Abstract
Objective
Our objective was to investigate how postprandial processing of intact proinsulin is influenced by different pharmacological strategies in type 2 diabetes mellitus (T2DM).
Materials/Methods
This exploratory, nonrandomized, cross-sectional study recruited T2DM patients and healthy subjects. Upon recruitment, eligible T2DM patients had been treated for ≥6 months with insulin glargine (GLA) plus metformin (MET), sulfonylureas (SU) plus MET, or dipeptidyl-peptidase-4 inhibitors (DPP-4-I) plus MET. Blood samples were drawn from study participants after an 8 h fast and at regular intervals for up to 5 h after consumption of a standardized meal. Study endpoints included postprandial intact proinsulin and insulin levels and the insulin/proinsulin ratio.
Results
As expected, postprandial secretion of proinsulin was greater in all T2DM treatment groups than in healthy subjects (p < .01 for all comparisons). Postprandial release of proinsulin was significantly greater in T2DM patients treated with SU plus MET than in those treated with GLA plus MET (p = .003). Treatment with DPP-4-I plus MET was associated with reduced proinsulin secretion versus SU plus MET and an increased insulin/proinsulin ratio versus the other T2DM groups.
Conclusions
Treatment of T2DM with GLA plus MET or DPP-4-I plus MET was associated with a more physiological postprandial secretion pattern of the β cell compared with those treated with SU plus MET.
Keywords: dipeptidyl-peptidase-4 inhibitors, insulin glargine, postprandial proinsulin, sulfonylureas
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease characterized by a progressive loss of β-cell function and a steady increase in the intact proinsulin/insulin ratio. Since the early 2000s, the predictive value of intact proinsulin levels alone and the potential pathogenic effects of this prohormone have been extensively investigated. For example, preclinical and clinical studies of T2DM have established that proinsulin is both a marker of decreasing β-cell function and a predictor of increased β-cell loss.1–3 In addition, a close association between elevated proinsulin levels and the development of cardiovascular disease could be demonstrated in subjects with and without diabetes.4–10
Discovery of the potential pathogenic role of proinsulin has triggered interest in the effects of different pharma-cological interventions in T2DM on this prohormone. For example, treatment with sulfonylureas (SU) increases secretion of intact proinsulin in patients with T2DM.11 We have shown that treatment with basal insulin in combination with metformin (MET) effectively reduced intact proinsulin levels and that insulin glargine (GLA) plus MET was superior to neutral protamine Hagedorn insulin plus MET in controlling the postprandial release of intact proinsulin.12 Other data suggest that glucagon-like peptide-1 (GLP-1)-based therapies, including GLP-1 receptor agonists and dipeptidyl-peptidase-4 inhibitors (DPP-4-I), may increase conversion of intact proinsulin to insulin and C-peptide, thereby reducing the levels of circulating intact proinsulin.13 To provide further insight on how postprandial release of intact proinsulin is influenced by the therapeutic strategy, we compared the effects of GLA plus MET combination therapy with that of SU plus MET and DPP-4-I plus MET on the postprandial release of intact proinsulin and other related variables.
Methods
Study Objectives
The primary objective of the study was to compare the effects of treatment with GLA plus MET versus those of SU plus MET therapy on plasma proinsulin levels in patients with T2DM for 5 h after consumption of a standardized meal. Secondary objectives included comparison of (1) postprandial plasma proinsulin levels in T2DM patients treated with DPP-4-I plus MET versus those in patients receiving GLA plus MET or SU plus MET; (2) postprandial plasma proinsulin levels in each of the three T2DM groups (GLA plus MET, SU plus MET, and DPP-4-I plus MET) versus those in healthy subjects; and (3) postprandial plasma insulin, insulin/proinsulin ratios, and blood glucose levels among the T2DM groups and between each T2DM group and the healthy subject group.
Study Participants
Patients with T2DM and healthy subjects were recruited. Eligible participants were male or female, were aged 40 to 75 years, and had a body mass index of 20 to 35 kg/m2. Inclusion criteria for patients with T2DM were T2DM duration of 3 to 15 years, glycated hemoglobin (HbA1c) ≤7.5%, treated with GLA plus MET or SU plus MET during the past 6 to 12 months or with DPP-4-I plus MET during the past 6 months, and treated with a stable antidiabetic dosage during the past 3 months. Inclusion criteria for healthy subjects only were fasting blood glucose ≤ 100 mg/dl (5.6 mmol/liter) and an oral glucose tolerance test (OGTT) that revealed no impaired glucose tolerance or T2DM.
