Abstract
Obesity is the most common nutritional disorder of cats and is a risk factor for diabetes. Similar to developments in obese people, obese cats show peripheral tissue insulin resistance and may demonstrate glucose intolerance when challenged with pharmacological amounts of glucose. However, they compensate well for the insulin resistance and do not show elevated glucose concentrations when monitored during their regular daily routine, including postprandial periods. This is possible because obese cats in the fasted and postprandial state are able to maintain hepatic insulin sensitivity and decrease endogenous glucose production, which allows them to maintain normoglycemia. Also dissimilar to what is seen in many obese humans, cats do not develop atherosclerosis and clinical hypertension. The time course for progression to overt diabetes of obese cats is unknown. One might speculate that diabetes develops when the liver finally becomes insulin resistant and/or insulin secretion becomes too low to overcome increased glucose production. In addition, amyloid, demonstrated to be deposited in islet of chronically obese cats, may contribute to a reduction in insulin secretion by reducing functional β-cell mass.
Keywords: amyloid, glucose tolerance, insulin sensitivity, metabolic syndrome
Introduction
Obesity is a risk factor for diabetes in both humans and cats.1,2 Obesity is well defined in people. In cats, obesity can be assessed subjectively using the body scoring system developed by Laflamme.3 Objective ways to judge obesity in cats also exist but are not usually perfomed in clinical practice. Body mass index (BMI) is well known from human medicine, where it is used ubiquitously to assess adiposity. It can be calculated in cats according to the following formula:4
where height is the distance from the point of the shoulder through the point of the elbow to the proximal boundary of the metacarpal pad and length is the distance from the point of the shoulder to the tuber ischium. The feline body mass index is calculated according to the following formula:
where rib cage is the rib cage circumference in centimeters and LIM is the length of the lower leg from the middle of the patella to the dorsal tip of the calcaneal process in centimeters.5 Obesity also can be assessed by measuring the girth circumference immediately caudal to the last rib.6 Both girth and BMI measurements do not need any specialized equipment. Highly sophisticated evaluations of adiposity include dual energy X-ray absorptiometry (DEXA) and magnetic resonance imaging.7,8 The latter allows the exact localization of fat depots in the body, which is not possible with DEXA. We have found that BMI and girth correlate well with DEXA results and are excellent objective markers of adiposity that could be used by clinicians. When cats become obese, both abdominal subcutaneous and visceral fat mass increase to the same extent,7 which contrasts with human subjects, where obesity is usually associated with a larger increase in abdominal visceral than abdominal subcutaneous obesity.9 There are no published data on veterinary standards for lean, overweight, or obese cats for any of the objective measurements to date. However, results of objective obesity measurements have been described in lean and obese cats, which have been used in research projects (e.g., Reference 7); they were mostly domestic shorthair cats.
The prevalence of feline obesity seems to be increasing and so is the prevalence of diabetes. Obesity is now seen in approximately 35% to 50% of cats.2,10 Feline diabetes has increased from 0.08% to 1.2 % in three decades in the United States.11 A prevalence of 0.4% was recorded in the United Kingdom.12 Diabetes is usually seen in older cats implying that pathophysiological mechanisms involved in the progression from obesity to diabetes may require years to develop. Obviously, not all obese cats become diabetic and, similar to other species, genetic, environmental, and metabolic factors may significantly influence the response to long-term obesity.
Glucose homeostasis depends on β-cell function, endogenous glucose production (EGP) by the liver, and glucose utilization in peripheral tissues, primarily muscle. The hallmark of diabetes in people is hyperglycemia, which develops when peripheral tissues and the liver become resistant to the effect of insulin, when EGP increases, and when insulin secretion becomes abnormal.13 It would be advantageous to find markers that forebode impending diabetes before overt hyperglycemia is obvious. Pursuant to this goal, the obese cat has been used in several studies as a model to elucidate factors contributing to progression from obesity to diabetes.
Insulin Sensitivity/Resistance Is Tissue Specific
Peripheral insulin resistance usually describes the loss of insulin action in muscle and adipose tissue. Obese cats have increased amounts of intramyocellular and extra-myocellular fat,7 which has been associated in people with a loss of insulin sensitivity.14 The higher amount of fat in muscle is due to a shift in the expression and activity of lipoprotein lipase. This enzyme responsible for uptake of fatty acids into tissues is lower in adipose tissue but higher in muscle tissue, leading to a partitioning of fatty acids to muscle.15 It has also been shown that tumor necrosis factor-a expression is higher in adipose tissue in obese cats,15 and this cytokine has been purported to downregulate lipoprotein lipase.16,17 The loss of insulin sensitivity in muscle tissue is not always accompanied by a loss of insulin sensitivity in fat tissue. During the euglycemic hyperinsulinemic clamp (EHC), nonesterified fatty acids were suppressed to a significantly higher degree in cats on a high-carbohydrate diet but not in those on a high-protein diet, indicating that insulin sensitivity is regulated in a tissue-specific way and can be influenced by diet.7 The greater insulin sensitivity of adipose tissue in that study, however, did not lead to a greater fat deposition, likely because the high insulin concentrations achieved during the clamp exceed the physiological insulin response after regular food intake. It is also possible that diet influenced nonesterified fatty acid metabolism independently of the action of insulin. A similar suppression of nonesterified fatty acids has also been seen during the intravenous glucose tolerance test (IVGTT).18
