Abstract
DNA represents an ideal vaccine platform for HIV and many infectious diseases because of its safety, stability, and ease of manufacture. However, the immunogenicity of DNA vaccines has traditionally been low compared with viral vectors, recombinant protein, and live attenuated vaccines. The immunogenicity of DNA vaccines has been significantly enhanced by delivery with in vivo electroporation. Further improvements now allow electroporation to be performed in the dermis, which could potentially improve patient tolerability and may further enhance immunogenicity. In this study we examined how the current of intradermal vaccination impacts antigen expression, inflammation, and the induction of both humoral and cellular immunity in guinea pigs and nonhuman primates. We observed that a lower (0.1 A) current reduced inflammation and improved antigen expression compared with a 0.2 A current. The improved antigen expression resulted in a trend toward higher cellular immune responses but no impact on HIV- and influenza-specific binding titers. This study highlights the need for optimization of electroporation conditions in vivo in order to balance enhanced plasmid transfection with a loss of expression due to tissue inflammation and necrosis. These results suggest that a lower, 0.1-A current may not only improve patient tolerability but also improve immunogenicity.
Hutnick and colleagues examine how the current used for in vivo electroporation intradermal vaccination impacts antigen expression, inflammation, and the induction of both humoral and cellular immunity in guinea pigs and nonhuman primates. They find that a lower current reduces inflammation and improves antigen expression and that the improved antigen expression results in a trend toward higher cellular immune responses but has no impact on HIV- and influenza-specific binding titers.
Introduction
Enhanced DNA vaccination with electroporation (E-DNA) may be an important platform for HIV vaccine development. Compared with virus-based vectors, DNA has an unparalleled safety profile, is easy to manipulate and manufacture, and does not generate any antivector immunity, which allows for repeat homologous vaccination (Kutzler and Weiner, 2008). In addition, although naked DNA induced primarily cellular immunity, E-DNA induces both cellular and humoral responses, a feature likely necessary for an effective HIV vaccine (Hirao et al., 2011; Yin et al., 2011). The promising results of E-DNA have extended beyond preclinical models and demonstrated robust HIV-specific T cell responses in humans (HVTN 080; Kalams, 2010). Previous studies with DNA in humans have focused primarily on intramuscular delivery; however, intradermal delivery may be an alternative method to further improve E-DNA tolerability while maintaining robust immunogenicity (Hirao et al., 2008; Roos et al., 2006).
There are many different modes of in vivo electroporation technology, each of which has specific features pertaining to their application. In this paper, we focus on constant-current intradermal electroporation delivery. Intradermal vaccination is an attractive clinical method for a number of reasons. First, the skin is a large and accessible target organ. Second, and perhaps more importantly, it is extremely immunocompetent. Last, development of smaller electrode arrays, lower current, and reduced muscle stimulation reduces pain and discomfort during vaccine delivery. In addition, intradermal delivery may improve interpatient variability (Mir et al., 1999; Bloquel et al., 2004). However, a downside to this choice of tissue target is the relatively high tissue variability between different treatment sites and different patients. The race, age, and hydration state of subjects are some factors that can dramatically affect the thickness and physiology of the skin. These inherent variations in skin will directly affect the resistance of the tissue. This has major implications on the parameters used for in vivo electroporation. The specific benefit of this particular mode of constant-current electroporation is the ability of the device (CELLECTRA; Inovio Pharmaceuticals, Blue Bell, PA) to maintain the square-wave pulse form in the target tissue irrespective of changes in tissue resistance (Khan et al., 2003).
In a study assessing the immune responses to influenza DNA vaccines at various doses, the highest hemagglutinin inhibition (HAI) titers were observed in the group treated with 0.2 A current (A.S. Khan, Inovio Pharmaceuticals, unpublished data). For this reason, a 2×2 pulse sequence at 0.2 A constant current was used in an extensive nonhuman primate study addressing the protective effects of a smallpox DNA vaccine delivered by intradermal electroporation (Hirao et al., 2011). After immunization with a multivalent smallpox DNA vaccine enhanced by electroporation, using this device set at the 0.2 A parameters, 100% of cynomolgus macaques were protected from lethal monkeypox challenge. In addition to the smallpox study, multiple preclinical studies have confirmed that the 0.2 A parameter setting was efficacious in eliciting immune responses in a number of animal models across a number of vaccine targets (Hirao et al., 2008, 2010; Laddy et al., 2009). However, crucial to the applicability of electroporation for prophylactic targets in the clinic is both the efficacy of this methodology and the tolerability of the procedure.
To expand on these promising results, we sought to determine whether we could improve tolerability while maintaining immunogenicity by lowering the current of intradermal electroporation (EP) delivery from 0.2 A down to 0.1 A. Although E-DNA theoretically represents a vital part of an improved vaccine platform, additional in vivo optimization of this platform is imperative not only for tolerability but also for efficiency. EP settings are often calculated using complex algorithms that account for needle distance, current, and tissue resistance. Likewise, the final conditions used in the clinic are oftentimes a balance of optimal immunogenicity and patient tolerability.
