Abstract
Cancer-targeting dual-gene virotherapy (CTGVT-DG) is an important modification of CTGVT, in which two suitable genes are used to obtain an excellent antitumor effect. A key problem is to join the two genes to form one fused gene, and then to clone it into the oncolytic viral vector so that only one investigational new drug application, instead of two, is required for clinical use. Many linkers (e.g., internal ribosome entry site) are used to join two genes together, but they are not all equally efficacious. Here, we describe finding the best linker, that is, sequence encoding the four amino acids IETD, to join the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene and the second mitochondria-derived activator of caspase (Smac) gene to form TRAIL-IETD-Smac and inserting it into oncolytic viral vector ZD55 to construct ZD55-TRAIL-IETD-Smac, which matched ZD55-TRAIL plus ZD55-Smac in completely eliminating xenograft hepatoma. ZD55-TRAIL-IETD-Smac works by quantitative cleavage at IETD↓by inducing caspase-8; activation or inhibition of caspase-8 could up- or downregulate cleavage, respectively. The cleaved product, TRAIL-IETD, does not affect the function of TRAIL. Numerous experiments have shown that the combined use of ZD55-TRAIL plus ZD55-X could completely eradicate many xenograft tumors, and therefore the IETD is potentially a useful linker to construct many antitumor drugs, for example, ZD55-TRAIL-IETD-X, where X has a compensative or synergetic effect on TRAIL. We found that the antitumor effect of ZD55-IL-24-IETD-TRAIL also has an equivalent antitumor effect compared with the combined use of ZD55-IL-24 plus ZD55-TRAIL, because ZD55-IL-24 could also induce caspase-8. This means that IETD, as a two-gene linker, may have broad use.
Wang and colleagues identify the amino acid sequence IETD as the most effective linker to mediate cancer-targeting dual gene virotherapy. IETD-linked tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and second mitochondria-derived activator of caspase (Smac) were cloned into the ZD55 oncolytic viral vector. ZD55-TRAIL-IETD-Smac completely eliminated xenografted hepatomas in nude mice.
Introduction
Cancer-targeting gene virotherapy (CTGVT) was initiated in 1999–2001 (Zhang et al., 2003) by inserting a strong antitumor gene into an oncolytic viral vector (i.e., OV-gene). CTGVT (OV-gene) has a much better antitumor effect than either the respective gene therapy or virotherapy used alone. We have studied the CTGVT (OV-gene) strategy since that initial publication, producing numerous papers on the subject, and have watched CTGVT (OV-gene) become a topic of general interest in research. The biotechnology giant Amgen (Thousand Oaks, CA) paid 1 billion USD to purchase the CTGVT (OV-gene) product OncoHSV-GM-CSF (OV from herpes simplex virus) from BioVex (Evans, 2011). JX-594, made by Jennerex (San Francisco, CA), has entered phase 2 clinical trials and produced encouraging results by intravenous administration, as described in the journal Nature (Breitbach et al., 2011). Furthermore, CTGVT was modified by the use of two genes, to form cancer-targeting dual-gene virotherapy (CTGVT-DG). Because two genes may have a compensative or synergetic effect, the antitumor effect of CTGVT-DG is always much better than that of CTGVT with one gene (Pan et al., 1997; Liu et al., 2005; Chu et al., 2006; Zhang et al., 2006, 2009). If two suitable genes are chosen, CTGVT-DG could completely eradicate all the xenografts of liver, pancreas, gastric and colorectal cancer, and so on (Pan et al., 1997; Liu et al., 2005; Chu et al., 2006; Zhang et al., 2006, 2009).
Human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL, Apo-2L) has attracted attention in the field of cancer therapy, not only because of its higher ability to effectively kill cancer cells but also because it has little cytotoxic effect on normal human cells. Therefore, TRAIL has been widely used in the oncolytic system (Lin et al., 2002). TRAIL interacts with two death-inducing receptors, DR4 and DR5, and with three “decoy receptors,” DcR1, DcR2, and the soluble osteoprotegerin (Pan et al., 1997; Schneider et al., 1997; Sheridan et al., 1997). In humans, TRAIL can induce tumor cell-selective killing by activating the death receptor-mediated apoptotic pathway through binding with TRAIL–R1/DR4 or TRAIL–R2/DR5 receptors (Pan et al., 1997; Sheridan et al., 1997). In this process, a death-inducing signaling complex (DISC) is formed. The adaptor protein FADD (Fas-associated death domain) is then recruited to the complex, facilitating induction of the initiator procaspase-8 prodomain into the DISC (Schneider et al., 1997). This leads to activation of procaspase-8, which becomes cleaved caspase-8, a potent activator of downstream effector caspases (Pan et al., 1997; Ashkenazi, 2002). Although many cancers undergo TRAIL-induced apoptosis, some develop resistance because of high levels of inhibitor of apoptosis proteins (IAPs). Cellular sensitivity to TRAIL-induced apoptosis can be modulated at many levels. The mitochondrial pathway is engaged by the release of apoptogenic factor, such as the second mitochondria-derived activator of caspase (Smac) from mitochondria into the cytosol (Saelens et al., 2004; Kroemer et al., 2007). Smac promotes apoptosis by neutralizing the action of IAPs (Deng et al., 2002; Saelens et al., 2004).
Smac promotes caspase activation in the cytochrome c/Apaf-1/caspase-9 pathway. Smac promotes caspase-9 activation by binding with IAPs and removing their inhibitory function. Smac is normally a mitochondrial protein but is released into the cytosol when cells undergo apoptosis. Mitochondrial import and the cleavage signal peptide of IAPs are required for Smac to exert its apoptotic function. Overexpression of Smac increases cell sensitivity to apoptotic stimuli (Chai et al., 2000; Du et al., 2000; Shi, 2002).
In this study, we attempted to generate a novel E1B-55K-deleted oncolytic adenovirus harboring both the TRAIL and Smac genes (ZD55-TRAIL-IETD-Smac). ZD55, an oncolytic adenovirus, is similar to ONYX-015, which has been demonstrated to replicate selectively in tumor cells in a p53-dependent (Bischoff et al., 1996) or p53-independent manner (Dix et al., 2001). In our vector, sequence encoding a caspase-8 cleavage site (IETD) was introduced between the TRAIL and Smac genes because it was reported that caspase-8 induced by TRAIL can cleave EGFP-IETD↓-Smac at the arrow position (Srinivasula et al., 2000). However, we do not know whether ZD55-TRAIL-IETD-Smac could be cut with enough cleavage efficacy to give equivalent amounts of TRAIL and Smac; if so, then the antitumor effect of ZD55-TRAIL-IETD-Smac will be equal to the combined use of ZD55-TRAIL plus ZD55-Smac. We also do not know whether the expressed and cleaved product TRAIL-IETD will equal the function of TRAIL. Answers to these questions will decide whether IETD is an acceptable linker or not. We have used CTGVT to achieve complete eradication of xenograft tumors, for example, ZD55-TRAIL plus ZD55-k5 for colorectal cancer (Zhang et al., 2003), ZD55-TRAIL plus ZD55-Smac for hepatoma (Evans, 2011), ZD55-TRAIL plus manganese superoxide dismutase (MnSOD) for colon cancer (Breitbach et al., 2011), ZD55-TRAIL plus ZD55-hSSTr2 for pancreatic cancer (Liu et al., 2005), ZD55-TRAIL plus ZD55-Cyld for colorectal cancer (Pei et al., 2004), ZD55-TRAIL plus ZD55-IL-24 for colorectal cancer (Zhang et al., 2006), and for hepatoma and gastric cancer (data not shown). If IETD turns out to be a reliable linker, all the previously mentioned gene pairs can be joined by IETD according to the CTGVT-DG strategy to produce antitumor drugs for clinical use.
