Abstract
Malaria transmission-blocking vaccination can effectively reduce and/or eliminate transmission of parasites from the human host to the mosquito vector. The immunity achieved by inducing an antibody response to surface antigens of male and female gametes and parasite stages in the mosquito. Our laboratory has developed DNA vaccine constructs, based on Pfs25 (a Plasmodium falciparum surface protein of 25 kDa), that induce a transmission-blocking immune response in mice (C. A. Lobo, R. Dhar, and N. Kumar, Infect. Immun. 67:1688-1693, 1999). To evaluate the safety, immunogenicity, and efficacy of the Pfs25 DNA vaccine in nonhuman primates, we immunized rhesus macaques (Macaca mulatta) with a DNA vaccine plasmid encoding Pfs25 or a Pfg27-Pfs25 hybrid or with the plasmid (empty plasmid) alone. Immunization with four doses of these DNA vaccine constructs elicited antibody titers that were high but nonetheless unable to reduce the parasite's infectivity in membrane feeding assays. Further boosting of the antibody response with recombinant Pfs25 formulated in Montanide ISA-720 increased antibody titers (30-fold) and significantly blocked transmission of P. falciparum gametocytes to Anopheles mosquitoes (∼90% reduction in oocyst numbers in the midgut). Our data show that a DNA prime-protein boost regimen holds promise for achieving transmission-blocking immunity in areas where malaria is endemic and could be effective in eradicating malaria in isolated areas where the level of malaria endemicity is low.
Plasmodium falciparum, one of the deadliest of the malaria-causing species, continues to threaten humans, especially children and pregnant women, in many parts of the world (8). The available drugs and the vector control campaigns used to date have not had a significant impact on the transmission of malaria from humans to mosquitoes. When a female Anopheles mosquito bites an infected human, the male and female gametocytes (formed during the erythrocytic phase of the malaria life cycle) are taken up in the blood meal and rapidly undergo gametogenesis and fertilization. Oocysts and eventually infective sporozoites are formed, thus completing parasite development. It has been shown that the crucial link for malaria transmission, i.e., infectivity of male and female gametocytes, can be blocked in the mosquito vector by antibodies directed against sexual-stage-specific surface antigens when they are ingested along with the parasites in the blood meal (5, 12, 18). It is believed that transmission-blocking immunity will play a significant role in reducing the emergence of vaccine-resistant strains. Such strains could be selected by vaccines targeting erythrocytic asexual forms. Likewise, spread of drug resistance could be diminished by reducing overall malaria transmission (4).
P. falciparum zygote-ookinete surface protein 25 (Pfs25) is one of the most promising candidates identified so far for the development of P. falciparum transmission-blocking vaccines. Pfs25 (a 25-kDa surface protein) is expressed at the onset of gametogenesis in the mosquito midgut, and its expression continues through zygote-ookinete transformation (14), It was previously shown that Pfs25 expressed in recombinant vaccinia virus or a yeast secretory system could elicit transmission-blocking antibodies that recognized conformational epitopes (2, 15, 16). Recently, our laboratory developed a DNA-based vaccine against Pfs25. This vaccine is highly immunogenic in mice. Antibodies induced were effective blockers of infectivity of P. falciparum gametocytes in mosquitoes (97% reduction in oocyst numbers in mosquito midguts and 75% reduction in the rate of infection) (19). As a novel vaccination approach, DNA-based vaccines are capable of inducing both humoral and cellular immune responses and offer an alternate way to produce multistage-multiantigen vaccines for complex parasites like those that cause malaria (26). Another advantage of DNA vaccines is the ease of their production and their ability to induce immune responses without any exogenous adjuvants, which is an absolute requirement for protein-based vaccine formulations.
As an extension of our studies with mice, we have evaluated the immunogenicity of transmission-blocking DNA vaccines encoding Pfs25 in nonhuman primates. It is now well recognized that DNA vaccines are poorly immunogenic in nonhuman primates and humans compared to mice (3, 7, 17, 25, 27). Immunostimulatory CpG oligodeoxynucleotides and coexpression of cytokines, e.g., granulocyte-macrophage colony-stimulating factor, interleukin-2 (IL-2), IL-10, IL-12, costimulatory molecules (B7), adhesion molecules (ICAM-1), and various delivery systems (cationic lipids, liposomes, microspheres, and lipid cochleate forms), have been used to increase the immunogenicity of DNA vaccines (17, 23, 27). Another approach is to use a heterologous boost with recombinant poxviruses (9, 21) or with recombinant protein formulated in a suitable adjuvant (11).
In our study, we first evaluated the immunogenicity of Pfs25 DNA vaccines alone, followed by boosting with recombinant protein (Pfs25) formulated in the adjuvant Montanide ISA-720. Our results showed that DNA immunizations alone, while giving considerable antibody responses, did not block transmission in membrane feeding assays. On the other hand, heterologous immunization with recombinant protein (Pfs25) boosted antibody responses both quantitatively and qualitatively that blocked the infectivity of P. falciparum gametocytes, as revealed by an ∼90% reduction in the oocyst numbers in Anopheles mosquitoes. We report here for the first time the safety and efficacy of a DNA prime-protein boost immunization regimen in nonhuman primates for the development of P. falciparum transmission-blocking vaccines.
MATERIALS AND METHODS
DNA vaccine plasmids.
