Abstract
Streptococcus pneumoniae infection is a frequent cause of pneumonia, otitis media, meningitis, and septicemia. Pneumococcal surface protein A (PspA) is an important virulence factor on the pathogen surface, and it is known to interfere with complement activation. In this study, flow cytometry was used to study the effects of PspA and antibodies to PspA on the deposition of complement C3 on the surface of a capsular type 3 strain, WU2, and its PspA− mutant, JY1119. Using naive mouse serum as a complement source, measurable deposition of C3 was observed within 4 min on PspA− pneumococci, and the amount of surface-bound C3 accumulated rapidly as the amount of serum was increased. In contrast, very little C3 was deposited on the PspA+ strain. In nonimmune mouse serum, the classical pathway was the dominant activation pathway triggered by PspA− pneumococci. Accordingly, EGTA blocked almost all of the complement activation. Moreover, a significant amount of C3 was still deposited on the PspA− strain when serum from factor B-deficient mice was used. This deposition was not observed on the PspA+ pneumococci, indicating that PspA may inhibit complement deposition via the classical pathway. Furthermore, under the conditions we tested, PspA also inhibited C3 deposition when the classical pathway was initiated by antibodies to capsular polysaccharide. Antibodies to PspA could overcome the anticomplementary effect of PspA, allowing for increased complement activation and C3 deposition onto PspA+ bacteria.
Streptococcus pneumoniae, a gram-positive coccus, is one of the most common causes of bacterial pneumonia, otitis media, meningitis, and septicemia (22). It is also an occasional cause of the hemolytic uremic syndrome, endocarditis, and peritonitis (27, 33). Pneumococcal infections can occur at any age but are more frequent in infants, the elderly, and immunocompromised patients. Despite the development of effective treatments, the pneumococcus has remained a significant cause of morbidity and mortality worldwide (15).
Complement is one of the most essential components of host immunity in eliminating invasive pneumococci (10). The complement system is composed of more than 30 plasma or membrane proteins, which together play a vital role in host innate and adaptive immunity against bacterial infections (26, 29). To mediate antibacterial activity, complement needs to be activated (26, 29). Three overlapping pathways of activation have been identified: the classical pathway, the alternative pathway, and the mannan (or mannose)-binding lectin (MBL) pathway (14). All three pathways converge on activation of C3 (31), and ultimately on activation of C5, leading to assembly of the terminal membrane attack complex. The complement system is known to play several roles in host defense. Some gram-negative bacteria and viruses can be directly lysed and thus killed by the membrane attack complex. Foreign particles (bacteria, viruses, toxins, etc.) coated with C3 fragments can be taken up by phagocytic cells via complement receptors. During inflammation, neutrophils, macrophages, and mast cells can be stimulated by the anaphylatoxins, the small peptide end products of complement activation.
Pneumococci have evolved several strategies to protect themselves from complement attack. The rigid gram-positive cell wall of S. pneumoniae prevents them from being lysed by the membrane attack complex. The capsular polysaccharide is resistant to complement deposition and is thought to mask cell wall-associated complement from being recognized by the complement receptors on phagocytes (10). Angel et al. showed that cell wall-associated enzyme activity of pneumococci can cause the degradation of native C3 (2). Other surface proteins are also involved in reducing C3 deposition on the bacterial surface. It has been reported that pneumococcal surface protein C (PspC) (6, 9, 23) and the factor H-binding inhibitor of complement (Hic), encoded by the pspC locus (19, 20), bind the complement regulatory component factor H, which inhibits the formation of C3 convertase and accelerates the degradation of C3b. Pneumococcal surface protein A (PspA) has also been shown to interfere with C3 deposition on the pneumococcal surface (1, 30, 35). Pneumolysin is released from pneumococci and acts at a distance to activate C3 and thereby make it unavailable for deposition on the bacterial surface (3, 28).
PspA is a choline-binding protein, tethered to the pneumococcal surface through its proline-rich and choline-binding domains (6). The importance of PspA in virulence has been well established in murine infection models with pneumococcal mutants that no longer express cell-surface PspA (8, 21). Reports from our laboratory and others have shown that PspA leads to reduced complement activation in vivo and in vitro. Tu et al. reported that a PspA− strain, which is cleared from the blood more rapidly than its PspA+ isogenic parent, also consumes more circulating host C3 than the PspA+ strain (35). The difference in virulence of the PspA+ and PspA− strains disappears when C3- or factor B-deficient mice are infected (35). Furthermore, PspA− strains have also been shown to bind more C3 than PspA+ strains in vitro (1, 35). Using a bystander hemolysis complement assay, Neeleman et al. also showed that PspA interferes with complement activation (23). Based on its sequence diversity, PspA has been classified into two major families (16). Recently, we showed that PspAs from both families exhibit the same inhibitory effect on activation and deposition of human complement C3 and the same effect on virulence in mice (30).
In this study, we used a highly reproducible and reliable flow cytometry-based method to detect the effects of PspA and antibodies to PspA on the deposition of C3 onto the pneumococcal surface. In our previous studies of the effects of PspA on human complement deposition, the results were complicated by the fact that virtually all human sera contain antibodies to PspA and other pneumococcal antigens. Here, we used normal mouse serum (NMS) isolated from naive mouse as the complement source because the mice have no natural exposure to S. pneumoniae and their serum should contain no specific antibodies reactive with pneumococci. We extended the previous studies by investigating the influence of the amount of serum and the time of incubation on complement activation. In addition, we investigated the role of PspA on complement activation initiated via different complement pathways. Our results suggest that PspA's inhibitory effect on complement deposition onto pneumococci may be through inhibition of classical pathway activation. Antibodies to PspA overcome the inhibitory action of PspA and increase complement deposition, indicating a mechanism to explain the ability of antibodies to PspA to protect against pneumococcal infections.