As stated earlier, glucose homeostasis not only depends on peripheral glucose uptake, but also on the EGP by the liver. In order to evaluate glucose output by the liver, a noninvasive approach employing nuclear magnetic resonance spectroscopy was used. This method has been used in humans,19 rats,20 and mice21 to investigate metabolic pathways in glucose production using a triple tracer method. Applying this method, it is possible to detect key steps in glucose metabolism (glucose turnover and gluconeogenic fluxes) with a single blood sample using stable, nonradioactive isotopes [2H2O, (U-13C3) propionate, and (3,4-13C2) glucose]. We utilized this methodology to study the metabolic pathways involved in glucose metabolism in lean and obese neutered male and female cats. We hypothesized that the insulin resistance of obese cats will be reflected in the activity of different metabolic pathways leading to hepatic glucose production. However, we found that obese cats compensate well for the peripheral insulin resistance by maintaining insulin sensitivity in the liver, allowing them to decrease EGP and to maintain normal blood glucose concentrations not only in the fasted, but also in the postprandial state.22,23 This was partially accomplished through pyruvate cycling. This futile cycle was higher in obese cats. Gluconeogenesis was the main contributor to EGP in fasted and postprandial cats. This was expected. It was, however, interesting that glycogenolysis accounted for approximately 35% of fluxes contributing to EGP in fasted cats and approximately 40% in postprandial cats. With the exception of one study,24 it has always been thought that cats do not produce much glycogen,25,26 however, this is clearly not the case. The hepatic glycogen content of 24 h fasted cats is similar to those found in people after a similar fast.27 Therefore, both gluconeogenic and glycogenolytic contributions to glucose production in the liver of cats (lean and obese) are similar to what has been documented in people. Histologically, glycogen seems to be abundant in the liver, and biochemical measurements show that, in 24 h fasted cats, approximately 5% of liver weight is from glycogen.23
Changes in Insulin Secretion during the Development of Insulin Resistance and Diabetes
Only one study has followed the insulin secretory pattern in response to an intravenous glucose load during the development of diabetes in cats. It shows that there is a distinct change in the secretory pattern of insulin, and one can distinguish different phases in the progression toward diabetes.28 Healthy lean cats have a biphasic insulin secretion pattern when stimulated with 1 g of glucose per kilogram body weight during an IVGTT. The glucose and insulin concentrations return to baseline at 120 min with the high dose of glucose (Figure 1). With the development of insulin resistance, there are changes in insulin secretion. Plasma insulin concentra-tions increase to a level that is high enough to allow the maintenance of normal glucose tolerance. With continued insulin resistance, the second phase of insulin secretion becomes even more prominent, and there is approximately 50% more insulin secreted during that phase compared with when the cats were insulin sensitive. Glucose clearance is delayed in those cats; however, baseline glucose is still normal (Figure 2). As insulin resistance continues and progresses, glucose clearance becomes abnormal, even in the fasted basal state, and insulin secretion becomes erratic and much lower than in normal or insulin-resistant cats. These cats are now diabetic (Figure 3). What leads to the deterioration of insulin secretion is clearly not glucose toxicity, because insulin secretion is abnormal long before glucose levels rise in the fasted state and long before glucose clearance becomes abnormal. In addition, one needs to consider that glucose concentrations in cats during their daily routine, including food intake, do not reach the blood glucose levels that are seen with the pharmacological amounts of glucose that are administered in an IVGTT, as described earlier.
Figure 1.
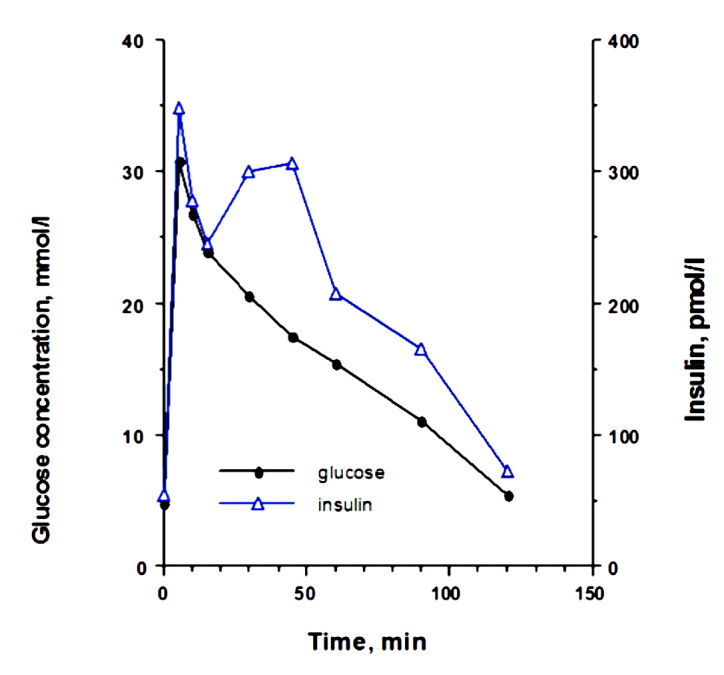
Plasma glucose and insulin concentrations in eight cats after intravenous administration of glucose before partial pancreatectomy and treatment with growth hormone and dexamethasone (mean ± standard deviation). Reprinted with permission from American Journal of Pathology.28
Figure 2.
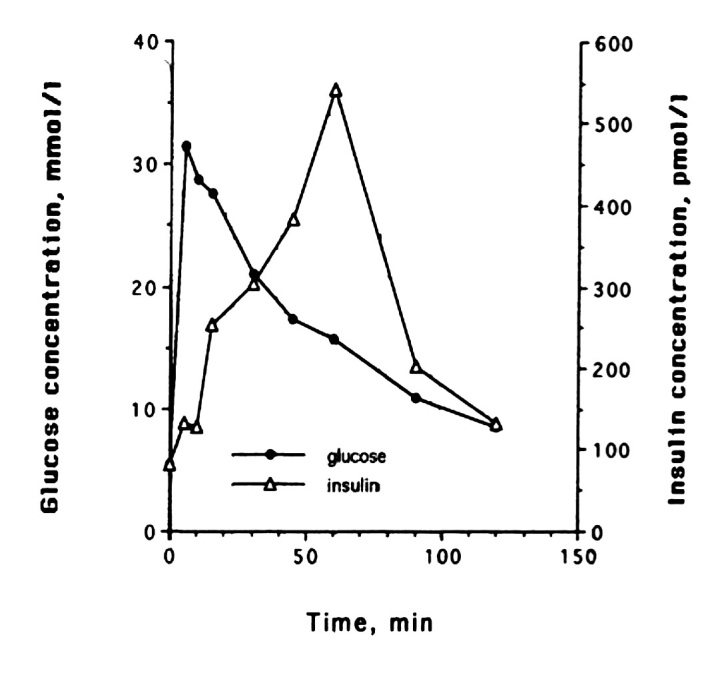
Representative example of changes in glucose and insulin release in one cat during the diabetes induction with growth hormone and dexamethasone. After 2 months, delayed glucose clearance is seen, but baseline glucose concentrations are still normal. Reprinted with permission from American Journal of Pathology.28
Figure 3.
