Abstract
Echinococcus multilocularis metacestodes are fluid-filled, vesicle-like organisms, which are characterized by continuous asexual proliferation via external budding of daughter vesicles, predominantly in the livers of infected individuals. Tumor-like growth eventually leads to the disease alveolar echinococcosis (AE). We employed the monoclonal antibody (MAb) E492/G1, previously shown to be directed against a carbohydrate-rich, immunomodulatory fraction of Echinococcus granulosus, to characterize potentially related components in E. multilocularis. Immunofluorescence studies demonstrated that MAb E492/G1-reactive epitopes were found predominantly on the laminated layer and in the periphery of developing brood capsules. The respective molecules were continuously released into the exterior medium and were also found in the parasite vesicle fluid. The MAb E492/G1-reactive fraction in E. multilocularis, named Em492 antigen, was isolated by immunoaffinity chromatography. Em492 antigen had a protein/carbohydrate ratio of 0.25, reacted with a series of lectins, and is related to the laminated layer-associated Em2(G11) antigen. The epitope recognized by MAb E492/G1 was sensitive to sodium periodate but was not affected by protease treatment. Anti-Em492 immunoglobulin G1 (IgG1) and IgG2 and, at lower levels, IgG3 were found in sera of mice suffering from experimentally induced secondary, but not primary, AE. However, with regard to cellular immunity, a suppressive effect on concanavalin A- or crude parasite extract-induced splenocyte proliferation in these mice was observed upon addition of Em492 antigen, but trypan blue exclusion tests and transmission electron microscopy failed to reveal any cytotoxic effect in Em492 antigen-treated spleen cells. This indicated that Em492 antigen could be modulating the periparasitic cellular environment during E. multilocularis infection through as yet unidentified mechanisms and could be one of the factors contributing to immunosuppressive events that occur at the host-parasite interface.
Alveolar echinococcosis (AE) is caused by the metacestode (larval) stage of Echinococcus multilocularis and is a rare but life-threatening disease, which is confined to the northern hemisphere (14, 15). The adult tapeworm exists as an enteric parasite in the fox and in a few other carnivores, such as wolves, cats, and dogs. Through fecal shedding, parasite eggs are released into the environment. The eggs contain oncospheres, which upon ingestion by a suitable intermediate host and subsequent passage through stomach and intestine get activated, penetrate the mucosa, enter blood and lymphatic vessels, and end up in the liver. In the liver parenchyma, oncospheres develop over time to form mature, asexually proliferating metacestodes, which are characterized by tumor-like growth. Metastasis formation in other organs has been reported (38). Mice and other small mammals act as natural intermediate hosts for E. multilocularis, while humans acquire AE by accidentally ingesting viable parasite eggs.
As for many other helminths, E. multilocularis metacestodes persist in their host for long periods of time, mostly lifelong. Thus, these parasites have evolved an outstanding arsenal of mechanisms by which they are capable of modulating and/or suppressing the host's abilities to target intruders (10, 29, 35, 43, 47, 58). For E. multilocularis, studies on the immune response in the experimental murine model as well as in humans have shown that cellular immunity induced by a Th-1-type cytokine secretion was able to successfully kill the metacestode at the initial stage of development but that antigenic- or nonantigenic proteins and carbohydrates of the oncosphere and/or metacestodes interfered with antigen presentation and cell activation, leading to a mixed Th1/Th2-type response at the later stage of infection (reviewed in reference 58). Thus, despite the fact that infection elicits a profound immune response, these parasites are able to survive and proliferate and can cause disease.
Several mechanisms which allow E. multilocularis metacestodes to avoid their host's defense have been described, and they involve either cell-cell contact or secretory products which could inhibit and/or modulate the immune response (13, 30, 45, 58). In this respect, the laminated layer plays a key role, and a wide range of possible functions has been attributed to it. It was proposed that, by acting as a physical barrier, the laminated layer protected the parasite from nitric oxide produced by periparasitic macrophages and dendritic cells (8, 27), and it has also been postulated that this layer prevents immune recognition by surrounding T cells (19), all this by virtue of its unusual physical and chemical stability (27). The laminated layer, more specifically the immunodominant Em2 antigen, also appears to be involved in the modulation of antigen recognition, T-cell activation, and antibody maturation and thus strongly influences cytokine production at the host-parasite interface (9).
Many immunomodulatory antigens in parasites have been described, and a large number of respective molecules are of a carbohydrate nature (2, 9, 16, 24, 39, 41, 46, 47, 54; P. M. Rudd, M. Butler, I. A. Wilson, J. Jaeken, and R. A. Dwek, Letter, Glycobiology 11:10, 2001). Several biological activities of pathogen carbohydrates have been described, such as antigenic activity (23, 39, 48, 50), inhibition of cellular proliferation (32, 44, 53, 54), mimicry of host components (37, 55), and other immunosuppressive and immunomodulatory effects (9, 11, 40, 56). Dematteis et al. (11) have isolated a carbohydrate-rich fraction named E4+ from Echinococcus granulosus protoscolices, which may have a role in the induction and maintenance of the Th2-type response during experimental E. granulosus infection in a murine model. E4+ is defined through immunoreactivity with the monoclonal antibody (MAb) E492/G1, and more-recent studies on the immune response in humans have suggested a possible role for E4+ during the course of infection with E. granulosus (7).
In this paper, a MAb E492/G1-binding fraction of the E. multilocularis metacestode, subsequently designated the Em492 antigen, was isolated from in vitro- and in vivo-generated parasites and was further characterized. Our study suggests that Em492 antigen is secreted and then transiently localized on the metacestode surface before it is released and thus could be one of the factors contributing to the modulation of cellular immunity during murine AE, and possibly also in humans.
MATERIALS AND METHODS
Biochemicals.
If not indicated otherwise, cell culture reagents were purchased from InVitroGen (Basel, Switzerland) and biochemicals were obtained from Sigma (St. Louis, Mo.).
Primary and secondary infection of mice.
Primary infection of C57BL/6 mice with E. multilocularis eggs was performed by oral inoculation of 2,000 eggs in 100 μl of phosphate-buffered saline (PBS) with a stomach tube. Eggs had been collected from fresh fox intestine at the Institute of Parasitology at the University of Hohenheim (Hohenheim, Germany) and had been stored for not longer than 6 weeks at 4°C prior to use. Parasites were allowed to grow for a period of 12 weeks. For secondary infection (intraperitoneal inoculation of vesicle suspension), the cloned E. multilocularis isolate KF5 (17, 18) was used. Metacestodes were maintained routinely either in C57BL/6 mice or gerbils by serial transplantation passages as described previously (21). After 6 to 8 weeks, animals were euthanized by CO2, and infected tissue was removed from the peritoneal cavity, was cut into tissue blocks of approximately 0.5 to 1 cm3, and was either frozen immediately at −80°C or processed for in vitro culture.
In vitro cultivation of E. multilocularis metacestodes.
In vitro culture of E. multilocularis was carried out as described previously (22). Cultures were obtained from both vesicle suspensions and tissue blocks. Harvesting of individual vesicles was carried out as described by Stettler et al. (52).
