., 2010). We focused our studies on equol and resveratrol because of the consistent and potent synergistic effects in combination with AdΔΔ both in AR-positive and -negative prostate cancer cell lines. Interestingly, it was previously reported that both equol and resveratrol can act as anti-androgens by down-regulating the AR, either via inhibition of the Raf/MEK/ERK pathway, inhibition of 5α-reductase, and/or by acting as potent estrogen receptor agonists (Gao et al., 2004). Our data suggest that additional mechanisms must be involved in the synergistic responses in the AR-negative PC-3 and DU145 cells. Importantly, in the virus- and chemotherapy-insensitive PC-3 cells, three- to eightfold reductions in viral EC50 values were achieved in combination with equol and resveratrol, with further decreases at higher doses (15 μM) of resveratrol.
We found that viral replication was initially attenuated by both resveratrol and equol in all cell lines. However, replication did increase over time, and while the absolute levels of new progeny particles were lower up to 72 hr after infection compared to untreated cells, these lower levels of replication were sufficient to contribute to the synergistic cell killing in all three cell lines. In contrast, E1A was still potently expressed under all conditions. These findings suggest that E1A might play a role as a sensitizer to the phytochemicals, as previously demonstrated in combination with chemotherapeutics (Liao et al., 2004; Öberg et al., 2010; Radhakrishnan et al., 2010). Furthermore, we recently found that nonreplicating adenoviral mutants expressing only the small E1A protein also interacted synergistically with various cytotoxic compounds, including equol and resveratrol, albeit with lower efficacy than with replicating mutants (Adam et al., unpublished data; Miranda et al., unpublished data). A factor contributing to the potent expression of E1A, but not fully explaining the synergistic effects, was the increased viral uptake in the presence of equol and resveratrol. The improved infectability was caused by parallel increases in at least one viral receptor, either CAR or αVβ3 and αVβ5 integrins. Phytochemicals have been reported to act on cellular pathways, such as the MAPK cascade, that promote cell surface expression of the viral receptors (Hedlund et al., 2003; Signorelli and Ghidoni, 2005). In light of the poor infectability of PC-3 cells with serotype C adenoviruses such as Ad5, it would be interesting to explore whether uptake of serotype B viruses, which utilize the ubiquitously expressed CD46 receptor, would also be enhanced by the phytochemicals. PC-3 cells have been shown to have both high levels of CD46 and are sensitive to the type B mutant Ad11p (Sandberg et al., 2009). Nevertheless, it is interesting to note that even in the presence of increased viral uptake in response to equol and resveratrol, viral replication was decreased, while S-phase entry was increased in all prostate cancer cells (data not shown). In combination-treated cells, the S-phase population was further increased both with Ad5 and AdΔΔ, suggesting that causes for the attenuated viral replication involved mechanisms at stages beyond S-phase entry. Our findings indicate that equol and resveratrol support rather than prevent early viral gene expression that is essential for the synergistic effects.
To achieve potent antitumor efficacy in vivo it is essential that oncolytic mutants also replicate and spread within the tumor in order to induce direct viral lysis in addition to E1A-mediated cytotoxic interactions with the phytochemicals. We demonstrated that, despite lower levels of viral replication at early time points, late viral gene expression and necrotic tissue were detected up to 26 days after administration of the combination treatments in PC-3 xenografts, indicating that viral replication progressed and virus could spread and infect adjacent tumor cells. We and others have previously demonstrated that despite a potent inhibition of initial viral replication by gemcitabine and other cytotoxic drugs in tumor cells, AdΔ19K, AdΔΔ, and Ad5 remain in the cells and resume replication, both in cell culture and in xenografts in vivo once the drug has been metabolized, efficiently reducing growth of tumor xenografts (Raki et al., 2005; Leitner et al., 2009; Öberg et al., 2010). Our findings with equol and resveratrol demonstrated that replication is not prevented but rather attenuated immediately after phytochemical addition, and can proceed, reaching high levels at later time points even in vivo. Furthermore, all replicating viral mutants used in this study had an intact E3-region, allowing expression of E3B genes that we previously demonstrated is essential for viral propagation in the presence of the host immune system in vivo (Öberg et al., 2010; Wang et al., 2003).
