Abstract
Antibodies directed against Pfs25, a protein present on the surface of zygotes and ookinetes of Plasmodium falciparum, completely block pathogen transmission. We evaluated the immunomodulatory effect of CpG oligodeoxynucleotides (ODN) on the immunogenicity of recombinant Pfs25 (rPfs25) formulated in alum (Al). Immunization of mice with rPfs25 plus CpG ODN improved both the antibody titer (a 30-fold-higher antibody response than that with rPfs25-Al alone) and avidity. Coadministration of CpG ODN dramatically enhanced the titer of immunoglobulin G2A (IgG2a) compared to the titer of the IgG1-dominant response caused by rPfs25-Al alone, and the sera from the CpG ODN-coadministered group completely blocked the transmission of P. falciparum parasites to mosquitoes, as determined by membrane feeding assays. However, transmission-blocking experiments revealed that blocking efficacy was dependent on high-titer antibody levels, independent of isotypes. These results suggest that CpG ODN can be used as an adjuvant to enhance the immunogenicity of rPfs25 as a malaria transmission-blocking vaccine.
Transmission-blocking immunity against malaria targets sexual stages, including gametocytes that are formed during the erythrocytic phase of the malaria life cycle and further developmental stages of the parasites in the mosquitoes. The male and female gametocytes ingested in the blood meal rapidly undergo gametogenesis and fertilization and develop into oocysts that eventually produce infective sporozoites. Thus, antibodies directed against sexual-stage-specific surface antigens, when ingested in the blood meal, can block the parasite development in the mosquito vector (3, 12, 21).
Plasmodium falciparum surface protein 25 (Pfs25) is one of the leading candidates for the development of malaria transmission-blocking vaccines (1, 2, 6, 28). Pfs25, a cysteine-rich 25-kDa surface protein with four tandem epidermal growth factor-like domains, is expressed at the onset of gametogenesis in the mosquito midgut and the expression continues through zygote-ookinete transformation (14). Immunization in mice and nonhuman primates by using Pfs25 expressed in recombinant vaccinia virus or in a yeast secretory system (known as TBV25H) elicited antibodies that recognized critical conformational epitopes and completely blocked infectivity of gametocytes in the mosquitoes (1, 15, 16, 32).
There are however, several obstacles to be overcome for the development of vaccines based on recombinant Pfs25 (rPfs25). First of all, since it is expressed in the parasite stages occurring in the mosquitoes, antibodies in the vertebrate host against Pfs25 need to be present at high titer and to be long lasting, due to lack of natural boosting. Second, epitopes recognized by blocking antibodies are reduction sensitive and conformation dependent, thus requiring expression in nonbacterial systems (1, 15). Third, rPfs25 expressed in Saccharomyces cerevisiae (TBV25H), when adsorbed to aluminum hydroxide as a clinical-grade vaccine for human use, produced a low level of antibodies that did not block transmission of P. falciparum to mosquitoes (31).
Our laboratory has tried to overcome some of the above problems and improve the immunogenicity and efficacy of Pfs25 vaccines by using a DNA-based vaccination strategy in mice and nonhuman primates (5a, 22). It is widely believed that the immunogenicity of DNA vaccines is in part due to the presence of immunostimulatory sequences containing CpG motifs. It is also well established that the synthetic oligodeoxynucleotides containing unmethylated cytosine-phosphate-guanosine motifs (CpG ODN) act as an adjuvant improving the immunogenicity of protein antigens as well as reducing the amount of antigen required (4, 19, 23, 35). Coadministration of CpG ODN together with protein antigens has produced promising results against several microbial diseases (4, 9, 11, 19, 23, 25, 35). The CpG ODN, which are underrepresented and methylated in vertebrate DNA, cause direct activation of B cells, natural killer cells, and professional antigen-presenting cells, so that they proliferate and/or secrete cytokines, chemokines, and immunoglobulins (reviewed in references 17, 20, and 34). It has recently been shown that cellular responses to CpG DNA are mediated via Toll-like receptor 9 (8, 10, 33).