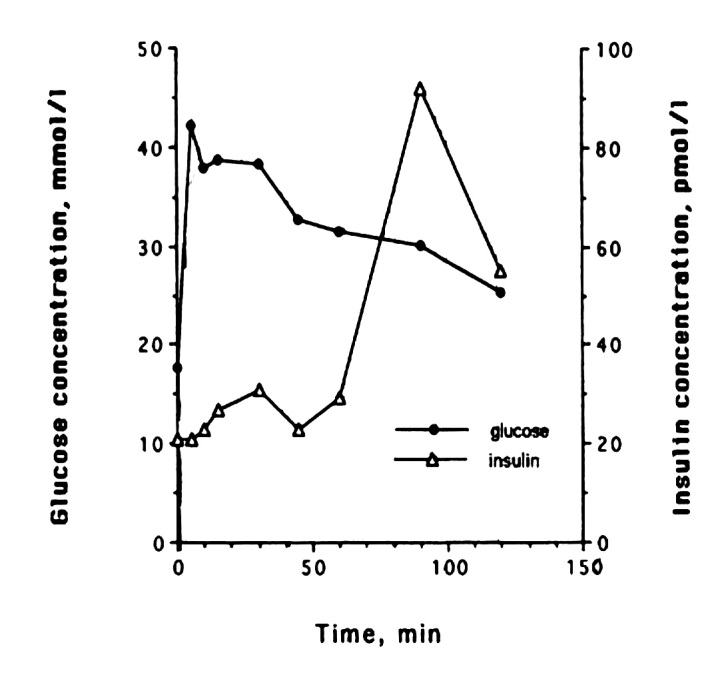
Representative example of changes in glucose and insulin release in one cat during the diabetes induction with growth hormone and dexamethasone. As insulin resistance continues and progresses, glucose clearance becomes abnormal, even in the fasted basal state. Insulin secretion becomes erratic and is much lower than in normal or insulin-resistant cats. These cats are now diabetic. Reprinted with permission from American Journal of Pathology.28
It is known that hypersecretion of insulin leads to hypersecretion of the hormone islet amyloid polypeptide, also called amylin (for review, see Reference 29). Many obese cats are hyperinsulinemic for many years and also show hyperamylinemia.30,31 In one study, we found that long-term obese old cats had fewer, but larger, pancreatic islets, often with pronounced amyloid deposition within the pancreatic islets.32 Fasting glucose concentrations were still normal in these cats. It has previously been shown that cats with impaired glucose tolerance have higher amyloid depositions;33,34 however, no change was seen in the insulin content of β cells in those cats as determined by immunohistochemistry. Therefore, with time, long-term hypersecretion of insulin and amylin resulting from insulin resistance leads to amyloid deposition and β-cell loss, likely through apoptosis. It is not known what amount of amyloid must be present before insulin secretion becomes low and erratic. It is also known that not all cats with diabetes have pancreatic amyloid and that diabetes can be transient despite the presence of amyloid. Nelson and colleagues34 have shown that 3 of 5 cats with transient diabetes had islet amyloid, whereas 2 of 5 age-matched control cats also had islet amyloid, although to a much lesser degree.
One might therefore speculate that progression from the obese to the diabetic state is due to the following: (1) a decrease in insulin secretion either because of islet amyloid leading to islet destruction or some other pathophysiological mechanism leading to β-cell failure, the hypoinsulinemia then would lead to increased EGP production by the liver because the suppressive effect of insulin is no longer present, or (2) EGP increases because of hepatic insulin resistance overwhelming the β-cell secretory machinery and leading to β-cell exhaustion. A combination of both events could also occur simultaneously. The higher blood glucose leads to toxic changes of the β cells, which already have been described over 60 years ago by Dohan and Lukens.35
Evaluating Insulin Sensitivity and Glucose Tolerance
Several studies have reported that obese cats show insulin resistance of peripheral tissues, although only one group of investigators has used the EHC in obese cats, which is considered the gold standard method for assessing insulin resistance in people.12,36,37 The EHC is a method that measures the amount of glucose necessary to compensate for a constant level of hyperinsulinemia without causing hypoglycemia. This experimental procedure was first introduced to feline research by Petrus and coworkers.38 This test does not rely on a feline-specific insulin assay, because it is regular recombinant human insulin, which is infused in high amounts during the test. An insulin-sensitive cat needs more glucose than an insulin-resistant cat to maintain euglycemia. Using this method, it was documented that obese cats had insulin resistance and lower glucose effectiveness, i.e., glucose uptake that is insulin independent.7,37 It had also previously been demonstrated that the development of feline obesity is associated with a decrease in the insulin-dependent glucose transporter expression, glucose transporter-4, in muscle and subcutaneous fat, whereas the insulin-independent glucose transporter, glucose transporter-1, expression is not altered.39
Other tests have been used in cats to evaluate insulin sensitivity. The response to a fixed and small amount of insulin (0.1 U/kg) has been examined.40,41 Unfortunately, this amount of insulin leads to hypoglycemia. It has been cautioned by Brehm and associates42 that hypoglycemia and the subsequent release of insulin-antagonistic hormones will lead to overestimation of insulin resistance.