Separation of vesicle fluid and tissue and 6 M urea extraction of metacestodes from in vitro cultures.
In vitro-cultured E. multilocularis metacestodes 1 to 5 mm in diameter were carefully broken up, and vesicle fluid and parasite tissue were separated by centrifugation at 9,000 × g for 20 min at 4°C. Vesicle fluid was centrifuged again at higher speed (12,000 × g) and was aliquoted and frozen at −80°C prior to use. The tissue (approximately 50 to 100 vesicles/tube, corresponding to approximately 100 μl of tissue material) was washed in 500 μl of PBS. Following centrifugation at 4,000 × g for 5 min at 4°C, PBS was removed, and 500 μl of 6 M urea in PBS was added. Preparations were incubated for 15 min at room temperature, and during this time the samples were extensively vortexed. After centrifugation at 9,000 × g for 20 min at 20°C, the supernatant containing the extracted components was collected and dialyzed extensively at 4°C against binding buffer (0.1 M sodium phosphate dihydrate, 0.1 M NaCl, 5 mM EDTA) with dialysis tubes with a cutoff of 12 to 14 kDa (Merck Eurolab, Dietikon, Switzerland). The dialyzed fraction was then centrifuged at 12,000 × g for 30 min at 4°C and was used for MAb E492/G1 immunoaffinity chromatography.
Urea (6 M) extraction of metacestode-infected tissue.
Tissue blocks obtained from mice (approximately 0.5 to 1 cm3) were washed twice in PBS and were extracted in 6 M urea in PBS as described above with a threefold volume of extraction medium (3 ml of PBS-urea/ml of tissue). Following centrifugation, the extracted fraction was dialyzed as described above and used for MAb E492/G1 immunoaffinity chromatography.
MAb E492/G1 immunoaffinity chromatography.
The coupling of MAb E492/G1 to CNBr-activated Sepharose (Amersham Pharmacia Biotech, Dübendorf, Switzerland) was done as described by Dematteis et al. (11). Bound parasite molecules were eluted with 0.1 M glycine-HCl, pH 2.8. The eluted fraction, now designated Em492 antigen, was collected into 2 M Tris, pH 11, and dialyzed overnight at 4°C against Milli-Q water and freeze-dried. Em492 antigen was then resuspended in PBS. Protein concentrations were determined by the Bio-Rad (Münich, Germany) protein assay, and carbohydrate contents were assessed as previously described (39).
Immunohistochemistry.
For immunolocalization of binding sites of MAb E492/G1 on sections of in vitro-cultured E. multilocularis metacestodes, vesicles were embedded in LR-White resin (20) and sections 1 to 2 μm in thickness were prepared. Sections of embedded tissue were loaded onto poly-l-lysine-coated coverslips and air dried. The sections were then incubated for 1 h in blocking buffer (1% bovine serum albumin [BSA] in PBS). Subsequently they were rinsed in PBS and incubated for 1 h with the biotinylated MAb E492/G1 (11) diluted 1:50 in PBS containing 0.1% BSA. Following a washing in PBS, sections were incubated with rabbit antiserum directed against E. multilocularis tissue (diluted 1:250 in PBS-0.1% BSA) (28) for 20 min, washed, and incubated with goat anti-rabbit rhodamine conjugate (1:100) and streptavidin-fluorescein isothiocyanate (1:100), both diluted in PBS-0.1% BSA, for 30 min at room temperature. Following a washing in PBS, the sections were incubated in the DNA-specific fluorescent dye Hoechst 32558 (1 μg/ml in PBS) for 2 min, rinsed in water, embedded in Fluoroprep (bioMérieux, Genèva, Switzerland), and viewed on a Nikon Eclipse E 800 digital confocal fluorescence microscope. Processing of images was performed with the Openlab, version 2.0.7, software (Improvision, Heidelberg, Germany).
Em492 ELISA.
Enzyme-linked immunsorbent assay (ELISA) strips (Maxisorb immunostrips; Nunc, Roskilde, Denmark) were coated overnight at room temperature with either the 6 M urea-soluble metacestode fraction (3 μg of carbohydrate/ml), vesicle fluid (5 μg of protein/ml), the excreted or secreted fraction (5 μg of protein/ml), or the MAb E492/G1 affinity-purified Em492 antigen (3 μg of carbohydrate/ml). In some experiments, coated wells were treated with either 50 mM NaIO4 in 40 mM sodium acetate buffer (pH 5.5) for 1 h at 37°C or 50 μg of proteinase K/ml for 1 h at 37°C to disrupt carbohydrate and protein epitopes, respectively. Following the blocking of unspecific binding sites (1% BSA in PBS, 1 h), plates were incubated with biotinylated MAb E492/G1 diluted 1:1,000 in PBS-0.05% Tween 20-0.05% BSA for 1 h at 37°C as described by Dematteis et al. (11). After being washed, wells were incubated with streptavidin-alkaline phosphatase conjugate (PharMingen, San Diego Calif.) diluted 1:500 in PBS-0.3% Tween 20-0.05% BSA. After 1 mg of the appropriate substrate (p-nitrophenylphosphate) was added into 1 ml of substrate buffer (0.5 M ethanolamine, 0.5 mM MgCl2, pH 9.8), plates were incubated at room temperature for 15 min, and the optical density at 405 nm was measured on a Dynatech MR7000 ELISA reader.
To study cross-reactivity of immunoaffinity-purified Em2(G11) antigen (12) and Em492 antigen, ELISA wells were coated with equal amounts (3 μg of carbohydrate/ml) of Em492 and Em2(G11) antigen, respectively. Reactivity with biotinylated MAb E492/G1 was assessed as described above, and MAb G11 reactivity was tested as described by Dai et al. (9).
Lectin ELISA.
For the detection of Em492 antigen lectin-binding sites, the following biotinylated lectins were used: Ulex europaeus agglutinin I (UEA-I) and Tetragonolobus purpurea agglutinin (TGA) (specificity for α-d-fucosyl residues), Artocarpus integrifolia agglutinin (jacalin; high affinity for the core structure of O-linked carbohydrate chains and methyl galactopyranose), Arachis hypogaea and Glycine max agglutinin (PNA and SBA, respectively; affinity for d-N-acetylgalactosamine), Triticum vulgaris agglutinin (WGA; affinity for d-N-acetylglucosamine dimers and sialic acid residues), and Canavalia ensiformis agglutinin (concanavalin A [ConA]; affinity for N-linked carbohydrate chains and methyl-α-d-mannopyranose). Ninety-six-well ELISA plates were coated with purified Em492 antigen as described above. After unspecific binding sites were blocked with 20 mM Tris (pH 7.4)-145 mM NaCl-0.3% Tween 20 for 2 h at room temperature, they were incubated for 1 h with lectins diluted in lectin binding buffer (20 mM Tris, 145 mM NaCl, 1 mM Mn2+, 1 mMCa2+, 1 mM Mg2+, pH 7.4) at a final concentration of 5 μg of lectin/ml. Parallel to all lectin experiments, control incubations in the presence of a 500 mM concentration of the corresponding inhibitory sugar were performed. These included methyl-α-d-mannopyranose for ConA, N-acetylglucosamine for WGA, N-acetylgalactosamine for PNA and SBA, methylgalactopyranose for jacalin, and α-d-fucose for UEA and TGA, respectively. Finally the plates were incubated with a streptavidin-alkaline phosphatase conjugate, and absorbance at 405 nm was read (see above).
