Abstract
Objective
We aimed to investigate the basal rate and bolus doses in children and adolescents at the start of insulin pump therapy and after 1 year of use.
Patients and Methods
Case records from 29 children and adolescents were examined. All pumps were started with rapid-acting insulin (Humalog). Patients were aged 13.1 ± 3.9 years, with a diabetes duration of 5.4 ± 4.1 years at pump start. Sixteen pumps were started for high hemoglobin A1c (HbA1c; >8.8%, 73 mmol/mol) and 13 for other reasons.
Results
Basal rates declined in both groups by 20% at 3 days after pump start. The bolus doses were reduced by 25–30% when the indication was high HbA1c and by 15% in the others. After 1 year, there was a significant difference in the basal rate between age groups. The 3–9-year-old age group had higher basal rates during the late evening (10:00 PM–12:00 AM), while the 15–21-year-old age group had higher basal rates in the early morning (3:00 AM–7:00 AM).
Conclusions
Insulin doses are reduced considerably when starting with a pump in pediatric practice. Younger children needed higher basal rates late in the evening (reversed dawn phenomenon), while older teenagers seem to need an increase in the morning, which may correspond to a true dawn phenomenon.
Keywords: basal rate, bolus doses, children, dawn phenomenon, diabetes, insulin pump
Introduction
The use of insulin pumps in the pediatric diabetes population is rapidly increasing1–4 and is approaching 35% in Sweden.5 When starting with an insulin pump, the basal rate and bolus doses are calculated from the current dose on injection therapy. Several randomized studies have shown a decrease in the daily insulin dose when comparing before and after pump start.6
A decrease of the total daily dose (TDD) of 10% when using regular insulin prepump, but no decrease in the case of rapid-acting insulin prepump, has been suggested unless the patient has frequent hypoglycemia before pump start.7 Boland and colleagues8 found a decrease from 1.2 to 1.0 U/kg/day (a decrease of 17%) with >90% of patients using regular insulin in the pump. Ahern and associates4 noted a decrease from 1.3 to 0.9 U/kg/day in adolescents (a decrease of 30%) but no significant change in other age groups when rapid-acting insulin was used.4 In the very young age group (1–5 years), Litton and coworkers9 found no change in total daily insulin dose.9 A randomized study by Doyle and colleagues,10 comparing multiple daily injections (MDI) with glargine insulin and pump (both with aspart insulin), showed that the TDD decreased from 1.4 to 0.9 U/kg/day (a decrease of 36%) in the pump group while it remained unchanged in the glargine group (1.1 versus 1.2 U/kg/day).
“Dawn phenomenon” is defined by Carroll and Schade11 as “hyperglycemia or an increase in the amount of insulin needed to maintain normoglycemia, occurring in the absence of antecedent hypoglycemia or waning insulin levels, during the early morning hours.”
To be clinically relevant, they suggest that the magnitude of the dawn increase in blood glucose level should be more than 10 mg/dl or the increase in insulin requirement should be at least 20% from the overnight nadir. Approximately 54% of patients with type 1 diabetes experience the dawn phenomenon with this definition.11 Most likely, the dawn phenomenon is growth-hormone-mediated impairment of insulin sensitivity in the liver and muscles.12,13
Age-specific patterns for the basal rate have been identified in a large German–Austrian registry study, with both a dawn and a dusk phenomenon, but with a different magnitude and time pattern depending on the age groups.14 However, as the authors point out, recommendations for the basal rate setting at pump start in different age groups were published in 2004,15 and these, in turn, were based on algorithms published earlier for adults using pumps.16 This may have biased the registry results, as the basal rates to a high degree will mirror previously published recommendations. In contrast, an American study found the highest basal rate to be during the whole night in both the 3–10 and the 11–20 years age groups.17 The aim of our study was to investigate retrospectively the basal rate and bolus doses in children and adolescents both when starting with insulin pump therapy and after 1 year of use in a pump-naïve population.