Materials and Methods
Cell lines and culture conditions
Human hepatoma cell lines Huh-7, PLC, and Bel-7404, human lung cancer cell line NCI-H460, human cervical cancer cell line HeLa, human colorectal cancer cell line SW620, human breast cancer cell line Bcap37, normal human liver cell line QSG-7701, and normal human fetal lung fibroblast cell line WI38 were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). HEK293 (a human embryonic kidney cell line containing the E1A region of adenovirus serotype 5 [Ad5]) was obtained from the American Type Culture Collection (ATCC, Manassas, VA).
Huh-7, Bel-7404, NCI-H460, HeLa, SW620, and Bcap37 cell lines were cultured in Dulbecco's modified Eagle's medium (Gibco brand DMEM; Life Technologies, Carlsbad, CA) supplemented with 5% heat-inactivated fetal bovine serum (FBS). The QSG-7701, WI38, and HEK293 cell lines were cultured in DMEM supplemented with 10% FBS. All cells were incubated at 37°C in a humidified air atmosphere with 5% CO2.
Plasmids
Adenoviral shuttle vector pCA13 and adenoviral packaging vector pBHGE3 were preserved in our laboratory. pZD55 was constructed by our group previously. The TRAIL-(IETD)-Smac recombinant gene was assembled and amplified by overlap PCR using A1 (5′-ACG CGT CGA CAT GGC TAT GAT GGA GGT CCA-3′), A2 (5′-CGC GTC TGT CTC AAT GCC AAC TAA AAA GGC-3′), B1 (5′-GGC ATT GAG ACA GAC GCG GTT CCT ATT GCA-3′), and B2 (5′-GCT CTA GAT CAA TCC TCA CGC AGG TAG G-3′) primers. The PCR products were digested with SalI and XbalI and then cloned into pCA13 to construct pCA13-TRAIL-(IETD)-Smac. The TRAIL-IETD gene was subcloned onto EcoRI and XbaI sites of pCA13 to construct pCA13-TRAIL-IETD. pZD55-TRAIL-(IETD)-Smac (or pZD55-TRAIL-IETD) was constructed by inserting the whole TRAIL-(IETD)-Smac (or TRAIL-IETD) expression cassette derived from pCA13-TRAIL-(IETD)-Smac (or pCA13-TRAIL-IETD), using BglII, into the corresponding site of pZD55. All plasmid constructs were confirmed by restriction enzyme digestion, PCR, and DNA sequencing.
Construction of Ad-TRAIL-IETD-Smac
The TRAIL-IETD-Smac expression cassette was first cloned into a shuttle vector, pShuttle. The resultant plasmid was linearized by digestion with restriction endonuclease PmeI and subsequently contransformed with an adenoviral backbone plasmid, pAdEasy, into Escherichia coli BJ5183 cells. Recombinants were selected for kanamycin resistance, and recombination was confirmed by restriction endonuclease analyses. Last, the linearized recombinant plasmid was transfected into adenovirus-packaging cell line 293. Recombinant adenovirus was typically generated within 7 to 12 days.
Generation, identification, purification, and titration of adenovirus
ZD55-TRAIL-(IETD)-Smac and ZD55-TRAIL-IETD were generated by homologous recombination of pZD55-TRAIL-(IETD)-Smac and pZD55-TRAIL-(IETD) with adenoviral packaging vector PBHGE3 in HEK293 cells. Individual plaques were picked and used to infect HEK293 cells. After observing apparent cytopathic effect, the cell culture medium was collected and viral genomic DNA was extracted. Contamination with wild-type adenovirus and the presence of foreign gene expression cassettes were then identified by PCR using primer pairs targeting the E1B region or exogenous gene. Recombinant adenovirus was amplified by infecting HEK293 cells and purified by cesium chloride gradient ultracentrifugation. Moreover, viral titers were determined by TCID50 (median tissue culture infective dose) assay in HEK293 cells. ZD55, ZD55-TRAIL, and ZD55-Smac were preserved in our laboratory.
Cell viability assay
Cells were plated on 96-well plates and treated with various recombinant adenoviruses at 10 multiplicities of infection (MOI) at various time points (24, 48, 72, and 96 hr) and then 20 μl of methylthiazolyl diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) solution (5 mg/ml) was added to each well. Cells were incubated at 37°C for 4 hr. The supernatant of each well was drawn off carefully and then an equal volume (150 μl) of dimethyl sulfoxide (DMSO) was added to each well and mixed thoroughly on a concentrating table for 10 min. The absorbance from the plates was read at 595 nm with a DNA microplate reader (GENios model; Tecan Group, Maennedorf, Switzerland).
Cytopathic effect assay
Bel-7404, Huh-7, PLC, and SW620 tumor cell lines, as well as the normal QSG-7701 and WI38 cell lines, were grown to subconfluence and infected with adenovirus at various MOIs. Six days after infection, cells were exposed to 2% crystal violet in 20% methanol for 15 min and then washed with distilled water and documented by photography.
Western blot analysis
Cells were harvested from the plates and resuspended in lysis buffer. Protein concentrations were determined with the Bio-Rad (Hercules, CA) protein assay system, and Western blotting was carried out according to standard procedures. Caspase-3 and caspase-8 antibodies were purchased from Cell Signaling Technology (Danvers, MA). TRAIL, Smac, E1A, poly(ADP-ribose) polymerase (PARP), and actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Hoechst 33342 staining
For the Hoechst staining assay, Bel-7404, NCI-H460, Huh-7, and Bcap37 cells were seeded in 6-well culture plates and then infected with ZD55-TRAIL-(IETD)-Smac at an MOI of 10; uninfected cells served as control. After 48 hr, cells were stained with an apoptosis-Hoechst 33342 staining kit (Beyotime, Shanghai, China) for 20 min as described in the manufacturer's protocol, washed with phosphate-buffered saline (PBS) twice, and observed by fluorescence microscopy.
Flow cytometric analysis
Cells infected with the vectors were trypsinized and washed once with complete medium. Aliquots of cells were resuspended in 500 ml of binding buffer and stained with fluorescein isothiocyanate (FITC)-labeled annexin V (BioVision, Palo Alto, CA) according to the manufacturer's instructions. A fluorescence-activated cell-sorting (FACS; BD Biosciences, San Jose, CA) assay was performed immediately after staining.
Antitumor experiments in vivo
Animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, D.C.). Female BALB/c nude mice (4–5 weeks old) were purchased from the Shanghai Experimental Animal Center (Shanghai, China). Bel-7404 cells were injected subcutaneously into the lower right flank of nude mice to establish the tumor xenograft model. Each group was composed of at least eight animals. When the tumors grew to 80–120 mm3 in size, mice were randomized into six groups and a daily dose of 5×108 plaque-forming units (PFU) of various adenoviruses in 100 μl was administered intratumorally for four successive days. Tumor growth was measured with a Vernier caliper every 3 days. Tumor volume (V) was calculated according to the formula V (mm3)=0.5×length (mm)×width (mm)2. On day 7 after viral injection, the tumors were harvested for immunohistochemical and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis.
Histopathology and immunohistochemical study
Tumors were harvested and fixed in 4% paraformaldehyde, embedded in paraffin, and cut in 4-μm sections. For histopathology analysis, the paraffin sections of tumors were stained with hematoxylin and eosin (H&E). For immunohistochemical (IHC) analysis, sections were stained with rabbit monoclonal anti-TRAIL and anti-Smac antibodies, each at a 1:500 dilution. The slides were then washed with PBS and incubated with avidin–biotin–peroxidase complex reagent (Vector Laboratories, Burlingame, CA) and detected with diaminobenzidine tetrahydrochloride solution containing 0.006% hydrogen peroxide. Hematoxylin was used as a counterstain. Tissue sections stained without primary antibodies were used as negative controls.