A T2DM patient was excluded if any of the following criteria were met: treatment in the past 6 to 12 months with any other insulin other than GLA or any oral antidiabetic drug (OAD) other than MET (for patients in the GLA plus MET group), treatment in the past 3 months with any insulin or any OAD other than SU or MET (SU plus MET group), treatment in the past 3 months with any insulin or any OAD other than DPP-4-I or MET (DPP-4-I plus MET group), or major microvascular or macrovascular complications as judged by the investigator. The study was performed in compliance with Good Clinical Practice and all applicable national laws and regulations. All patients provided written informed consent, and the study was approved by the appropriate independent ethics committee (Ethik-Kommission der Landesärztekammer Rheinland-Pfalz, Mainz, Germany) and regulatory authority (The Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn, Germany).
Study Design
This was a single-center, open-label, four-arm, exploratory study comprising a screening visit (visit 1) and, 1 to 14 days later, a test meal visit (visit 2). On the day of visit 2, participants entered the clinic after an 8 h fast. An intravenous catheter was inserted into a superficial vein of the forearm, and the first blood sample was drawn. After 30 min, another sample was drawn and a standardized test meal (27 g protein, 15 g fat, and 48 g carbohydrates; 434 kcal) was consumed. After the test meal, blood samples were drawn every 30 min for the measurement of blood glucose, insulin, and intact proinsulin.
Assignment to the three T2DM groups and the healthy subject group was predetermined according to the study eligibility criteria. Patients with T2DM took their usual antidiabetic medications throughout the study, including on the day of visit 2. All participants continued to use other concomitant medications, provided that these medications were not covered by the exclusion criteria.
Blood Sample Analyses and Study End Points
Enzyme-linked immunosorbent assays were used to measure plasma proinsulin. Plasma insulin and blood glucose levels were determined using a chemiluminescence immunoassay and an electrochemical biosensor, respectively. The trapezoidal method was used to calculate the area under the plasma concentration time curve from 0 to 300 min (AUC0–300 min) for plasma proinsulin and insulin and for blood glucose. In addition, the insulin/proinsulin ratio was calculated.
Statistics
Data are reported for the per protocol set, defined as all study participants who completed the study without major protocol violations. All end points were evaluated using primarily descriptive statistics. Inferential statistics, including a two-sided Student's t-test, were employed for more detailed analysis of between-group differences. A p value of <0.05 in the two-sided t-test indicated statistical significance.
Results
Participant Disposition and Demographics
A total of 104 potential participants were screened for possible inclusion in the study. Of these, 24 failed screening while 80 were considered eligible and returned for visit 2. Overall, 65 participants were included in the per protocol set (GLA plus MET, n = 14; all other groups, n = 17).
Baseline demographics of the per protocol set are shown in Table 1. In general, baseline demographics were well balanced among the four groups. However, while there were almost equal numbers of male and female healthy subjects, there was a preponderance of males in the T2DM groups. In addition, patients in the DPP-4-I group were slightly younger and had lower HbA1c levels than those in the other T2DM groups.
Table 1.