MATERIALS AND METHODS
Pneumococcal strains.
Capsular type 3 pneumococcal strain WU2 (PspA+) and its PspA− mutant, JY1119 (1, 35, 36), were used. Bacterial stocks were frozen at −80°C in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) containing 10% glycerol. For flow cytometry, pneumococci from frozen stocks were grown in THY to exponential growth phase and then diluted to an optical density at 600 nm (OD600) of less than 0.1. The diluted bacteria were regrown to an OD600 of 0.45 and then used immediately. To maintain the inactivating insert in its pspA gene, JY1119 was grown in culture medium containing erythromycin (0.3 μg/ml).
Mice.
Female CBA/CAHN-XID/J (CBA/N), BALB/cByJ (BALB/c), and wild-type C57BL/6J mice (WT) (FB+/+) were obtained from Jackson Laboratories (Bar Harbor, Maine). C57BL/6J mutant mice unable to express factor B (FB−/−) were from our own colonies and have been described (35).
Sera and buffers.
Fresh sera from 5 to 10 mice of each type were pooled, and aliquots were made and stored at −80°C until use. Heat-inactivated serum was prepared by heating at 56°C for 30 min. Dulbecco's phosphate-buffered saline PBS without additives (Gibco, Grand Island, N.Y.) was used for each washing step. For detecting complement activation by all complement pathways or by only the classical pathway, gelatin Veronal buffer with calcium (0.15 mM) and magnesium (0.5 mM) (GVB2+; Sigma, St. Louis, Mo.) was used to dilute NMS or FB−/− mouse serum. For blockade of all three complement pathways by chelation of calcium and magnesium ions, gelatin Veronal buffer-EDTA (GVB-EDTA; Sigma) was used to dilute the serum, and extra EDTA was added to maintain an effective concentration of 10 mM. To selectively block the classical and MBL pathways by chelating calcium, gelatin Veronal buffer with magnesium and EGTA (0.1% gelatin, 10 mM barbital, 145 mM NaCl, 0.5 mM MgCl2, 10 mM EGTA) was used. All buffers had a pH of 7.3 to 7.4.
Complement deposition.
A volume of 150 μl of bacterial culture (OD600 = 0.45) was washed twice with PBS and resuspended in the appropriate buffer with or without EDTA or EGTA. Serum at the desired concentrations was added in a total volume of 50 μl, and the mixture was incubated for 30 min, unless otherwise indicated. To study the influence of the amount of serum, pooled CBA/N serum was used at concentrations from 0 to 10%, with an incubation time of 30 min. To study C3 deposition kinetics, 10% NMS of CBA/N mice was used, and the samples were incubated for 15 s, 30 s, 1 min, 2 min, 4 min, 8 min, 15 min, 30 min, or 60 min. To study the influence of PspA on classical pathway activation, bacteria were pretreated with different concentrations of ascites fluid containing monoclonal antibody (MAb) 16.3 to type 3 capsule (4, 35) in the presence of EDTA for 30 min, washed, and then incubated with 10% FB−/− or WT (FB+/+) serum. To study the impact of antibodies to PspA on complement activation, bacteria were pretreated with 10% pooled mouse anti-PspA immune serum in the presence of EDTA, washed, and then incubated with 10% WT (FB+/+) or FB−/− serum. All incubations were performed at 37°C. After incubation, samples were washed twice with 1 ml of ice-cold PBS and the pellet was resuspended in 50 μl of PBS.
Analysis of deposition of C3 on the pneumococcal surface by flow cytometry.
Goat anti-mouse complement C3 immunoglobulin G (IgG) (ICN, Costa Mesa, Calif.) was biotinylated by using a biotin-labeling kit according to the manufacturer's instructions (Boehringer Mannheim GmbH, Mannheim, Germany). After incubation with mouse serum, 50 μl of bacterial suspension was incubated with biotin-labeled goat anti-mouse C3 IgG (1:100 dilution in PBS) at 37°C for 30 min. There were three binding controls. In one sample, bacteria were also incubated with serum but PBS was used in place of the biotin-labeled antibodies. Another control consisted of incubating with PBS in place of serum followed by incubation with the biotin-labeled antibodies. A third control consisted of replacing both the serum and biotin-labeled antibodies with PBS. All three controls gave similar low backgrounds. Then, the bacteria were washed with PBS and incubated with Alexa Fluor 488-conjugated streptavidin (Molecular Probes, Eugene, Oreg.; 10 μg/ml) in PBS for 30 min on ice. The washed bacteria were resuspended in 300 μl of 2% paraformaldehyde. Flow cytometric analysis was conducted using a flow cytometer, FACScan instrument (Becton-Dickinson, Mountain View, Calif.). Forward and side scatter were used to exclude debris and aggregates, and 20,000 gated events were recorded. The mean fluorescence intensity and percentage of fluorescent bacteria (brighter than 10 fluorescence intensity units on the FL1 axis) were calculated for each sample.
Measurement of binding of anticapsule Ab to pneumococci.
A volume of 150 μl of culture (OD600 = 0.45) of WU2 or JY1119 was collected, washed, and then incubated with various amount of anticapsule MAb 16.3 for 30 min at 37°C. The bacteria were washed and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Southern Biotechnology Associates, Inc., Birmingham, Ala.). The binding of MAb on pneumococci was examined by measuring the fluorescence intensity with a flow cytometer.