TUNEL assay
Apoptotic cells in tumor tissue sections were assessed by TUNEL staining with a TACS TdT kit in situ apoptosis detection kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. All sections were counterstained with hematoxylin.
Statistical analysis
The data reported in this paper represent the means of three independent experiments, and error bars show the standard deviation. The Student t test was used to calculate the statistical significance of the experimental results. Results were considered statistically significant when p<0.05.
Results
Construction and characterization of ZD55-TRAIL-IETD-Smac
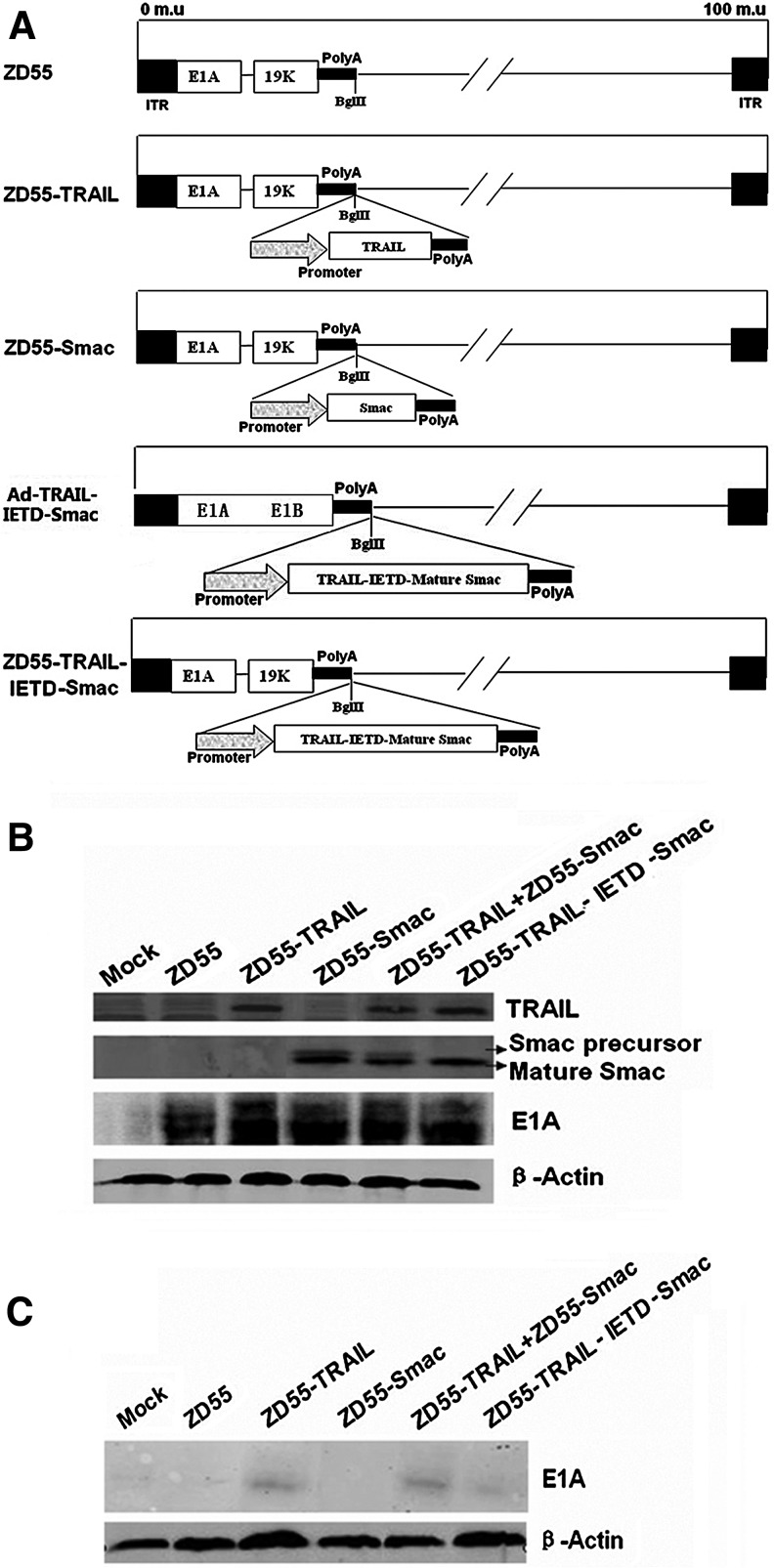
ZD55-TRAIL-IETD-Smac, Ad-TRAIL-IETD-Smac, ZD55-TRAIL, ZD55-Smac, and ZD55 were constructed as shown in Fig. 1A. With the goal of simultaneous delivery and expression of TRAIL and Smac by one oncolytic virus (i.e., cancer-targeting dual-gene virotherapy), a TRAIL-IETD-Smac expression cassette was inserted into ZD55 to construct ZD55-TRAIL-IETD-Smac. In the hepatocellular carcinoma (HCC) cell line (Bel-7404), infection with ZD55-TRAIL, ZD55-Smac, ZD55-TRAIL plus ZD55-Smac, and ZD55-TRAIL-IETD-Smac at the indicated MOIs resulted in significant production of adenovirus protein E1A (Fig. 1B). Treatment with ZD55-TRAIL, ZD55-TRAIL-IETD-Smac, or ZD55-TRAIL combined with ZD55-Smac showed equal production of TRAIL, and similar expression of Smac protein was observed in cells treated with ZD55-Smac, ZD55-TRAIL-IETD-Smac, or a combination with ZD55-TRAIL and ZD55-Smac. In the normal human liver cell line (QSG-7701), little or no expression of E1A was detected after treatment with all tested OV(s) (Fig. 1C). These results indicated that ZD55-TRAIL-IETD-Smac had cancer cell-selective replication properties, and that TRAIL and Smac transgenes were effectively expressed in cancer cell line.
FIG. 1.
Characterization of ZD55-TRAIL-IETD-Smac and its selective replication in tumor cells. (A) A schematic drawing of the oncolytic adenovirus ZD55-TRAIL-IETD-Smac and other recombinant adenoviruses. ITR, inverted terminal repeat. (B) Identification of various recombinant adenoviruses by Western blot analysis. Bel-7404 cells were treated with ZD55, ZD55-TRAIL, ZD55-Smac, and ZD55-TRAIL-IETD-Smac at an MOI of 10, or with ZD55-TRAIL combined with ZD55-Smac at an MOI of 5. After 48 hr, cell lysates were prepared for analyzing the expression of TRAIL, Smac, and E1A proteins. Mock-infected cells were included as a control. β-Actin was used as a protein loading control. (C) E1A protein expression in normal human liver cells (QSG-7701) was detected by Western blotting after treatment with various recombinant adenoviruses at the dose described previously. Data are from three independent experiments.