Baseline Demographic Characteristics of Patients with Type 2 Diabetes Mellitus and Healthy Subjectsa
| Patients with T2DM | ||||
|---|---|---|---|---|
| GLA + MET (n = 14) | SU + MET (n = 17) | DPP-4-I + MET (n = 17) | Healthy subjects (n = 17) | |
| Male n (%) | 11 (78.6) | 11 (64.7) | 10 (58.8) | 9 (52.9) |
| Age (years) | 65.5 (6.5) | 64.9 (6.5) | 62.6 (8.4) | 64.9 (6.3) |
| Weight (kg) | 88.5 (10.6) | 90.6 (14.9) | 86.4 (12.7) | 80.7 (16.0) |
| Height (cm) | 170.9 (7.1) | 168.8 (10.8) | 169.1 (7.2) | 172.5 (10.4) |
| Body mass index (kg/m2) | 30.3 (2.7) | 31.6 (2.7) | 30.1 (3.2) | 26.9 (3.7) |
| HbA1c (%) | 7.1 (0.3) | 7.0 (0.5) | 6.6 (0.4) | 5.6 (0.3) |
| Duration of diabetes (years) | 8.9 (3.3) | 7.9 (4.2) | 6.9 (3.9) | NA |
| Fasting glucose (mmol/liter) | 7.3 (1.6)b | 7.5 (1.0)b | 7.6 (1.7)b | 5.5 (0.5) |
| Fasting insulin (pmol/liter) | 14.9 (7.8) | 27.5 (21.9)b | 15.3 (7.3)b | 9.2 (3.9) |
| Fasting proinsulin (pmol/liter) | 7.0 (3.4)b | 14.2 (8.3)b,c,d | 5.6 (3.2)b | 4.5 (1.9) |
Values are mean (standard deviation) unless otherwise stated. NA, not applicable.
p < .05 versus healthy subjects.
p < .05 versus GLA plus MET.
p < .05 versus DPP-4-I plus MET.
Fasting glucose and proinsulin levels at baseline were greater in all participants with T2DM than in healthy subjects, while fasting insulin levels were greater in the SU plus MET and DPP-4-I plus MET groups (Table 1). Fasting proinsulin levels in the SU plus MET group were significantly greater than in all other groups.
Effects on Postprandial Plasma Proinsulin
Fasting intact proinsulin levels were significantly higher in patients treated with SU plus MET compared with all other groups (Figure 1). Postprandial secretion of proinsulin was significantly greater in patients with T2DM who were treated with SU plus MET than in patients receiving GLA plus MET or in those patients receiving DPP-4-I plus MET (Table 2 and Figure 1). Postprandial secretion of proinsulin was significantly greater in all T2DM groups compared with healthy subjects (p < .01 for all comparisons).
Figure 1.
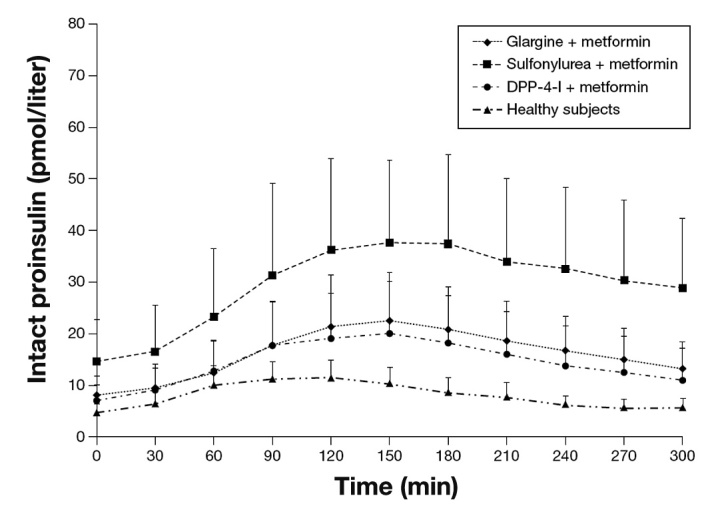
Mean (+standard deviation) preprandial and postprandial plasma levels of intact proinsulin before and after a standardized meal in T2DM patients and healthy subjects.
Table 2.
Postprandial Plasma Levels of Proinsulin, Insulin, and Blood Glucose after a Standardized Meal in Patients with Type 2 Diabetes Mellitus and Healthy Subjectsa
| Patients with T2DM | ||||
|---|---|---|---|---|
| GLA + MET (n = 14) | SU + MET (n = 17) | DPP-4-I + MET (n = 17) | Healthy subjects (n = 17) | |
| Proinsulin AUC0–300 min (pmol/liter) | 4462 (3522–6363)b | 8256 (5662–10270)c,d,e | 4589 (3112–5645)b | 2758 (2152–3354) |
| Insulin AUC0–300 min (pmol/liter) | 7605 (5292–10990) | 14870 (11250–18863)b,d | 10200 (7700–13410)f | 6755 (5120–9923) |
| Blood glucose AUC0–300 min (mmol/liter) | 49330 (47195–56300)g | 48405 (44183–52168)g | 47640 (40350–52400)g | 33400 (31315–35355) |
Values are median (interquartile range).
p < .01 versus healthy subjects.
p = .003 versus GLA plus MET.
p = .01 versus DPP-4-I plus MET.
p = .034 versus DPP-4-I plus MET.
p = .011 versus healthy subjects.
p < .001 versus healthy subjects.