Mouse immunization with recombinant PspA.
To produce immune sera to PspA, groups of 10 CBA/N or BALB/c mice were immunized with purified recombinant PspA protein, UAB103, which contains the full α-helical domain and partial proline-rich domain of PspA from strain Rx1 (amino acids 1 to 370) (9). Preimmune serum was collected and pooled on day 0. Mice were then immunized subcutaneously with 2 μg of purified protein emulsified with 50 μg of alum (Pierce, Rockford, Ill.) in 0.1 ml of Ringer's saline on days 1, 8, and 22. Postimmune serum was collected and pooled on day 29. Pre- and postimmune sera were stored at −80°C.
RESULTS
Analysis of complement deposition on PspA+ and PspA− pneumococci by flow cytometry.
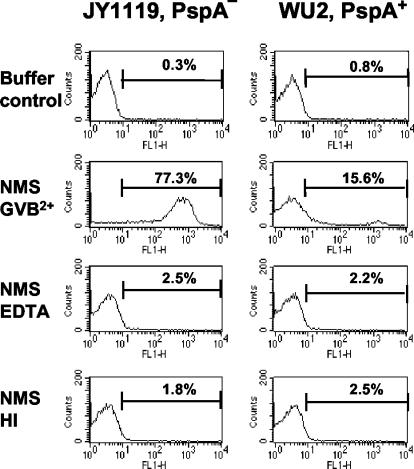
To determine the level of C3 deposition on the pneumococcal surface, PspA+ and PspA− bacteria were incubated with 10% NMS, washed, and incubated with biotin-labeled anti-C3 antibodies and fluorescence-conjugated streptavidin, and then the bacterial surface-bound C3 was detected by flow cytometry. Similar to previous findings obtained using enzyme-linked immunosorbent assay (ELISA) or immunoblotting (1, 35), with 10% NMS from naive CBA/N mice, the observed level of C3 deposition was low on the surface of PspA+ bacteria and high on the surface of PspA− bacteria (Fig. 1). The effect of EDTA on complement activation was evaluated by incubating bacteria in 10% CBA/N NMS for 30 min with and without 10 mM EDTA. Complement C3 deposition was totally abolished by 10 mM EDTA, which chelates both Mg2+ and Ca2+ and thus blocks all three complement activation pathways. Complement deposition was also not observed with heat-inactivated mouse serum. These results show that the deposition of C3 that we observed on the pneumococci requires complement activation; i.e., the native or denatured form of C3 present in normal or heat-inactivated mouse serum does not deposit onto pneumococci.
FIG. 1.
C3 deposition on the surfaces of PspA+ and PspA− strains. WU2 (PspA+) and JY1119 (PspA−) were incubated at 37°C for 30 min with 10% naive CBA/N mouse serum (NMS) diluted with GVB2+, 10% NMS with 10 mM EDTA, or 10% heat-inactivated (HI) serum diluted with GVB2+. The buffer control samples were incubated with GVB2+ instead of 10% serum. The percentage of C3-positive bacteria (intensity greater than 10 on axis of FL1) is indicated for each sample. The data shown are representative of at least three experiments.
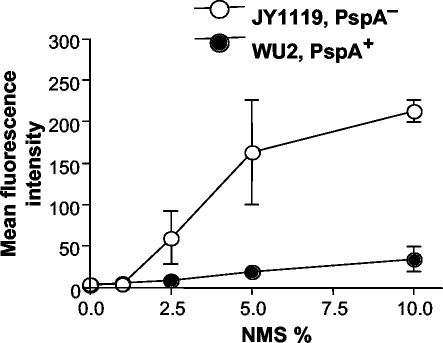
To determine the influence of the amount of serum on complement deposition on the pneumococcal surface, bacteria were incubated with various amounts of NMS for 30 min. We observed that significant C3 deposition occurred on PspA− pneumococci at serum concentrations as low as 2.5% and that the amount of C3 deposited increased significantly with increasing serum concentration (Fig. 2). When 2.5% NMS was incubated with PspA− pneumococci, 4.5% of bacteria were C3 positive, and 76% of PspA− pneumococci became C3 positive when the NMS concentration was increased to 10%. In stark contrast, little C3 was deposited on PspA+ bacteria, and increasing concentrations of serum yielded little increase in the mean intensity of fluorescence or in the C3-positive proportion of bacteria.
FIG. 2.
Influence of serum concentrations on C3 deposition. Different concentrations of NMS (from naive CBA/N mice) diluted with GVB2+ were incubated with PspA+ or PspA− pneumococci. In each case, the bacteria were incubated with serum for 30 min at 37°C. Error bars indicate the standard errors of three experiments.
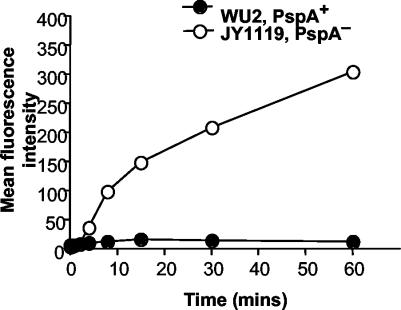
To determine the influence of incubation time on complement deposition on the pneumococcal surface, bacteria were incubated in 10% CBA/N NMS and the reactions were terminated after various times of incubation. C3 deposition onto PspA− pneumococci could be observed after 4 min of incubation and steadily increased over the 60-min incubation period. This increase in complement deposition over time was apparent based on both the mean intensity of fluorescence (Fig. 3) and the C3-positive proportion of PspA− bacteria (data not shown). Very little C3 was deposited on PspA+ pneumococci, and the numbers of C3-bearing PspA+ bacteria did not show a consistent increase over time.