Effects of activation and inhibition of caspase-8 on expression of TRAIL and Smac transgenes
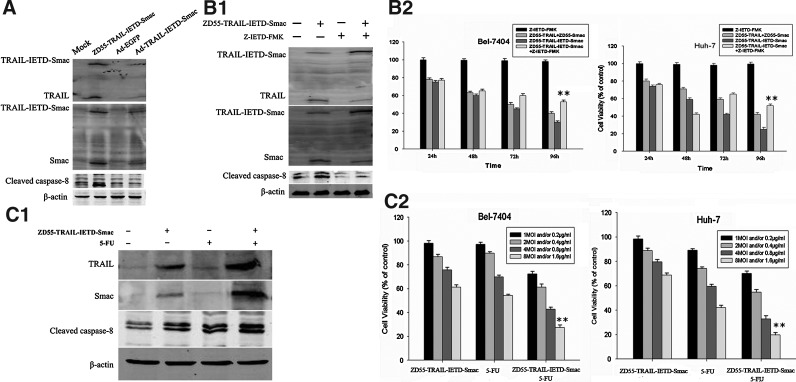
First, Ad-TRAIL-IETD-Smac (Ad is a replication-deficient adenovirus) was constructed. Bel-7404 cells were treated with Ad-TRAIL-IETD-Smac or ZD55-TRAIL-IETD-Smac (MOI of 10), and it was found that expression of TRAIL-IETD-Smac and its cleavage by caspase-8 to produce TRAIL-IETD and Smac in the Ad-TRAIL-IETD group were much less than that of ZD55-TRAIL-IETD-Smac group (Fig. 2A).
FIG. 2.
Activation and inhibition of caspase-8 affect cell viability with the expression of TRAIL and Smac transgenes. (A) Bel-7404 cells were treated with Ad-TRAIL-IETD-Smac or ZD55-TRAIL-IETD-Smac (10 MOI) and it was found that expression of TRAIL-IETD-Smac and its cleavage by caspase-8 to produce TRAIL-IETD and Smac in the Ad-TRAIL-IETD group were less than in the ZD55-TRAIL-IETD-Smac group. (B1) Bel-7404 cells were treated with ZD55-TRAIL-IETD-Smac (10 MOI) for 72 hr, either alone or in combination with caspase-8 inhibitor (Z-IETD-FMK, 20 μM). The cells were collected and cell lysates were analyzed by Western blotting for detection of expression of TRAIL and Smac, and activation of caspase-8. In addition, Ad-TRAIL-IETD-Smac was used as a control to prove that the fusion protein TRAIL-IETD-Smac is cleaved by caspase-8 activated by oncolytic virus infection. (B2) Two cancer cell lines were treated with Z-IETD-FMK (20 μM), ZD55-TRAIL (5 MOI) plus ZD55-Smac (5 MOI), and ZD55-TRAIL-IETD-Smac (10 MOI) with or without Z-IETD-FMK for the indicated times. Cell viability was determined by MTT assay. Data are presented as means±SD of three independent experiments. **p<0.01 versus ZD55-TRAIL-IETD-Smac. (C1) Expression of both TRAIL and Smac, and caspase-8 cleavage, were detected by Western blot analysis when Bel-7404 cells were treated with ZD55-TRAIL-IETD-Smac and/or 5-fluorouracil (5-FU) (0.8 μg/ml) for 72 hr. (C2) The MTT assay was used to determine the viability of Bel-7404 and Huh-7 cells treated with ZD55-TRAIL-IETD-Smac, 5-FU, or ZD55-TRAIL-IETD-Smac plus 5-FU at the indicated doses for 48 hr. Data are presented as means±SD of three independent experiments. The Student t test was used to determine significant differences, **p<0.01 versus ZD55-TRAIL-IETD-Smac.
In theory, infection with ZD55-TRAIL-IETD-Smac allows expression of a cytosolic TRAIL-IETD-Smac fusion protein that can be cleaved by caspase-8 to generate TRAIL-IETD and mature Smac. Thus we investigated whether activation or inhibition of caspase-8 will affect the production of TRAIL and Smac in cancer cells infected with ZD55-TRAIL-IETD-Smac. Hepatoma cells (Bel-7404) were treated with ZD55-TRAIL-IETD-Smac at an MOI of 10 for 72 hr, either alone or in combination with Z-IETD-FMK (a caspase-8 inhibitor) at 20 μM. Expression of transgene TRAIL, Smac, and cleaved caspase-8 was significantly downregulated by Z-IETD-FMK (Fig. 2B1). In addition, cotreatment with Z-IETD-FMK inhibited virus-induced cytotoxicity against various cancer cell lines (Fig. 2B2). Because 5-fluorouracil (5-FU) has been shown to induce upregulation of caspase-8 in tumor cells (Ehrhardt et al., 2008), we examined whether 5-FU increases expression of TRAIL and Smac transgenes or not. As shown in Fig. 2C1, combined treatment with ZD55-TRAIL-IETD-Smac and 5-FU (0.8 μg/ml) resulted in higher levels of cleaved caspase-8 and TRAIL and Smac expression compared with cells treated with this virus alone. As expected, 5-FU also sensitized human cancer cell lines Bel-7404 and Huh-7 to ZD55-TRAIL-IETD-Smac-induced cell death (Fig. 2C2).
Comparison of antitumor activity of ZD55-TRAIL and ZD55-TRAIL-IETD
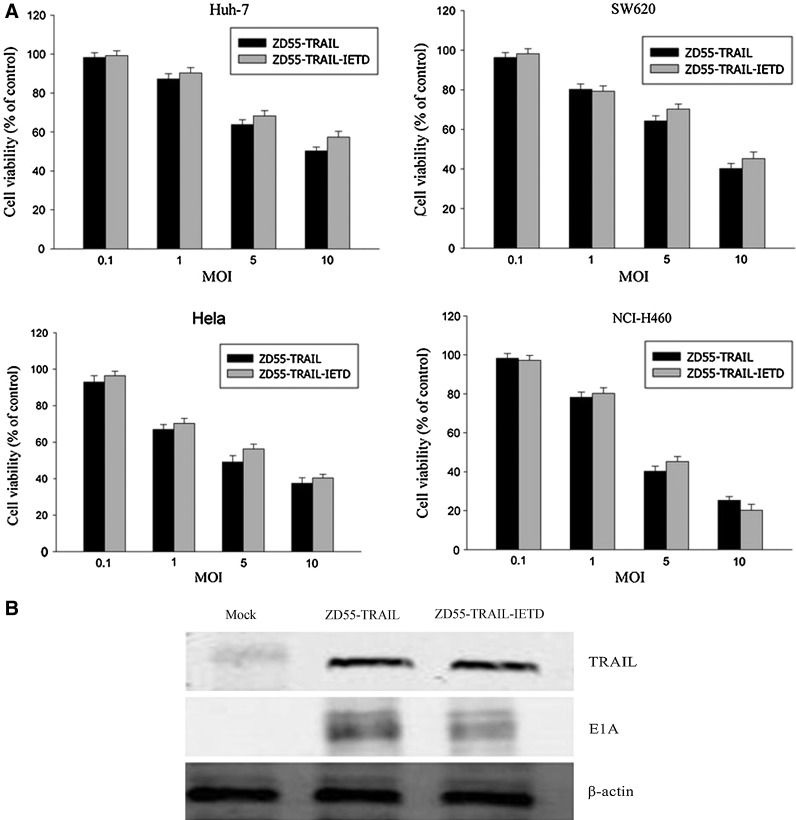
Because the TRAIL-IETD↓-Smac fusion protein could be cleaved at the arrow position to produce TRAIL-IETD and Smac after the action of caspase-8, it was necessary to determine whether IETD residues in the C terminus of TRAIL could influence the antitumor activity of TRAIL. Huh-7, SW620, HeLa, and NCI-H460 cells were tested in the presence of ZD55-TRAIL and ZD55-TRAIL-IETD by MTT assay, respectively, at various doses for 4 days. Results showed that both ZD55-TRAIL and ZD55-TRAIL-IETD could induce cytotoxicity with equal efficiency in all tested cancer cell lines in a dose-dependent manner (Fig. 3A). Also, the protein levels of TRAIL and E1A were unchanged in NCI-H460 cells on treatment with ZD55-TRAIL and ZD55-TRAIL-IETD at an MOI of 10 for 48 hr (Fig. 3B). These results indicate that the IETD residues in the C terminus of TRAIL do not affect its function.