Equol and resveratrol have been reported to activate numerous intracellular cell-signaling pathways. We found that both phytochemicals induced caspase-dependent apoptosis in the prostate cancer cells tested in this study. Resveratrol was previously reported to induce apoptosis and/or autophagy, acting on numerous intracellular effectors including inhibiting ERK, NF-κB, and cyclin D; activating p38 MAPK; down-regulating Bcl-2; and up-regulating p21, p27, and Bax (Signorelli and Ghidoni, 2005; Ulrich et al., 2005). Far less is known about the mechanisms of action for equol, although effects on apoptosis have been reported via the inhibition of NF-κB, MEK/ERK, and Akt (Hedlund et al., 2003). Adenoviral mutants have been implicated in numerous types of cell killing such as necrosis-like, apoptosis-like, and autophagy-mediated pathways depending on the cell line, and no definite mechanism has yet been convincingly identified (O'Shea et al., 2005; Cherubini et al., 2006; Ito et al., 2006; Jiang et al., 2011; Libertini et al., 2011). We and others have previously demonstrated that caspase-dependent apoptosis does not appear to be involved in Ad5 cell killing, although mutants deleted in the anti-apoptotic E1B19K gene can cause apoptotic death in some cell lines including pancreatic and prostate cancer cells (Abou El Hassan et al., 2004; Leitner et al., 2009; Öberg et al., 2010). The apoptosis-inducing properties of E1A have been well established, both alone and in combination with other cytoxic agents. However, during the course of infection, the viral anti-apoptotic proteins E1B19K and E1B55K counteract E1A-induced apoptosis. Furthermore, the viral E1A proteins interact with a plethora of cellular signaling molecules (Frisch and Mymryk, 2002). It is possible that these interactions also interfere with key signaling factors to turn the balance towards apoptosis (Ogier-Denis et al., 2000; Arico et al., 2001; O'Shea et al., 2005; Fimia and Piacentini, 2010). However, extensive pathway analysis would be required to delineate the underlying cellular responses to equol and resveratrol and determine how these mechanisms might be altered by concurrent infection with adenoviral mutants.
Our data also suggest that the exact molecular events are dependent on the specific gene alterations in each cell line. In DU145 cells, sensitization was caused by increased apoptosis that was efficiently blocked by a pan-caspase inhibitor, while in PC-3 cells only partial inhibition was obtained in response to the inhibitor. In contrast, in 22Rv1 cells there was no increase in caspase-3 activation in combination with virus, despite increased cell killing with the combination treatments. Preliminary findings also indicate that in 22Rv1 cells, autophagy-related pathways are potently activated by equol and resveratrol and might play a role in the synergistically enhanced cell killing (data not shown). Future in-depth studies would be necessary to identify the activated cell death pathways in response to the combinations in 22Rv1. In addition, the specific responses are likely to be dependent on the doses of equol and resveratrol and in this study have only been explored at low doses.
Earlier studies and epidemiological reports have suggested that numerous phytochemicals might function as potent agents for cancer prevention, especially for prostate, breast, and colon cancers (Surh, 2003; de Souza et al., 2010). We found that several of these dietary compounds also have direct anti-cancer efficacy in preclinical prostate cancer models and that efficacy could be significantly improved in combination with the potent replication-selective mutant AdΔΔ. Based on our findings presented in this report, we propose that nontoxic doses of equol or resveratrol in combination with cancer-selective adenoviral mutants warrants further investigation for development as a new promising strategy to treat prostate cancer.