Assessment of the humoral immune response against Em492 antigen during primary and secondary infection in mice.
ELISA strips were coated with purified Em492 antigen or with crude E. multilocularis antigen (9), and unspecific binding sites were blocked as described above. Sera were diluted 1:100 in PBS-0.05% Tween 20-1% BSA as previously described (8-10), and bound antibodies were detected with immunoglobulin G (IgG) subclass-specific alkaline phosphatase conjugates (Southern Biotechnology Associates Inc., Birmingham, Ala.). Specific IgG1, IgG2a, and IgG3 binding was determined.
Lymphocyte proliferation assays.
Spleen cells from experimentally infected and noninfected mice were removed, and cell suspensions were depleted of erythrocytes by adding 0.83% NH4Cl in 0.01 M Tris-HCl (pH 7.2) and resuspended in medium (RPMI 1640 containing 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 100 μg of streptomycin and 100 U of penicillin/ml). Cells (2 × 105 per well) were cultured in 96-well round-bottom plates (Sarstedt, Newton, Mass.) and stimulated with crude parasite Emc antigen (50 μg of protein/ml) and purified Em492 (2 μg of carbohydrate/ml) or left unstimulated as negative controls. As an internal stimulation control, ConA (2 μg/ml) was used. All tests were performed in quadruplicate. After 96 h of cultivation, cells were pulsed with 1 μCi of [3H]thymidine/well and were harvested after 18 to 20 h. [3H]thymidine incorporation was measured as described by Dai et al. (9). The significance of the differences between the control (stimulated by concanavalin A or Emc antigenic extract) and experimental (ConA or Emc plus Em492 antigen) assays was determined by nonparametric Mann-Whitney U test. P values <0.05 were considered statistically significant. Statistical analyses were performed with JMPIn statistical package software.
TEM.
Splenocytes from secondarily infected mice or control mice were stimulated with either ConA, crude Emc antigen, Em492 antigen, or ConA and Emc antigen in the presence of Em492 antigen as described above without the addition of [3H]thymidine. Processing for transmission electron microscopy (TEM) was done at room temperature (51). Cells were carefully resuspended in electron microscopy fixation buffer (100 mM sodium cacodylate, pH 7.2) containing 2.5% glutaraldehyde. Following fixation for 2 h, specimens were washed in buffer and postfixed in 2% OsO4 in cacodylate buffer for 2 h. They were then washed in water, incubated in 1% uranyl acetate for 1 h, washed in water, and dehydrated in a graded series of ethanol. Specimens were embedded in Epon 812 epoxy resin and polymerized, and sections were cut and stained with lead citrate and uranyl acetate as described previously (20).
RESULTS
Identification, localization, and immunochemical characterization of Em492 antigen.
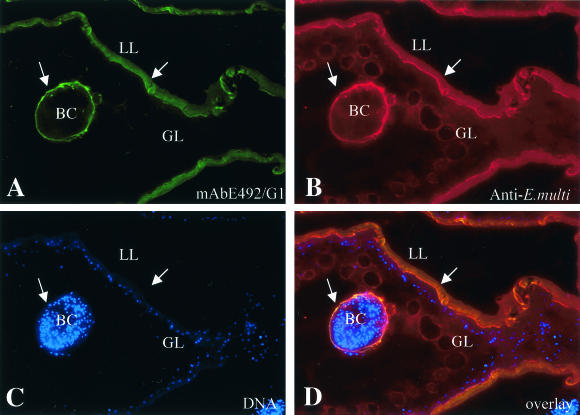
MAb E492/G1, recognizing the carbohydrate moiety Galα(1,4)-Gal, has been previously employed for the characterization of antigenic components of E. granulosus protoscolices (4), and the corresponding immunoaffinity-purified fraction, E4+, could be involved in immunosuppression phenomena during immunization with E. granulosus protoscolex antigens (11). In this study, MAb E492/G1 was initially used to investigate the localization of potentially related molecules in E. multilocularis metacestodes. For this, sections of LR-White-embedded, in vitro-cultured metacestodes were labeled with MAb E492/G1. Respective epitopes were found predominantly on the laminated layer and at the periphery of brood capsules. The actual metacestode tissue and the interior of protoscolices remained largely unstained by MAb E492/G1 (Fig. 1).
FIG. 1.
Immunofluorescence localization of MAb E492/G1-reactive epitopes in E. multilocularis metacestodes. Sections of LR-White-embedded parasite tissue were stained with MAb E492/G1 and a fluorescein isothiocyanate-conjugated secondary antibody (A), followed by polyclonal rabbit anti-E. multilocularis metacestode antiserum and rhodamine-conjugated goat anti-rabbit IgG (B). The parasite nuclei were stained with Hoechst 22358 to indicate the living parasite tissue (C). (D) Overlay of all three stainings. Arrows indicate the presence of MAb E492/G2-reactivity on the laminated layer (LL) and at the periphery of brood capsules (BC), while the germinal layer (GL) remains unstained.
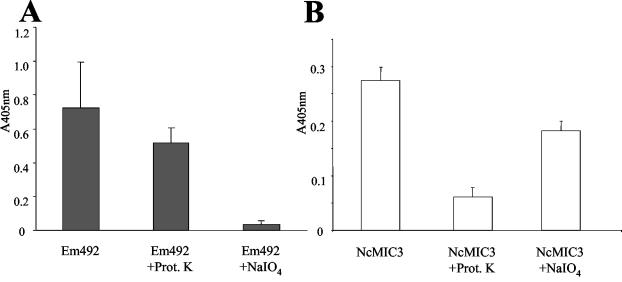
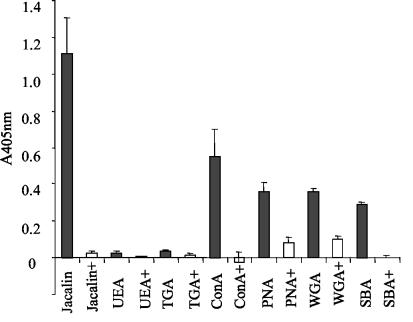
To purify those E. multilocularis metacestode antigens interacting with MAb E492/G1, in vitro-cultured metacestode material was extracted with 6 M urea, which resulted in an insoluble, purified laminated layer fraction largely devoid of MAb E492/G1 reactivity (data not shown). The 6 M urea-soluble fraction was dialyzed and centrifuged to remove aggregates, and those components reacting with MAb E492/G1 were purified by immunoaffinity chromatography (11). This purified fraction was designated Em492 antigen. By ELISA, both the 6 M urea extract and Em492 antigen reacted readily with MAb E492/G1 (data not shown). Determination of protein and carbohydrate concentrations in 6 M urea extract and purified Em492 showed that the purified Em492 fraction was mainly of carbohydrate nature, with a protein/carbohydrate ratio of 0.25 (data not shown). The predominant presence of carbohydrate epitopes within the Em492 fraction was further confirmed by other experiments involving MAb E492/G1 ELISA. Treatment of Em492 antigen with NaIO4 resulted in a substantial reduction of MAb E492/G1 reactivity. In contrast, treatment of Em492 with proteinase K had no effect (Fig. 2A), while control protease K treatment of the recombinant protein antigen recNcMIC3 (6) substantially diminished the reactivity of anti-NcMIC3 antibodies, demonstrating the efficacy of the protease treatment (Fig. 2B). To obtain further information on the type of carbohydrate residues present in the purified Em492 antigen fraction, lectin ELISAs were performed using a panel of biotinylated lectins with affinities for selected carbohydrates (Fig. 3). Of those lectins tested, Em492 reacted most strongly with jacalin and to a lesser extent with ConA, PNA, WGA, and SBA. Respective control incubations, carried out in parallel and incorporating 500 mM corresponding inhibitory sugars, showed that the reactivities of these lectins were truly based on lectin-carbohydrate interactions. UEA and TGA exhibited no reactivity.