DNA vaccine vector VR1020 (Vical, Inc., San Diego, Calif.) encoding Pfs25 or a Pfg27-Pfs25 hybrid has been described earlier (19). The Pfs25 (GenBank accession no. X07802) construct was created as a truncated version by removing putative signal and anchor sequences, whereas the entire coding sequence of Pfg27 (GenBank accession no. M38286) was used. Both genes were amplified by PCR using P. falciparum (3D7), a clone of NF54, genomic DNA as the template and cloned into VR1020 (19). Recombinant DNA plasmids were purified by using endotoxin-free plasmid purification kits (Qiagen Inc., Valencia, Calif.). The endotoxin levels of the purified DNA were determined by using a Limulus amoebocyte lysate assay (BioWhittaker, Inc., Walkersville, Md.) in accordance with the manufacturer's instructions. The DNA plasmid solution containing endotoxin levels higher than 50 EU/mg of DNA were further purified with Triton X-114 (1). The purity of plasmid DNA was checked by agarose gel electrophoresis and PCR analysis of inserts. Plasmid DNA was diluted in endotoxin-free PBS for immunizations.
Animals.
Nineteen 4- to 6-year-old rhesus macaques (Macaca mulatta) of Chinese origin housed at the Tulane National Primate Research Center were used in the study. Tulane National Primate Research Center animal care facilities are accredited by the American Association for Accreditation of Laboratory Animal Care and licensed by the U.S. Department of Agriculture. All animals were routinely cared for in accordance with the guidelines prescribed by the National Institutes of Health Guide to Laboratory Animal Care. Prior to immunization, sera from the monkeys were tested to detect any possible reactivity to Pfs25 by enzyme-linked immunosorbent assay (ELISA) and for any transmission-blocking activity to P. falciparum gametocytes by membrane feeding assays (see below). Animals that did not have any reactivity or blocking activity were chosen.
Immunizations.
The monkeys were assigned to five groups randomly as follows. Group 1 (M1, M2, M3, and M4) received 0.5 mg of VR1020 encoding Pfs25 (VR1020/25) DNA vaccine, group 2 (M5, M6, M7, and M8) received 1.0 mg of VR1020/25 DNA vaccine, group 3 (M9, M10, M11, and M12) received 0.5 mg of VR1020 encoding the Pfg27-Pfs25 hybrid (VR1020/27-25) DNA vaccine, group 4 (M13, M14, M15, and M16) received 1.0 mg of VR1020/27-25 hybrid DNA vaccine, and group 5 (M17, M18, M19) received 0.5 mg of the VR1020 vector alone. All groups contained four monkeys, except the vector-alone group (three monkeys). Animals were immunized four times with DNA vaccine intramuscularly (i.m.) at four sites (triceps, tibialis anterior, deltoid, and quadriceps in a total volume 1 ml of phosphate-buffered saline [PBS]) at weeks 0, 4, 12, and 24. Twenty weeks after the last DNA immunization, all of the animals were immunized i.m. with 25 μg of Saccharomyces cerevisiae-derived recombinant protein Pfs25 (kindly supplied by the Malaria Vaccine Development Unit, National Institute of Allergy and Infectious Diseases, National Institutes of Health) emulsified with the adjuvant Montanide ISA-720 (Seppic, Inc., Paris, France) (3 volumes of protein and 7 volumes of adjuvant). Details of recombinant Pfs25 expression have been described elsewhere (13, 24). The coding sequence used for expression was optimized by deletion of the N-terminal signal peptide and the C-terminal GPI anchor sequences and mutation (N→Q) of all three potential N-glycosylation sites. Sera were collected before and after each immunization and stored at −20°C until use.
ELISA.
Serum antibody analysis was conducted by ELISA as previously described (6, 19). Briefly, 96-well Immulon 2 plates were coated with recombinant Pfs25 at 2 μg/ml in bicarbonate buffer (4 mM Na2CO3, 8 mM NaHCO3, pH 9.6) and incubated overnight at 4°C. After blocking with 5% nonfat milk in PBS plus 0.05% Tween 20, 100-μl volumes of serum dilutions were added to duplicate wells and incubated for 2 h at room temperature. Plates were washed with PBS plus 0.05% Tween 20 between incubations and incubated with horseradish peroxidase-conjugated goat anti-monkey immunoglobulin G (IgG; Kirkegaard & Perry Laboratories, Gaithersburg, Md.). To examine the IgG subclasses of rhesus sera, plates were incubated with horseradish peroxidase-conjugated sheep anti-human IgG1, IgG2, IgG3, and IgG4 (The Binding Site, Birmingham, United Kingdom) at a 1:2,000 dilution (22). Finally, plates were developed with the 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) single-reagent substrate (Kirkegaard & Perry Laboratories) and absorbance was read at 405 nm. Endpoint titers were defined as serum dilutions giving an absorbance higher than the average optical density (OD) at 405 nm of preimmune serum plus 3 standard deviations (SD).
To test antibody avidity, various concentrations (0, 1, 2, 4, and 6 M) of sodium thiocyanate (NaSCN) washes were used in the standard ELISA protocol to disrupt antigen-antibody binding as described previously (19, 20). Recombinant Pfs25-coated plates were incubated with various serum dilutions (linear portion of the serum dilution curve). After three washes with PBS plus 0.05% Tween 20, different concentrations of the chaotropic agent NaSCN were added. Plates were allowed to stand at room temperature for 15 min and washed extensively (six times). Subsequent steps were done as described above in the ELISA protocol.
Western blot analysis.