Effects on Postprandial Plasma Insulin, Insulin/Proinsulin Ratio, and Blood Glucose
Postprandial insulin levels were higher in the three T2DM groups than in healthy subjects (Table 2). These increases were statistically significant in the SU plus MET and DPP-4-I plus MET groups (p = .001 and .011, respectively, versus healthy subjects) but not in the GLA plus MET group. This lack of statistical significance was likely due, at least in part, to the large variability in insulin AUC0–300 min values observed in the latter group. Comparisons among T2DM groups showed that postprandial insulin levels were higher in the SU plus MET group than the other T2DM groups (Table 2), although only the difference between the SU plus MET and DPP-4-I plus MET groups was statistically significant (p = .034).
Postprandial insulin/proinsulin ratios were significantly higher in healthy subjects (19.6 ± 7.3) compared with the GLA plus MET (13.3 ± 6.4; p = .017) and SU plus MET (13.1 ± 5.3; p = .006) groups but not versus the DPP-4-I group (17.0 ± 4.7). This ratio was numerically higher in the DPP-4-I plus MET group than in the other T2DM groups, but only the difference with the SU plus MET group was statistically significant (p = .032).
Postprandial blood glucose levels were higher in the three T2DM groups than in the healthy subjects (p < .001 for all comparisons; Table 2). No significant differences in postprandial blood glucose levels were observed among the three T2DM groups (Table 2).
Discussion
In this study, treatment with GLA plus MET was associated with positive effects on two important measures of β-cell function in comparison with SU plus MET. Firstly, patients treated with GLA plus MET experienced a statistically significant reduction in postprandial intact proinsulin secretion. In addition, a trend toward a decrease in postprandial insulin levels was observed in the GLA plus MET group. These results show that GLA plus MET, compared with SU plus MET, reduces the postprandial work load for the β cell associated with an improvement in the capacity to convert intact proinsulin into insulin and C-peptide. The study also showed that DPP-4-I plus MET decreased the postprandial secretion of proinsulin and insulin compared with SU plus MET therapy. Furthermore, the insulin/proinsulin ratio was higher with DPP-4-I plus MET compared with SU plus MET or GLA plus MET, indicating an improved conversion rate during treatment with DPP-4-I.
Measurement of intact proinsulin from the β cell is used as an indicator of β-cell dysfunction and has been reported to be related to a polymorphism in three different risk alleles (HHEX, CDKN2A/B, and IGF2BP2) that may be predictive for the risk of β-cell failure and development of T2DM in individual subjects.14 The insulin/proinsulin ratio was introduced as a marker for the capability of the β cell to convert intact proinsulin into insulin and C-peptide.15 In addition, elevated plasma levels of intact proinsulin were shown to be predictive for cardiovascular risk estimation in subjects with and without diabetes. Indeed, elevated levels of this prohormone have been linked to increased cardiovascular risk4–10 and the severity of angiographically characterized coronary heart disease.16 The atherogenic potential of proinsulin was highlighted some years ago in two clinical trials investigating the therapeutic potential of human proinsulin in T2DM, where an eight-fold increase in cardiovascular disease events was observed during treatment with human proinsulin versus human regular insulin.17,18
Subsequent investigations have indicated that measuring intact proinsulin release from the β cell after stimulation by OGTT or food intake might further increase the sensitivity and predictive value of this biomarker.19–21
The mechanisms via which proinsulin contributes to atherogenesis have not been fully elucidated; however, it is known that levels of plasminogen activator inhibitor-1 (PAI-1) are elevated in vitro after administration of pro-insulin.22 Studies have also indicated that the atherogenic effects of proinsulin may be linked to increased plasma PAI-1 concentrations and the subsequent inhibition of fibrinolysis and augmentation of thrombogenic potency.23–25 Initiation of insulin treatment, meanwhile, leads to a decline in plasma levels of intact proinsulin and PAI-1, suggesting that insulin therapy not only protects residual β-cell function, but may also provide sophisticated effects on vascular biology and the development of vascular complications in patients with T2DM.26–28
Treatment with SU was associated with a marked post-prandial increase in intact proinsulin levels in our study, while treatment with GLA or DPP-4-I was associated with much lower elevations and was more reflective of the physiological situation in nondiabetic subjects. Importantly, this β-cell relieving effect was achieved despite comparable postprandial blood glucose control among the three T2DM groups. It should be noted that patients in the DPP-4-I group were younger and had lower HbA1c levels than patients in the other T2DM groups. Since this was a cross-sectional, nonrandomized study, such differences might reflect a tendency to use DPP-4-I therapy in patients at an earlier stage of β-cell failure. Furthermore, GLP-1 has been shown to stimulate the conversion of intact proinsulin into insulin and C-peptide.29 Such effects might have contributed to the improved insulin/proinsulin ratio observed in the DPP-4-I plus MET group compared with the other treatment groups.