In the present study we evaluated the effect of CpG ODN on the immunogenicity of rPfs25 expressed in S. cerevisiae. In addition, we also wished to evaluate the effect of immunoglobulin G (IgG) isotypes affected by CpG ODN (4, 19) on P. falciparum transmission-blocking activity.
CpG ODN enhances anti-Pfs25-specific antibody responses.
To test the immunomodulatory effect of CpG ODN, 5 μg of yeast-derived rPfs25 (TBVPfs25H) kindly provided by the Malaria Vaccine Development Unit (National Institutes of Health) under MTA (13, 32) was chosen for immunizations based on the previous studies that used 1 to 25 μg of rPfs25 (7, 32). ODN were synthesized as described earlier (18). CpG ODN had the sequences GCTAGACGTTAGCGT and TCAACGTTGA, where CpG motifs are underlined. Control ODN (non-CpG ODN) had the same sequences, except that the CpG motifs were switched to GpC.
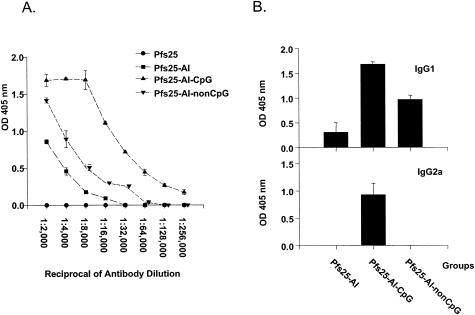
Female (6- to 8-week-old) BALB/c mice (five mice per group) were immunized intraperitoneally as follows: 5 μg of rPfs25 in phosphate-buffered saline alone, formulated with alum (Al) (aluminum hydroxide gel; protein/Al ratio = 1/16), with Al plus 50 μg of CpG ODN (equal amounts of two CpG ODN), and with Al plus 50 μg of non-CpG ODN and only 50 μg of CpG ODN plus Al without protein in 100 μl of phosphate-buffered saline solution. The protein was adsorbed to Al by dropwise addition while being vortexed gently, followed by incubation for 30 min at room temperature before injections. Mice were boosted 4 and 12 weeks later with the same dose regimen, and sera were pooled from all five mice within a group for enzyme-linked immunosorbent assays (ELISA) and transmission-blocking assays (see below; smaller serum volumes prohibited analysis for each animal individually) (5, 22). Two weeks after the first boost, the Pfs25-Al + CpG ODN-immunized group had 30- and 15-fold-higher antibody responses than did the Pfs25-Al and Pfs25-Al + non-CpG ODN groups (1,024,000 reciprocal serum dilutions versus 32,000 and 64,000, respectively) (Fig. 1A). Moreover, the elevated antibody response in the CpG ODN-coadministered group was detectable even after primary immunization (Table 1). Sera from Pfs25 alone (no Al) or CpG ODN + Al-immunized mice showed no detectable Pfs25-specific antibody responses (Fig. 1A). Consistent with the previous reports (4, 19), only the CpG ODN-coadministered group had elevated levels of IgG2a (Fig. 1B), whereas Pfs25-Al + non-CpG ODN immunization elicited a dominant IgG1 response similar to that for the Pfs25-Al-immunized group.
FIG. 1.
Pfs25-specific antibody responses in mice. BALB/c mice (five/group) were immunized intraperitoneally (details in Materials and Methods). Four weeks later each group was boosted with the same dose regimen and sera were collected 2 weeks after each boost, pooled, and analyzed by ELISA. (A) Pfs25-specific total IgG responses. The results are expressed as mean ± standard deviation of duplicate wells. End point titers were defined as the highest serum dilution giving optical density readings at 405 nm (OD 405 nm) greater than the optical density of preimmune serum + 2 standard deviations. (B) Isotype analysis of Pfs25-specific antibodies. Pooled mouse sera from each group were studied at 1:10,000 dilutions. The results are expressed as mean ± standard deviation of triplicate wells.