FIG. 3.
Time course of C3 deposition. PpsA+ and PspA− pneumococci were incubated with 10% NMS from CBA/N mice, and the reactions were stopped at various time points using ice-cold PBS buffer containing 10 mM EDTA. The mean fluorescence intensity for a representative experiment (of three) is shown.
Comparison of C3 deposition using normal serum from different mouse strains.
For the studies for which results are shown in Fig. 1 through 3, NMS from CBA/N mice was used. This serum has been shown to lack protective antibodies to phosphocholine (7). Non-CBA/N mouse serum contains natural antibodies reactive with teichoic acids (12, 13). These antibodies, especially those of the IgG isotypes can be protective (4, 5). It was thus important to compare the results obtained with CBA/N serum to those obtained using serum from immunologically “normal” mice. With NMS from BALB/c and C57BL/6J mice, we obtained similar results, i.e., strong C3 deposition on PspA− pneumococci and very little C3 deposition on the PspA+ pneumococci.
Contribution of the classical and alternative pathways to complement activation and C3 deposition in the presence and absence of PspA.
The complement system utilizes two main pathways for activation, the classical pathway and the alternative pathway. The MBL pathway can be considered a modification of the classical pathway. To investigate the effect of PspA on each of these pathways, we used EGTA to block the classical pathway (including the MBL pathway) and used serum from FB−/− mice to eliminate the alternative pathway.
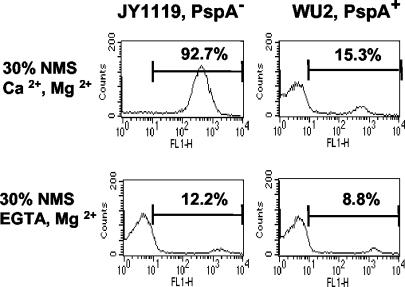
With 10% CBA/N NMS diluted in buffer containing EGTA, negligible C3 deposition was observed on either PspA+ or PspA− strains (data not shown). Even with a higher serum concentration (30%) and a longer time of incubation (60 min), PspA's effect was negligible, as very little complement was deposited on either strain in the presence of EGTA (Fig. 4). In the absence of EGTA, the effect of PspA on C3 deposition was easily detected, as was shown in Fig. 1 through 3. The amount of C3 deposited on the PspA− strain in the presence of EGTA was only one-eighth of that deposited following incubation in the absence of EGTA (Fig. 4, left panels). For the PspA+ strain, the positive proportion of bacteria incubated with NMS-GVB2+ was only twofold greater than that of bacteria incubated with NMS-EGTA (Fig. 4, right panels). These results showed that the classical pathway and/or the MBL pathway is required for the much greater complement deposition that is seen in the absence of PspA.
FIG. 4.
Complement activation and C3 deposition onto pneumococci via the alternative pathway. PspA+ and PspA− strains were incubated with 30% NMS from CBA/N mice in the presence of Ca2+ and Mg2+ or in the presence of EGTA (blocking the activation of the classical/MBL pathway) and Mg2+. The result of a representative experiment of three is shown.
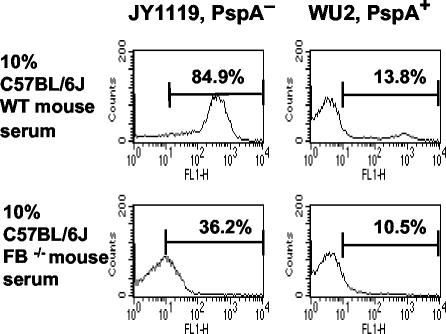
We also examined the effect of PspA on C3 deposition in the absence of the alternative complement pathway. The absence of the alternative pathway in FB−/− serum reduced the intensity of C3 deposition onto bacteria but did not eliminate it (Fig. 5). It is important, however, that even without the alternative pathway, there was still significant deposition of complement on the PspA− bacteria compared with that on PspA+ bacteria (P = 0.02, two-tailed t test). In the presence of 10% FB−/− serum, the deposition of C3 was three times higher on PspA− bacteria than on PspA+ bacteria (Fig. 5). Together with the results shown in Fig. 4, these data suggest that while the alternative pathway is clearly important for amplification of complement activation and deposition on the pneumococcal surface, the classical (or MBL) pathway is absolutely required.
FIG. 5.
Complement activation and C3 deposition in the absence of the alternative pathway. Pneumococci were incubated with 10% serum from C57BL/6J WT or FB−/− mice. The result of a representative experiment of three is shown.
PspA can inhibit C3 deposition via the classical pathway triggered by MAbs to the type 3 capsular polysaccharide.
With the naive FB−/− mouse serum, the level of complement activation initiated by the classical pathway was relatively low because the amount of available natural nonspecific antibodies is limited. To further investigate the effect of PspA on C3 deposition via the classical pathway, we used the IgG3 MAb to the type 3 capsule, 16.3, to initiate the classical pathway. Pneumococci were first incubated with MAb 16.3 at different concentrations in the presence of EDTA and then exposed to FB−/− mouse serum after the nonbound antibodies and EDTA had been washed away. FB−/− mouse serum was used to ensure that all C3 deposition was dependent on the classical or MBL pathway.