No significant differences in postprandial blood glucose levels between the three T2DM groups were discernable. This finding is of interest against the background of the lower insulin levels observed in the GLA plus MET and DPP-4-I plus MET groups versus the SU plus MET group.
In summary, this cross-sectional, exploratory study indicates the restoration of a more physiological secretion pattern of the β cell in patients treated with GLA plus MET or DPP-4-I plus MET compared with those receiving SU plus MET therapy. It might be assumed that the reduced postprandial release of intact proinsulin reflects a reduced postprandial β-cell stress and a less atherogenic postprandial state in patients with T2DM. The results of this cross-sectional study should be interpreted in an exploratory sense. A prospective, randomized study would need to be performed to further address the effects of the different treatment modalities on β-cell function and vascular risk in patients with T2DM.
Acknowledgments
Editorial support was provided by Tom Claus, Ph.D., of PPSI (a PAREXEL company) and was funded by Sanofi. Stefan Pscherer, Martin Larbig, and Berndt von Stritsky contributed to study design, interpretation of study results, and preparation of the manuscript. Andreas Pfützner and Thomas Forst contributed to study design, clinical performance of the study, interpretation of study results, and preparation of the manuscript.
Glossary
Abbreviations
- (AUC0–300)
area under the concentration time curve from 0 to 300 min
- (DPP-4-I)
dipeptidyl-peptidase-4 inhibitors
- (GLA)
insulin glargine
- (GLP-1)
glucagon-like peptide-1
- (HbA1c)
glycated hemoglobin
- (MET)
metformin
- (OAD)
oral antidiabetic drug
- (OGTT)
oral glucose tolerance test
- (PAI-1)
plasminogen activator inhibitor-1
- (SU)
sulfonylureas
- (T2DM)
type 2 diabetes mellitus
Funding
This study (clinical trials registration number EUDRA-CT 2009-015993-37) was supported by Sanofi.
Disclosures
Stefan Pscherer, Andreas Pfützner, and Thomas Forst received speaker and advisory fees from Sanofi. Martin Larbig and Berndt von Stritsky were employees of Sanofi.