Patients and Methods
To accomplish this, case records from 29 consecutive children and adolescents that had started pump therapy during four years (1997–2001) at our department were examined. All pumps were started with rapid-acting insulin (Humalog). All patients were on MDI, and previous basal insulins were NPH (12), Ultralente (16), and glargine (1). Previous bolus insulins were (using insulin pens) lispro (26), aspart (2), and regular (1). The TDD on MDI was used for calculations of the pump doses. The TDD was decreased around 20%, more in those with high hemoglobin A1c (HbA1c) as indication and less for the other indications. When adjusting pump basal rates, the overnight dose was adjusted to the morning plasma glucose (PG) readings, with the help of 3:00 AM PG. During the day we did not use fasting, instead the basal rate was adjusted with the help of preprandial PG. The basal rate was divided into five blocks: 12:00 AM–3:00 AM, 3:00 AM–7:00 AM, 7:00 AM–12:00 PM, 12:00 PM–6:00 PM, and 6:00 PM–12:00 AM. When starting, nighttime basal was slightly lower than daytime. The dose 12:00 AM–3:00 AM was 0.1–0.2 U/h higher or lower than 3:00 AM–7:00 AM, depending on individual tendency for nighttime hypo-glycemia. The bolus doses were adjusted with the help of the difference between preprandial and 2 h postprandial PG. Hemoglobin A1c was measured with DCA 2000 (Ames, Elkhart, IN), calibrated according to the national Swedish standard, which is approximately 1% below the Diabetes Control and Complications Trial.18 In this study, the HbA1c results were recalculated to Diabetes Control and Complications Trial numbers (%) and the new International Federation of Clinical Chemistry units (mmol/mol). Severe hypoglycemia was defined as help from other person and ketoacidosis as pH < 7.30. Unless otherwise stated, Student's two-sided t-test was used for statistical comparisons.
Results
The patients were aged 13.1 ± 3.9 years (± standard deviation, range 3–21), with a diabetes duration of 5.4 ± 4.1 years (0.8–15) at pump start. Their insulin requirements on MDI were 1.1 ± 0.3 U/kg/day (0.6–1.7) with 52±11% as basal insulin. At pump start, 56% of the TDD was given as basal rate in the 3–9-year-olds, 54% in the 10–15-year-olds, and 57% in the 15–21-year-olds. Compared with MDI, the bolus doses were decreased by 22.4% at start and the basal dose by 8.7%. Figure 1 shows this data divided by indication for starting with the pump. In the group with high HbA1c, the prescribed dose was often not the taken dose, due to often forgetting primarily bolus doses. Therefore, the bolus doses of this group was lowered more when starting with the pump, and even more during the following days. The total insulin dose/24 h after 3 days was 78% of the prepump dose in the age group 3–9 years, 78% in the age group 10–15 years, and 76% in the age group 16–21 years.
Figure 1.
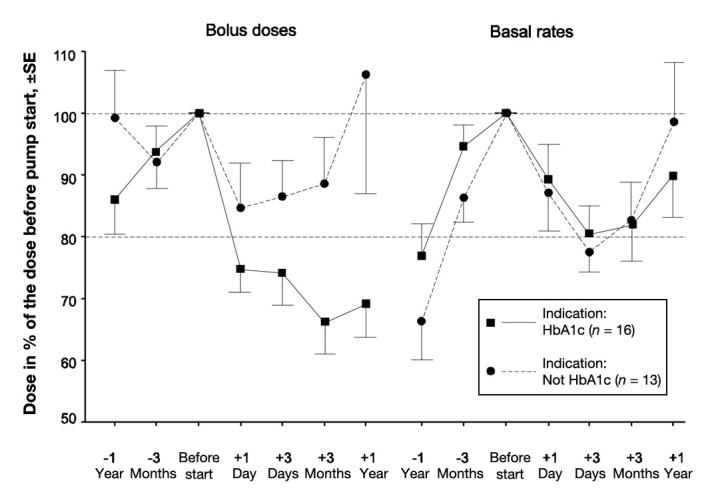
Change in insulin doses in relation to indication for starting with the pump. The dose with MDI before pump start is defined as 100%. The HbA1c indication was defined as HbA1c > 8.8% at pump start. SE, standard error.
The patients were divided into two groups regarding the indication for pump: high HbA1c at the time of pump start (HbA1c > 8.8%, 73 mmol/mol, n = 16, age 13.9 ± 2.6 years, duration 6.3 ± 4.2 years), and “other reasons” [n = 13, (age 12.2 ± 2.6 years, duration 4.2 ± 3.7 years); 6 for life quality reasons, 4 because of painful injections, 2 due to fluctuating glucose, and 1 because of dawn phenomenon]. Age and diabetes duration was not significantly different between the two groups. Basal rates declined similarly in both groups by approximately 20% at 3 days after pump start (Figure 1). The bolus doses were reduced by 25–30% when the indication was high HbA1c and by approximately 15% in the other cases.