One advantage of EP for vaccine delivery is not only the increase in antigen expression, but also the adjuvanting effect of EP, which leads to enhanced immunogenicity (Babiuk et al., 2004). However, the increased immunogenicity needs to be balanced with effectiveness and tolerability. Low voltages often have reduced immunogenicity although they induce less tissue damage and are more tolerable (Roos et al., 2006). Increasing the voltage can increase immunogenicity; however, voltage that is too high results in decreased tolerability, tissue damage, decreased antigen expression, and a reduction in immunogenicity (Mir et al., 1999). Therefore, it is critical to test the delivery conditions that maximize antigen expression and immunogenicity.
In this study, we report that lowering the current did not negatively impact the magnitude of the T or B cell response in both nonhuman primates and guinea pigs. Importantly, the lower voltage actually trended toward enhancing cellular immune responses. Studies in the guinea pig demonstrated that EP with the lower 0.1 A current results in higher antigen expression and reduced skin inflammation. The data in these studies demonstrate that lower current settings for EP are able to elicit comparable cellular and humoral immune responses. Thus, optimizing the EP conditions may result in equivalent immunogenicity at a lower energy level, increasing the efficiency of the procedure and potentially improving tolerability.
Materials and Methods
Macaque vaccination
Twenty-four Indian rhesus macaques (age range, 4–7 years) were housed at the Children's Hospital of Philadelphia (CHOP, Philadelphia, PA) according to the standards of the American Association for Assessment and Accreditation of Laboratory Animal Care International. Five animals each received 1.0 mg of pHIV consensus M Pol/M Gag and 1.5 mg of consensus envelope B in sterile water delivered by intradermal injection followed by in vivo electroporation at 0.2 or 0.1 A. Electroporation was performed with the constant-current CELLECTRA device and 3P needle array (Inovio Pharmaceuticals). Four control monkeys received an irrelevant vaccine.
Blood collection
Animals were bled before vaccination and 2 weeks after each immunization. Blood (20 ml at each time point) was collected in EDTA tubes and peripheral blood mononuclear cells (PBMCs) were isolated using a standard Ficoll-Hypaque procedure with ACCUSPIN tubes (Sigma-Aldrich, St. Louis, MO).
Rectal biopsies
Rectal punch biopsies were performed on week 20 after the final DNA immunization to assess mucosal immunity. Twenty biopsies were obtained with an alligator jaw-style biopsy punch. To isolate intraepithelial lymphocytes (IELs), biopsies were washed three times for 30 min at 37°C on a platform at 200 rpm in Hanks' balanced salt solution (HBSS) containing 75 mM EDTA, penicillin (100 U/ml), 25 mM HEPES buffer, and 10% fetal bovine serum (FBS). Biopsies were passed through a 100-μm filter in between each wash and supernatants were centrifuged twice for 15 min at 1200 rpm. To isolate lamina propria lymphocytes (LPLs), biopsies were washed twice for 30 min at 37°C on a platform at 200 rpm in RPMI 1640 medium with collagenase type II (0.5 mg/ml), penicillin (100 U/ml), 25 mM HEPES buffer, and 10% FBS. After each incubation, biopsies were drawn six times through a 20-, 18-, or 16-gauge blunt needle and passed through a 100-μm filter. Supernatants were centrifuged twice at 1200 rpm for 15 min. All LPL and IEL fractions were combined for flow cytometry.
ELISA
The ELISA was performed as previously described, using HIV Gag p24 (1 μg/ml; Immune Technology, New York, NY) in PBS-T (PBS with 0.5% Tween 20) (Shedlock et al., 2011). End-point titers were determined as previously reported (Frey et al., 1998). Briefly, the upper prediction limit of Gag-specific IgG antibodies was calculated using the Student t distribution, where the mathematical formula that defines the upper prediction limit is expressed as the standard deviation multiplied by a factor based on the number of naive controls and a 95% confidence interval. The end-point titer is reported as the reciprocal of the lowest dilution that remained above the upper prediction limit.
Enzyme-linked immunospot assay
Peptides were obtained from the AIDS Research and Reference Reagents Program (Division of AIDS, NIAID, and NIH). HIV consensus B Gag peptides (no. 8117), HIV consensus B Pol peptides (no. 6208), and HIV consensus envelope B (no. 9480) were resuspended in dimethyl sulfoxide (DMSO) and pooled at an approximate final concentration of 1 mg/ml for each peptide. Cellular responses were measured with a monkey interferon (IFN)-γ ELISPOT PRO kit with precoated plates (MabTech, Nacka Strand, Sweden) in accordance with the manufacturer's instructions. Samples were run in triplicate with an R10 (RPMI 1640 containing l-glutamine with 10% heat-inactivated FBS and 1% penicillin/streptomycin) and phorbol myristate acetate (PMA)–ionomycin (PMA, 0.1 μg/ml; and ionomycin, 0.5 μg/ml) control.