References
- 1.Pfützner A, Kunt T, Hohberg C, Mondok A, Pahler S, Konrad T, Lübben G, Forst T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004;27(3):682–687. doi: 10.2337/diacare.27.3.682. [DOI] [PubMed] [Google Scholar]
- 2.Røder ME, Dinesen B, Hartling SG, Houssa P, Vestergaard H, Sodoyez-Goffaux F, Binder C. Intact proinsulin and beta-cell function in lean and obese subjects with and without type 2 diabetes. Diabetes Care. 1999;22(4):609–614. doi: 10.2337/diacare.22.4.609. [DOI] [PubMed] [Google Scholar]
- 3.Uchizono Y, Alarcón C, Wicksteed BL, Marsh BJ, Rhodes CJ. The balance between proinsulin biosynthesis and insulin secretion: where can imbalance lead? Diabetes Obes Metab. 2007;9(Suppl 2):56–66. doi: 10.1111/j.1463-1326.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 4.Alssema M, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ, Hoorn Study Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care. 2005;28(4):860–865. doi: 10.2337/diacare.28.4.860. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl B, Dinesen B, Eliasson M, Røder M, Hallmans G, Stegmayr B. High proinsulin levels precede first-ever stroke in a nondiabetic population. Stroke. 2000;31(12):2936–2941. doi: 10.1161/01.str.31.12.2936. [DOI] [PubMed] [Google Scholar]
- 6.Oh JY, Barrett-Connor E, Wedick NM. Sex differences in the association between proinsulin and intact insulin with coronary heart disease in nondiabetic older adults: the Rancho Bernardo Study. Circulation. 2002;105(11):1311–1316. doi: 10.1161/hc1102.105565. [DOI] [PubMed] [Google Scholar]
- 7.Wohlin M, Sundström J, Arnlöv J, Andrén B, Zethelius B, Lind L. Impaired insulin sensitivity is an independent predictor of common carotid intima-media thickness in a population sample of elderly men. Atherosclerosis. 2003;170(1):181–185. doi: 10.1016/s0021-9150(03)00283-1. [DOI] [PubMed] [Google Scholar]
- 8.Yudkin JS, May M, Elwood P, Yarnell JW, Greenwood R, Davey Smith G, Caerphilly Study Concentrations of proinsulin like molecules predict coronary heart disease risk independently of insulin: prospective data from the Caerphilly Study. Diabetologia. 2002;45(3):327–336. doi: 10.1007/s00125-001-0756-7. [DOI] [PubMed] [Google Scholar]
- 9.Zethelius B, Byberg L, Hales CN, Lithell H, Berne C. Proinsulin is an independent predictor of coronary heart disease: report from a 27-year follow-up study. Circulation. 2002;105(18):2153–2158. doi: 10.1161/01.cir.0000015855.04844.e7. [DOI] [PubMed] [Google Scholar]
- 10.Zethelius B, Lithell H, Hales CN, Berne C. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia. 2005;48(5):862–867. doi: 10.1007/s00125-005-1711-9. [DOI] [PubMed] [Google Scholar]
- 11.Inoguchi T, Umeda F, Kakimoto M, Sako Y, Ishii H, Noda K, Kunisaki M, Imamura M, Yu HY, Etoh T, Yoshikawa H, Aoki T, Hashimoto T, Nawata H. Chronic sulfonylurea treatment and hyperglycemia aggravate disproportionately elevated plasma proinsulin levels in patients with type 2 diabetes. Endocr J. 2000;47(6):763–770. doi: 10.1507/endocrj.47.763. [DOI] [PubMed] [Google Scholar]
- 12.Forst T, Larbig M, Hohberg C, Sorst S, Diessel S, Borchert M, Roth W, Pfützner A. Adding insulin glargine vs. NPH insulin to metformin results in a more efficient postprandial beta-cell protection in individuals with type 2 diabetes. Diabetes Obes Metab. 2010;12(5):437–441. doi: 10.1111/j.1463-1326.2010.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratley RE, Schweizer A, Rosenstock J, Foley JE, Banerji MA, Pi-Sunyer FX, Mills D, Dejager S. Robust improvements in fasting and prandial measures of beta-cell function with vildagliptin in drug-naïve patients: analysis of pooled vildagliptin monotherapy database. Diabetes Obes Metab. 2008;10(10):931–938. doi: 10.1111/j.1463-1326.2007.00835.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff K, Machicao F, Haupt A, Schäfer SA, Tschritter O, Staiger H, Stefan N, Häring HU, Fritsche A. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51(4):597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 15.Fritsche A, Madaus A, Stefan N, Tschritter O, Maerker E, Teigeler A, Häring H, Stumvoll M. Relationships among age, proinsulin conversion, and beta-cell function in nondiabetic humans. Diabetes. 2002;51(Suppl 1):S234–9. doi: 10.2337/diabetes.51.2007.s234. [DOI] [PubMed] [Google Scholar]