In vitro-cultured mature P. falciparum (NF54) gametocytes were induced for exflagellation and gametogenesis as described earlier (19). Briefly, gametes and zygotes were purified by using a Nycodenz gradient and extracts were run on a sodium dodecyl sulfate-12.5% polyacrylamide gel under nonreducing conditions. The gel was transferred to nitrocellulose membrane and probed with pooled monkey sera (at a 1:2,000 dilution) from each group. The bands were detected by ECL-plus (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) detection system.
Membrane feeding transmission-blocking assays.
Mature gametocytes (14 to 18 days old) of P. falciparum strain NF54 were produced in vitro as reported previously (10). Membrane feeding assays were performed to test the infectivity of the P. falciparum gametocytes for Anopheles mosquitoes in the presence of monkey sera as previously described (19). Briefly, the sera that had higher antibody titers from each group were mixed at various dilutions with freshly washed human erythrocytes (50% final hematocrit), human sera, and mature gametocyte cultures (0.3 to 0.4% final gametocytemia). These mixtures were fed to starved (5 to 6 h) mosquitoes (Anopheles stephensi or A. gambiae) housed in cages. Mosquitoes were allowed to engorge blood and serum mixtures for 15 min through a Parafilm membrane warmed to 39°C with a glass water jacket. After a blood meal, mosquitoes were maintained at 26°C and 60 to 80% relative humidity. Seven to 9 days later, midguts were stained with 0.1% mercurochrome and examined for the presence of oocysts by microscopy.
Assessment of vaccine safety.
To assess vaccine safety, animals were examined clinically, and clinical laboratory tests composed of complete blood cell counts, serum chemistries, and urinalyses were performed. In addition, anti-DNA antibody was quantified by using a commercially available ELISA (Hemagen Diagnostics, Inc., Waltham, Mass.) to detect antibodies to double-stranded DNA. Clinical examinations were performed at the time of serum specimen collection, i.e., every 2 weeks, starting at the time of the first DNA immunization, until week 16 thereafter. During this same period, clinical laboratory tests were performed every 4 weeks. After week 16, clinical examinations, laboratory tests, and serum collection were performed approximately every 8 weeks. After the first protein immunization, animals were assessed as described above every 3 weeks until week 78. The serum specimens collected from all 19 animals at weeks, 0, 16, 37, and 78 were tested for the presence of antibodies to double-stranded DNA.
Statistical analysis.
Statistically significant differences in antibody responses between groups were analyzed with a one-way analysis of variance (ANOVA). Overall antibody responses between monkeys after each immunization were analyzed by using a mixed ANOVA. Membrane feeding assay results were analyzed by using both Kruskal-Wallis one-way analysis and the Mann-Whitney U test. P < 0.05 was considered to be statistically significant.
RESULTS
Effect of DNA prime-DNA boost regimen on Pfs25-specific antibody response.
To evaluate the immunogenicity and efficacy of the Pfs25 DNA vaccine in nonhuman primates, 19 rhesus macaques were divided into five groups and immunized with two different constructs (VR1020/25 and VR1020/27-25) at two different doses (0.5 and 1.0 mg). Animals in the control group were immunized with vector VR1020 alone (0.5 mg). Immunizations with DNA constructs were repeated 1 month after administration of the priming dose and two more times at 3-month intervals after the second DNA immunization. Sera obtained after each immunization were tested for antibody levels by ELISA. Table 1 gives the ELISA results of all of the sera obtained 7 weeks after the fourth immunization. Antigen-specific antibody responses against Pfs25 were elevated up to a reciprocal titer of 32,000 in some monkeys (M5, M6, M9, and M15), although the mean reciprocal titers were 12,125 for group 1, 21,000 for group 2, 16,250 for group 3, and 12,500 for group 4. The overall mean antibody titers after each DNA immunization are shown in Fig. 1. Regardless of the DNA dose (0.5 or 1 mg) or the plasmid construct (VR1020/25 or VR1020/27-25) used, antibody titers among the various groups were not significantly different (P > 0.05, one-way ANOVA). Some monkeys from different immunization groups had low antibody titers (M1, M12, M13, and M14). As expected, animals in the control group (group 5) did not have detectable Pfs25-specific antibodies at any time.
TABLE 1.