FIG. 2.
MAb E492/G1 recognizes a carbohydrate epitope present in Em492 antigen. (A) ELISA wells were coated with purified Em492 antigen, and fractions were treated either with proteinase K or with 50 mM NaIO4. Note the significant decrease in MAb E492/G1 reactivity upon removal of carbohydrates. (B) Control experiment with a recombinant protein antigen (NcMIC3) and respective anti-NcMIC3 antibodies. Note the decrease in reactivity of anti-NcMIC3 upon protease digestion.
FIG. 3.
Lectin reactivities of Em492 antigen. ELISA wells were coated with Em492 antigen and were incubated with a panel of biotinylated lectins as indicated. Binding of lectins was assessed by streptavidin-alkaline phosphatase reaction. Black columns, reactivity of lectins; white columns, outcome of the control incubation, where 500 mM corresponding inhibitory sugar was added. Note the specific carbohydrate-mediated binding of jacalin, ConA, PNA, WGA, and SBA.
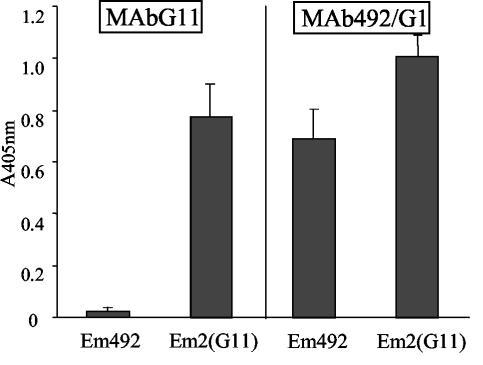
Since Em492 antigen was localized on the laminated layer, we investigated the cross-reactivity of the major laminated layer-associated antigen Em2(G11), which was obtained through immunoaffinity purification using the Em2-specific MAb G11 (12) with Em492 antigen, by comparing the reactivities with MAb G11 and MAb E492/G1, respectively. While MAb G11 did not recognize purified Em492 antigen, MAb E492/G1 reacted with both Em492 and Em2(G11) antigens (Fig. 4). Thus, Em492 and the Em2(G11) antigen are immunologically related but are not identical antigenic fractions.
FIG. 4.
Relationship of Em492 and Em2(G11) antigens. ELISA wells were coated with either purified Em492 or purified Em2(G11) antigen, both at 3 μg of carbohydrate/ml. Wells were then incubated either with MAb G11 or with MAb E492/G1. Note that the MAb G11 does not react with purified Em492 antigen but that MAb Eb492/G1 labels both Em492 and Em2(G11). One out of three experiments is shown, with all three yielding identical results.
Em492 and host-parasite interactions.
Assessments of E. multilocularis vesicle fluid antigen and of secretory and excretory fractions of in vitro-cultured E. multilocularis metacestodes showed that Em492 antigen appears to be continuously released into the exterior medium, as well as into the interior vesicle fluid, during in vitro maintenance of metacestodes (data not shown).
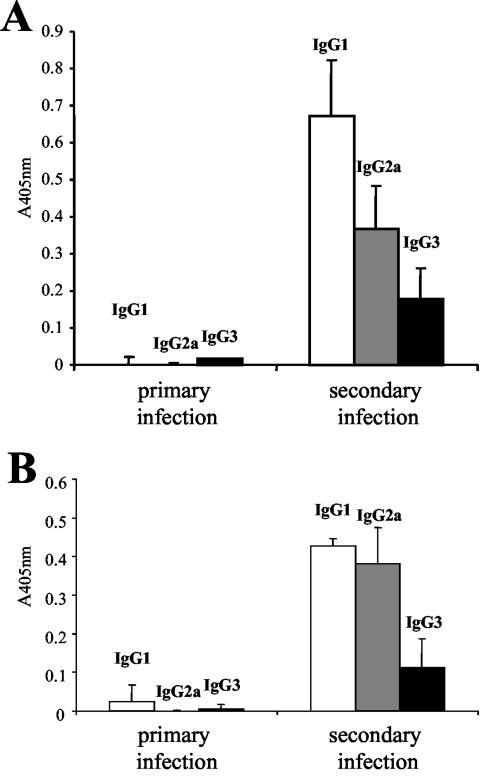
Sera from mice which were experimentally infected with E. multilocularis metacestodes (secondary infection) were shown to elicit a strong humoral anti-Em492 antigen immune response, with high levels of both IgG1 and IgG2a and, to a lesser extent, IgG3 (Fig. 5B). Upon primary (egg) infection, the antibody response in mice was virtually undetectable. These results point to the relevance of Em492 as a secreted or excreted antigenic component potentially associated with a profound mixed Th1/Th2-type-mediated antibody response during primary and secondary infection. In contrast, the same sera, when tested against crude E. multilocularis extract, exhibited a profound IgG1-biased antibody response (Fig. 5A).
FIG. 5.
Reactivity of sera from eight mice infected with E. multilocularis through primary (oral, egg) and secondary (intraperitoneal, metacestode) routes with crude E. multilocularis antigen (A) and purified Em492 antigen (B). IgG1, IgG2a, and IgG3 were assessed. No reactivity was found in sera obtained from primarily infected mice. In secondary infection, note the relatively polarized IgG1 antibody response directed against crude antigen (A), while Em492 antigen is recognized by both IgG1 and IgG2a at similar levels (B). Reactive IgG3 antibodies are found in both antigenic fractions. Data presented correspond to the means for eight mice plus standard deviations.