Flow cytometry
PBMCs and gut biopsies (1–2×106) were stimulated for 6 hr in a 96-well U-bottom plate with Gag, Pol, and Env peptide pools containing approximately 1 μg of each peptide, R10, or PMA (0.1 μg/ml) and ionomycin (0.5 μg/ml) in the presence of the secretion inhibitors brefeldin A (1 μg/ml; BD Biosciences, San Jose, CA) and monensin (1 μg/ml; BD Biosciences). After stimulation, cells were washed in phosphate-buffered saline (PBS) and stained with a Live/Dead stain kit with a violet amine-reactive dye (Life Technologies, Carlsbad, CA) for 5 min at room temperature, surface stained for 30 min at room temperature, washed in PBS, fixed, permeabilized with BD Cytofix/Cytoperm (BD Biosciences) for 15 min at room temperature, and washed twice in BD Perm/Wash buffer (BD Biosciences). Cells were then stained with intracellular antibodies for 1 hr at room temperature, washed in BD Perm/Wash buffer, and fixed with 2% paraformaldehyde. Cells were analyzed with a flow cytometer (modified BD LSR II; BD Biosciences) and analyzed with FlowJo 9.2 (Tree Star, Ashland, OR).
Statistics
Statistical analysis was performed with PASW SPSS Statistics 18 (IBM, Armonk, NY). Analysis among groups was performed with an independent t test and a Mann–Whitney test depending on normalcy of data. All data are expressed as means±SEM. A p value less than 0.05 was considered statistically significant. Animals with responses that were 2 standard deviations above the mean were considered outliers and were removed from the reported results.
Guinea pig vaccination
Female Hartley guinea pigs (4 weeks of age), weighing between 300 and 350 g, were used in the immune response and plasmid expression studies. All animals were housed and handled at BioTox Sciences (San Diego, CA) in accordance with the standards of the Institutional Animal Care and Use Committee (IACUC).
Immunization and electroporation in guinea pigs
Guinea pigs were randomized into two group (n=5). After 1 week of acclimation, animals were sedated with inhaled isoflurane and sites on the flanks of the animals were prepared (shaved and cleaned) for intradermal injection and electroporation. DNA vaccine pGX2001 expressing the H5HA influenza antigen (100 μg in a total volume of 50 μl of PBS) was injected intradermally followed immediately by electroporation at various current settings (either 0.1 or 0.2 A). Animals were bled through the jugular vein every 2 weeks. A boost immunization was conducted at week 3. Sera from blood samples were harvested for ELISA.
ELISA for detection of antibody end-point titers
Antibody responses against H5HA were evaluated by ELISA, using serum from immunized guinea pigs as previously described (Lin et al., 2011). Briefly, Costar 96-well EIA/RIA plates were coated with recombinant H5HA (0.3 μg/ml; Immune Technology) at 4°C overnight. After washing, the plates were blocked for nonspecific binding by adding 200 μl of PBS with 0.5% bovine serum albumin (BSA) for 1 hr at 37°C. Serum samples were diluted 1:50 in dilution buffer (PBS with 0.2% BSA and 0.05% Tween 20) and then serial 1:3 dilutions were performed in the plate from the first row for each sample. After incubation for 2 hr at 37°C and subsequent washing, anti-guinea pig IgG (H+L)–biotin (Jackson Laboratory, Bar Harbor, ME) was diluted 1:20,000 and added and incubated for 1 hr at 37°C before washing. This was followed by adding 50 μl of streptavidin–horseradish peroxidase (HRP) (Southern Biotech, Birmingham, AL), diluted 1:2000, to each well and incubated for 1 hr at 37°C before washing. Fifty microliters of HRP substrate (P-9187; Sigma-Aldrich) was added to the wells and incubated at room temperature in the dark for 10 min before reading the optical density (OD) at 450 nm. A reading was considered positive if the OD was higher than the average OD+3×SD from prebleed serum. The positive titer was plotted as the end-point titer.
Green fluorescent protein localization studies
Guinea pigs were anesthetized with isoflurane (5 and 3% for induction and maintenance, respectively). Sites on the flank of each animal were shaved and cleaned carefully. Plasmid expressing green fluorescent protein (gWiz-GFP; Aldevron, Fargo, ND) at a dose of 1.0 mg/ml was injected into the skin intradermally (50-μl injection volume), using a standard Mantoux method followed with or without EP, using the CELLECTRA-3P device set at either 0.2 or 0.1 A. The guinea pigs were killed on day 3 and skin samples were removed postmortem and frozen at −20°C. Skin samples expressing GFP were imaged and captured with an OV-100 fluorescence microscope (AntiCancer, San Diego, CA).
Histological examination of skin at EP sites
Guinea pigs were injected intradermally with gWiz-GFP plasmid (50 μl per injection/1.0-mg/ml dose) followed without or with EP at various currents. Guinea pigs were killed humanely on day 3, and skin samples were harvested by skin punch (8 mm) and fixed in 4% paraformaldehyde overnight for cryosectioning. Twenty-micrometer sections were cut and the tissue sections were stained with the BBC Histo-Perfect H&E staining system (BBC Biochemical, Seattle, WA) according to the manufacturer's protocol. In the H&E staining, hematoxylin stains nuclei blue whereas cytoplasm is stained pink with eosin. The presentation of the total area of blue nuclear staining is a crude method to determine inflammatory cell infiltration. An infiltration of inflammatory cells into skin results in an increase in the area of blue staining roughly proportional to the severity of the response. Skin sections were viewed and captured in visible light, using the ×10 objective of an Olympus BX51 microscope.