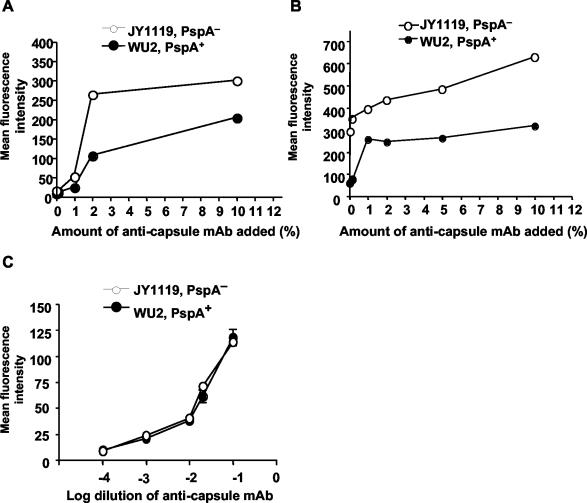
When the antibodies present in ascites fluid at a concentration as low as 1% were added, C3 deposition increased relative to buffer controls on both PspA− and PspA+ pneumococci (Fig. 6A), and the mean intensity of C3 deposition increased as the concentration of antibodies was increased. At each antibody concentration tested, the amount of C3 on the PspA− strain was always greater than that on the PspA+ strain (Fig. 6A). To verify that equivalent amounts of antibody were bound to the surfaces of PspA− and PspA+ strains for a given antibody concentration, the levels of the surface-bound antibody were also measured. Comparable amounts of antibody were detected as being bound to the capsule for these two strains (Fig. 6C). Thus, our results suggest that PspA can act to inhibit the complement deposition triggered via the antibody-dependent classical pathway. When WT (FB+/+) serum was incubated with the bacteria, the difference of C3 deposition between PspA+ and PspA− pneumococci was more obvious than with FB−/− mouse serum (Fig. 6B).
FIG. 6.
Effect of PspA on C3 deposition via the classical pathway triggered by anticapsule MAb. WU2 (PspA+) and JY1119 (PspA−) were pretreated with different amounts of anticapsule MAb, washed, and then incubated with 10% FB−/− (A) or WT (FB+/+) (B) serum diluted with GVB2+. The data are representative of three experiments. (C) The amount of anticapsule MAb bound on PspA+ and PspA− strains. WU2 (PspA+) and JY1119 (PspA−) were incubated with different dilutions of anticapsule MAb, and the surface binding of MAb was detected with Fluorescein isothiocyanate-conjugated goat anti-mouse IgG. Error bars indicate the standard errors of duplicate samples.
Mouse anti-PspA antibodies enhance C3 deposition onto PspA+ pneumococci.
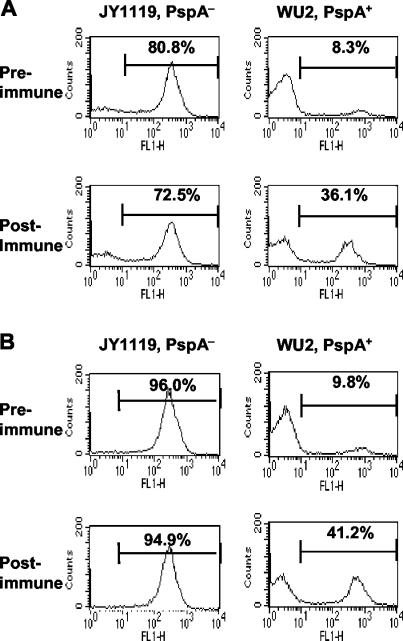
To determine the influence of anti-PspA antibodies on C3 deposition on the pneumococcal surface, CBA/N mice were immunized with purified recombinant PspA. Sera from the mice were collected before and after immunization and pooled separately for use in the study as the source of antibodies and complement. Pneumococci were incubated with 10% pre- or postimmune sera. The same amount of C3 was deposited on the PspA− bacteria regardless of which serum pool was used, as would be expected since these bacteria lacked PspA (Fig. 7A, left panels). However, C3 deposition on the PspA+ strain was significantly greater (P < 0.0001, chi-square test) when the bacteria were incubated with the anti-PspA immune serum as compared with the preimmune serum (Fig. 7A, right panels). To exclude the possibility that the differences in C3 deposition could have been due to variations of complement activity between preimmune and postimmune sera, bacteria were pretreated with 10% pre- or postimmune serum (as the antibody source) in the presence of EDTA, washed, and then incubated with the naive NMS (as a complement source) (Fig. 7B). This study yielded statistically significant results very similar to those described above. These findings were further confirmed by using pre- and postimmune BALB/c mouse sera prepared similarly to the CBA/N sera described above. When incubated with preimmune BALB/c serum, 18.8% of WU2 were C3 positive, and the C3-positive percentage increased to 45.3% when WU2 was incubated with postimmune BALB/c serum. Thus, we have obtained the same general results by doing this experiment with both BALB/c and CBA/N sera and with CBA/N sera by two different methods. Therefore, this finding appears to be highly consistent. Even though these three experiments differed somewhat in actual design, the results were so similar that we analyzed them together using a paired t test, which gave a P value of 0.003.
FIG. 7.
Comparison of C3 deposition on PspA+ and PspA− pneumococci incubated with pre- or postimmune serum against recombinant PspA protein. (A) Pneumococci were incubated with 10% pre- or postimmune serum from CBA/N mice. (B) Pneumococci were pretreated with 10% pre- or postimmune serum from CBA/N mice in the presence of EDTA, washed, and then incubated with 10% pooled naive NMS.