FIG. 3.
Comparison of the antitumor activity of ZD55-TRAIL and ZD55-TRAIL-IETD. (A) Huh-7, SW620, HeLa, and NCI-H460 cells were infected with ZD55-TRAIL or ZD55-TRAIL-IETD at MOIs of 0.1, 1, 5, and 10. After 96 hr, cell survival was determined by MTT assay. Results are expressed as a percentage relative to untreated controls and data represent the mean±SD of three independent experiments. (B) Lysates of NCI-H460 cells infected with the recombinant adenoviruses were prepared to analyze the expression of TRAIL and E1A proteins. Mock-infected cells were included as a control. β-Actin was used as a protein loading control. The result shown is representative of three separate experiments.
Broad antitumor activity of ZD55-TRAIL-IETD-Smac in vitro
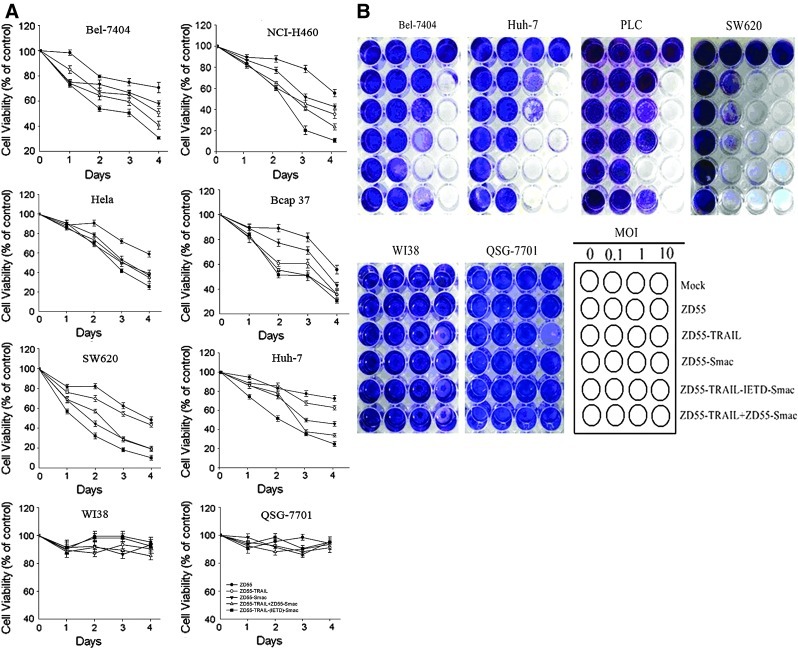
To investigate the effect of ZD55-TRAIL-IETD-Smac on cell viability, and also to confirm the toxicity results, various human cancer cell lines (Bel-7404, NCI-H460, HeLa, Bcap37, SW620, Huh-7) and normal human cell lines (QSG-7701 and WI38) were infected with ZD55, ZD55-TRAIL, ZD55-Smac, and ZD55-TRAIL-IETD-Smac at an MOI of 10. Cotreatment with ZD55-TRAIL and ZD55-Smac, each at an MOI of 5, was also evaluated. Cell proliferation was measured by MTT assay. The assays showed that ZD55-TRAIL-IETD-Smac induced cell death of about 69–89% of all the infected cancer cell lines within 96 hr postinfection and that the cytotoxicity of ZD55-TRAIL-IETD-Smac was stronger than that of ZD55-TRAIL and ZD55-Smac in all cancer cell lines, suggesting a broad antitumor effect. In contrast, 86–99% of two normal human cell lines were still viable at that dose of virus (Fig. 4A). Similar results were obtained when crystal violet staining of cancer cell lines treated with the various adenoviruses for 6 days was performed. As shown in Fig. 4B, the antitumor potency of ZD55-TRAIL-IETD-Smac against Bel-7404, Huh-7, and PLC cells was about 10-fold higher than that of ZD55-TRAIL and ZD55-Smac.
FIG. 4.
Broad antitumor activity of ZD55-TRAIL-IETD-Smac in vitro. (A) Human cancer cell lines (Bel-7404, NCI-H460, HeLa, Bcap37, SW620, and Huh-7) and normal human cell lines (QSG-7701 and WI38) were infected with ZD55, ZD55-TRAIL, ZD55-Smac, and ZD55-TRAIL-IETD-Smac at an MOI of 10 or with ZD55-TRAIL combined with ZD55-Smac at an MOI of 5. On days 1, 2, 3, and 4 postinfection, cell viability was examined by MTT colorimetric assay. Data shown (means±SD) are representative of three experiments. (B) Tumor cells (Bel-7404, Huh-7, PLC, and SW620) and normal human cells (WI38 and QSG-7701) were seeded in 24-well plates at a density of 5×104 cells per well and infected with various oncolytic adenoviruses at a series of MOIs. In combination therapy, ZD55-TRAIL and ZD55-Smac were used at a corresponding half-dose. Six days later, cells were stained with crystal violet. Color images available online at www.liebertpub.com/hum
Mechanism of action of ZD55-TRAIL-IETD-Smac
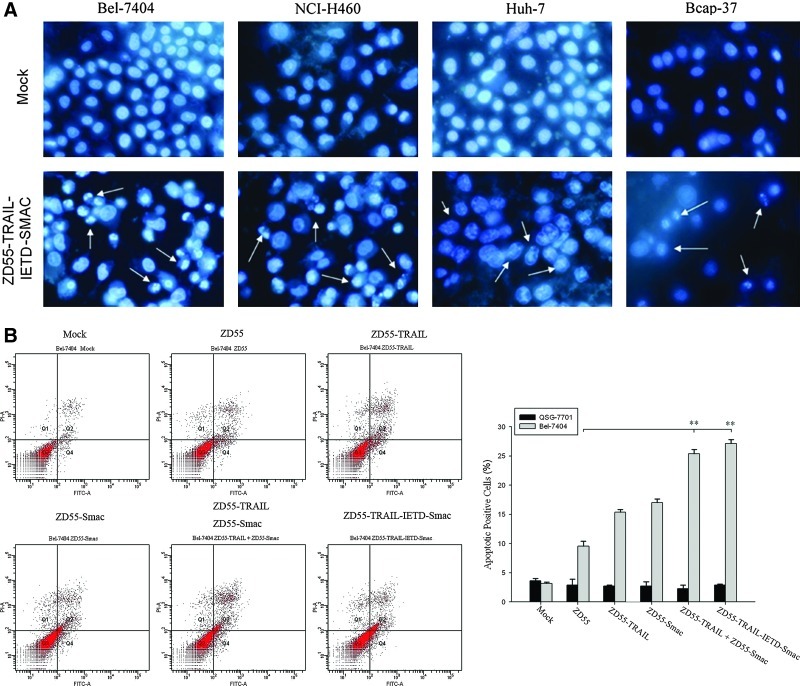
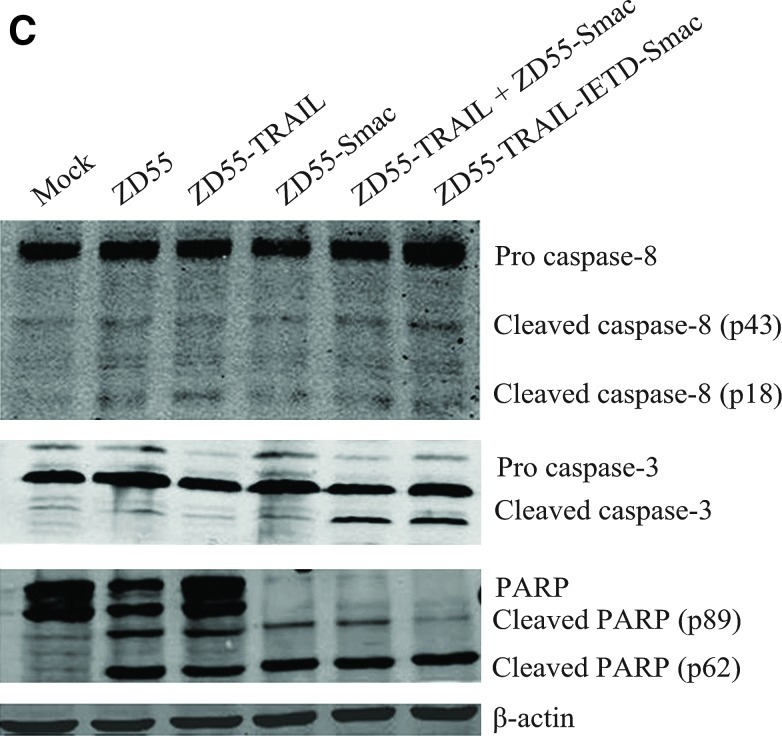
The Hoechst assay was performed on cancer cell lines Bel-7404, NCI-H460, Huh-7, and Bcap37. The results showed that treatment with ZD55-TRAIL-IETD-Smac led to massive apoptosis featured by chromatin condensation, nuclear fragmentation, and apoptotic bodies (Fig. 5A). The apoptosis induced by ZD55-TRAIL-IETD-Smac was also detected by cytometry assay on hepatoma cells (Bel-7404) and normal cells (QSG-7701) with ZD55, ZD55-TRAIL, ZD55-Smac, a combination of ZD55-TRAIL and ZD55-Smac (at a 1:1 ratio), and ZD55-TRAIL-IETD-Smac at an MOI of 10 for 48 hr. The apoptosis following treatment with ZD55-TRAIL plus ZD55-Smac or with ZD55-TRAIL-IETD-Smac was much greater than that following treatment with ZD55-TRAIL, ZD55-Smac, or ZD55, as shown in Fig. 5B (left). The column drawing in Fig. 5B (right) represents the mean values of three cytometric assays. Consistent with the above findings, Western blotting analysis showed increased activation of caspase-8, caspase-3, and PARP in the ZD55-TRAIL-IETD-Smac-treated group (Fig. 5C).