Results
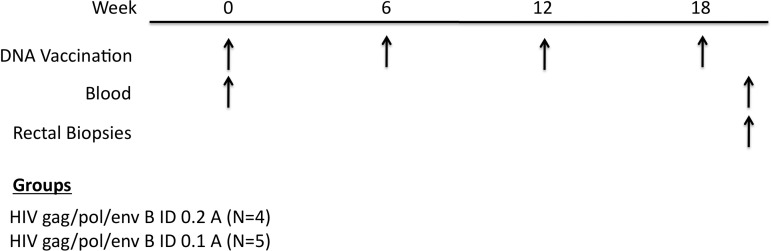
E-DNA vaccine delivery to the dermis represents a novel method to improve vaccine immunity, due to the large number of skin-resident antigen-presenting cells (APCs). To study the impact of electric current on DNA vaccination, we immunized two groups of five Indian rhesus macaques with a consensus pHIV gag/pol/env B vaccine administered intradermally at either 0.1 or 0.2 A. Animals were vaccinated at weeks 0, 6, 12, and 18 with blood drawn before vaccination and 2 weeks after each dose (Fig. 1). To access mucosal immune responses we also performed rectal biopsies 2 weeks after the final immunization.
FIG. 1.
Study outline. To examine how electrical current affects the HIV-1-specific immune response we intradermally vaccinated two groups of Indian rhesus macaques with consensus pHIV-1 gag/pol/env B with in vivo electroporation (E-DNA) at either 0.1 or 0.2 A. E-DNA was done at weeks 0, 6, 12, and 18 with blood drawn before and after vaccination. Rectal biopsies were also performed at week 20 to access mucosal immunity. ID, intradermal.
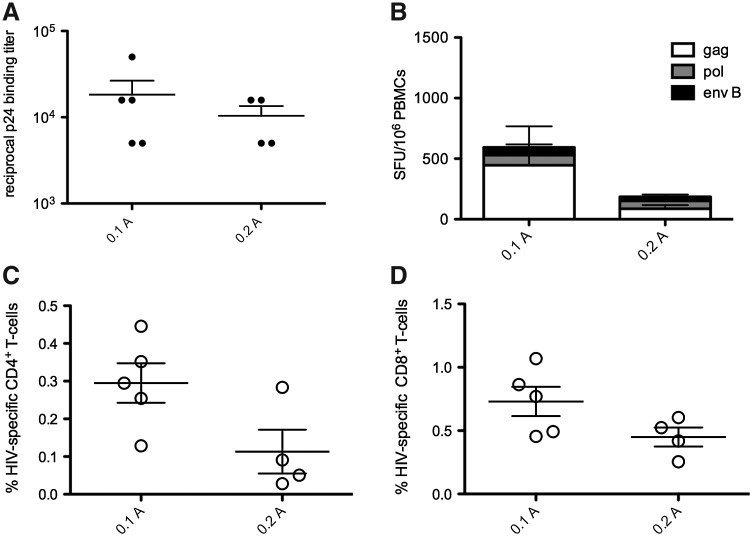
After the final vaccination, we observed that EP delivery with both 0.1 and 0.2 A resulted in similar HIV-specific cellular and humoral immune responses. We measured antibody responses by ELISA and observed no difference in Gag-specific IgG binding titers after EP delivery with either 0.1 or 0.2 A (Fig. 2A). We assessed the impact of EP current on cellular immunity, using both an IFN-γ enzyme-linked immunospot assay (ELISPOT) as well as by flow cytometry. The total IFN-γ ELISPOT response was 712.34±328.7 spot-forming units (SFU)/106 PBMCs after 0.1 A E-DNA vaccination compared with 157.8±78.9 SFU/106 PBMCs after 0.2 A E-DNA vaccination (Fig. 2B). When we examined the proportion of the cellular response by flow cytometry, we again observed a similar percentage of CD4+ and CD8+ cells producing interleukin (IL)-2, tumor necrosis factor (TNF)-α, or IFN-γ in monkeys receiving EP at 0.2 and 0.1 A EP (Fig. 2C and D). Unexpectedly, both ELISPOT and flow cytometry showed a trend toward an overall higher magnitude of cellular immunity with the lower current (0.1 A). Together, these data suggest that intradermal E-DNA delivered at a lower electrical current drives cellular responses that were either as good as or better than a higher current.
FIG. 2.
Current does not impact the magnitude of HIV-specific immune responses. Vaccine immunogenicity was accessed 2 weeks after the final vaccination. (A) Gag p24-specific IgG binding titers measured by ELISA. (B) IFN-γ ELISPOT after the final vaccination. (C) Total CD4+ response of cells making IFN-γ, IL-2, and/or TNF-α was measured by flow cytometry. (D) Total CD8+ response of cells making IFN-γ, IL-2, and/or TNF-α was measured by flow cytometry.