The frequently sampled IVGTT combined with an insulin injection at 20 min (called modified frequently sampled IVGTT) has been compared with the EHC in cats36,40 and was found to overestimate insulin resistance, and results were highly variable.36 Results from this test are analyzed with minimal model analysis. In people, the analysis is often performed using a computer program, the MinMod Millennium.43 The utility of this program for the accurate analysis of insulin sensitivity in cats has not been critically evaluated. One of the protocols, which has been described in cats,44 may lead to hypoglycemia in non-diabetic cats. Hypoglycemia, however, as stated earlier, needs to be avoided because it has been shown that the counterregulatory reflex associated with hypo-glycemia leads to an incorrect estimation of insulin sensitivity.42,45 The IVGTT without insulin administration has been frequently performed in cats to assess primarily glucose disposal and occasionally to assess insulin sensitivity.40,41,46–49 This is a dynamic test where glucose is injected intravenously as a bolus and blood samples are taken at various intervals usually for 120 min or longer. Depending on the dose of glucose that is administered, glucose and insulin concentrations return to baseline levels between 60 and 90 min (0.5 g/kg body weight) or 90 and 120 min (1 g/kg body weight) in lean cats, whereas insulin concentrations remain higher in obese cats throughout the test, but glucose clearance is usually still normal.41,47 Because it is a dynamic test, it is better suited to examine the insulin secretory capacity of the β cells, especially during the earlier time points, rather than as a measure of insulin sensitivity, and it is difficult to distinguish secretion from action with this testing method unless specific mathematical methods are employed for the analysis.
Other simpler and less work-intensive methods to assess insulin sensitivity are available in human medicine. The first was the homeostatic model assessment (HOMA),50 and a later method is the quantitative insulin sensitivity check.51 Both methods employ fasting glucose and insulin concentrations to calculate insulin sensitivity, and in people, both correlate reasonably with the results of clamping studies. These tests are based on the assumption that higher insulin concentrations are needed to maintain basal glucose concentrations in the normal range in a person with insulin resistance. However, it has been pointed out that fasting insulin concentrations not only indicate insulin sensitivity, but also reflect insulin secretion as well as metabolic clearance of the hormone. Therefore, they do not accurately reflect insulin sensitivity in patients with β-cell dysfunction.52 The HOMA has not been validated for use in cats or other animals, and according to Wallace and associates,53 “such use violates the assumptions of the model.” It has also been shown in the dog that HOMA of insulin resistance is inadequate to reflect changes in insulin sensitivity, and the authors concluded that it was necessary to use other, accurate indices to measure changes in insulin sensitivity.54
Lastly, the hyperglycemic clamp has been used to evaluate insulin sensitivity in cats.55 This method is usually employed to examine the β-cell secretory response to glucose in people (β-cell sensitivity). There, it has also been shown to correlate well with other indices of insulin sensitivity.56 A comparison of this method with the EHC has not been conducted in cats.
It is obvious that much more work is needed to validate many of the tests that are employed in human medicine for use in cats, and any conclusions based on results from tests that have not been validated for cats need to be examined with caution. A confounding problem for assessment of insulin sensitivity is the lack of a specific and sensitive feline insulin assay to measure endogenous insulin concentrations.
A variety of questions arise from our current state of knowledge about assessment of the progression toward diabetes in cats: What do we learn from any of these tests? Can they be used to predict progression from obesity to diabetes in cats? Does peripheral insulin resistance predict the timeline for the development of overt diabetes? Would progression in cats not be indicated by an increase in fasting blood glucose concentrations and/or an increase in postprandial glucose concentrations similar to the diagnostic threshold for type 2 diabetes in people? Why are veterinarians not using fasting glucose concentrations or oral glucose tolerance tests as indicators for progression in cats?
In human medicine, fasting blood glucose concentrations repeatedly higher than 126 mg/dl are diagnostic for diabetes mellitus. In addition, the oral glucose tolerance test is one of the most frequently used tests to evaluate a person's ability to dispose of a glucose load in a timely fashion. Interpretation of this test is based on the assumption that a healthy adult person should be able to metabolize a standard amount of glucose taken orally within a defined period of time. This is a more physiological assessment than, for example, the IVGTT, because glucose is normally presented to the body by ingestion, and the same mechanisms, including the incretin response, are initiated, which are responsible for the maintenance of glucose concentrations after consumption of a meal.57 Diabetes is diagnosed if a person has a blood glucose over 200 mg/dl 2 h after the ingestion of glucose.
Evaluating fasting glucose concentrations in cats is problematic. Many client-owned cats have a high incidence of stress hyperglycemia58 regardless of body condition. This is likely different in colony cats that have been well adjusted to blood sampling. It is known from those research populations that cats do not show an increase in fasting blood glucose concentrations even with long-term obesity or drug-induced insulin resistance22,28 and do not show a change in fasting blood glucose until insulin secretion becomes low and erratic.28 Results from the oral glucose tolerance test have been reported in experimental cats.59 Unfortunately, the response to glucose was variable, and a clear distinction between individual lean and obese cats was not possible. High variability of the results has also been described in dogs.60 This test, therefore, cannot be recommended as a routine test to examine the risk of developing diabetes in individual cats as it is used in people.
A different approach has been recently taken to see if obese cats with documented peripheral insulin resistance based on EHC and IVGTT showed a difference in glucose concentrations when monitored over several days, including the postprandial periods. Measurement of blood glucose concentrations using a laboratory reference method and a handheld glucometer and evaluation of interstitial glucose concentrations using a continuous glucose monitoring system were performed for 7 days during the normal daily routine of six lean and eight long-term obese old cats who had documented peripheral insulin resistance for many years. It was found that there was no difference in glucose concentrations between lean and 7 of 8 long-term obese and insulin-resistant cats over this 7-day recording period. Only 1 of 8 cats could be identified as prediabetic (blood glucose values were approximately 25% higher than in the lean and 7 of 8 obese cats).61 This indicates that tests used to assess peripheral insulin resistance or IVGTTs are of little value in the prediction of daily glucose homeostasis in cats, even in cats that have been severely obese and insulin-resistant for several years.