- 16.Jia EZ, Yang ZJ, Zhu TB, Wang LS, Chen B, Cao KJ, Huang J, Ma WZ. Proinsulin is an independent predictor of the angio-graphical characteristics of coronary atherosclerosis. Cardiology. 2008;110(2):106–111. doi: 10.1159/000110488. [DOI] [PubMed] [Google Scholar]
- 17.Galloway JA, Hooper SA, Spradlin CT, Howey DC, Frank BH, Bowsher RR, Anderson JH. Biosynthetic human proinsulin. Review of chemistry, in vitro and in vivo receptor binding, animal and human pharmacology studies, and clinical trial experience. Diabetes Care. 1992;15(5):666–692. doi: 10.2337/diacare.15.5.666. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl B, Dinesen B, Eliasson M, Røder M, Jansson JH, Huhtasaari F, Hallmans G. High proinsulin concentration precedes acute myocardial infarction in a nondiabetic population. Metabolism. 1999;48(9):1197–1202. doi: 10.1016/s0026-0495(99)90138-5. [DOI] [PubMed] [Google Scholar]
- 19.Fritsche A, Schweitzer MA, Häring HU, 4001 Study Group Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138(12):952–959. doi: 10.7326/0003-4819-138-12-200306170-00006. [DOI] [PubMed] [Google Scholar]
- 20.Larsson H, Ahrén B. Relative hyperproinsulinemia as a sign of islet dysfunction in women with impaired glucose tolerance. J Clin Endocrinol Metab. 1999;84(6):2068–2074. doi: 10.1210/jcem.84.6.5717. [DOI] [PubMed] [Google Scholar]
- 21.Forst T, Pfützner A, Lübben G, Weber M, Marx N, Karagiannis E, Koehler C, Baurecht W, Hohberg C, Hanefeld M. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk--the PIOSTAT Study. Metabolism. 2007;56(4):491–496. doi: 10.1016/j.metabol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Nordt TK, Schneider DJ, Sobel BE. Augmentation of the synthesis of plasminogen activator inhibitor type-1 by precursors of insulin. A potential risk factor for vascular disease. Circulation. 1994;89(1):321–330. doi: 10.1161/01.cir.89.1.321. [DOI] [PubMed] [Google Scholar]
- 23.Festa A, D'Agostino R, Jr, Mykkänen L, Tracy RP, Zaccaro DJ, Hales CN, Haffner SM. Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance. The Insulin Resistance Atherosclerosis Study (IRAS) Arterioscler Thromb Vasc Biol. 1999;19(3):562–568. doi: 10.1161/01.atv.19.3.562. [DOI] [PubMed] [Google Scholar]
- 24.Lyon CJ, Hsueh WA. Effect of plasminogen activator inhibitor-1 in diabetes mellitus and cardiovascular disease. Am J Med. 2003;115(Suppl 8A):62S–68S. doi: 10.1016/j.amjmed.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Nordt TK, Bode C, Sobel BE. Stimulation in vivo of expression of intra-abdominal adipose tissue plasminogen activator inhibitor type I by proinsulin. Diabetologia. 2001;44(9):1121–1124. doi: 10.1007/s001250100618. [DOI] [PubMed] [Google Scholar]
- 26.Hohberg C, Forst T, Larbig M, Safinowski M, Diessel S, Hehenwarter S, Weber MM, Schöndorf T, Pfützner A. Effect of insulin glulisine on microvascular blood flow and endothelial function in the postprandial state. Diabetes Care. 2008;31(5):1021–1025. doi: 10.2337/dc07-2185. [DOI] [PubMed] [Google Scholar]
- 27.Jain SK, Nagi DK, Slavin BM, Lumb PJ, Yudkin JS. Insulin therapy in type 2 diabetic subjects suppresses plasminogen activator inhibitor (PAI-1) activity and proinsulin-like molecules independently of glycaemic control. Diabet Med. 1993;10(1):27–32. doi: 10.1111/j.1464-5491.1993.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 28.Pfützner A, Lorra B, Abdollahnia MR, Kann PH, Mathieu D, Pehnert C, Oligschleger C, Kaiser M, Forst T. The switch from sulfonylurea to preprandial short- acting insulin analog substitution has an immediate and comprehensive beta-cell protective effect in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2006;8(3):375–384. doi: 10.1089/dia.2006.8.375. [DOI] [PubMed] [Google Scholar]
- 29.Stumvoll M, Fritsche A, Stefan N, Hardt E, Häring H. Evidence against a rate-limiting role of proinsulin processing for maximal insulin secretion in subjects with impaired glucose tolerance and beta-cell dysfunction. J Clin Endocrinol Metab. 2001;86(3):1235–1239. doi: 10.1210/jcem.86.3.7331. [DOI] [PubMed] [Google Scholar]