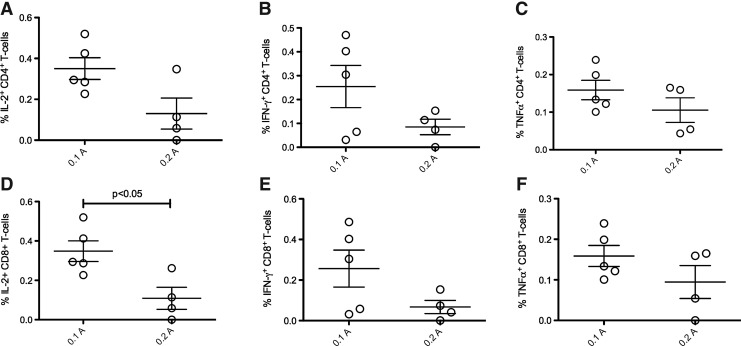
To further examine how current may affect the functionality of HIV-specific cellular immune responses, we measured the percentage of CD4+ and CD8+ T cells producing IL-2, IFN-γ, or TNF-α by flow cytometry. There was no difference in the percentage of CD4+ T cells producing IL-2 (Fig. 3A), IFN-γ (Fig. 3B), or TNF-α (Fig. 3C) after 0.1 or 0.2 A delivery. We again observed a trend toward higher CD4+ responses with the lower, 0.1 A delivery. In contrast to CD4+ T cells, the percentage of IL-2+ CD8+ T cells was significantly higher after EP at 0.1 A (Fig. 3D; p<0.08). However, there was no difference in the percentage of both IFN-γ+ (Fig. 3E) and TNF-α+ (Fig. 3F) CD8+ T cells after EP at either current setting despite a trend toward increased responses with 0.1 A delivery.
FIG. 3.
Electroporation (EP) current does not skew cellular immune function. Two weeks after the final vaccination, PBMCs were stimulated with overlapping 15-mers of HIV-1 consensus Gag, Pol, and clade B envelope for 6 hr and cytokine production was measured by flow cytometry. (A) CD4+ T cells producing IL-2. (B) CD4+ T cells producing IFN-γ. (C) CD4+ T cells producing TNF-α. (D) CD8+ T cells producing IL-2. (E) CD8+ T cells producing IFN-γ. (F) CD4+ T cells producing TNF-α.
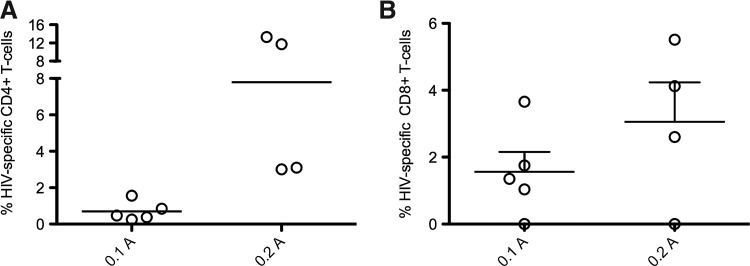
One critical feature of an effective HIV vaccine is the ability to induce mucosal immunity. Therefore, we decided to examine whether a lower current setting would impact the magnitude of mucosal T cell responses in rectal biopsies. We were able to detect robust HIV-specific CD4+ and CD8+ T cell responses after both 0.1 and 0.2 A EP delivery. Again, we observed no significant difference in the magnitude of HIV-specific CD4+ (Fig. 4A) or CD8+ (Fig. 4B) T cell responses in rectal biopsies. In contrast to systemic results, we observed a trend toward enhanced CD4+ and CD8+ responses with 0.2 A, suggesting higher current is needed to drive immune responses in secondary tissues.
FIG. 4.
Low current maintains mucosal cellular responses. Rectal biopsies were taken 2 weeks after the final vaccination and IEL and LPLs were isolated. Isolated cells were stimulated for 6 hr with overlapping Pol peptide pools and the total response was measured by the production of IFN-γ, IL-2, and/or TNF-α. (A) Pol-specific mucosal CD4+ T cells. (B) Pol-specific mucosal CD8+ T cells.
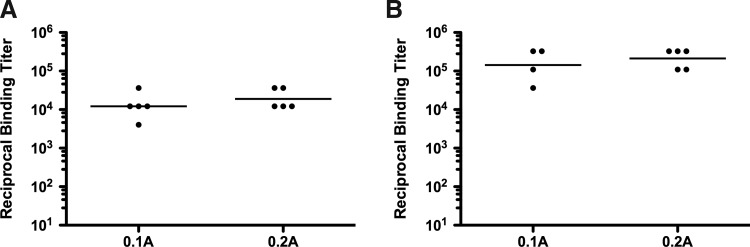
To ascertain whether these results with varying current settings were a general feature of EP and applicable to other species and plasmids, we performed additional studies in guinea pigs with a plasmid expressing GFP to visualize antigen expression and an H5 influenza plasmid to characterize humoral immunity. Similar to the antibody responses in nonhuman primates (NHPs), we observed no significant differences in the magnitude of H5-specific IgG after both the first (Fig. 5A) and second (Fig. 5B) vaccinations with delivery at either 0.1 or 0.2 A. These data confirm our finding in NHPs that E-DNA vaccination at a lower current (0.1 A) is just as immunogenic as the standard 0.2 A delivery.