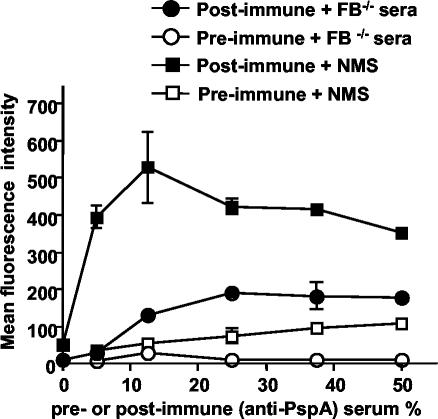
To determine if antibodies to PspA trigger the activation of the classical pathway, we incubated the WU2 strain of S. pneumoniae with FB−/− serum in the presence of anti-PspA antibodies. Different amounts of pre- or postimmune CBA/N mouse serum were incubated with the PspA+ strain in the presence of EDTA (to block complement activation during antibody deposition). The bacteria were then washed and incubated with 10% FB−/− serum. When FB−/− serum was used as the complement source, the anti-PspA antibodies were able to increase the amount of complement deposited as compared with the amount of deposition observed following pretreatment with the preimmune control serum. The maximum level of complement deposition was reached at 25% serum. Higher concentrations of serum provided no further increase in complement deposition (Fig. 8), indicating that the PspA sites on the WU2 strain had probably been saturated.
FIG. 8.
Effect of antibodies to PspA on C3 deposition on PspA+ pneumococci. Strain WU2 was pretreated with different concentrations of pre- (open symbols) or postimmune (filled symbols) serum against recombinant PspA from CBA/N mice (in the presence of EDTA), washed, and then incubated with 10% serum from C57BL/6J FB−/− (circle) or WT (square) mice. Data are presented as mean intensities ± standard errors of the means of duplicate samples in one of three different experiments.
When the WU2 strain was incubated with NMS from WT (FB+/+) C57BL/6J mice, a much higher level of C3 was bound on the bacterial surface than with FB−/− serum for a given concentration of anti-PspA immune serum. For example, using FB+/+ serum as the complement source, the maximum level of C3 deposition (mean intensity = 530) was reached with 12.5% anti-PspA immune serum. This compares with a mean intensity of 200 using 25% anti-PspA immune serum when FB−/− serum was used as the complement source. These data indicate that the effect of antibodies to PspA on complement activation is through the classical pathway and that the alternative pathway serves to amplify this effect.
DISCUSSION
The earlier studies demonstrating that PspA− strains fixed more complement than PspA+ strains were conducted using immunoblot or ELISA (1, 35). More recently, we have found that flow cytometry provides a more appropriate way to analyze the amount of C3 deposited onto the bacterial surface (30). This approach is not only more quantitative, but because it looks at recent complement deposition on intact and live pneumococci, the results may be more relevant than the results from earlier techniques. By judicious gating of forward and side scatter, one can exclude the lysed or aggregated bacteria. Since data are recorded for each bacterium studied, we can make direct comparisons between PspA+ and PspA− strains without having to worry about potential differences in the attachment of different mutants to microtiter plates or the number of bacteria loaded into the sodium dodecyl sulfate-polyacrylamide gels (21, 30, 35). In addition to confirming the earlier results based on ELISA and immunoblotting, our results obtained using flow cytometry have added new information about the influence of PspA and anti-PspA on C3 deposition by the classical and alternative pathways. Our new in vitro results are also consistent with the previous in vivo findings, showing that nonimmune mice cleared the PspA− strain from their blood relatively more rapidly than they cleared the PspA+ strain. Most of the PspA− mutants of the WU2 strain were eliminated from the blood within 4 h, while the PspA+ bacteria of the WU2 strain exhibited continuous growth in vivo until the mice died (30, 35). This difference in bacterial clearance between the PspA+ and PspA− strains was complement dependent (35).
The deposition of C3 on the PspA− strain was totally abolished when EDTA was added to the serum to block all C3 activation or when the serum was heat inactivated, a process that inactivates complement activity. Therefore, the majority of surface-bound C3 that we observed must be the activated forms of C3 (C3b, iC3b, or C3d/g), in which the thioester group interacts with the pneumococcal surface structure (17, 25). Although Smith and Hostetter have reported that the pneumococcal surface protein CbpA can directly bind native C3 (32), we observed so little C3 binding on the PspA+ WU2 strain in the presence of EDTA that any C3 binding effect of CbpA must be small compared with the amount of C3 deposited through complement activation.
Our results regarding the effects of PspA on complement deposition did not appear to be dependent on the use of a particular mouse serum, since similar results were obtained with sera from CBA/N, BALB/c, and C57BL/6J mice. All three sera gave very similar results. Thus, neither the absence of antibodies to phosphocholine in the CBA/N sera nor any complement activity differences that might exist between different mouse strains appeared to be required for PspA to inhibit complement deposition.
Complement plays a central role in innate immune defenses, providing a system for the rapid identification and destruction of a wide range of invading microorganisms. The activation steps of the complement system have been well described (29). An appreciation of the significance of our findings requires an understanding of the events that lead to the activation and deposition of complement. The classical pathway can be triggered by antibodies bound to foreign particle surfaces. This activation is initiated by the binding of the C1 complex to two or more antibody Fc regions in close proximity. In the presence of Ca2+, activated C1s can enzymatically cleave C4 into C4a and C4b. The latter fragment has an exposed thioester group, which binds covalently to the activating surface. Membrane-bound C4b then interacts with C2 in the presence of Mg2+. The classical pathway-derived C4bC2a complex can cleave C3 into C3a and C3b, and like C4b, C3b binds to the activating bacterial surface.