TABLE 1.
Kinetics of the Pfs25-specific IgG antibodiesa
| Immunization group | Value after:
|
|||
|---|---|---|---|---|
| Prime | 1st boost | 2nd boost | 6 mo | |
| Pfs25 alone | 0 | 0 | 0 | 0 |
| Pfs25-A1 | 0 | 32,000b | 512,000 | 320,000 |
| Pfs25-A1 + CpG | 8,000 | 1,024,000 | 1,024,000 | >640,000 |
| Pfs25-A1 + non-CpG | 0 | 64,000 | 1,024,000 | >640,000 |
| CpG + A1 | 0 | 0 | 0 | 0 |
Sera were studied 2 weeks after each immunization.
Numbers represent end point ELISA titers.
CpG ODN alter the avidity of Pfs25-specific antibodies.
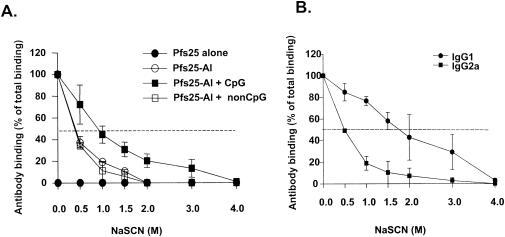
To determine the quality of antibody responses (reflected in the affinity and avidity) in each group, we conducted a sodium thiocyanate (NaSCN, a chaotropic agent) avidity assay. To test antibody avidity, various concentrations (0, 0.5, 1, 1.5, 2, 3, and 4 M) of sodium thiocyanate washes were used in the standard ELISA protocol as described elsewhere (22). The initial optical density without an NaSCN wash represented effective total binding of specific IgG, and subsequent optical densities in the presence of various concentrations of NaCSN were converted to the percentage of total bound IgGs. The binding of antibodies with lesser avidity to antigen is disrupted at concentrations of NaSCN lower than for antibodies with greater avidity. The 50% effective dose of NaSCN in the Pfs25-Al + CpG ODN-immunized group was greater than those for the Pfs25-Al- or Pfs25-Al + non-CpG ODN-immunized group (1.0 versus <0.5 M, respectively) (Fig. 2). To analyze whether higher avidity of antibodies from the Pfs25-Al + CpG ODN-immunized group was due to a higher level of IgG2a antibodies, we also studied the avidity of isotype-specific immunoglobulins. We found that the avidity of the IgG1 isotype antibody in the Pfs25-Al + CpG ODN-immunized group was greater than that of the IgG1 isotype antibody in other groups (data not shown). However, different antibody isotypes within the Pfs25-Al + CpG ODN-immunized group had distinctive avidity binding. The IgG2a isotype antibody had lower avidity than did IgG1-type isotypes (0.5 and 1.5 M, respectively) (Fig. 2B).
FIG. 2.
Avidity analysis of anti-Pfs25-specific antibodies. Binding of antibody to antigen on an ELISA plate was assessed after brief treatment in the presence of 0, 0.5, 1, 1.5, 2, 3, and 4 M NaSCN. Initial optical density without NaSCN was assumed to represent effective total binding of specific immunoglobulin, and subsequent optical densities after treatment with various concentrations of NaSCN were converted to the percentage of the total bound immunoglobulins. (A) The avidity of anti-Pfs25 IgG antibodies from various sera. Two weeks after the first boost, pooled sera from each group were evaluated at dilutions that were within the linear range of the dilution curve. The results are expressed as mean ± standard deviation of two different experiments. The dotted line represents 50% of total immunoglobulin bound for each group of serum. (B) The avidity of different isotypes (IgG1 and IgG2a) of the rPfs25-Al + CpG ODN-immunized group. Serum was studied 2 weeks after the first boost. The results are expressed as mean ± standard deviation of two different experiments. The dotted line represents 50% of total immunoglobulin bound for each isotype.