FIG. 5.
Current does not affect H5-specific IgG in guinea pigs after intradermal E-DNA vaccination. Groups of five guinea pigs received pGX-H5HA at weeks 0 and 3 with intradermal EP at either 0.1 or 0.2 A. Individual end-point titers (circles) and the geometric mean titer (horizontal lines) are graphed. (A) H5-specific IgG titers 2 weeks after the first vaccination. (B) H5HA-specific IgG 2 weeks after the second vaccination.
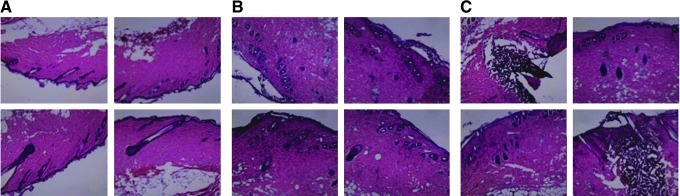
To investigate the effect the current setting has on antigen expression, we immunized guinea pigs with a GFP-expressing plasmid. Three days after vaccination, we observed higher levels of GFP expression after 0.1 A E-DNA delivery (Fig. 6A and B). The site of vaccination also appeared less inflamed compared with areas vaccinated with the higher 0.2 A EP (Fig. 6C). We hypothesized that the trend toward increased immune responses and increased antigen expression may be a result of less tissue damage and inflammation, allowing longer antigen expression. To confirm this hypothesis, we performed H&E staining on skin section samples 3 days posttreatment to allow assessment of infiltration and tissue damage. There was significantly less local damage to the site of vaccination with 0.1 A E-DNA compared with 0.2 A E-DNA (Fig. 7). Taken together, these studies demonstrate that the E-DNA vaccination at a lower current setting not only improves tolerability but also induces immune responses, similar to animals treated at higher current settings, by reducing inflammation and likely facilitating antigen expression.
FIG. 6.
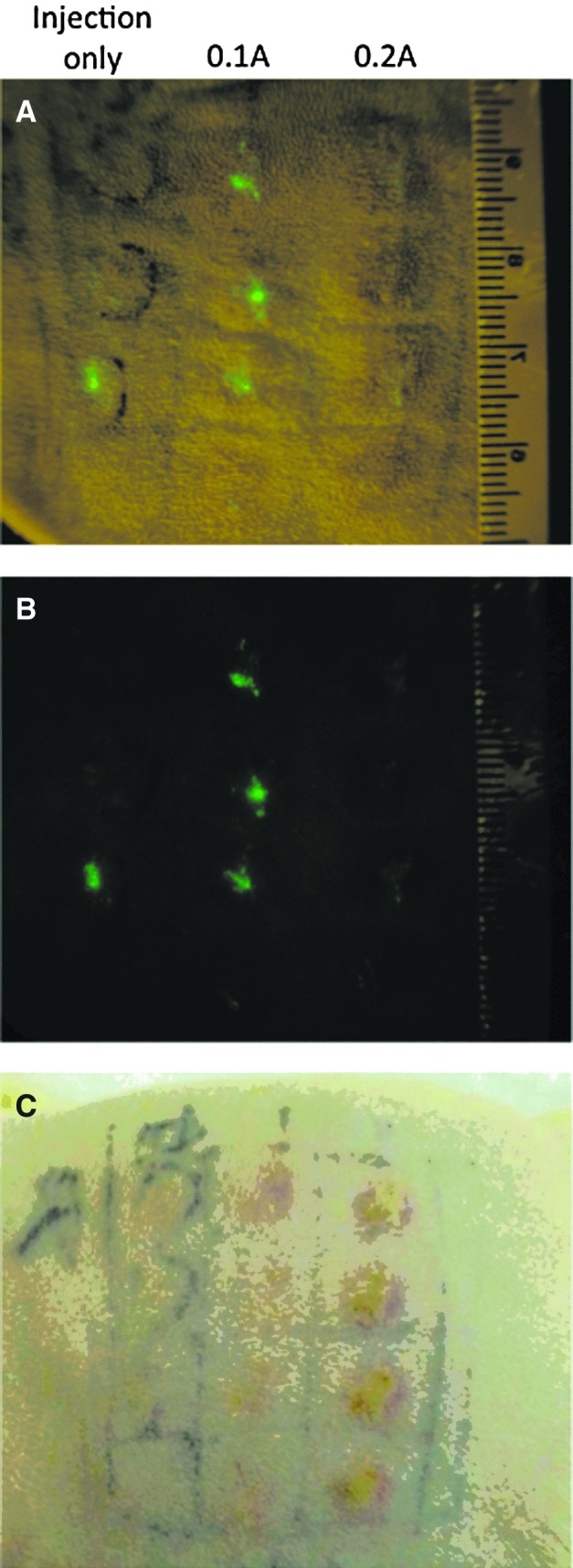
GFP expression and inflammation in the skin of guinea pigs 3 days posttreatment. (A) GFP expression on the surface of skin under visible and blue light. (B) GFP expression under blue light. (C) Surface inflammation at treated sites under visible light.