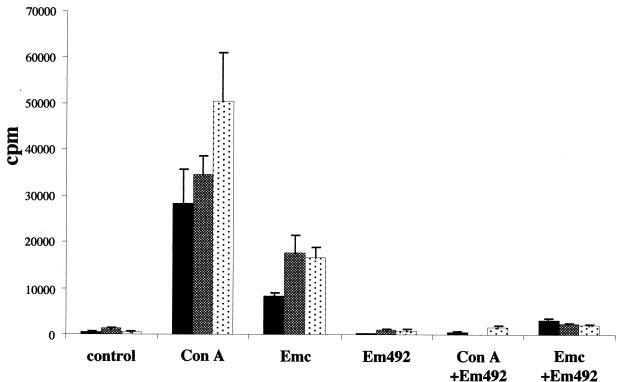
The effects of Em492 antigen on the cellular immune response were analyzed by splenocyte proliferation assays. While the results are shown only for Em492 antigen isolated from in vitro-cultured metacestodes (Fig. 6 and 7), identical findings were obtained for Em492 antigen isolated from metacestode tissue which had not been previously cultured in vitro (data not shown). Spleen cells from either primarily or secondarily infected mice or from noninfected mice were cultured in vitro and stimulated with ConA. This resulted in splenocyte proliferation in all groups. Upon stimulation with crude metacestode extract (Emc), proliferative responses in splenocytes originating from infected mice were observed. Upon stimulation with purified Em492 antigen, no proliferation could be measured in any of the groups, and ConA- and Emc antigen-mediated splenocyte proliferation was completely abolished upon addition of Em492 antigen in all groups. Thus, the addition of Em492 antigen completely suppresses the cellular proliferation of splenocytes induced by either mitogenic or antigenic stimulation in vitro (Fig. 6). Suppression of spleen cell proliferation was also evident in a heterologous-murine-infection model, with mice which had been infected with the protozoan parasite Neospora caninum (data not shown).
FIG. 6.
Impairment of splenocyte proliferative responses by the addition of Em492 antigen. Splenocytes of uninfected mice (black columns), primarily infected mice (grey columns), and secondarily infected mice (dotted columns) were cultured in vitro. They were left unstimulated (control), or proliferation was induced by the mitogen ConA or with crude E. multilocularis extract (Emc). Stimulation with purified Em492 antigen did not result in any proliferation at all, and splenocyte proliferation was severely inhibited by the addition of Em492 antigen to ConA (ConA+Em492) or to Emc (Emc+Em492). Data presented are the means of quadruplicate experiments plus standard deviations.
FIG. 7.
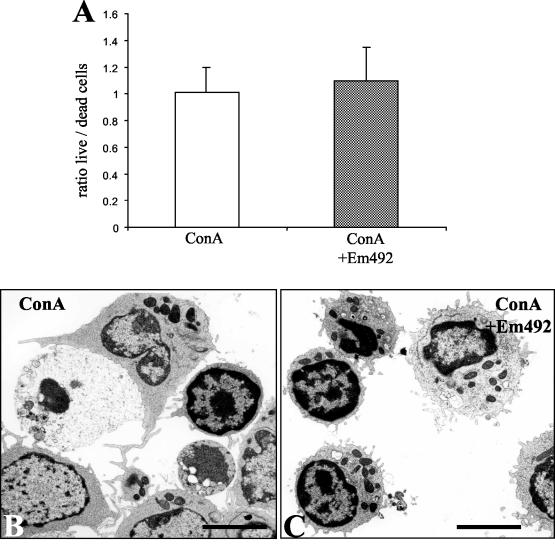
Em492 antigen does not exhibit a cytotoxic effect on splenocyte populations. (A) In vitro-cultured splenocytes were stimulated either with ConA alone or with ConA in the presence of Em492 antigen. Following trypan blue staining of cells, the numbers of dead and live splenocytes were determined microscopically in a Neubauer chamber by inspection of all nine quadrants of the chamber and determination of the ratio between dead and live cells. The data shown correspond to one out of three experiments, with all three yielding largely identical results. (B) TEM of ConA-stimulated splenocytes. Note the macrophage in the process of phagocytosing a dead cell. (C) TEM of splenocytes incubated in the presence of ConA plus Em492 antigen. Bars = 28 μm.
Two assessments indicated that the negative impact of Em492 antigen on spleen cell proliferation was not due to cytotoxicity (Fig. 7). First, a trypan blue exclusion test did not indicate that the percentage of nonviable spleen cells was significantly higher in Em492 antigen-treated splenocyte populations than in untreated ones (Fig. 7A). Second, inspection of Em492 antigen-treated and untreated splenocytes by TEM did not reveal any significantly altered cells in treated versus untreated samples (Fig. 7B and C).
DISCUSSION
This study describes a carbohydrate-rich fraction in the E. multilocularis metacestode, designated Em492 antigen, which is defined through its reactivity with MAb E492/G1 (4). MAb E492/G1 had been used earlier for immunoaffinity isolation of a carbohydrate-rich fraction from E. granulosus protoscolices called E4+. E4+ in turn was shown to modulate the cellular immune response in E. granulosus-infected or immunized BALB/c mice (11), and this study suggests that E. multilocularis Em492 antigen and E. granulosus E4+ are closely related in terms of antigenic properties and function, in that they both are highly glycosylated and both exhibit cellular proliferative suppression.
The epitope recognized by MAb E492/G1 was shown to be the Galα(1,4)-Gal moiety (11). This motif is, e.g., also present on P1 antigen, which is a soluble glycoprotein of E. granulosus that exhibits P1 blood group reactivity (5). P1 antigen is synthesized in the protoscolex tegument and stored in the cyst fluid and then diffuses through the laminated layer of E. granulosus hydatid cysts before it is released (36). In the E. multilocularis Em492 antigen, the epitope recognized by MAb E492/G1 is vulnerable to NaIO4 treatment, but the binding of MAb E492/G1 to Em492 antigen is not affected by protease treatment (Fig. 2A). The fact that jacalin, a lectin with a high affinity for methylgalactopyranose, which represents the core structure of O-linked carbohydrate chains, binds efficiently to affinity-purified Em492 antigen indicates that a predominant fraction of the Em492 antigen glycopeptides are O linked. However, the (albeit weaker) interaction with ConA and WGA shows that Em492 antigen also contains N-linked sugars of the hybrid and possibly high-mannose-type core structures (31). The binding to SBA points to the presence of d-N-acetylgalactosamine residues. Thus, Em492 antigen appears to be a heterogeneous fraction, and the Galα(1,4)-Gal moiety is likely to be present on a number of different glycoconjugates.
MAb E492/G1 ELISA showed that this Galα(1,4)-Gal epitope, or a closely related one, is also present on the Em2(G11) antigen, an integral component of the outer acellular matrix of E. multilocularis metacestodes, whose localization in metacestode tissue is strictly confined to the laminated layer. The Em2(G11) antigen was purified through MAb G11 affinity chromatography and was similar to Em492 antigen, also detected in excreted and secreted fractions of in vitro-cultured E. multilocularis metacestodes (12, 21). In contrast, the Em2(G11) antigen-specific MAb G11 does not bind to MAb E492/G1-purified Em492 antigen. However, while Em2(G11) was recently demonstrated to be a mucin-type glycoconjugate (25), the exact composition of the MAb G11-reactive epitope has not been elucidated to date. In any case, our results indicate that the two antigens are immunologically related. The distinction between cross-reactive antigen and similar epitopes is rather difficult to demonstrate with carbohydrate epitopes, for which a complete characterization (chemical and spatial) would be required. In any case, if the MAb E492/G1-reactive epitopes are similar in both spatial conformation and function, this could be equally relevant. Our results suggest that, possibly, Em492 antigen represents a breakdown product or a subfraction of Em2(G11) antigen.