Do Obese Cats Develop the Metabolic Syndrome?
Metabolic syndrome is the name for a cluster of risk factors for atherosclerosis, coronary artery disease, stroke, and diabetes mellitus.13 Increased weight and insulin resistance are probably the most important risk factors in people.62 They are associated with alterations in several hormones and lipoproteins, including elevated cholesterol, triglycerides, and apolipoprotein B concentrations, as well as higher very-low-density lipoprotein (VLDL), higher low-density lipoprotein (LDL), and lower high-density lipoprotein (HDL) cholesterol levels.62 It has been shown that the number of lipoprotein particles and their size that determine risk for disease.63–65 In people, large VLDL and small LDL and HDL particles are associated with insulin resistance and associated cardiovascular problems. In cats, increased weight and insulin resistance are also associated with lipid changes similar to what has been reported in humans. Obese cats showed an increase in nonesterified fatty acids, VLDL, and plasma triglycerides, primarily originating from VLDL. In fact, the increase in triglycerides in the VLDL fraction was, on average, 500% higher in obese cats than in lean controls. Overproduction of VLDL has been associated with decreased expression of peroxisome proliferator-activated receptor a. Peroxisome proliferator-activated receptor a is involved in adipocyte mitochondrial biogenesis and the upregulation of genes involved in fatty acid oxidation66 and is low in obese cats.67
Despite high VLDL concentrations, obese cats had no change in baseline LDL concentrations, suggesting that VLDL was metabolized rapidly to LDL, and LDL clearance was increased to maintain normal levels. The over-production of VLDL in cats was associated with an increase in the VLDL particle number. The particles were of large and medium size, which, in people, has been associated with cardiovascular disease.63 Especially large triglyceride-enriched VLDL can bind to LDL receptors and lead to the formation of cholesterol-rich foam cells in people;68,69 however, this has not been documented in cats. Large VLDL particles are linked with small LDL and HDL particles, and it has been suggested that the high triglyceride contents of large VLDL is the major predictor of LDL size in type 2 diabetes patients. Increased levels of small, dense LDL have been shown to be strongly associated with coronary artery disease risk in people.70,71 Very small and medium small LDL particles were almost three-fold increased in obese cats, whereas only large particles were detected in lean cats.72 Small HDL particles have also been associated with cardiovascular disease.73 As in obese people, small particle concentrations were significantly higher in obese cats.72 Despite all these changes in lipoprotein concentrations, particle number and size, atherosclerosis, coronary artery disease, and stroke or clinical hypertension have not been described in obese or diabetic cats.
Hormonal Changes Associated with Obesity in Cats
The changes in metabolism that are seen in obese cats may, in part, be caused by alterations of hormones involved in metabolic regulation. Adiponectin7,74 and leptin7,75,76 have been studied in cats in more detail. Adiponectin is a hormone that is secreted by adipocytes. This hormone has beneficial effects on glucose and lipid metabolism.77 It stimulates fatty acid oxidation and suppresses hepatic gluconeogenesis. It inhibits inflammatory responses that, in people, have been associated with insulin resistance and the metabolic syndrome. In obese cats, adiponectin levels are inversely related to the degree of adiposity, and weight loss leads to an increase in adiponectin7 to levels that are not different from those seen in lean cats.
Leptin is also secreted from fat cells. It acts by binding to specific receptors in the hypothalamus, where it alters the expression of several neuropeptides involved in the regulation of neuroendocrine function, energy intake, and expenditure.78 Obese cats are leptin-resistant, indicated by the fact that leptin concentrations are several-fold higher in obese compared with lean cats without causing the appropriate physiological response.7 Fortunately, with weight loss, leptin levels in obese cats normalize, and leptin is therefore a good indicator of fat mass.7,75 We have recently shown that both leptin and insulin are higher in old lean cats compared with young lean cats, despite similar body fat mass, suggesting development of both insulin and leptin resistance with aging. Thyroid hormone resistance has been postulated by the observation of an increase in free79 and, sometimes, total thyroxine22 in obese cats.
Conclusions
Obese cats have many similarities and dissimilarities to obese people. The major dissimilarity is the fact that cats do not develop atherosclerosis and clinical hypertension. The main similarity is insulin resistance. However, cats seem to compensate well for the insulin resistance by lowering their glucose output from the liver and are able to maintain normal glucose concentrations, even postprandial, for many years, despite peripheral insulin resistance. Measurement of insulin resistance alone, therefore, will not allow us to predict progression to diabetes, neither will glucose tolerance testing with pharmacological amounts of glucose. Only an increase in glucose concentrations during their daily routine will indicate progression. Measures of long-term glucose control might, therefore, be better indicators if it can be shown that the progression from the insulin resistant/glucose tolerant state to overtly diabetic state develops slowly but may also not be useful if it develops rapidly. The time course is not known and needs to be investigated in a large-scale prospective study, which ideally should span over many years.