Anti-Pfs25 antibody responses of sera from immunized rhesus monkeys determined by ELISAa
| Group (treatment) or parameter | Monkey no. | Antibody titer
|
||
|---|---|---|---|---|
| Post 4th DNA immunization | Post heterologous protein boost | 6 mo after heterologous protein boost | ||
| 1 (VR1020/25, 0.5 mg) | M1 | 500 | 80,000 | 40,000 |
| M2 | 16,000 | >1,280,000 | 320,000 | |
| M3 | 16,000 | >1,280,000 | 160,000 | |
| M4 | 16,000 | >1,280,000 | 20,000 | |
| Mean ± SEM | 12,125 ± 3,875 | 980,000 ± 300,000 | 135,000 ± 69,000 | |
| 2 (VR1020/25, 1.0 mg) | M5 | 32,000 | >1,280,000 | 160,000 |
| M6 | 32,000 | >1,280,000 | 80,000 | |
| M7 | 16,000 | 640,000 | 40,000 | |
| M8 | 4,000 | 320,000 | 160,000 | |
| Mean ± SEM | 21,000 ± 6,800 | 880,000 ± 240,000 | 110,000 ± 30,000 | |
| 3 (VR1020/27-25, 0.5 mg) | M9 | 32,000 | 640,000 | 80,000 |
| M10 | 16,000 | 320,000 | 80,000 | |
| M11 | 16,000 | >1,280,000 | 20,000 | |
| M12 | 1,000 | 320,000 | 10,000 | |
| Mean ± SEM | 16,250 ± 6,300 | 640,000 ± 226,300 | 47,500 ± 18,900 | |
| 4 (VR1020/27-25, 1.0 mg) | M13 | 1,000 | 640,000 | 40,000 |
| M14 | 1,000 | 320,000 | 10,000 | |
| M15 | 32,000 | >1,280,000 | 160,000 | |
| M16 | 16,000 | >1,280,000 | 160,000 | |
| Mean ± SEM | 12,500 ± 7,400 | 880,000 ± 240,000 | 92,500 ± 39,500 | |
| 5 (control) (VR1020, 0.5 mg) | M17 | 0 | 320,000 | 80,000 |
| M18 | 0 | 160,000 | 80,000 | |
| M19 | 0 | 160,000 | 40,000 | |
| Mean ± SEM | 213,333 ± 53,000 | 66,700 ± 13,000 | ||
ELISA was performed by using sera taken 7 weeks after the fourth DNA immunization (groups 1 to 5) and 3 weeks and 6 months after a protein boost (groups 1 to 5). Titers represent reciprocal serum dilutions. Endpoint titers were defined as serum dilutions giving an absorbance higher than the average OD at 405 nm of preimmune serum plus 3 SD. Comparison of antibody titers of each group to those of the control group was done by one-way ANOVA test. Comparison of antibody titers over the time for each monkey was done by mixed ANOVA.
FIG. 1.
Time course of Pfs25-specific antibody induction by DNA vaccine. Serum antibody levels were measured individually by ELISA before and/or after (4 to 7 weeks) each immunization. Results shown are the mean reciprocal serum dilutions of each monkey or group. Endpoint titers were defined as serum dilutions giving an absorbance higher than the average OD at 405 nm of preimmune serum plus 3 SD.
Effect of heterologous immunization with recombinant protein on Pfs25-specific antibody responses in rhesus monkeys immunized with DNA vaccines.
Previous studies have shown that the transmission-blocking activity of immune serum is closely related to the level of antibodies (16). In the present study, although DNA immunization alone elicited high antibody titers in all of the immunized monkeys, none of these animals contained antibody titers high enough to block the infectivity of P. falciparum gametocytes in membrane feeding assays (see below). Thus, we used recombinant Pfs25 protein formulated in Montanide ISA-720 to boost the antigen-specific antibody levels achieved by DNA vaccination. Our decision to boost with protein was based on similar studies with mice primed with a single dose of DNA vaccine, followed by highly effective boosting with protein emulsified in alum (unpublished results). All of the animals, including the controls, received an i.m. injection of 25 μg of recombinant protein Pfs25 emulsified in Montanide ISA-720. Three weeks after the protein injection, sera from animals in all of the previously immunized groups showed much higher Pfs25-specific antibody titers (mean reciprocal titers: group 1, 980,000; group 2, 880,000; group 3, 640,000; group 4, 880,000), including those from some monkeys that had reciprocal titers higher than 1,000,000 (Table 1). The protein emulsified in Montanide ISA-720 was found to induce a strong primary antibody response to Pfs25, as demonstrated by a mean reciprocal titer of 213,333 in the three monkeys of the control group. After protein immunization, the mean antibody titers of the groups were not significantly different from that of the control group (P > 0.05; one-way ANOVA). At 6 months after protein immunization, antibody levels decreased in all of the animals; however, they were still higher than those observed prior to the protein boost. The fold reductions in antibody titers were 7.25, 8, 13.4, 9.5, and 3.2 in groups 1 to 5, respectively, and the resulting mean antibody titers did not differ significantly among the groups (P > 0.05; one-way ANOVA).
These results demonstrate that DNA vaccine constructs based on Pfs25 and Pfg27-Pfs25 induce significant antibody titers in rhesus macaques. Administration of recombinant protein emulsified in Montanide ISA-720 resulted in further boosting of the immune response primed by DNA immunizations; thus, DNA prime-protein boost can be an effective strategy.
Isotypes and avidity of antibodies.
Our previous studies with mice have shown that both the IgG1 and IgG2a isotypes were elevated after immunization with the DNA plasmid constructs used in the present study (19). To address the question of whether certain isotypes contribute more than others to the transmission-blocking efficacy of the antibodies elicited, we analyzed the IgG isotypes in monkey sera on the basis of cross-reactivity with antibodies to human IgG isotypes (there are no reagents available for direct analysis of antibody isotypes in rhesus monkeys), except for IgG3 (22). On the basis of this analysis, we found that DNA immunization elevated mainly IgG1-type responses in all groups (data not shown). The IgG1 responses were increased further after a protein boost. Additionally, IgG2 and IgG4 isotype anti-Pfs25 antibodies also became detectable after a protein boost compared to the dominant IgG1 response seen after DNA immunization alone (data not shown).