Effectiveness of Pfs25-Al + CpG ODN-immunized sera in transmission-blocking assays.
The membrane feeding assay is the only method to assess the transmission-blocking activity of the sera against Plasmodium (12). Reduction of parasite transmission is reflected by the reduced oocyst formation in mosquito midguts. Pooled mouse sera from each group were mixed with in vitro-cultured mature P. falciparum (strain NF54) gametocytes (14 to 18 days old) and were fed to Anopheles stephensi mosquitoes (22). Mosquitoes were allowed to engorge blood and serum mixtures for 15 min thorough a Parafilm membrane warmed to 39°C by a glass water jacket. Blood-fed mosquitoes were maintained at 26°C and 60 to 80% relative humidity, and midguts were examined 7 to 9 days later for the presence of oocysts by microscopy. After one boost, the Pfs25-Al-immunized group had statistically significant blocking activity (∼73%), as determined by the reduced oocyst numbers (P < 0.001 with the Mann-Whitney U test) (Table 2). On the other hand, the antibodies in the Pfs25-Al+ CpG ODN-immunized sera completely blocked the infectivity of P. falciparum gametocytes to mosquitoes (100% blocking, P < 0.001 with Mann-Whitney U test) (in two independent membrane feeding assays, we found only one highly degenerated oocyst in a single mosquito). In contrast, transmission-blocking activity of sera from the Pfs25-Al+ non-CpG ODN group was only ∼58% (P = 0.016 with Mann-Whitney U test). Further twofold dilution of sera from the Pfs25-Al+ CpG ODN-immunized group (after the first boost) resulted in reduced blocking activity in a dilution-dependent manner (data not shown).
TABLE 2.
Transmission-blocking activity of sera from immunized mice 2 weeks after the first boosta
| Group | No. of infected mosquitoes/no. of dissected mosquitoes | Geometric mean no. of oocysts (range) | Infectivity (% of control)b |
|---|---|---|---|
| Preimmune mouse sera | 23/25 | 5 (0-14) | 100 |
| Pfs25-A1 | 14/19 | 1.4 (0-8)c | 27.5 |
| Pfs25-A1 + CpG | 0/19 | 0c,d | 0 |
| Pfs25-A1 + non-CpG | 15/20 | 2.1 (0-12)c | 42 |
P. falciparum (NF54 strain) gametocytes were fed to A. stephensi mosquitoes at 1:3 serum dilution. The table shows results from one representative experiment repeated twice.
The geometric mean oocyst number of the test group divided by that of the preimmune-serum group × 100.
Statistically significant by the Mann-Whitney U test (P < 0.001 for the Pfs25-A1 and Pfs25-A1 + CpG groups and P = 0.016 for the Pfs25-A1 + non-CpG group).
Statistically significant difference compared to Pfs25-A1 and Pfs25-A1 + non-CpG-immunized groups by the Mann-Whitney U test (P < 0.001).
To evaluate whether the effect of CpG ODN on the blocking activity was due to high IgG2a isotype levels or simply total IgG levels, all groups of animals were boosted once more with the same dose regimen. The sera obtained 2 weeks after the second boost were studied for the antibody responses by ELISA. As shown in Table 1, antibody levels were boosted further in the mice immunized with Pfs25-Al and Pfs25-Al + non-CpG ODN. The mean antibody titer in the non-CpG ODN-coadministered group was as high as that in the CpG ODN-coadministered group, remaining comparable in both groups even 6 months after the second boost (Table 1). The isotype profile in all groups did not change after the second boost (data not shown).
In view of the observed increase in the antibody responses after the second boost, we evaluated them in membrane feeding assays at three different dilutions (1:6, 1:12, and 1:24). As shown in Table 3, sera from the Pfs25-Al + non-CpG ODN-immunized group (2 weeks after the second boost) also resulted in significant transmission reduction similar to that in the Pfs25-Al + CpG ODN-immunized group at 1:6, 1:12, and 1:24 serum dilutions (P < 0.001 with the Mann-Whitney U test) (Table 3). Once again the blocking activity was concentration dependent, as revealed by higher infectivity at 1:12 and 1:24 dilutions.