Immunofluorescence localization showed that Em492 is abundant at the periphery of the protoscolices of E. multilocularis metacestodes, as well as enriched within the laminated layer (Fig. 1). In this context it is interesting that previously two other laminated layer-associated molecules were shown to exhibit a localization identical to that of Em492 antigen. First, EmP2 (28), a 116-kDa, most likely nonglycosylated protein predominantly localized at the tegument-laminated layer interface. Second, the E. multilocularis alkaline phosphatase (EmAP), which is highly glycosylated, was shown to be localized on the laminated layer and on the periphery of brood capsules and developing protoscolices. Anti-EmAP antibodies cross-reacted with purified Em2(G11) antigen, and MAb G11 reacted with purified EmAP (33). In any case, the exact relationship between Em492 antigen and Em2(G11) antigen, and between EmP2 and EmAP, needs to be investigated in more detail.
In contrast to the Em2(G11) antigen (27, 26), Em492 does not appear to be an integral part of the laminated layer but rather is just associated with it, as it was solubilized by 6 M urea extraction, a procedure normally used for the purification of the laminated layer (27, 26). In addition, Em492 antigen was progressively released during metacestode in vitro culture and accumulated in the medium supernatant with increasing time of culture. Thus, according to localization and fractionation studies we conclude that Em492 antigen appears to be synthesized within the parasite germinal layer and secreted by the parasite, either into its interior (vesicle fluid) or toward its exterior. When secreted toward the exterior, Em492 antigen is transiently stored in the laminated layer and subsequently released into the surrounding medium.
The importance of secreted parasite-derived components had already been demonstrated in previous studies on parasitic helminths. These studies have shown that surface-associated or secreted metabolic products exhibit immunomodulatory effects and often strongly influence the outcome of an infection in favor of the parasite (1, 34, 42, 49, 57). The same could account for Em492 antigen. First, upon secondary infection, a profound IgG1/IgG2a anti-Em492 antigen immune response is elicited, which is indicative for a mixed Th1/Th2 immune response. The mixed Th1/Th2 cellular immunity has been associated with immunosuppressive phenomenon during AE (58). It is interesting, though, that the same sera, when tested against crude antigen extract, exhibited a rather IgG1-dominated humoral immune response (Fig. 5). In any case, primary (egg) infection of mice did not elicit a detectable IgG response, either against crude antigen or against Em492. It is very likely that different antigens used during priming may result in different responses at later time points of infection. This means that eggs and metacestodes are surely different with regard to antigenicity and thus differ in quality and quantity of antigens, including Em492. These differences may be reflected in chronic infection in the antibody responses during primary versus secondary infection. However, the situation is clearly different with regard to cellular immunity (Fig. 6).
Observations similar to those reported here for Em492 antigen have been reported earlier for antibody responses directed against Em2(G11) antigen (9). In addition, Dai et al. (9) also showed that anti-Em2(G11) antigen antibodies lacked avidity maturation during increasing time of infection and provided evidence that Em2(G11) represents a T-cell-independent antigen and could thus contribute to the immunosuppression phenomenon observed during progressive E. multilocularis infection. To what extent Em492 antigen might contribute to modulating antibody production during infection will require further investigations.
What is evident from our studies is that secretion of Em492 antigen by metacestodes could potentially have a significant impact on those immune cell populations which are relevant for success or failure of an infection, namely, the lymphoid cells in the periparasitic compartment. This is suggested by experiments showing that the presence of Em492 antigen during splenocyte proliferation assays resulted in a marked depression of proliferative responses upon mitogenic (ConA) or antigenic (Emc antigen) stimulation (Fig. 6). Thus, Em492 antigen suppresses a homologous Th2-type (Echinococcus), as well as a heterologous Th1-type (Neospora), spleen cell proliferative response in vitro. This suppressive effect was not due to cytotoxicity or cell death, as evidenced by trypan blue staining. In addition, TEM failed to detect signs of increased necrosis or apoptosis in spleen cell populations which had been exposed to Em492 antigen (Fig. 7).
At present it is not clear how this proliferation-inhibitory effect is mediated. One possibility for the observed inhibition of the mitogenic properties of ConA and parasite extracts could be a blockade of interleukin-2 (IL-2) synthesis. This would lead to a decreased growth of T cells (44). In E. granulosus infections, splenocytes from E4+-immunized mice not only showed suppressed proliferative response to ConA but also exhibited induced IL-10 production. This may contribute to the induction of the type 2 cytokine response established in early experimental infection (11). For Taenia solium, studies on murine spleen cells stimulated with ConA in the presence or absence of a metacestode factor (MF) showed that, due to MF, cells produced significantly less IL-2, gamma interferon, and IL-4 than those stimulated with ConA alone. Also production of tumor necrosis factor alpha by macrophages was decreased by MF. The authors concluded that MF inhibited the production of a multitude of cytokines, regardless of the cell type (3). In a recent study by Jenne et al. (30) it was shown that the maturation of dendritic cells was inhibited in the presence of a crude E. multilocularis antigen mixture. Further studies are in progress to investigate the mode of action and the effects of Em492 antigen on cytokine production in different immune cell populations.
In conclusion, our results suggest that Em492 antigen may be an important molecule in the survival strategies employed by E. multilocularis metacestodes. Furthermore our results are in line with other studies that show that glycosylated moieties from different parasitic preparations of E. multilocularis and of E. granulosus induce significant humoral immune responses in experimental infection models (9, 16, 39, 48). The present study serves as an initial step for further investigations on the biological relevance of the Em492 antigen during E. multilocularis infection.
Acknowledgments
Many thanks are addressed to Norbert Müller and Claus Wedekind for many pieces of advice and for critical reading of the manuscript. We also thank Maja Suter and Toni Wyler (Institute of Veterinary Pathology and Institute of Zoology, University of Berne, respectively), as well as Phillippe Tregenna-Piggott and Beatrice Frey (Department of Chemistry and Biochemistry, University of Berne), for access to their electron microscopy facilities and Renate Fink for superb technical assistance. Peter Deplazes, Hansueli Ochs, and Manuela Schnyder (Institute of Parasitology, Zürich) are thanked for the maintenance of E. multilocularis IM280 in vivo. The primary egg infection experiments were performed in collaboration with Michael Merli and Ute Mackenstedt from the Institute of Parasitology, University of Hohenheim, Hohenheim, Germany.