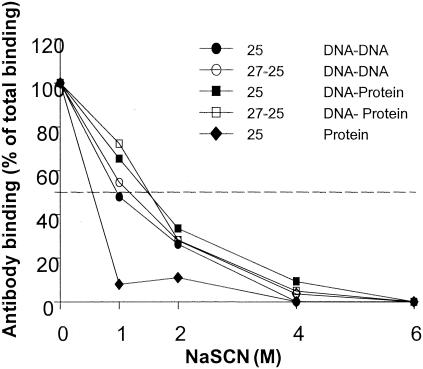
Further analysis was carried out to compare levels of antibody-antigen binding with various sera before and after boosting with protein. We hypothesized that the protein boost would have not only a quantitative effect (reflected in the titers) but also a qualitative effect (reflected in the affinity and avidity) on the specific antibodies. We investigated antibody binding in ELISAs after a brief treatment with various concentrations (0 to 6 M) of NaSCN, a chaotropic agent that disrupts antigen-antibody interaction (Fig. 2). The binding of antibodies with less avidity to antigen is disrupted at lower concentrations of NaSCN than that of antibodies with greater avidity. The ODs after incubation in the presence of various concentrations of NaSCN were converted to percentages of the total bound IgGs represented by OD without washing with NaSCN. The 50% effective doses of NaSCN in the VR1020/25 DNA-DNA (mean of M5 and M6)- and VR1020/27-25 DNA-DNA (mean of M15 and M16)-vaccinated groups, after four immunizations with DNA vaccine alone, were similar (∼1 and ∼1.25 M, respectively). These concentrations of NaSCN resulted in more than 90% loss of bound antibody in the sera from monkeys immunized only once with recombinant protein (M17 and M18), suggesting a qualitatively superior antibody response in DNA-immunized monkeys. After the protein boost, the 50% effective dose (∼1.5 M NaSCN) was increased for all of the monkeys in both the VR1020/25 and VR1020/27-25 DNA-protein groups that were previously immunized with DNA vaccine. These results indicate that the avidity of antibodies induced by DNA immunizations is higher than after a single protein immunization and that a heterologous immunization with protein further improves antibody avidity.
FIG. 2.
Avidity analysis of Pfs25-specific antibodies. ELISA plates were coated with recombinant Pfs25. Binding of antibody in the sera from the monkeys (two monkeys from each group that had high antibody titers) was assessed after a brief treatment with 0, 1, 2, 4, or 6 M NaSCN. Sera from various groups were tested at a single dilution from the linear portion of the dilution curve. The initial optical density without NaSCN was assumed to represent the effective total binding of specific IgG, and subsequent ODs after treatment with various concentrations of NaSCN were converted to the percentage of the total bound IgGs. Results are shown as the mean value of two different monkey sera from each group.
Recognition of native Pfs25 antigen of P. falciparum gametes and zygotes.
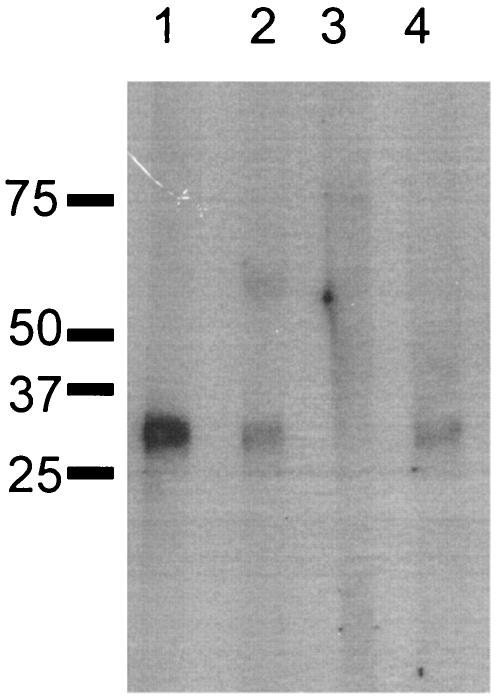
To analyze whether the antibodies raised by DNA vaccines in rhesus monkeys would recognize native antigen Pfs25 on purified P. falciparum gametes and zygotes, Western blot analysis was performed. Figure 3 shows that the pooled sera from monkeys immunized with VR1020/25 or VR1020/27-25 recognized the parasite antigen under nonreducing conditions (Pfs25 runs anomalously under nonreducing conditions) (19). A similar reactivity was obtained with sera from mice immunized with DNA vaccine plasmids and recombinant Pfs25 (data not shown). Sera from monkeys immunized with the VR1020 control plasmid did not recognize any specific parasite antigen.
FIG. 3.
Recognition of native Pfs25 antigen from purified gametes and zygotes of P. falciparum by Western blot analysis. Electrophoresis was performed under nonreducing conditions and blotted with pooled monkey sera (1:2,000 dilution). Lanes: 1, VR1020/25 DNA-DNA; 2, VR1020/27-25 DNA-DNA; 3, VR1020 alone; 4, recombinant Pfs25 alone. The sera used were collected 7 weeks after four DNA immunizations and 3 weeks after one protein immunization (group 5). The values on the left are molecular sizes in kilodaltons.
Transmission-blocking efficacy assessed by membrane feeding assays.
Membrane feeding assays were carried out to determine the ability of the sera to block oocyst formation in mosquito midguts and thus block malaria transmission. Individual monkey sera were mixed with in vitro-cultured mature P. falciparum gametocytes and fed to A. stephensi or A. gambiae mosquitoes. Seven to 9 days later, mosquitoes were dissected, midguts were stained with mercurochrome, and oocysts were enumerated under a light microscope. Table 2 shows the results obtained with sera from individual monkeys that had the highest antibody responses from groups 2, 4, and 5. Animals in groups 2 and 4 received 1.0-mg doses of DNA vaccine. While antibodies in sera collected after four DNA immunizations did not block the infectivity of P. falciparum gametocytes to mosquitoes, the sera obtained after a subsequent single protein boost effectively blocked parasite infectivity. This occurred with serum specimens obtained from monkeys M5 and M6 (95 and 89%, respectively; P < 0.05 with Kruskal-Wallis one-way analysis) and monkeys M15 and M16 (88 and 83%, respectively; P < 0.05 with Kruskal-Wallis one-way analysis). Both vaccine constructs (Pfs25 and Pfg27-Pfs25) were comparable in their transmission blocking-inducing efficacy after a single protein boost. The control group (animal M17, which had highest antibody titer) also showed 61% reduction of transmission after a single protein immunization (P < 0.05 with Kruskal-Wallis one-way analysis); however, monkey M18 from this group, which had a lower antibody titer than monkey M17, did not show any oocyst reduction compared to preimmune control sera (P > 0.05 with Kruskal-Wallis one-way analysis).