The activation of the recently described MBL pathway is triggered by the binding of the MBL to mannose and/or N-acetylglucosamine residues in the bacterial cell wall. This pathway is dependent on MBL-associated serine proteases (MASPs) (34). The formation of the MBL-MASP complex is Ca2+ dependent and activates C4 in a manner like the C1 complex does, leading to the activation of the rest of the classical pathway.
The alternative pathway does not require antibodies or MBL. Rather, initiation of alternative pathway activation on a surface occurs spontaneously. Native C3 in plasma is hydrolyzed continuously at a low rate to form C3(H2O), which binds factor B in an Mg2+ dependent manner and presents factor B for cleavage by factor D. C3(H2O)Bb is the initiation convertase, which can cleave C3 into C3b and allow the final convertase, C3bBb, to form. However, the C3b generated via the classical/MBL pathway can directly bind factor B and can then form the alternative pathway convertase, C3bBb. The C3bBb in turn activates more C3, which generates more C3b. Thus, the alternative pathway can act as an amplification loop for the complement activation of the classical/MBL pathway.
Under the conditions of our experiments we did not distinguish between the MBL and classical pathways. Although the MBL pathway has been reported to enhance complement activation during opsonization of Staphylococcus aureus (24), no direct evidence has been found that the MBL pathway plays an important role in host defense against pneumococcal infection. In fact, MBL has been reported to have a very low affinity for binding to S. pneumoniae (18). Recently, Brown et al. reported that, compared with the other two pathways, the MBL pathway plays only a minor role in complement activation by S. pneumoniae (11). Moreover, based on results obtained using mice deficient in different complement components, they concluded that the classical pathway was the dominant pathway for innate immunity against pneumococcal infection (11). Thus, the C3 activation that we observed in FB−/− sera is likely to be the result of activation by the classical rather than the MBL pathway. In the remainder of our discussion of complement activation, although we will generally refer only to the classical pathway, it must be remembered that participation by the MBL pathway has not been formally excluded.
By using EGTA, NMS, and serum from FB−/− mice, we were able to distinguish the roles of the alternative and classical pathways of complement activation in the complement deposition on pneumococci that we observed. For the PspA− strain, little C3 deposition was observed with serum in the presence of EGTA (blocks the classical but not the alternative pathway), and this result was not changed even when the serum concentration was increased from 10 to 30%. In contrast, a significant amount of C3 was deposited on the PspA− strain when only 10% FB−/− mouse serum was used. However, when both pathways exist, the deposition of C3 on the PspA− strain was much greater than with either pathway alone. Our results thus indicate that although amplification by the alternative pathway is required for maximal C3 deposition, the classical pathway was required for complement activation and deposition on PspA− pneumococci.
Additional results indicating that PspA inhibits classical pathway-mediated C3 deposition came from studies comparing complement deposition in nonimmune C57BL/6J FB−/− mouse serum. Those studies showed greater complement deposition on PspA− than PspA+ pneumococci in FB−/− serum. Our observations are consistent with the results of others, who demonstrated that in the absence of specific antibodies, the low levels of complement deposition on wild-type bacteria are also dependent on the classical pathway (11). In our study, the naive sera used as complement sources were from mice with no known contact with pneumococci, and some of the sera were from CBA/N mice, which also lack antibodies to phosphocholine in their sera. As a result, if the classical pathway activation we observed is dependent on antibodies, it must be due to nonspecific immunoglobulins or cross-reactive antibodies elicited in response to other environmental antigens. The classical pathway of complement activation might also be initiated by one or more antibody-independent mechanisms (29). Evidence that PspA could inhibit the classical pathway of complement activation mediated by antibodies to pneumococcal surface antigens was obtained by using MAbs to the type 3 capsular polysaccharide to trigger the classical pathway in FB−/− serum.
In the present study, we were not able to detect the effect of PspA on the alternative pathway when the classical pathway was blocked. It is still possible, however, that PspA may interfere with the ability of the alternative pathway to amplify complement activation initiated by the classical pathway. In a previous report, we had concluded that PspA interfered specifically with C3 convertase formation in the alternative pathway because the PspA− strain became as virulent as the PspA+ strain in FB−/− mice (1, 35). From the results in the present study, it now seems likely that the conclusion of our previous report should have been that alternative pathway amplification is required to achieve maximal complement deposition in the absence of PspA.
Mouse antibodies to PspA increased the complement deposition onto the PspA+ strain. There may be two possible protective roles for antibodies to PspA, both ultimately elaborated through increasing the amount of complement activated on the surface of the PspA+ strain. One role would consist of the Fc fragment of bacterial-surface-bound antibodies binding complement C1q, which would then activate complement through the classical pathway. The second role might be the binding of important domains or epitopes of PspA and thus the blocking of its anticomplementary function. PspA is an important virulence factor of pneumococci and is a very promising candidate for a pneumococcal protein-based vaccine. Information regarding the exact mechanisms by which PspA inhibits complement deposition and by which antibodies to PspA increase complement deposition is germane to the use of PspA as a vaccine antigen.
Acknowledgments
We thank John F. Kearney and Moon H. Nahm for their assistance with the flow cytometry analysis, Gunnar Lindahl and Scott Barnum for their review of our work and helpful suggestions, and Susan B. Kniebes for editing of the presubmission manuscript.
This work was supported by NIH grants AI42183 (to A.J.S.) and AI21548 (to D.E.B.) and by the Carsten Cole Buckley Pediatric Meningitis Research Fund (to D.E.B.).