TABLE 3.
Transmission-blocking activity of sera from immunized mice 2 weeks after the second boosta
| Group | Dilu- tion | No. of infected mosquitoes/no. of dissected mosquitoes | Geometric mean no. of oocysts (range) | Infectivity (% of control)b |
|---|---|---|---|---|
| Preimmune mouse sera | 1:6 | 18/20 | 4.1 (0-17) | 100 |
| Pfs25-A1 | 1:6 | 12/21 | 0.8 (0-4)c | 20 |
| 1:12 | 13/15 | 3.2 (0-9) | 77 | |
| 1:24 | 26/29 | 3.2 (0-21) | 79 | |
| Pfs25-A1 + CpG | 1:6 | 2/16 | 0.1 (0-1)c | 2 |
| 1:12 | 5/15 | 0.3 (0-2)c | 8 | |
| 1:24 | 4/9 | 0.7 (0-7)c | 17 | |
| Pfs25-A1 + non-CpG | 1:6 | 5/10 | 0.5 (0-2)c | 11 |
| 1:12 | 11/21 | 0.8 (0-3)c | 19 | |
| 1:24 | 18/28 | 1.4 (0-9)c | 34 |
P. falciparum (NF54 strain) gametocytes were fed to A. stephensi mosquitoes at various serum dilutions. The table shows results from one representative experiment repeated four times.
The geometric mean oocyst number of the test group divided by that of the preimmune-serum group × 100.
Statistically significant by the Mann-Whitney U test (P < 0.001).
In this study, we showed that the coadministration of CpG ODN with Pfs25 formulated in Al elevated antibody responses even after primary immunization (Table 1). Al is needed to cross-link the ODN to the antigen, without which the ODN quickly migrate from the site of administration (19, 23, 24, 26). This enhancement of the immunogenicity was further revealed by much higher titers and avidity of Pfs25-specific antibodies in the animals immunized with Pfs25-Al + CpG ODN. Consequently, the transmission-blocking activity in this group was superior to that in the other two groups. This may be a reflection of the immunomodulatory effect of CpG ODN resulting in antibody avidity maturation and possibly isotype switching. The antibody levels in the CpG ODN-coadministered group rapidly reached a plateau after the first boost and did not change after the second boost, but transmission-blocking activity of antibodies remained effective after the second boost even at higher dilutions.
One of the major aims in this study was to take advantage of the Th1-biased immune effect of CpG ODN and to explore the possible role of IgG isotypes in transmission-blocking immunity. It has been shown previously that IgG2a is an important isotype for transmission-blocking monoclonal antibodies recognizing another target antigen, Pfs230 (29). Immunoglobulin isotypes after the first boost showed a great discrepancy between groups (IgG2a and IgG1 for Pfs25-Al + CpG ODN-immunized group, whereas IgG1 was predominant for Pfs25-Al- and Pfs25-Al + non-CpG ODN-immunized groups). Overall, the IgG1 isotype antibodies in the CpG ODN-administered group had higher avidity than did those in the other groups (data not shown), and the avidity of IgG2a isotypes was lower than that of IgG1 isotypes in the CpG ODN-coadministered group (Fig. 2B). To evaluate the possible effect of increased IgG2a levels on transmission-blocking activity, we boosted mice in all the three groups once more with the same regimen. The level of IgG antibody in the Pfs25-Al + non-CpG ODN-immunized group increased to levels comparable to those in the Pfs25-Al + CpG ODN-immunized group, although the isotype profiles in the various groups remained similar to that before the boost (data not shown). Evaluation of sera after the second boost suggested that, despite the differences in the isotype distribution among the three groups, sera from all the three groups possessed transmission-blocking activity. Our results suggest that the high level of antibody responses is a more critical determinant for transmission-blocking activity irrespective of isotypes.