TABLE 2.
Transmission-blocking activity of sera from immunized rhesus monkeys determined by membrane feeding assaya
| Monkey no. treatment (group) or control | No. of infected mosquitoes/no. dissectedb | Geometric mean no. of oocysts (range) | Infectivity (% of control)c |
|---|---|---|---|
| Normal human sera | 32/33 | 21.2 (0-66) | |
| Preimmune sera | 39/40 | 17.5 (0-55) | 100 |
| M5, DNA-DNA (2) | 35/36 | 12.9 (0-89) | 74 |
| M6, DNA-DNA (2) | 42/45 | 17.3 (0-90) | 99 |
| M5, DNA-protein (2) | 21/39 | 0.9 (0-7)d | 5 |
| M6, DNA-protein (2) | 18/26 | 1.9 (0-10)d | 11 |
| M15, DNA-DNA (4) | 28/28 | 16.6 (3-48) | 95 |
| M16, DNA-DNA (4) | 46/47 | 14.8 (0-52) | 85 |
| M15, DNA-protein (4) | 33/46 | 2.1 (0-13)d | 12 |
| M16, DNA-protein (4) | 41/47 | 3.1 (0-17)d | 17 |
| M17, protein (5) | 34/35 | 6.9 (0-27)d | 39 |
| M18, protein (5) | 33/35 | 21 (0-93) | 120 |
Cultured gametocytes of P. falciparum strain NF54 were fed to A. stephensi mosquitoes at a 1:2 serum dilution. Results shown are pooled from two experiments and represent similar overall findings in six individual assays. The sera tested were collected after a fourth DNA immunization (DNA-DNA) or after a subsequent protein boost (DNA-protein).
Number of infected mosquito midguts/total number of mosquitoes dissected.
Calculated as the geometric mean oocyst number of the test group divided by that of the preimmune serum-fed group × 100.
Significantly different from preimmune serum at P < 0.05 by Mann-Whitney U test and Kruskal-Wallis one-way analysis.
In subsequent membrane feeding assays, we tested sera at 1:4 and 1:8 dilutions and found that the blocking activity (the oocyst numbers in the midguts) was inversely correlated with the serum dilutions (Table 3). We performed dilution experiments with two different laboratory-adapted mosquito species, A. gambiae and A. stephensi, and found similar blocking activities of sera in both species of malaria vectors. These results once again emphasize the importance of antigen-specific antibody titers as a critical requirement for transmission-blocking immunity.
TABLE 3.
Transmission-blocking activity of sera from immunized rhesus monkeys at various dilutions determined by membrane feeding assaya
| Monkey no. (treatment) or control | Dilution | No. of infected mosquitoes/no. dissectedb | Geometric mean no. of oocysts (range) | Infectivity (% of control)c |
|---|---|---|---|---|
| Normal human sera | 38/39 | 13.4 (0-46) | ||
| Preimmune sera | 38/38 | 16.9 (3-53) | 100 | |
| M5 (DNA-protein) | 1:4 | 29/32 | 3.5 (0-20)d | 21 |
| 1:8 | 12/16 | 3.9 (0-16)d | 23 | |
| M6 (DNA-protein) | 1:4 | 5/5 | 6.3 (4-11) | 37 |
| 1:8 | 19/21 | 3.2 (0-11)d | 19 | |
| M15 (DNA-protein) | 1:4 | 28/35 | 2.4 (0-24)d | 14 |
| 1:8 | 16/16 | 9.4 (0-32) | 55 | |
| M17 (protein) | 1:4 | 29/31 | 6.4 (0-23) | 38 |
| 1:8 | 26/27 | 10.4 (0-38) | 62 |
Cultured gametocytes of P. falciparum strain NF54 were fed to A. gambiae mosquitoes at serum dilutions of 1:4 and 1:8. Results shown here are pooled from two experiments and represent three individual experiments. The sera tested were collected 3 weeks after a protein boost (DNA-protein). Similar results were obtained with A. stephensi mosquitoes.
Number of infected mosquito midguts/total number of mosquitoes dissected.
Calculated as the geometric mean oocyst number of the test group divided by that of the preimmune-serum-fed group × 100.
Significant at P < 0.05 by Mann-Whitney U test and Kruskal-Wallis one-way analysis.
Assessment of vaccine safety.
Vaccine safety was assessed in all animals as described in Materials and Methods. At all time points throughout the study, animals appeared clinically healthy. No local or systemic effects of the vaccine were observed. Similarly, the results of clinical laboratory tests were normal at all of the times at which they were performed and no anti-DNA antibodies were detected. By these criteria, therefore, the DNA vaccine per se and its combination with protein immunization appeared safe.