Glossary
Abbreviations
- (BMI)
body mass index
- (DEXA)
dual energy X-ray absorptiometry
- (EGP)
endogenous glucose production
- (EHC)
euglycemic hyperinsulinemic clamp
- (HDL)
high-density lipoprotein
- (HOMA)
homeostatic model assessment
- (IVGTT)
intravenous glucose tolerance test
- (LDL)
low-density lipoprotein
- (VLDL)
very-low-density lipoprotein
References
- 1.Courcier EA, O'Higgins R, Mellor DJ, Yam PS. Prevalence and risk factors for feline obesity in a first opinion practice in Glasgow, Scotland. J Feline Med Surg. 2010;12(10):746–753. doi: 10.1016/j.jfms.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc. 1998;212(11):1725–1731. [PubMed] [Google Scholar]
- 3.Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract. 1997;25(5-6):13–18. [Google Scholar]
- 4.Nelson RW, Himsel CA, Feldman EC, Bottoms GD. Glucose tolerance and insulin response in normal-weight and obese cats. Am J Vet Res. 1990;51(9):1357–1362. [PubMed] [Google Scholar]
- 5.Hawthorne A, Butterwick R. Predicting the body composition of cats: development of a zoometric measurement for estimation of percentage body fat in cats. J Vet Intern Med. 2000;14(3):365. [Google Scholar]
- 6.Wilkins C, Long RC, Jr, Waldron M, Ferguson DC, Hoenig M. Assessment of the influence of fatty acids on indices of insulin sensitivity and myocellular lipid content by use of magnetic resonance spectroscopy in cats. Am J Vet Res. 2004;65(8):1090–1099. doi: 10.2460/ajvr.2004.65.1090. [DOI] [PubMed] [Google Scholar]
- 7.Hoenig M, Thomaseth K, Waldron M, Ferguson DC. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R227–34. doi: 10.1152/ajpregu.00313.2006. [DOI] [PubMed] [Google Scholar]
- 8.Speakman JR, Booles D, Butterwick R. Validation of dual energy X-ray absorptiometry (DXA) by comparison with chemical analysis of dogs and cats. Int J Obes Relat Metab Disord. 2001;25(3):439–447. doi: 10.1038/sj.ijo.0801544. [DOI] [PubMed] [Google Scholar]
- 9.Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52(10):2490–2496. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 10.Russell K, Sabin R, Holt S, Bradley R, Harper EJ. Influence of feeding regimen on body condition in the cat. J Small Anim Pract. 2000;41(1):12–17. doi: 10.1111/j.1748-5827.2000.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 11.Prahl A, Guptill L, Glickman NW, Tetrick M, Glickman LT. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J Feline Med Surg. 2007;9(5):351–358. doi: 10.1016/j.jfms.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCann TM, Simpson KE, Shaw DJ, Butt JA, Gunn-Moore DA. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire-based putative risk factor analysis. J Feline Med Surg. 2007;9(4):289–299. doi: 10.1016/j.jfms.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 14.Schrauwen-Hinderling VB, Hesselink MK, Schrauwen P, Kooi ME. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring) 2006;14(3):357–367. doi: 10.1038/oby.2006.47. [DOI] [PubMed] [Google Scholar]
- 15.Hoenig M, McGoldrick JB, deBeer M, Demacker PN, Ferguson DC. Activity and tissue-specific expression of lipases and tumor-necrosis factor alpha in lean and obese cats. Domest Anim Endocrinol. 2006;30(4):333–344. doi: 10.1016/j.domaniend.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Porat O. The effect of tumor necrosis factor alpha on the activity of lipoprotein lipase in adipose tissue. Lymphokine Res. 1989;8(4):459–469. [PubMed] [Google Scholar]
- 17.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95(5):2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoenig M, Ferguson DC. Effect of darglitazone on glucose clearance and lipid metabolism in obese cats. Am J Vet Res. 2003;64(11):1409–1413. doi: 10.2460/ajvr.2003.64.1409. [DOI] [PubMed] [Google Scholar]
- 19.Jones JG, Solomon MA, Sherry AD, Jeffrey FM, Malloy CR. 13C NMR measurements of human gluconeogenic fluxes after ingestion of [U-13C]propionate, phenylacetate, and acetaminophen. Am J Physiol. 1998;275(5 Pt 1):E843–52. doi: 10.1152/ajpendo.1998.275.5.E843. [DOI] [PubMed] [Google Scholar]
- 20.Jin ES, Burgess SC, Merritt ME, Sherry AD, Malloy CR. Differing mechanisms of hepatic glucose overproduction in triiodothyronine-treated rats vs. Zucker diabetic fatty rats by NMR analysis of plasma glucose. Am J Physiol Endocrinol Metab. 2005;288(4):E654–62. doi: 10.1152/ajpendo.00365.2004. [DOI] [PubMed] [Google Scholar]
- 21.Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J Biol Chem. 2006;281(28):19000–19008. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kley S, Hoenig M, Glushka J, Jin ES, Burgess SC, Waldron M, Jordan ET, Prestegard JH, Ferguson DC, Wu S, Olson DE. The impact of obesity, sex, and diet on hepatic glucose production in cats. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R936–43. doi: 10.1152/ajpregu.90771.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoenig M, Jordan ET, Glushka J, Kley S, Patil A, Waldron M, Prestegard JH, Ferguson DC, Wu S, Olson DE. Effect of macro-nutrients, age, and obesity on 6 and 24-hour post-prandial glucose metabolism in cats. Am J Physiol Regul Integr Comp Physiol. 2011;301(6):R1798–807. doi: 10.1152/ajpregu.00342.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kettelhut IC, Foss MC, Migliorini RH. Glucose homeostasis in a carnivorous animal (cat) and in rats fed a high-protein diet. Am J Physiol. 1980;239(5):R437–44. doi: 10.1152/ajpregu.1980.239.5.R437. [DOI] [PubMed] [Google Scholar]
- 25.Ballard FJ. Glucose utilization in mammalian liver. Comp Biochem Physiol. 1965;14:437–443. doi: 10.1016/0010-406x(65)90218-5. [DOI] [PubMed] [Google Scholar]