We gratefully acknowledge the financial support of the Swiss National Science Foundation (3100-063615.00), the Stanley Thomas Johnson Foundation, the Novartis Research Foundation, the Stiftung für Leberkrankheiten (Switzerland), Conicyt Clemente Estable no. 4002, and CSIC Uruguay.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Allen, J. E., and A. S. MacDonald. 1998. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 20:241-247. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk, B. J., I. van Die, S. J. van Vliet, C. Vandenbroucke-Grauls, T. B. H. Geijtenbeek, and Y. van Kooyk. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. [DOI] [PubMed] [Google Scholar]
- 3.Arechavaleta, F., J. L. Molinari, and P. Tato. 1998. A Taenia solium metacestode factor nonspecifically inhibits cytokine production. Parasitol. Res. 84:117-122. [DOI] [PubMed] [Google Scholar]
- 4.Baz, A., A. Richieri, A. Puglia, A. Nieto, and S. Dematteis. 1999. Antibody response in CD4-depleted mice after immunization or during early infection with Echinococcus granulosus. Parasite Immunol. 21:141-150. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, G. L., and J. M. Staveley. 1957. Blood group-P substance in hydatid cyst fluids. Nature 179:147-148. [DOI] [PubMed] [Google Scholar]
- 6.Cannas, A., A. Naguleswaran, N. Müller, B. Gottstein, and A. Hemphill. 2003. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and Ribi adjuvant. J. Parasitol. 89:44-50. [DOI] [PubMed] [Google Scholar]
- 7.Cardozo, G., P. Tucci, and A. Hernandez. 2002. Characterization of the immune response induced by a carbohydrate enriched fraction from Echinococcus granulosus protoscoleces in patients with cystic hydatid disease. Parasitol. Res. 88:984-990. [DOI] [PubMed] [Google Scholar]
- 8.Dai, W. J., and B. Gottstein. 1999. Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology 97:107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai, W. J., A. Hemphill, A. Waldvogel, K. Ingold, P. Deplazes, H. Mossmann, and B. Gottstein. 2001. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of αβ+ CD4+ T cells. Infect. Immun. 69:6074-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai, W. J., A. Waldvogel, T. Jungi, M. Stettler, and B. Gottstein. 2003. Inducible nitric oxide synthase deficiency in mice increases resistance to chronic infection with Echinococcus multilocularis. Immunology 108:238-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dematteis, S., F. Pirotto, J. Marques, A. Nieto, A. Orn, and A. Baz. 2001. Modulation of the cellular immune response by a carbohydrate rich fraction from Echinococcus granulosus protoscoleces in infected or immunized BALB/c mice. Parasite Immunol. 23:1-9. [DOI] [PubMed] [Google Scholar]
- 12.Deplazes, P., and B. Gottstein. 1991. A monoclonal-antibody against Echinococcus multilocularis Em2 antigen. Parasitology 103:41-49. [DOI] [PubMed] [Google Scholar]
- 13.Dixon, J. B. 1997. Echinococcosis. Comp. Immunol. Microbiol. Infect. Dis. 20:87-94. [DOI] [PubMed] [Google Scholar]
- 14.Eckert, J., and P. Deplazes. 1999. Alveolar echinococcosis in humans: the current situation in Central Europe and the need for countermeasures. Parasitol. Today 15:315-319. [DOI] [PubMed] [Google Scholar]
- 15.Eckert, J., F. J. Conraths, and K. Tackmann. 2000. Echinococcosis—an emerging or re-emerging zoonosis. Int. J. Parasitol. 30:1283-1294. [DOI] [PubMed] [Google Scholar]
- 16.Ferragut, G., and A. Nieto. 1996. Antibody response of Echinococcus granulosus infected mice: recognition of glucidic and peptidic epitopes and lack of avidity maturation. Parasite Immunol. 18:393-402. [DOI] [PubMed] [Google Scholar]
- 17.Gottstein, B. 1992. Echinococcus multilocularis infection—immunology and immunodiagnosis. Adv. Parasitol. 31:321-380. [DOI] [PubMed] [Google Scholar]
- 18.Gottstein, B. 1992. Molecular and immunological diagnosis of echinococcosis. Clin. Microbiol. Rev. 5:248-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottstein, B., and A. Hemphill. 1997. Immunopathology of echinococcosis. Chem. Immunol. 66:177-208. [DOI] [PubMed] [Google Scholar]
- 20.Hemphill, A., and S. L. Croft. 1997. Electron microscopy in parasitology, p. 227-268. In M. Rogan (ed.), Analytical parasitology. Springer Verlag, Heidelberg, Germany.
- 21.Hemphill, A., and B. Gottstein. 1995. Immunology and morphology studies on the proliferation of in vitro cultivated Echinococcus multilocularis metacestodes. Parasitol. Res. 81:605-614. [DOI] [PubMed] [Google Scholar]
- 22.Hemphill, A., M. Stettler, M. Walker, M. Siles-Lucas, R. Fink, and B. Gottstein. 2003. In vitro culture of Echinococcus multilocularis and Echinococcus vogeli metacestodes: studies on the host-parasite interface. Acta Trop. 85:145-155. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez, A., and A. Nieto. 1994. Induction of protective immunity against murine secondary hydatidosis. Parasite Immunol. 16:537-544. [DOI] [PubMed] [Google Scholar]
- 24.Hokke, C. H., and A. M. Deelder. 2001. Schistosome glycoconjugates in host-parasite interplay. Glycoconj. J. 18:573-587. [DOI] [PubMed] [Google Scholar]
- 25.Hulsmeier, A. J., P. M. Gehrig, R. Geyer, R. Sack, B. Gottstein, P. Deplazes, and P. Kohler. 2002. A major Echinococcus multilocularis antigen is a mucin-type glycoprotein. J. Biol. Chem. 277:5742-5748. [DOI] [PubMed] [Google Scholar]
- 26.Ingold, K., W. Dai, R. L. Rausch, B. Gottstein, and A. Hemphill. 2001. Characterization of the laminated layer of in vitro cultivated Echinococcus vogeli metacestodes. J. Parasitol. 87:55-64. [DOI] [PubMed] [Google Scholar]
- 27.Ingold, K., B. Gottstein, and A. Hemphill. 2000. High molecular mass glycans are major structural elements associated with the laminated layer of in vitro cultivated Echinococcus multilocularis metacestodes. Int. J. Parasitol. 30:207-214. [DOI] [PubMed] [Google Scholar]
- 28.Ingold, K., B. Gottstein, and A. Hemphill. 1998. Identification of a laminated layer-associated protein in Echinococcus multilocularis metacestodes. Parasitology 116:363-372. [DOI] [PubMed] [Google Scholar]
- 29.Janssen, D., M. C. Rueda, P. H. DeRycke, and A. Osuna. 1997. Immunomodulation by hydatid cyst fluid toxin (E. granulosus). Parasite Immunol. 19:149-160. [DOI] [PubMed] [Google Scholar]
- 30.Jenne, L., J. F. Arrighi, B. Sauter, and P. Kern. 2001. Dendritic cells pulsed with unfractionated helminthic proteins to generate antiparasitic cytotoxic T lymphocyte. Parasite Immunol. 23:195-201. [DOI] [PubMed] [Google Scholar]