Editor: D. L. Burns
REFERENCES
- 1.Abeyta, M. 1999. Pneumococcal surface protein A and capsular polysaccharide in virulence of Streptococcus pneumoniae. Ph D. thesis. University of Alabama at Birmingham, Birmingham.
- 2.Angel, C. S., M. Ruzek, and M. K. Hostetter. 1994. Degradation of C3 by Streptococcus pneumoniae. J. Infect. Dis. 170:600-608. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briles, D. E., J. L. Claflin, K. Schroer, and C. Forman. 1981. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature 294:88-90. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D. E., C. Forman, J. C. Horowitz, J. E. Volanakis, W. H. Benjamin, Jr., L. S. McDaniel, J. Eldridge, and J. Brooks. 1989. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect. Immun. 57:1457-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles, D. E., S. K. Hollingshead, E. Swiatlo, A. Brooks-Walter, A. Szalai, A. Virolainen, L. S. McDaniel, K. A. Benton, P. White, K. Prellner, A. Hermansson, P. C. Aerts, H. Van Dijk, and M. J. Crain. 1997. PspA and PspC: their potential for use as pneumococcal vaccines. Microb. Drug Resist. 3:401-408. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., J. Horowitz, L. S. McDaniel, W. H. Benjamin, Jr., J. L. Claflin, C. L. Booker, G. Scott, and C. Forman. 1986. Genetic control of susceptibility to pneumococcal infection. Curr. Top. Microbiol. Immunol. 124:103-120. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., J. Yother, and L. S. McDaniel. 1988. Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev. Infect. Dis. 10(Suppl. 2):S372-S374. [DOI] [PubMed] [Google Scholar]
- 9.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5(Suppl. 4):S797-S805. [DOI] [PubMed] [Google Scholar]
- 11.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claflin, J. L. 1976. Uniformity in the clonal repertoire of the immune response to phosphorylcholine in mice. Eur. J. Immunol. 6:669-674. [DOI] [PubMed] [Google Scholar]
- 13.Claflin, J. L., R. Lieberman, and J. M. Davie. 1974. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J. Exp Med. 139:58-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita, T. 2002. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2:346-353. [DOI] [PubMed] [Google Scholar]
- 15.Garau, J. 2002. Treatment of drug-resistant pneumococcal pneumonia. Lancet Infect. Dis. 2:404-415. [DOI] [PubMed] [Google Scholar]
- 16.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hostetter, M. K., R. A. Krueger, and D. J. Schmeling. 1984. The biochemistry of opsonization: central role of the reactive thiolester of the third component of complement. J. Infect. Dis. 150:653-661. [DOI] [PubMed] [Google Scholar]
- 18.Jack, D. L., N. J. Klein, and M. W. Turner. 2001. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol. Rev. 180:86-99. [DOI] [PubMed] [Google Scholar]
- 19.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257-37263. [DOI] [PubMed] [Google Scholar]
- 20.Jarva, H., R. Janulczyk, J. Hellwage, P. F. Zipfel, L. Bjorck, and S. Meri. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J. Immunol. 168:1886-1894. [DOI] [PubMed] [Google Scholar]
- 21.McDaniel, L. S., J. Yother, M. Vijayakumar, L. McGarry, W. R. Guild, and D. E. Briles. 1987. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J. Exp. Med. 165:381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-807. [DOI] [PubMed] [Google Scholar]
- 23.Neeleman, C., S. P. Geelen, P. C. Aerts, M. R. Daha, T. E. Mollnes, J. J. Roord, G. Posthuma, H. van Dijk, and A. Fleer. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67:4517-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neth, O., D. L. Jack, M. Johnson, N. J. Klein, and M. W. Turner. 2002. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J. Immunol. 169:4430-4436. [DOI] [PubMed] [Google Scholar]
- 25.Newman, S. L., and L. K. Mikus. 1985. Deposition of C3b and iC3b onto particulate activators of the human complement system. Quantitation with monoclonal antibodies to human C3. J. Exp. Med. 161:1414-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen, C. H., and R. G. Leslie. 2002. Complement's participation in acquired immunity. J. Leukoc. Biol. 72:249-261. [PubMed] [Google Scholar]
- 27.Novak, R. W., C. R. Martin, and E. N. Orsini. 1983. Hemolytic-uremic syndrome and T-cryptantigen exposure by neuraminidase-producing pneumococci: an emerging problem? Pediatr. Pathol. 1:409-413. [DOI] [PubMed] [Google Scholar]
- 28.Paton, J. C. 1996. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 4:103-106. [DOI] [PubMed] [Google Scholar]
- 29.Prodinger, W. M., R. Wurzner, A. Erdei, and M. P. Dierich. 1999. Complement, p. 967-995. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 30.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahu, A., and J. D. Lambris. 2001. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 180:35-48. [DOI] [PubMed] [Google Scholar]
- 32.Smith, B. L., and M. K. Hostetter. 2000. C3 as substrate for adhesion of Streptococcus pneumoniae. J. Infect. Dis. 182:497-508. [DOI] [PubMed] [Google Scholar]
- 33.Svanbom, M., and T. Strandell. 1978. Bacterial endocarditis. I. A prospective study of etiology, underlying factors and foci of infection. Scand. J. Infect. Dis. 10:193-202. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi, M., Y. Endo, T. Fujita, and M. Matsushita. 1999. A truncated form of mannose-binding lectin-associated serine protease (MASP)-2 expressed by alternative polyadenylation is a component of the lectin complement pathway. Int. Immunol. 11:859-863. [DOI] [PubMed] [Google Scholar]
- 35.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]