FIG. 5.
Apoptosis and activation of the caspase pathway by ZD55-TRAIL-IETD-Smac. (A) Bel-7404, NCI-H460, Huh-7, and Becap-37 cells were infected with ZD55-TRAIL-IETD-Smac at an MOI of 10 for 48 hr. Nuclei were stained with Hoechst 33342 (5 μg/ml) to visualize chromatin condensation, nuclear shrinkage, or fragmentation (markers of apoptosis; arrows) by fluorescence microscopy (original magnification,×200). (B) Left: Percent apoptotic cell death was determined by FACS 48 hr after treatment of Bel-7404 and QSG-7701 cell groups with ZD55, ZD55-TRAIL, ZD55-Smac, a combination of ZD55-TRAIL and ZD55-Smac (at a ratio of 1:1), and ZD55-TRAIL-IETD-Smac at 10 MOI. Right: A column drawing representing the mean values of three cytometric assays. **p<0.01 versus ZD55-TRAIL and ZD55-Smac. (C) Bel-7404 cells were infected with ZD55-TRAIL-IETD-Smac and the other adenoviruses for 48 hr at the dose described previously. Whole-cell extracts were prepared and immunoblotted to detect activation of the caspase pathway. β-Actin was used as a loading control. The data are representative of two determinations with identical results. PARP, poly(ADP-ribose) polymerase. Color images available online at www.liebertpub.com/hum
Complete eradication of xenograft hepatoma by both ZD55-TRAIL-IETD-Smac and cotreatment with ZD55-TRAIL with ZD55-Smac
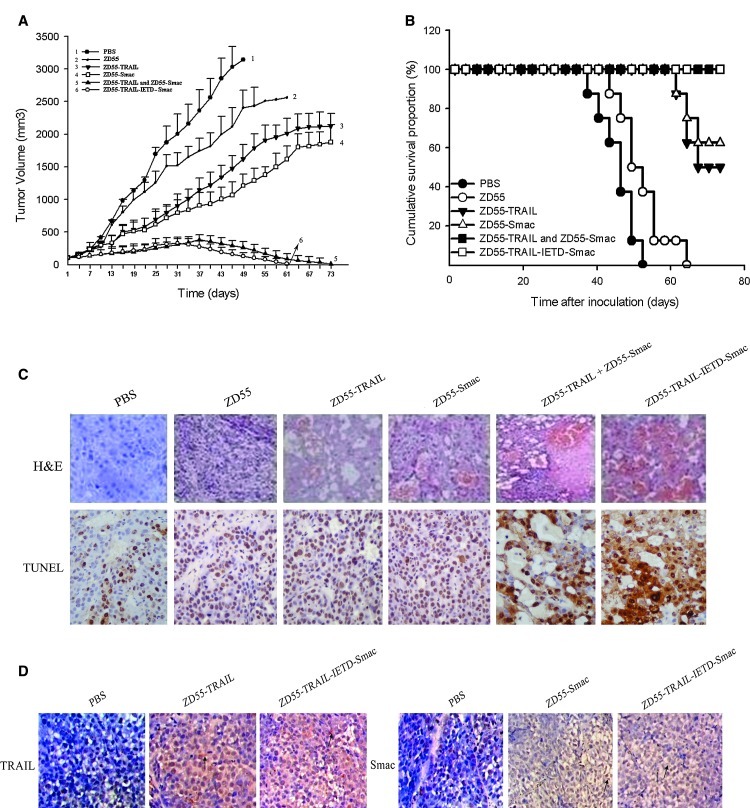
The antitumor efficacy of ZD55-TRAIL-IETD-Smac was evaluated in a subcutaneous Bel-7404 xenograft model in nude mice. In all studies, mice bearing established tumors (initial tumor volume, 80–100 mm3) received intratumoral injection of the viruses. ZD55, ZD55-TRAIL, ZD55-Smac, and ZD55-TRAIL-IETD-Smac at single doses of 5×108 PFU in a volume of 100 μl were administered each day successively for 4 days, totaling 2×109 PFU, to groups of mice (n=8), whereas in the combination group animals received 2.5×108 PFU of ZD55-TRAIL plus 2.5×108 PFU of ZD55-Smac per day. PBS was used as control. As shown in Fig. 6A, both the ZD55-TRAIL plus ZD55-Smac treatment group and the ZD55-TRAIL-IETD-Smac treatment group had significant antitumor effect until complete eradication of the xenograft hepatoma at 73 days after treatment, which is much better than the results with ZD55-TRAIL (p<0.0001 and p<0.0001, respectively) or ZD55-Smac (p<0.0001 and p<0.0001, respectively).
FIG. 6.
Antitumor efficacy of ZD55-TRAIL-IETD-Smac in nude mice. Female BALB/c nude mice were subcutaneously inoculated with Bel-7404 cells (1×107). When tumors reached a size of 80–100 mm3, the animals were treated with an intratumoral injection of ZD55-TRAIL-IETD-Smac or other recombinant adenovirus at the dose described in Materials and Methods. PBS was used as a control. (A) Tumor size was measured and tumor volume was monitored at various times after treatment. Data are presented as means±SD (n=8). (B) Kaplan–Meier survival curves of animals. The percentage of surviving mice was calculated by monitoring the death of mice over a period of 73 days. A pair-wise log-rank test was used to analyze survival rates in the various groups. (C and D) Representative histological changes and protein expression of TRAIL and Smac in xenografts on day 7 after treatment. Tumors were harvested and fixed in 4% paraformaldehyde, embedded in paraffin, cut into 4-μm sections, and then subjected to immunochemical staining, TUNEL assay, and H&E staining (original magnification,×200). Color images available online at www.liebertpub.com/hum
Kaplan–Meier survival curves were used to describe the survival and death of the animals. Nude mice in the ZD55-TRAIL plus ZD55-Smac group and the ZD55-TRAIL-IETD-Smac group were all alive at 73 days, whereas a significant proportion of mice in the other treatment groups died; all of the mice in the control group and ZD55 group died (Fig. 6B).