- 31.Kobata, A., and K. Yamashita. 1993. Fractionation of oligosaccharides by serial affinity chromatography with use of immobilized lectin columns, p. 103-125. In M. Fukuda and A. Kobata (ed.), Glycobiology: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 32.Ladisch, S., A. Hasegawa, R. X. Li, and M. Kiso. 1994. A chemically synthesized sialic acid-containing glycoconjugate, 2-(tetradecylhexadecyl)-O-(5-acetamido-3, 5-dideoxy-D-glycero-alpha-D-galacto-2-nonulopyranosylonic acid)-(2-3)-O-beta-D-galactopyrannosyl-(1-4)-beta-D-glucopyranoside, is a potent inhibitor of cellular immune responses. Biochem. Biophys. Res. Commun. 203:1102-1109. [DOI] [PubMed] [Google Scholar]
- 33.Lawton, P., A. Hemphill, P. Deplazes, B. Gottstein, and M. E. Sarciron. 1997. Echinococcus multilocularis metacestodes: immunological and immunocytochemical analysis of the relationships between alkaline phosphatase and the Em2 antigen. Exp. Parasitol. 87:142-149. [DOI] [PubMed] [Google Scholar]
- 34.Lightowlers, M. W., and M. D. Rickard. 1988. Excretory secretory products of helminth parasites—effects on host immune responses. Parasitology 96:S123-S166. [DOI] [PubMed] [Google Scholar]
- 35.Maizels, R. M., D. A. P. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth-parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 36.Makni, S., K. H. Ayed, A. M. Dalix, and R. Oriol. 1992. Immunological localization of blood Pl antigen in tissues of Echinococcus granulosus. Ann. Trop. Med. Parasitol. 86:87-88. [DOI] [PubMed] [Google Scholar]
- 37.Mandrell, R. E., R. McLaughlin, Y. A. Kwaik, A. Lesse, R. Yamasaki, B. Gibson, S. M. Spinola, and M. A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect. Immun. 60:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehlhorn, H., J. Eckert, and R. C. A. Thompson. 1983. Proliferation and metastases formation of larval Echinococcus multilocularis. II. Ultrastructural investigations. Z. Parasitenkd. 69:749-763. [DOI] [PubMed] [Google Scholar]
- 39.Miguez, M., A. Baz, and A. Nieto. 1996. Carbohydrates on the surface of Echinococcus granulosus protoscoleces are immunodominant in mice. Parasite Immunol. 18:559-569. [DOI] [PubMed] [Google Scholar]
- 40.Motran, C. C., F. L. Diaz, A. Gruppi, D. Slavin, B. Chatton, and J. L. Bocco. 2002. Human pregnancy-specific glycoprotein 1a (PSG1a) induces alternative activation in human and mouse monocytes and suppresses the accessory cell-dependent T cell proliferation. J. Leukoc. Biol. 72:512-521. [PubMed] [Google Scholar]
- 41.Nicod, L., S. Bressonhadni, D. A. Vuitton, I. Emery, B. Gottstein, H. Auer, and D. Lenys. 1994. Specific cellular and humoral immune responses induced by different antigen preparations of Echinococcus multilocularis metacestodes in patients with alveolar echinococcosis. Parasite 1:261-270. [DOI] [PubMed] [Google Scholar]
- 42.Pastrana, D. V., N. Raghavan, P. Fitzgerald, S. W. Eisinger, C. Metz, R. Bucala, R. P. Schleimer, C. Bickel, and A. L. Scott. 1998. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect. Immun. 66:5955-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paterson, J. C., P. Garside, M. W. Kennedy, and C. E. Lawrence. 2002. Modulation of a heterologous immune response by the products of Ascaris suum. Infect. Immun. 70:58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Persat, F., C. Vincent, D. Schmitt, and M. Mojon. 1996. Inhibition of human peripheral blood mononuclear cell proliferative response by glycosphingolipids from metacestodes of Echinococcus multilocularis. Infect. Immun. 64:3682-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakha, N. K., J. B. Dixon, S. D. Carter, S. D. Craig, P. Jenkins, and S. Folkard. 1991. Echinococcus multilocularis antigens modify accessory cell function of macrophages. Immunology 74:652-656. [PMC free article] [PubMed] [Google Scholar]
- 46.Schönemeyer, A., R. Lucius, B. Sonnenburg, N. Brattig, R. Sabat, K. Schilling, J. Bradley, and S. Hartmann. 2001. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J. Immunol. 167:3207-3215. [DOI] [PubMed] [Google Scholar]
- 47.Sestak, K., L. A. Ward, A. Sheoran, X. Feng, D. E. Akiyoshi, H. D. Ward, and S. Tzipori. 2002. Variability among Cryptosporidium parvum genotype 1 and 2 immunodominant surface glycoproteins. Parasite Immunol. 24:213-219. [DOI] [PubMed] [Google Scholar]
- 48.Severi, M. A., G. Ferragut, and A. Nieto. 1997. Antibody response of Echinococcus granulosus infected mice: protoscolex specific response during infection is associated with decreasing specific IgG1/IgG3 ratio as well as decreasing avidity. Parasite Immunol. 19:545-552. [DOI] [PubMed] [Google Scholar]
- 49.Spolski, R. J., J. Corson, P. G. Thomas, and R. E. Kuhn. 2000. Parasite-secreted products regulate the host response to larval Taenia crassiceps. Parasite Immunol. 22:297-305. [DOI] [PubMed] [Google Scholar]
- 50.Sterla, S., H. Sato, and A. Nieto. 1999. Echinococcus granulosus human infection stimulates low avidity anticarbohydrate IgG2 and high avidity antipeptide IgG4 antibodies. Parasite Immunol. 21:27-34. [DOI] [PubMed] [Google Scholar]
- 51.Stettler, M., R. Fink, M. Walker, B. Gottstein, T. G. Geary, J. F. Rossignol, and A. Hemphill. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 47:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stettler, M., M. Siles-Lucas, E. Sarciron, P. Lawton, B. Gottstein, and A. Hemphill. 2001. Echinococcus multilocularis alkaline phosphatase as a marker for metacestode damage induced by in vitro drug treatment with albendazole sulfoxide and albendazole sulfone. Antimicrob. Agents Chemother. 45:2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Syrokou, A., G. Tzanakakis, T. Tsegenidis, A. Hjerpe, and N. K. Karamanos. 1999. Effects of glycosaminoglycans on proliferation of epithelial and fibroblast human malignant mesothelioma cells: a structure-function relationship. Cell Prolif. 32:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terrazas, L. I., K. L. Walsh, D. Piskorska, E. McGuire, and D. A. Harn. 2001. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1+ cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4+ cells: a potential mechanism for immune polarization in helminth infections. J. Immunol. 167:5294-5303. [DOI] [PubMed] [Google Scholar]
- 55.Tsai, C. M., W. H. Chen, and P. A. Balakonis. 1998. Characterization of terminal NeuN Acalpha2-3Galbeta1-4GlcNAc sequence in lipooligosaccharides of Neisseria meningitidis. Glycobiology 8:359-365. [DOI] [PubMed] [Google Scholar]
- 56.Velupillai, P., and D. A. Harn. 1994. Oligosaccharide-specific induction of interleukin-10 production by B220+ cells from schistosome-infected mice—a mechanism for regulation of Cd4+ T-cell subsets. Proc. Natl. Acad. Sci. USA 91:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villa, O. F., and R. E. Kuhn. 1996. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant amergy and downregulation of Th1-associated phenomena. Parasitology 112:561-570. [DOI] [PubMed] [Google Scholar]
- 58.Vuitton, D. A. 2003. The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta Trop. 85:119-132. [DOI] [PubMed] [Google Scholar]