- 26.Engelking LR. Textbook of veterinary physiological chemistry. 2nd ed. Burlington: Academic Press; 2011. [Google Scholar]
- 27.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254(5031):573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 28.Hoenig M, Hall G, Ferguson D, Jordan K, Henson M, Johnson K, O'Brien T. A feline model of experimentally induced islet amyloidosis. Am J Pathol. 2000;157(6):2143–2150. doi: 10.1016/S0002-9440(10)64852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89(8):3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 30.Martin LJ, Siliart B, Lutz TA, Biourge V, Nguyen P, Dumon HJ. Postprandial response of plasma insulin, amylin and acylated ghrelin to various test meals in lean and obese cats. Br J Nutr. 2010;103(11):1610–1619. doi: 10.1017/S000711450999359X. [DOI] [PubMed] [Google Scholar]
- 31.Henson MS, Hegstad-Davies RL, Wang Q, Hardy RM, Armstrong PJ, Jordan K, Johnson KH, O'Brien TD. Evaluation of plasma islet amyloid polypeptide and serum glucose and insulin concentrations in nondiabetic cats classified by body condition score and in cats with naturally occurring diabetes mellitus. Am J Vet Res. 2011;72(8):1052–1058. doi: 10.2460/ajvr.72.8.1052. [DOI] [PubMed] [Google Scholar]
- 32.Gal A, Hoenig M, O'Brien TD, Wallig M, Singh K. Histopathology from life-long dietary-induced obese cats and lean controls. Vet Pathol. 2010;47(6S):150. (abstract) [Google Scholar]
- 33.Ma Z, Westermark GT, Johnson KH, O'Brien TD, Westermark P. Quantitative immunohistochemical analysis of islet amyloid polypeptide (IAPP) in normal, impaired glucose tolerant, and diabetic cats. Amyloid. 1998;5(4):255–261. doi: 10.3109/13506129809007298. [DOI] [PubMed] [Google Scholar]
- 34.Nelson RW, Griffey SM, Feldman EC, Ford SL. Transient clinical diabetes mellitus in cats: 10 cases (1989-1991) J Vet Intern Med. 1999;13(1):28–35. [PubMed] [Google Scholar]
- 35.Dohan FC, Lukens FD. Experimental diabetes produced by the administration of glucose. Endocrinology. 1948;42(4):244–262. doi: 10.1210/endo-42-4-244. [DOI] [PubMed] [Google Scholar]
- 36.Hoenig M, Thomaseth K, Brandao J, Waldron M, Ferguson DC. Assessment and mathematical modeling of glucose turnover and insulin sensitivity in lean and obese cats. Domest Anim Endocrinol. 2006;31(4):373–389. doi: 10.1016/j.domaniend.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Hoenig M, Thomaseth K, Waldron M, Ferguson DC. Fatty acid turnover, substrate oxidation, and heat production in lean and obese cats during the euglycemic hyperinsulinemic clamp. Domest Anim Endocrinol. 2007;32(4):329–338. doi: 10.1016/j.domaniend.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Petrus DJ, Jackson MW, Kemnitz JW, Finegood DT, Panciera D. Assessing insulin sensitivity in the cat: evaluation of the hyper-insulinemic euglycemic clamp and the minimal model analysis. Res Vet Sci. 1998;65(2):179–181. doi: 10.1016/s0034-5288(98)90174-6. [DOI] [PubMed] [Google Scholar]
- 39.Brennan CL, Hoenig M, Ferguson DC. GLUT4 but not GLUT1 expression decreases early in the development of feline obesity. Domest Anim Endocrinol. 2004;26(4):291–301. doi: 10.1016/j.domaniend.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Appleton DJ, Rand JS, Priest J, Sunvold GD. Determination of reference values for glucose tolerance, insulin tolerance, and insulin sensitivity tests in clinically normal cats. Am J Vet Res. 2001;62(4):630–636. doi: 10.2460/ajvr.2001.62.630. [DOI] [PubMed] [Google Scholar]
- 41.Backus RC, Cave NJ, Ganjam VK, Turner JB, Biourge VC. Age and body weight effects on glucose and insulin tolerance in colony cats maintained since weaning on high dietary carbohydrate. J Anim Physiol Anim Nutr (Berl) 2010;94(6):e318–28. doi: 10.1111/j.1439-0396.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 42.Brehm A, Thomaseth K, Bernroider E, Nowotny P, Waldhäusl W, Pacini G, Roden M. The role of endocrine counterregulation for estimating insulin sensitivity from intravenous glucose tolerance tests. J Clin Endocrinol Metab. 2006;91(6):2272–2278. doi: 10.1210/jc.2006-0019. [DOI] [PubMed] [Google Scholar]
- 43.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5(6):1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 44.Feldhahn JR, Rand JS, Martin G. Insulin sensitivity in normal and diabetic cats. J Feline Med Surg. 1999;1(2):107–115. doi: 10.1016/S1098-612X(99)90067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cryer PE, Tse TF, Clutter WE, Shah SD. Roles of glucagon and epinephrine in hypoglycemic and nonhypoglycemic glucose counter-regulation in humans. Am J Physiol. 1984;247(2 Pt 1):E198–205. doi: 10.1152/ajpendo.1984.247.2.E198. [DOI] [PubMed] [Google Scholar]
- 46.Biourge V, Nelson RW, Feldman EC, Willits NH, Morris JG, Rogers QR. Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. J Vet Intern Med. 1997;11(2):86–91. doi: 10.1111/j.1939-1676.1997.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 47.Hoenig M, Alexander S, Holson J, Ferguson DC. Influence of glucose dosage on interpretation of intravenous glucose tolerance tests in lean and obese cats. J Vet Intern Med. 2002;16(5):529–532. doi: 10.1892/0891-6640(2002)016<0529:iogdoi>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien TD, Hayden DW, Johnson KH, Stevens JB. High dose intravenous glucose tolerance test and serum insulin and glucagon levels in diabetic and non-diabetic cats: relationships to insular amyloidosis. Vet Pathol. 1985;22(3):250–261. doi: 10.1177/030098588502200308. [DOI] [PubMed] [Google Scholar]
- 49.Nelson RW, Himsel CA, Feldman EC, Bottoms GD. Glucose tolerance and insulin response in normal-weight and obese cats. Am J Vet Res. 1990;51(9):1357–1362. [PubMed] [Google Scholar]