DISCUSSION
Malaria transmission-blocking immunity is mediated by antibodies that inhibit parasite development in the mosquito midgut. Such antibodies have been elicited by immunization with recombinant proteins produced in a variety of expression systems (2, 15, 16, 24). Protein expressed in Escherichia coli, although immunogenic, did not elicit transmission-blocking antibodies (15). On the other hand, the same protein expressed in S. cerevisiae was not only immunogenic but also induced antibodies that were effective blockers of P. falciparum gametocyte infectivity (13, 15). This has been attributed to the conformation of expressed proteins, which is largely dependent on proper disulfide bond formation, in S. cerevisiae. Our rationale for DNA immunizations was based on the assumption that proteins expressed in mammalian cells (immunized host) would acquire a near-native conformation after posttranslational folding and thus should elicit appropriate blocking antibody responses. Indeed, in our previous studies on immunization of mice with DNA vaccines, we found that plasmids encoding Pfs25 were highly immunogenic and that antibodies were effective blockers of P. falciparum gametocyte infectivity in mosquitoes (19), findings that suggest that Pfs25 expressed after immunization with plasmid DNA had the proper conformation and thus induced transmission-blocking antibodies. A successful outcome of tests with higher mammals, including nonhuman primates, would pave the way for evaluation of vaccine constructs in humans.
The study described in this paper summarizes results from a trial of such DNA vaccine constructs in rhesus macaques. Animals were immunized with different DNA vaccine constructs at two different doses chosen on the basis of results from other studies (11, 26). In our previous studies with mice (average body weight, 25 to 30 g), a DNA dose of 25 to 50 μg (two doses) gave optimum immunogenicity. For our studies with rhesus monkeys weighing 3 to 4 kg, we chose to immunize with 0.5 and 1.0 mg of DNA per dose. However, our results revealed only marginal differences in overall immunogenicity between the two vaccine plasmids tested at the 0.5- and 1.0-mg doses. Plasmids at both doses induced significant antibody titers and were therefore found to be successful in inducing antigen-specific antibodies. This is in sharp contrast to other malarial antigens encoded by DNA vaccines (7), which were only poorly immunogenic in nonhuman primates, especially in terms of antibody responses. Although Pfs25 DNA vaccines induced reciprocal antibody titers of up to 32,000 in nonhuman primates, these levels were 10 to 20 times lower than the titers obtained in mice (19). Another aspect of the immunogenicity difference between mice and monkeys is the total number of DNA immunizations that were needed. In mice, only two doses were sufficient, whereas in rhesus macaques, at least four doses were required for maximal antibody titers. Since the transmission-blocking activity of antibodies depends not only on recognition of conformational epitopes but also on higher titers, it was not surprising to find that sera from monkeys immunized four times with DNA vaccines containing reduced titers compared to those in mice (19) were not effective blockers in membrane feeding assays.
In our mouse studies, we found that antibodies induced by the Pfg27-Pfs25 hybrid DNA vaccine construct had lower transmission-blocking activity than antibodies induced by the Pfs25 DNA vaccine construct, although the ELISA titers were very similar. This suggested that expression of the Pfg27-Pfs25 hybrid protein might affect the conformation or presentation of immunologically important epitopes in Pfs25 (19). In contrast, we show here that the titer and transmission-blocking activity of antibodies elicited by the hybrid construct in nonhuman primates did not differ from that of antibodies induced by the Pfs25 construct alone, suggesting another level of immune response difference between mice and monkeys.
In view of the fact that antibody titers after the fourth immunization with plasmids did not differ from the titer elicited by the third immunizations, we chose a heterologous immunization strategy to boost antibody titers by using recombinant Pfs25 formulated in Montanide ISA-720. Our choice also was based on the experience of other investigators (11) who found a heterologous prime-boost immunization strategy to be more efficacious in nonhuman primates than a DNA-DNA immunization strategy alone. A single administration of 25 μg of protein was sufficient to increase antibody titers between 50- and 150-fold in the various vaccine groups. This increase was not due to an independent primary immune response to epitopes in the recombinant Pfs25 protein. Animals that were immunized with the control plasmid (group 5), when given a protein dose in parallel to the other four groups, had three- to fivefold lower antibody titers. These studies thus demonstrate that an immune response primed by a Pfs25 DNA vaccine can be boosted in a specific manner by heterologous immunization with a recombinant protein (Pfs25). The protein boost improved antibody responses not only quantitatively (enhanced antibody titers) but also qualitatively and functionally. Antibodies after administration of the protein boost did appear to undergo further affinity maturation, as revealed by avidity assays and detection of the IgG2 and IgG4 isotypes among a dominant IgG1 response. The final distinction was provided by the membrane feeding assays, in which antibodies after administration of the protein boost resulted in dose-dependent inhibition of gametocyte infectivity in mosquitoes.
The results of a phase 1 clinical trial with humans (28) demonstrated that recombinant Pfs25 elicited specific immune responses but with a very low antibody titer. Results of the present study suggest the importance of priming with DNA vaccine as an alternative approach to recombinant protein vaccines in future vaccine trials with humans (28). Moreover, the DNA vaccines showed no apparent toxicity in nonhuman primates, either alone or in combination with a protein boost. As has been argued in numerous studies, DNA vaccines can facilitate the delivery of several antigens simultaneously and elicit both cellular and humoral immune responses. We propose that Pfs25-like transmission-blocking components should be included in a cocktail of malaria DNA vaccines depending on the desired endpoint in clinical trials.