FIG. 7.
H&E staining of skin 3 days after treatment with various current levels. All images were taken with a ×10 objective. (A) Control skin without any treatment; (B) skin injected with plasmid followed by EP at 0.1 A; (C) skin injected with plasmid followed by EP at 0.2 A.
Discussion
In this study we report that E-DNA vaccination at a lower current setting induced cellular, mucosal, and humoral immune responses at equivalent rates to the current setting of 0.2 A. In addition to preserving immunity, 0.1 A delivery can represent an improvement for patient tolerability and as such an expansion of the scope of E-DNA vaccination in human trials. In these animal studies we were able to demonstrate pediatric vaccination protocols. We demonstrated that a lower current setting reduced redness, inflammation, and local tissue damage at the site of vaccination. Reductions in inflammation appeared to correspond with enhanced levels of GFP antigen expression, possibly contributing to the trend toward enhanced immunogenicity with 0.1 A. However, the quenching effect of hemoglobin on GFP expression should also be taken into account (Lane and Smith, 1999).
Electroporation is an example of a physical method that involves the application of brief electrical square-wave pulses that result in disruption of the lipid bilayer membranes of mammalian cells. The destabilizing effect of these electrical pulses allows the passage of large molecules, such as DNA vaccines, to cross the cell membrane. In normal, stable states, the membrane would be less permeable to the transport of such large, charged molecules. Therefore, electroporation can increase both the uptake of, as well as the extent to which DNA is delivered to target tissues.
EP is rapidly gaining clinical acceptance as a vaccination methodology, with a number of completed and ongoing human trials (Daud et al., 2008; Vasan et al., 2011). However, both clinical staff and the general public may be unfamiliar with the technology and may have concerns about the tolerability of the procedure. To scientifically address this issue, a human tolerability study of EP, using the CELLECTRA device, was carried out in healthy subjects (Lee et al., 2010). Ten volunteers were electroporated with the constant-current device, using the 0.2 A setting (0.2 sec between pulses, 2×2 pulse pattern), and asked to score the sensation on a visual analog scale (VAS) where 0 cm represents no pain and 10 cm represents the worst pain imaginable. All 10 subjects completed the study, and there were no reported serious adverse events. The VAS mean of all subjects immediately after EP was 2.5 cm. This score decreased to 1 cm at the 5-min time point after the EP treatment. This demonstrated that the EP procedure is tolerable and any pain from the procedure is rapidly attenuated by as early as 5 min postprocedure to levels of discomfort well within those of a normal syringe injection. Although this study demonstrated that intradermal EP with the CELLECTRA device at the 0.2 A setting is clearly a tolerable procedure, there is room to improve the tolerability of the procedure further.
We believe that there is a direct correlation between applied current and perceived tolerability (Broderick et al., 2011; and our unpublished observation); this observation is based on the relationship between applied current/voltage and electric field strength. The optimal electric field for dermal delivery would be contained within the epidermis and dermis and not interfere with lower lying nerves and musculature. Therefore, we hypothesize that lower applied currents will result in increased tolerability. It is crucial, however, that the efficacy of the resulting immune response not be impacted negatively by changes in parameter settings.
In this study, we observed that lowering the current setting of electroporation from 0.2 to 0.1 A did not significantly impact either cellular or humoral immunity in NHPs and humoral immunity in guinea pigs. Guinea pigs are the most relevant model for intradermal delivery because their skin closely mimics human skin tissue in both thickness and vascularity (Mershon et al., 1990; Sueki, 2000). The confirmation of these results in the NHP model is critical because promising DNA vaccine immunogenicity in small animals has often failed to translate into larger mammals and humans (Wang et al., 1993; MacGregor et al., 1998). Taken together, these data suggest that the more tolerable and lower current setting for intradermal E-DNA delivery may maintain or improve the responses induced in human trials with DNA vaccines delivered by the intradermal route.
Optimizing electroporation conditions is a balance between increasing expression to drive immunogenicity and inducing inflammation that can negatively impact tolerability. Studies suggest that immunogenicity likely depends on improving the level of antigen expressed and recruiting APCs to present antigen. Electroporation alone at high voltage is proinflammatory and the local inflammation acts as an adjuvant to recruit APCs and expand the immune response. However, excessive inflammation can result in the death of transfected cells, reduced antigen expression, and lower patient tolerability. We believe that the findings of this study elegantly demonstrate these principles. Although higher current settings will theoretically enhance cellular transfection, in this study we observed higher inflammation at a higher, 0.2-A current as observed with H&E staining. We believe the increased inflammation actually worked to reduce immunogenicity due to the destruction of transfected cells and reduced antigen expression. Future studies will be required to elucidate the exact mechanism of reduced immunogenicity and aim at characterizing how inflammasome induction, APC trafficking, and activation impact vaccine-induced immunogenicity under various delivery conditions. Taken together, these results highlight the need to experimentally test theoretically determined protocols to evaluate antigen expression and immunogenicity.