Supplementary Material
Acknowledgments
The authors want to thank Dr. Daniel Öberg and Dr. Enrique Miranda for generating the replicating (AdΔΔ) and nonreplicating E1ACR2-deleted mutants, Dr. Gioia Cherubini for insightful discussions, and Dr. Subham Basu for invaluable suggestions and discussions (Molecular Oncology, Barts Cancer Institute). This project was supported by a CRUK PhD student fellowship (Dr. V. Adam) and grants from Barts & The London Charity, Cancer Research UK (C633-A6253/A6251 programme grant) and the Prostate Cancer Research Foundation (currently Prostate Action).
Author Disclosure Statement
No competing financial interests exist.
References
- Abou El Hassan M.A. van der Meulen-Muileman I. Abbas S. Kruyt F.A. Conditionally replicating adenoviruses kill tumor cells via a basic apoptotic machinery-independent mechanism that resembles necrosis-like programmed cell death. J. Virol. 2004;78:12243–12251. doi: 10.1128/JVI.78.22.12243-12251.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaza H. Miyanaga N. Takashima N., et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004;34:86–89. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- Anders M. Christian C. McMahon M., et al. Inhibition of the Raf/MEK/ERK pathway up-regulates expression of the coxsackievirus and adenovirus receptor in cancer cells. Cancer Res. 2003;63:2088–2095. [PubMed] [Google Scholar]
- Arico S. Petiot A. Bauvy C., et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- Bewley M.C. Springer K. Zhang Y.B., et al. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- Cheong S.C. Wang Y. Meng J.H., et al. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini G. Petouchoff T. Grossi M., et al. E1B55K-deleted adenovirus (ONYX-015) overrides G1/S and G2/M checkpoints and causes mitotic catastrophe and endoreduplication in p53-proficient normal cells. Cell Cycle. 2006;5:2244–2252. doi: 10.4161/cc.5.19.3263. [DOI] [PubMed] [Google Scholar]
- Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- de la Lastra C.A. Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol. Nutr. Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- de Souza P.L. Russell P.J. Kearsley J.H. Howes L.G. Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr. Rev. 2010;68:542–555. doi: 10.1111/j.1753-4887.2010.00314.x. [DOI] [PubMed] [Google Scholar]
- Dilley J. Reddy S. Ko D., et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- Ekblad M. Hallden G. Adenovirus-based therapy for prostate cancer. Curr. Opin. Mol. Ther. 2010;12:421–431. [PubMed] [Google Scholar]
- Fimia G.M. Piacentini M. Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol. Life Sci. 2010;67:1581–1588. doi: 10.1007/s00018-010-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag S.O. Stricker H. Peabody J., et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol. Ther. 2007;15:636–642. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- Frisch S.M. Mymryk J.S. Adenovirus-5 E1A: paradox and paradigm. Nat. Rev. Mol. Cell Biol. 2002;3:441–452. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- Gao S. Liu G.Z. Wang Z. Modulation of androgen receptor-dependent transcription by resveratrol and genistein in prostate cancer cells. Prostate. 2004;59:214–225. doi: 10.1002/pros.10375. [DOI] [PubMed] [Google Scholar]
- Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- Goel A. Aggarwal B.B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- Hedlund T.E. Johannes W.U. Miller G.J. Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2003;54:68–78. doi: 10.1002/pros.10137. [DOI] [PubMed] [Google Scholar]
- Hemminki A. Kanerva A. Liu B., et al. Modulation of coxsackie-adenovirus receptor expression for increased adenoviral transgene expression. Cancer Res. 2003;63:847–853. [PubMed] [Google Scholar]
- Ito H. Aoki H. Kuhnel F., et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J. Natl. Cancer Inst. 2006;98:625–636. doi: 10.1093/jnci/djj161. [DOI] [PubMed] [Google Scholar]