Adjuvants can strongly influence the outcome of an immune response. For example, Al is classified as a Th2-type adjuvant (high IgG1), whereas CpG ODN is a Th1-type adjuvant (high IgG2a) (4, 27). Recently, it has been found that ODN without CpG motifs can also function as adjuvant and elicit antigen-specific Th2 responses (24, 30). The latter was further confirmed in our studies: non-CpG ODN when administered together with rPfs25 plus Al induces antibody responses greater than does Pfs25-Al alone and antibodies (IgG1 dominant) are effective blockers of transmission of P. falciparum (Table 3). In general, CpG ODN has been found to be a safe and effective adjuvant in animals (11, 18, 35) and is currently in human clinical trials with promising results (information found at the Coley Pharmaceutical Group company website, http://www.coleypharma.com/wt/coley/clinical_program). Similarly, in our experiments we did not observe any apparent toxicity of CpG ODN when it was coadministered with rPfs25 and support further studies to evaluate CpG ODN as adjuvants for an rPfs25 transmission-blocking vaccine candidate.
Acknowledgments
We thank Mobolaji Okulate for help with laboratory mosquitoes.
This study was supported by research grants from the National Institutes of Health (AI47089 [N.K.]) and the WHO-TDR (N.K.). The supply of human erythrocytes for malaria culture is supported by NCCR OPD-GCRC RR00722.
Mice were housed at an Association for Assessment of Laboratory Animal Care-accredited animal facility and were used per an approved protocol.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Barr, P. J., K. M. Green, H. L. Gibson, I. C. Bathurst, I. A. Quakyi, and D. C. Kaslow. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 174:1203-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter, R., P. M. Graves, D. B. Keister, and I. A. Quakyi. 1990. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 12:587-603. [DOI] [PubMed] [Google Scholar]
- 3.Carter, R., P. M. Graves, I. A. Quakyi, and M. F. Good. 1989. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 169:135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coban, C., K. J. Ishii, D. J. Sullivan, and N. Kumar. 2002. Purified malaria pigment (hemozoin) enhances dendritic cell maturation and modulates the isotype of antibodies induced by a DNA vaccine. Infect. Immun. 70:3939-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Coban, C., M. T. Philipp, J. E. Purcell, D. B. Keister, M. Okulate, D. S. Martin, and M. Kumar. 2004. Induction of Plasmodium falciparium transmission-blocking antibodies in nonhuman primates by a combination of DNA and protein immunizations. Infect. Immun. 72:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy, P. E., and D. C. Kaslow. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 65:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozar, M. M., V. L. Price, and D. C. Kaslow. 1998. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect. Immun. 66:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, S. Akira, and P. Moingeon. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. (Erratum, 409: 646, 2001.) [DOI] [PubMed] [Google Scholar]
- 9.Hirunpetcharat, C., J. Wipasa, S. Sakkhachornphop, T. Nitkumhan, Y. Z. Zheng, S. Pichyangkul, A. M. Krieg, D. S. Walsh, D. G. Heppner, and M. F. Good. 2003. CpG oligodeoxynucleotide enhances immunity against blood-stage malaria infection in mice parenterally immunized with a yeast-expressed 19 kDa carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 (MSP1(19)) formulated in oil-based Montanides. Vaccine 21:2923-2932. [DOI] [PubMed] [Google Scholar]
- 10.Ishii, K. J., F. Takeshita, I. Gursel, M. Gursel, J. Conover, A. Nussenzweig, and D. M. Klinman. 2002. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J. Exp. Med. 196:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, T. R., N. Obaldia III, R. A. Gramzinski, Y. Charoenvit, N. Kolodny, S. Kitov, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 1999. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine 17:3065-3071. [DOI] [PubMed] [Google Scholar]