To confirm cell death from apoptosis and the expression of TRAIL and Smac in tumor tissues, H&E, TUNEL, and immunohistochemical staining with anti-TRAIL and Smac antibodies were performed on day 7 after viral injection. H&E staining showed the killing of tumor cells from virus-treated tumors, with ZD55-TRAIL-IETD-Smac and the combination therapy resulting in more severe cytopathic effects than ZD55-TRAIL or ZD55-Smac alone (Fig. 6C, top row). In addition, TUNEL staining further confirmed that ZD55-TRAIL-IETD-Smac induced more extensive apoptosis in tumors whereas other adenoviruses resulted in less apoptosis (Fig. 6C, bottom row). Immunohistochemical staining confirmed strong expression of both TRAIL and Smac in tumor tissues after treatment with ZD55-TRAIL-IETD-Smac (Fig. 6D), suggesting that IETD was successfully used to link two genes followed by complete cleavage into two perfectly functional genes.
Discussion
In previous studies the strong antitumor effect of cancer-targeting gene virotherapy (CTGVT) had often achieved complete eradication of many xenograft tumors, as follows: ZD55-TRAIL plus ZD55-k5 for colorectal cancer (Liu et al., 2005), ZD55-TRAIL plus ZD55-Smac for hepatoma (Pei et al., 2004), ZD55-TRAIL plus ZD55-MnSOD for colorectal cancer (Zhang et al., 2006), ZD55-hSSTr2 plus ZD55-TRAIL for pancreatic carcinoma (Zhang et al., 2009), ZD55-TRAIL plus ZD55-Cyld for colon cancer (Chu et al., 2006), ZD55-IL-24 plus ZD55-TRAIL for SW620 colorectal cancer (Zhao et al., 2006), and also for Bel-7404 hepatoma and SGC7901 gastric cancer (data not shown). In the above-cited experiments, resulting in completely eradicated xenograft tumors, ZD55-TRAIL was the essential component.
Our cancer-targeting dual-gene virotherapy (CTGVT-DG) strategy is to combine two suitable genes: OV-gene 1 and OV-gene 2. It is difficult to join two genes by a linker into one OV to form OV-gene 1-linker-OV-gene 2 and also show a similar strong antitumor effect. Many linkers are used to join two genes: (1) the internal ribosome entry site (IRES): there are many IRESs from viruses or cells. The first discovered IRES was from encephalomyocarditis virus (ECMV) and was about 450 bp in length (Ngoi et al., 2004), but there are many variations ranging from 9 nucleotides (Chappell et al., 2000) to 1.5 kb (Hudder and Werner, 2000). However, the expression of gene 1 in the OV-gene 1-IRES-OV-gene 2 construct is 10-fold higher than that of gene 2 (Mizuguchi et al., 2000). For example, expression of the H chain in the H-IRES-L construct (H and L represent the heavy and light chains of an antibody, such as vascular endothelial growth factor receptor-2 [VEGFR2] neutralization mAb) is 1.6 μg/ml, whereas for the L chain it is 0.1 μg/ml; this represents a 16-fold difference (Fang et al., 2005); (2) the foot-and-mouth disease virus (FMDV) 2A peptide to join two proteins such as the H and L chains of an antibody: the genome of FMDV is a positive-stranded RNA and is divided into 1A, 1B, 1C…2A, 2B, 2C…3A, 3B, 3C…(Carrillo et al., 2005). The commonly used 2A is a 24-amino acid peptide (APVKQTLNFDLLKLAGDVESNPG↓P) that is self-cleaved at the arrow position (Mattion et al., 1996) with high efficacy in eukaryotic cells but not in prokaryotic cells. FMDV 2A can be used to join the heavy and light chains of VEGFR2 neutralization mAb, but after self-cleavage there remain many amino acids of 2A in the H chain, although there remains only one amino acid residue in the L chain (Evans, 2011); (3) using 2A, Fang and colleagues added a furin (an enzyme universally existing in cells) cleaving site R↓AK↓R↓before 2A to form F·2A. In H-F2A-L, the F·2A can be nearly completely cleaved, with only one amino acid (arginine) in the H chain and one amino acid (proline) in the L chain remaining. F·2A is a good linker for joining the H and L chains of antibodies according to a paper published in Nature Biotechnology (Fang et al., 2005).
As for the TRAIL gene, we have developed a new method by using the four-amino acid IETD (isoleucine-aspartate-threonine-glutamate) linker. Srinivasula and colleagues found that GFP-IETD↓-Smac can be cleaved at the arrow position by caspase-8 induced by TRAIL (Srinivasula et al., 2000), but a study on ZD55-TRAIL-IETD-Smac has not been done. If true, the cleaved product TRAIL-IETD will influence the antitumor effect of TRAIL. Our data showed that the antitumor effect of ZD55-TRAIL-IETD-Smac concentrated 2-fold is quantitatively equal to that of the ZD55-TRAIL plus ZD55-Smac group and much better than that of the ZD55-TRAIL and ZD55-Smac groups, as detected by the MTT or crystal violet method in vitro (Fig. 4) and in an animal model in vivo (Fig. 6). These data also further proved that TRAIL-IETD does not influence the antitumor effect of TRAIL, as shown in Fig. 3. An inhibitor of caspase-8 both downregulates the expression of ZD55-TRAIL-IETD and ZD55-Smac as detected by Western blot and also decreases the antitumor effect of ZD55-TRAIL-IETD-Smac. All the above data showed that IETD is the best linker for TRAIL in the CTGVT-DG strategy to date.
Our data showed the following: (1) ZD55-IETD-Smac can be cleaved by caspase-8 as proved by the use of caspase-8 inhibitor or activator; (2) the cleaved product with IETD attached to TRAIL (TRAIL-IETD) does not affect the function of TRAIL; (3) the cleavage efficacy of ZD55-TRAIL-IETD-Smac and the functional cleaved products are quantitative (Figs. 3 and 6); and (4), the most important point, IETD is the smallest useful linker for ZD55-TRAIL-IETD-X drugs, which may completely eliminate various kinds of cancers.