Acknowledgments
We thank Sabra L. Klein for help with statistical analysis. We also thank Darin Kongkasuriyachai and Mrinal Bhattacharyya for various discussions.
These studies were supported by research grants from the National Institutes of Health, AI47089 (N.K.) and RR00164 (M.T.P.), and also from the WHO-TDR (N.K.). Supply of human erythrocytes for malaria culture is supported by NCCR OPD-GCRC RR00722.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aida, Y., and M. J. Pabst. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132:191-195. [DOI] [PubMed] [Google Scholar]
- 2.Barr, P. J., K. M. Green, H. L. Gibson, I. C. Bathurst, I. A. Quakyi, and D. C. Kaslow. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 174:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandstrom, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351:1320-1325. [DOI] [PubMed] [Google Scholar]
- 4.Carter, R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309-2314. [DOI] [PubMed] [Google Scholar]
- 5.Carter, R., P. M. Graves, I. A. Quakyi, and M. F. Good. 1989. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 169:135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coban, C., K. J. Ishii, D. J. Sullivan, and N. Kumar. 2002. Purified malaria pigment (hemozoin) enhances dendritic cell maturation and modulates the isotype of antibodies induced by a DNA vaccine. Infect. Immun. 70:3939-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doolan, D. L., and S. L. Hoffman. 2001. DNA-based vaccines against malaria: status and promise of the Multi-Stage Malaria DNA Vaccine Operation. Int. J. Parasitol. 31:753-762. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood, B., and T. Mutabingwa. 2002. Malaria in 2002. Nature 415:670-672. [DOI] [PubMed] [Google Scholar]
- 9.Hill, A. V., W. Reece, P. Gothard, V. Moorthy, M. Roberts, K. Flanagan, M. Plebanski, C. Hannan, J. T. Hu, R. Anderson, P. Degano, J. Schneider, E. Prieur, E. Sheu, and S. C. Gilbert. 2000. DNA-based vaccines for malaria: a heterologous prime-boost immunization strategy. Dev. Biol. 104:171-179. [PubMed] [Google Scholar]
- 10.Ifediba, T., and J. P. Vanderberg. 1981. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294:364-366. [DOI] [PubMed] [Google Scholar]
- 11.Jones, T. R., D. L. Narum, A. S. Gozalo, J. Aguiar, S. R. Fuhrmann, H. Liang, J. D. Haynes, J. K. Moch, C. Lucas, T. Luu, A. J. Magill, S. L. Hoffman, and B. K. Sim. 2001. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. J. Infect. Dis. 183:303-312. [DOI] [PubMed] [Google Scholar]
- 12.Kaslow, D. C. 1993. Transmission-blocking immunity against malaria and other vector-born diseases. Curr. Opin. Immunol. 5:557-565. [DOI] [PubMed] [Google Scholar]
- 13.Kaslow, D. C., and J. Shiloach. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Bio/Technology 12:494-499. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow, D. C., I. A. Quakyi, C. Syin, M. G. Raum, D. B. Keister, J. E. Coligan, T. F. McCutchan, and L. H. Miller. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74-80. [DOI] [PubMed] [Google Scholar]
- 15.Kaslow, D. C., I. C. Bathurst, T. Lensen, T. Ponnudurai, P. J. Barr, and D. B. Keister. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect. Immun. 62:5576-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaslow, D. C., S. N. Isaacs, I. A. Quakyi, R. W. Gwadz, B. Moss, and D. B. Keister. 1991. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science 252:1310-1313. [DOI] [PubMed] [Google Scholar]
- 17.Klinman, D. M., K. J. Ishii, and D. Verthelyi. 2000. CpG DNA augments the immunogenicity of plasmid DNA vaccines. Curr. Top. Microbiol. Immunol. 247:131-142. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, N., I. Ploton, G., Koski, C. Ann-Lobo, and C. Contreras. 1995. Malaria transmission-blocking immunity. Identification of epitopes and evaluation of immunogenicity. Adv. Exp. Med. Biol. 383:65-72. [PubMed] [Google Scholar]
- 19.Lobo, C. A., R. Dhar, and N. Kumar. 1999. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect. Immun. 67:1688-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 21.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shearer, M. H., R. D. Dark, J. Chodosh, and R. C. Kennedy. 1999. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin. Diagn. Lab. Immunol. 6:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sin, J. I., and D. B. Weiner. 2000. Improving DNA vaccines targeting viral infection. Intervirology 43:233-246. [DOI] [PubMed] [Google Scholar]
- 24.Stowers, A. W., D. B. Keister, O. Muratova, and D. C. Kaslow. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect. Immun. 68:5530-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 26.Wang, R., D. L. Doolan, Y. Charoenvit, R. C. Hedstrom, M. J. Gardner, P. Hobart, J. Tine, M. Sedegah, V. Fallarme, J. B. Sacci, Jr., M. Kaur, D. M. Klinman, S. L. Hoffman, and W. R. Weiss. 1998. Simultaneous induction of multiple antigen-specific cytotoxic T lymphocytes in nonhuman primates by immunization with a mixture of four Plasmodium falciparum DNA plasmids. Infect. Immun. 66:4193-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weeratna, R. D., M. J. McCluskie, L. Comanita, T. Wu, and H. L. Davis. 2000. Optimization strategies for DNA vaccines. Intervirology 43:218-226. [DOI] [PubMed] [Google Scholar]
- 28.Zou, L., A. P. Miles, J. Wang, and A. W. Stowers. 2003. Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine 21:1650-1657. [DOI] [PubMed] [Google Scholar]