- 12.Kaslow, D. C. 1993. Transmission-blocking immunity against malaria and other vector-borne diseases. Curr. Opin. Immunol. 5:557-565. [DOI] [PubMed] [Google Scholar]
- 13.Kaslow, D. C., and J. Shiloach. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Bio/Technology 12:494-499. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow, D. C., I. A. Quakyi, C. Syin, M. G. Raum, D. B. Keister, J. E. Coligan, T. F. McCutchan, and L. H. Miller. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74-80. [DOI] [PubMed] [Google Scholar]
- 15.Kaslow, D. C., I. C. Bathurst, T. Lensen, T. Ponnudurai, P. J. Barr, and D. B. Keister. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect. Immun. 62:5576-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaslow, D. C., S. N. Isaacs, I. A. Quakyi, R. W. Gwadz, B. Moss, and D. B. Keister. 1991. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science 252:1310-1313. [DOI] [PubMed] [Google Scholar]
- 17.Klinman, D. M., D. Verthelyi, F. Takeshita, and K. J. Ishii. 1999. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity 11:123-129. [DOI] [PubMed] [Google Scholar]
- 18.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinman, D. M., K. M. Barnhart, and J. Conover. 1999. CpG motifs as immune adjuvants. Vaccine 17:19-25. [DOI] [PubMed] [Google Scholar]
- 20.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, N., I. Ploton, G., Koski, C. Ann-Lobo, and C. Contreras. 1995. Malaria transmission-blocking immunity. Identification of epitopes and evaluation of immunogenicity. Adv. Exp. Med. Biol. 383:65-72. [PubMed] [Google Scholar]
- 22.Lobo, C. A., R. Dhar, and N. Kumar. 1999. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect. Immun. 67:1688-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCluskie, M. J., and H. L. Davis. 1998. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161:4463-4469. [PubMed] [Google Scholar]
- 24.McCluskie, M. J., and H. L. Davis. 2000. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine 19:413-422. [DOI] [PubMed] [Google Scholar]
- 25.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216-1224. [DOI] [PubMed] [Google Scholar]
- 26.Near, K. A., A. W. Stowers, D. Jankovic, and D. C. Kaslow. 2002. Improved immunogenicity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by the addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect. Immun. 70:692-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Hagan, D. T., M. L. MacKichan, and M. Singh. 2001. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 18:69-85. [DOI] [PubMed] [Google Scholar]
- 28.Quakyi, I. A., R. Carter, J. Rener, N. Kumar, M. F. Good, and L. H. Miller. 1987. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 139:4213-4220. [PubMed] [Google Scholar]
- 29.Roeffen, W., F. Geeraedts, W. Eling, P. Beckers, B. Wizel, N. Kumar, T. Lensen, and R. Sauerwein. 1995. Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230-specific antibodies is isotype dependent. Infect. Immun. 63:467-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sano, K., H. Shirota, T. Terui, T. Hattori, and G. Tamura. 2003. Oligodeoxynucleotides without CpG motifs work as adjuvant for the induction of Th2 differentiation in a sequence-independent manner. J. Immunol. 170:2367-2373. [DOI] [PubMed] [Google Scholar]
- 31.Stowers, A., and R. Carter. 2001. Current developments in malaria transmission-blocking vaccines. Expert Opin. Biol. Ther. 1:619-628. [DOI] [PubMed] [Google Scholar]
- 32.Stowers, A. W., D. B. Keister, O. Muratova, and D. C. Kaslow. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect. Immun. 68:5530-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeshita, F., C. A. Leifer, I. Gursel, K. J. Ishii, S. Takeshita, M. Gursel, and D. M. Klinman. 2001. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167:3555-3558. [DOI] [PubMed] [Google Scholar]
- 34.Wagner, H. 1999. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv. Immunol. 73:329-368. [DOI] [PubMed] [Google Scholar]
- 35.Weeratna, R. D., M. J. McCluskie, Y. Xu, and H. L. Davis. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18:1755-1762. [DOI] [PubMed] [Google Scholar]