The genomes of tumor cells are invariably altered at multiple sites and several important signaling pathways are always disrupted at the same time (Hanahan and Weinberg, 2000); a combination of genes that target totally different aspects of tumor biology would achieve more potent inhibition in a wide variety of tumors (Zhang et al., 2003, 2006; Pei et al., 2004; Liu et al., 2005; Breitbach et al., 2011; Evans, 2011). This point was supported by A.D. Levinson (2010), the chairman of Genentech (San Francisco, CA), and also by J. Kaiser in a special issue of Science (Cancer Crusade at 40; Marshall, 2011). In the past, the U.S. Food and Drug Administration (FDA) and Chinese State Food and Drug Administration (SFDA) allowed investigational new drug (IND) applications only for a single gene or single drug for clinical use. However, sometimes one drug or a single gene cannot exhibit its antitumoral effect. For example, the inhibitor of the enzyme MEK (mitogen-activated protein kinase kinase) has proven disappointing as a single agent against pancreatic and non-small cell lung cancer, despite frequent MEK pathway activation in these cancers, but the combination of MEK inhibitor with an agent that targets the phosphatidylinositol-3-kinase (PI3K) signaling pathway can result in substantial tumor regression. Therefore, the U.S. FDA in 2010 announced that they plan to draft new guidelines for testing and approving the use of multidrug treatment. A.D. Levinson also said that if we are to achieve breakthrough cancer therapies, current U.S. FDA regulations must change (Levinson, 2010). The cocktail method, that is, the use of several drugs in combination, is used for the treatment of AIDS, and C. Sawyer (one of the scientists who developed the well-known drug Gleevec) and many other researchers say: “There's no reason it [i.e., a cocktail] shouldn't work for cancer” (Marshall, 2011). In 2006, X.-Y. Liu suggested (in the Chinese Science and Technology Daily, June 23, 2006) the use of the cocktail method for cancer therapy. This point of view was also further supported in 2011 by J. Kaiser, who said that “to stretch the benefit over years, it might be necessary to devise one complex cocktail after another” (Marshall, 2011). For cancer therapy, CTGVT-DG is similar to a cocktail of two genes, with the two genes exerting a compensatory or synergistic effect. The combination of two genes into one fusion gene with a linker may play an important role in cancer therapy and IETD might be the best linker, which will be used to combine many two-gene combinations into one gene, such as ZD55-TRAIL-IETD-Smac. In addition, IETD can be used to link IL-24 and TRAIL to make ZD55-IL-24-IETD-TRAIL [Ad-sup-E1A(Δ24)E1B(Δ55)-IL-24-IETD-TRAIL] and produce an excellent antitumor effect, because IL-24 can also induce caspase-8 expression (our unpublished data). This means that, with just four amino acids, IETD may have broad use for linking two genes into one gene, for example, OV-gene 1-IETD-OV-gene 2, if gene 1 can induce caspase-8 expression and gene 2 has a compensative or synergetic affect with gene 1 to produce an antitumor effect.
Acknowledgments
The authors thank Gongchu Li, Kan Chen, Guoqing He, and Guoliang Xie for professional technical assistance. This work was supported by the National Basic Research Program of China (973 Program) (no. 2010CB529901), Zhejiang Sci-Tech University Study start-up grants (1016834-Y), National Natural Science Foundation of China grants (no. 81070419), and the Zhejiang Provincial Natural Science Foundation of China (no. R2090392).
Author Disclosure Statement
No competing financial interests exist.
References
- Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- Bischoff J.R. Kirn D.H. Williams A., et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Breitbach C.J. Burke J. Jonker D., et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- Carrillo C. Tulman E.R. Delhon G., et al. Comparative genomics of foot-and-mouth disease virus. J. Virol. 2005;79:6487–6504. doi: 10.1128/JVI.79.10.6487-6504.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J. Du C. Wu J.W., et al. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- Chappell S.A. Edelman G.M. Mauro V.P. A 9-nt segment of a cellular mRNA can function as an internal ribosome entry site (IRES) and when present in linked multiple copies greatly enhances IRES activity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1536–1541. doi: 10.1073/pnas.97.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L. Gu J. He Z., et al. Adenoviral vector expressing CYLD augments antitumor activity of TRAIL by suppression of NF-κB survival signaling in hepatocellular carcinoma. Cancer Biol. Ther. 2006;5:615–622. doi: 10.4161/cbt.5.6.2662. [DOI] [PubMed] [Google Scholar]
- Deng Y. Lin Y. Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix B.R. Edwards S.J. Braithwaite A.W. Does the antitumor adenovirus ONYX-015/dl1520 selectively target cells defective in the p53 pathway? J. Virol. 2001;75:5443–5447. doi: 10.1128/JVI.75.12.5443-5447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C. Fang M. Li Y., et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H. Hacker S. Wittmann S., et al. Cytotoxic drug-induced, p53-mediated upregulation of caspase-8 in tumor cells. Oncogene. 2008;27:783–793. doi: 10.1038/sj.onc.1210666. [DOI] [PubMed] [Google Scholar]
- Evans J. Recent deal highlights hopes for cancer-killing viruses. Nat. Med. 2011;17:268–269. doi: 10.1038/nm0311-268b. [DOI] [PubMed] [Google Scholar]
- Fang J. Qian J.J. Yi S., et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hudder A. Werner R. Analysis of a Charcot-Marie-Tooth disease mutation reveals an essential internal ribosome entry site element in the connexin-32 gene. J. Biol. Chem. 2000;275:34586–34591. doi: 10.1074/jbc.M005199200. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Galluzzi L. Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Levinson A.D. Cancer therapy reform. Science. 2010;328:137. doi: 10.1126/science.1189749. [DOI] [PubMed] [Google Scholar]
- Lin T. Gu J. Zhang L., et al. Targeted expression of green fluorescent protein/tumor necrosis factor-related apoptosis-inducing ligand fusion protein from human telomerase reverse transcriptase promoter elicits antitumor activity without toxic effects on primary human hepatocytes. Cancer Res. 2002;62:3620–3625. [PubMed] [Google Scholar]
- Liu X.Y. Qiu S.B. Zou W.G., et al. Effective gene-virotherapy for complete eradication of tumor mediated by the combination of hTRAIL (TNFSF10) and plasminogen k5. Mol. Ther. 2005;11:531–541. doi: 10.1016/j.ymthe.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Marshall E. Cancer research and the $90 billion metaphor. Science. 2011;331:1540–1541. doi: 10.1126/science.331.6024.1540-a. [DOI] [PubMed] [Google Scholar]
- Mattion N.M. Harnish E.C. Crowley J.C. Reilly P.A. Foot-and-mouth disease virus 2A protease mediates cleavage in attenuated Sabin 3 poliovirus vectors engineered for delivery of foreign antigens. J. Virol. 1996;70:8124–8127. doi: 10.1128/jvi.70.11.8124-8127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi H. Xu Z. Ishii-Watabe A., et al. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. 2000;1:376–382. doi: 10.1006/mthe.2000.0050. [DOI] [PubMed] [Google Scholar]
- Ngoi S.M. Chien A.C. Lee C.G. Exploiting internal ribosome entry sites in gene therapy vector design. Curr. Gene Ther. 2004;4:15–31. doi: 10.2174/1566523044578095. [DOI] [PubMed] [Google Scholar]
- Pan G. O'Rourke K. Chinnaiyan A.M., et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Pei Z. Chu L. Zou W., et al. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39:1371–1381. doi: 10.1002/hep.20203. [DOI] [PubMed] [Google Scholar]
- Saelens X. Festjens N. Vande Walle L., et al. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- Schneider P. Bodmer J.L. Thome M., et al. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- Sheridan J.P. Marsters S.A. Pitti R.M., et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- Srinivasula S.M. Datta P. Fan X.J., et al. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J. Biol. Chem. 2000;275:36152–36157. doi: 10.1074/jbc.C000533200. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Gu J. Zhao L., et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res. 2006;66:4291–4298. doi: 10.1158/0008-5472.CAN-05-1834. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Huang Y. Newman K., et al. Reexpression of human somatostatin receptor gene 2 gene mediated by oncolytic adenovirus increases antitumor activity of tumor necrosis factor-related apoptosis-inducing ligand against pancreatic cancer. Clin. Cancer Res. 2009;15:5154–5160. doi: 10.1158/1078-0432.CCR-09-0025. [DOI] [PubMed] [Google Scholar]
- Zhang Z.L. Zou W.G. Luo C.X., et al. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res. 2003;13:481–489. doi: 10.1038/sj.cr.7290191. [DOI] [PubMed] [Google Scholar]
- Zhao L. Dong A. Gu J., et al. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]