- Jang M. Cai L. Udeani G.O., et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jemal A. Bray F. Center M.M., et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jiang H. White E.J. Rios-Vicil C.I., et al. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J. Virol. 2011;85:4720–4729. doi: 10.1128/JVI.02032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan W. Duncan G. Van Patten C., et al. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr. Cancer. 2010;62:198–207. doi: 10.1080/01635580903305318. [DOI] [PubMed] [Google Scholar]
- Leitner S. Sweeney K. Oberg D., et al. Oncolytic adenoviral mutants with E1B19K gene deletions enhance gemcitabine-induced apoptosis in pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clin. Cancer Res. 2009;15:1730–1740. doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E. Stupack D. Klemke R., et al. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y. Zou Y.Y. Xia W.Y. Hung M.C. Enhanced paclitaxel cytotoxicity and prolonged animal survival rate by a nonviral-mediated systemic delivery of E1A gene in orthotopic xenograft human breast cancer. Cancer Gene Ther. 2004;11:594–602. doi: 10.1038/sj.cgt.7700743. [DOI] [PubMed] [Google Scholar]
- Libertini S. Abagnale A. Passaro C., et al. AZD1152 negatively affects the growth of anaplastic thyroid carcinoma cells and enhances the effects of oncolytic virus dl922-947. Endocr. Relat. Cancer. 2011;18:129–141. doi: 10.1677/ERC-10-0234. [DOI] [PubMed] [Google Scholar]
- Liu T.C. Galanis E. Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Öberg D. Yanover E. Sweeney K., et al. Improved potency and selectivity of an oncolytic E1ACR2 and E1B19K deleted adenoviral mutant in prostate and pancreatic cancers. Clin. Cancer Res. 2010;16:541–553. doi: 10.1158/1078-0432.CCR-09-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier-Denis E. Pattingre S. El Benna J. Codogno P. Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J. Biol. Chem. 2000;275:39090–39095. doi: 10.1074/jbc.M006198200. [DOI] [PubMed] [Google Scholar]
- O'Shea C.C. Johnson L. Bagus B., et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- O'Shea C.C. Choi S. McCormick F. Stokoe D. Adenovirus overrides cellular checkpoints for protein translation. Cell Cycle. 2005;4:883–888. doi: 10.4161/cc.4.7.1791. [DOI] [PubMed] [Google Scholar]
- Parato K.A. Senger D. Forsyth P.A. Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan S. Miranda E. Ekblad M., et al. Efficacy of oncolytic mutants targeting pRb and p53 pathways is synergistically enhanced when combined with cytotoxic drugs in prostate cancer cells and tumor xenografts. Hum. Gene Ther. 2010;21:1311–1325. doi: 10.1089/hum.2010.019. [DOI] [PubMed] [Google Scholar]
- Raki M. Kanerva A. Ristimaki A., et al. Combination of gemcitabine and Ad5/3-Delta24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther. 2005;12:1198–1205. doi: 10.1038/sj.gt.3302517. [DOI] [PubMed] [Google Scholar]
- Raykov Z. Georgieva P.B. Angelova A., et al. Anticancer effects of an oncolytic parvovirus combined with non-conventional therapeutics on pancreatic carcinoma cell lines. Acta Virol. 2009;53:57–60. doi: 10.4149/av_2009_01_57. [DOI] [PubMed] [Google Scholar]
- Sandberg L. Papareddy P. Silver J., et al. Replication-competent Ad11p vector (RCAd11p) efficiently transduces and replicates in hormone-refractory metastatic prostate cancer cells. Hum. Gene Ther. 2009;20:361–373. doi: 10.1089/hum.2007.124. [DOI] [PubMed] [Google Scholar]
- Signorelli P. Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J. Nutr. Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Small E.J. Carducci M.A. Burke J.M., et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol. Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Thomasset S.C. Berry D.P. Garcea G., et al. Dietary polyphenolic phytochemicals—promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- Ulrich S. Wolter F. Stein J.M. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol. Nutr. Food Res. 2005;49:452–461. doi: 10.1002/mnfr.200400081. [DOI] [PubMed] [Google Scholar]
- Wang Y. Hallden G. Hill R., et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat. Biotechnol. 2003;21:1328–1335. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- Yuan J.P. Wang J.H. Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora—implications for health. Mol. Nutr. Food Res. 2007;51:765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.