- 50.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 51.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 52.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 53.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 54.Stefanovski D, Ader M, Richey JM, Kim SP, Woolcott O, Harrison LN, Kolka CM, Ionut V, Dittmann J, Yae S, Catalano KJ, Hsu IR, Mooradian V, Chiu JD, Castro AV, Zheng D, Lottati M, Kabir M, Liu H, Bergman RN. 71st Meeting of the American Diabetes Association. San Diego, California: 2011. Failure of homeostatic model assessment of insulin resistance (HOMA-IR) to detect marked diet-induced insulin resistance in dogs. Abstract 1535. [Google Scholar]
- 55.Slingerland LI, Robben JH, van Haeften TW, Kooistra HS, Rijnberk A. Insulin sensitivity and beta-cell function in healthy cats: assessment with the use of the hyperglycemic glucose clamp. Horm Metab Res. 2007;39(5):341–346. doi: 10.1055/s-2007-976541. [DOI] [PubMed] [Google Scholar]
- 56.Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes Care. 1996;19(3):278–286. doi: 10.2337/diacare.19.3.278. [DOI] [PubMed] [Google Scholar]
- 57.Vilsbøll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47(3):357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 58.Opitz M. Stress hyperglycemia in cats. Berl Munch Tierarztl Wochenschr. 1990;103(5):151–158. [PubMed] [Google Scholar]
- 59.Hoenig M, Jordan ET, Ferguson DC, de Vries F. Oral glucose leads to a differential response in glucose, insulin, and GLP-1 in lean versus obese cats. Domest Anim Endocrinol. 2010;38(2):95–102. doi: 10.1016/j.domaniend.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Church DB. A comparison of intravenous and oral glucose tolerance tests in the dog. Res Vet Sci. 1980;29(3):353–359. [PubMed] [Google Scholar]
- 61.Hoenig M, Pach N, Thomaseth K, de Vries F, Ferguson DC. Evaluation of long-term glucose homeostasis in lean and obese cats using continuous glucose monitoring. Am J Vet Res. Forthcoming. [DOI] [PubMed]
- 62.Schindler C. The metabolic syndrome as an endocrine disease: is there an effective pharmacotherapeutic strategy optimally targeting the pathogenesis? Ther Adv Cardiovasc Dis. 2007;1(1):7–26. doi: 10.1177/1753944707082662. [DOI] [PubMed] [Google Scholar]
- 63.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(7):1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 64.Goff DC, Jr, D'Agostino RB, Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. results from the insulin resistance atherosclerosis study. Metabolism. 2005;54(2):264–270. doi: 10.1016/j.metabol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Griffin BA. Lipoprotein atherogenicity: an overview of current mechanisms. Proc Nutr Soc. 1999;58(1):163–169. doi: 10.1079/pns19990022. [DOI] [PubMed] [Google Scholar]
- 66.Li P, Zhu Z, Lu Y, Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-alpha. Am J Physiol Endocrinol Metab. 2005;289(4):E617–26. doi: 10.1152/ajpendo.00010.2005. [DOI] [PubMed] [Google Scholar]
- 67.Hoenig M, Caffall Z, Ferguson DC. Triiodothyronine differentially regulates key metabolic factors in lean and obese cats. Domest Anim Endocrinol. 2008;34(3):229–237. doi: 10.1016/j.domaniend.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 69.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40(1):1–16. [PubMed] [Google Scholar]
- 70.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. prospective results from the quebec cardiovascular study. Circulation. 1997;95(1):69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 71.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106(15):1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 72.Jordan E, Kley S, Le NA, Waldron M, Hoenig M. Dyslipidemia in obese cats. Domest Anim Endocrinol. 2008;35(3):290–299. doi: 10.1016/j.domaniend.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Arsenault BJ, Lemieux I, Després JP, Gagnon P, Wareham NJ, Stroes ES, Kastelein JJ, Khaw KT, Boekholdt SM. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-norfolk prospective population study. Atherosclerosis. 2009;206(1):276–281. doi: 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 74.Ishioka K, Omachi A, Sasaki N, Kimura K, Saito M. Feline adiponectin: molecular structures and plasma concentrations in obese cats. J Vet Med Sci. 2009;71(2):189–194. doi: 10.1292/jvms.71.189. [DOI] [PubMed] [Google Scholar]
- 75.Backus RC, Havel PJ, Gingerich RL, Rogers QR. Relationship between serum leptin immunoreactivity and body fat mass as estimated by use of a novel gas-phase Fourier transform infrared spectroscopy deuterium dilution method in cats. Am J Vet Res. 2000;61(7):796–801. doi: 10.2460/ajvr.2000.61.796. [DOI] [PubMed] [Google Scholar]
- 76.Shibata H, Sasaki N, Honjoh T, Ohishi I, Takiguchi M, Ishioka K, Ahmed M, Soliman M, Kimura K, Saito M. Feline leptin: immunogenic and biological activities of the recombinant protein, and its measurement by ELISA. J Vet Med Sci. 2003;65(11):1207–1211. doi: 10.1292/jvms.65.1207. [DOI] [PubMed] [Google Scholar]
- 77.Ahima RS. Metabolic actions of adipocyte hormones: focus on adiponectin. Obesity (Silver Spring) 2006;14(Suppl 1):9S–15S. doi: 10.1038/oby.2006.276. [DOI] [PubMed] [Google Scholar]
- 78.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 79.Ferguson DC, Caffall Z, Hoenig M. Obesity increases free thyroxine proportionally to nonesterified fatty acid concentrations in adult neutered female cats. J Endocrinol. 2007;194(2):267–273. doi: 10.1677/JOE-07-0064. [DOI] [PubMed] [Google Scholar]