Acknowledgments
The authors acknowledge Dr. Phillip R. Johnson for kindly providing rhesus macaques and space at the Children's Hospital of Philadelphia, and Mary Connell for assistance in nonhuman primate care and sampling.
Author Disclosure Statement
For disclosure purposes, D.B.W. notes that he and his laboratory have had several commercial relationships with companies in the area of vaccines. These include that he receives consulting fees, or received stock ownership, for Advisory Board/Review Board Service, or received speaking support or research support from commercial entities including the following: Inovio, BMS, VGXI, Pfizer, Virxsys, J & J, Merck, Sanofi Pasteur, Althea, Novo Nordisk, SSI, Aldevron, Novartis, Incyte, and possibly others. No writing assistance was used in the production of this manuscript.
References
- Babiuk S. Baca-Estrada M.E. Foldvari M., et al. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J. Biotechnol. 2004;110:1–10. doi: 10.1016/j.jbiotec.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Bloquel C. Fabre E. Bureau M.F. Scherman D. Plasmid DNA electrotransfer for intracellular and secreted proteins expression: New methodological developments and applications. J. Gene Med. 2004;6(Suppl. 1):S11–S23. doi: 10.1002/jgm.508. [DOI] [PubMed] [Google Scholar]
- Broderick K.E. Shen X. Soderholm J., et al. Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther. 2011;18:258–265. doi: 10.1038/gt.2010.137. [DOI] [PubMed] [Google Scholar]
- Daud A.I. Deconti R.C. Andrews S., et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008;26:5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A. Di Canzio J. Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods. 1998;221:35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- Hirao L.A. Wu L. Khan A.S., et al. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Hirao L.A. Wu L. Satishchandran A., et al. Comparative analysis of immune responses induced by vaccination with SIV antigens by recombinant Ad5 vector or plasmid DNA in rhesus macaques. Mol. Ther. 2010;18:1568–1576. doi: 10.1038/mt.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao L.A. Draghia-Akli R. Prigge J.T., et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2011;203:95–102. doi: 10.1093/infdis/jiq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams S. HIV Vaccine Trials Network: shocking results from HVTN 080. 2010. hvtn.org/meeting/ppt/nov10/P7_Kalams.ppt. [Jun 18;2012 ]. hvtn.org/meeting/ppt/nov10/P7_Kalams.ppt
- Khan A.S. Smith L.C. Abruzzese R.V., et al. Optimization of electroporation parameters for the intramuscular delivery of plasmids in pigs. DNA Cell Biol. 2003;22:807–814. doi: 10.1089/104454903322625019. [DOI] [PubMed] [Google Scholar]
- Kutzler M.A. Weiner D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddy D.J. Yan J. Khan A.S., et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J. Virol. 2009;83:4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M.C. Smith W.C. The origins of primitive blood in Xenopus: Implications for axial patterning. Development. 1999;126:423–434. doi: 10.1242/dev.126.3.423. [DOI] [PubMed] [Google Scholar]
- Lee J. Khan A. Giffear M., et al. Tolerability of EP by Cellectra device following intradermal administration of saline in healthy volunteers. Poster Presentation. DNA Vaccine Conference 2010; New Orleans LA. 2010. [Google Scholar]
- Lin F. Shen X. McCoy J.R., et al. A novel prototype device for electroporation-enhanced DNA vaccine delivery simultaneously to both skin and muscle. Vaccine. 2011;29:6771–6780. doi: 10.1016/j.vaccine.2010.12.057. [DOI] [PubMed] [Google Scholar]
- MacGregor R.R. Boyer J.D. Ugen K.E., et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- Mershon M.M. Mitcheltree L.W. Petrali J.P., et al. Hairless guinea pig bioassay model for vesicant vapor exposures. Fundam. Appl. Toxicol. 1990;15:622–630. doi: 10.1016/0272-0590(90)90046-m. [DOI] [PubMed] [Google Scholar]
- Mir L.M. Bureau M.F. Gehl J., et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A.K. Moreno S. Leder C., et al. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol. Ther. 2006;13:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Shedlock D.J. Talbott K.T. Cress C., et al. A highly optimized DNA vaccine confers complete protective immunity against high-dose lethal lymphocytic choriomeningitis virus challenge. Vaccine. 2011;29:6755–6762. doi: 10.1016/j.vaccine.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueki D. Hairless guinea pig skin: Anatomical basis for studies of cutaneous biology. Eur. J. Dermatol. 2000;10:577. [PubMed] [Google Scholar]
- Vasan S. Hurley A. Schlesinger S.J., et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011;6:e19252. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. Ugen K.E. Srikantan V., et al. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. Dai A. Lecureux J., et al. High antibody and cellular responses induced to HIV-1 clade C envelope following DNA vaccines delivered by electroporation. Vaccine. 2011;29:6763–6770. doi: 10.1016/j.vaccine.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]