After 1 year with the pump, HbA1c was significantly lower than before starting with the pump for those in the high HbA1c group (8.0% versus 9.2%, 64 versus 77 mmol/mol, p < .001, t-test), while there was no difference in the other group (7.2% versus 7.2%, 55 versus 55 mmol/mol). The insulin requirements decreased in both groups (Table 1). After 1 year, there was a significant difference between the age groups in the percentage of basal rate in the late evening and during the night (Figure 2). The younger 3–9 year-old age group had higher basal rates late (10:00 PM–12:00 AM), while the oldest age group had a slight increase in basal rates in the early morning (3:00 AM–7:00 AM). When comparing basal rates between the group with HbA1c indication and the group with other indications, the HbA1c group had a higher basal rate in the afternoon (12:00 PM–6:00 PM, 112% versus 100%, p = .017) and lower in the early morning (3:00 AM–7:00 AM, 81% versus 99%, p = .013).
Table 1.
Differences in Total Insulin Doses (Basal and Bolus) in Units/Kilograms/Day for the Two Groups of Different Indications for Pump Start before and after Pump Therapy
| Group | Prepump | After 1 year | Difference | p |
|---|---|---|---|---|
| High HbA1c | 1.2 | 0.8 | 0.4 | <0.001 |
| Other indications | 1.0 | 0.8 | 0.2 | 0.011 |
Figure 2.
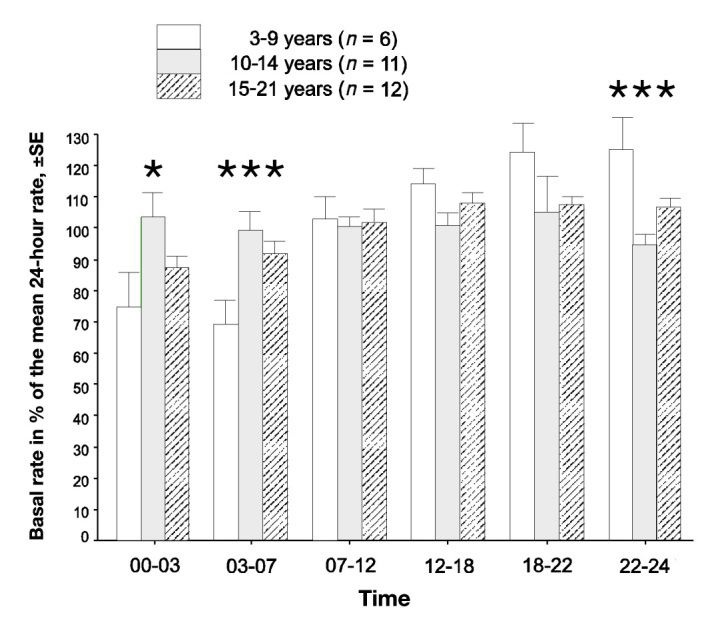
Basal rates in the different age groups in relation to the mean hourly basal rate 1 year after pump start. The mean total basal rate per 24 h divided by 24 is defined as 100%. There was a significant difference in the distribution of the basal rate between the age groups during the night and the late evening (one-way analysis of variance). The asterisk represents p < .05, and three asterisks represent p < .001). SE, standard error.
The total number of episodes with severe hypoglycemia was nine during the year before pump (one patient with three and six patients with one episode), while it was four during the year after pump (four patients with one episode). There were no episodes of ketoacidosis during the year before pump start, while three patients had one episode of ketoacidosis during the year after pump start.
Discussion
This retrospective study describes a case study of two groups (high HbA1c and other indications for starting the pump) using insulin pumps. We found that both the basal insulin rate and the bolus doses were decreased considerably in both groups. When divided into age groups, younger children needed higher basal rates late in the evening and early in the night (reversed dawn phenomenon) while older teenagers needed a higher dose in the late night, corresponding to a true dawn phenomenon. For the younger children, we divided the 6:00 PM–12:00 AM basal block into 6:00 PM–10:00 PM and 10:00 PM–12:00 AM blocks to accomplish a higher basal rate before midnight.
To our knowledge, this study is the first to show both the basal insulin dose and the bolus dose 1 year before treatment start with continuous subcutaneous insulin infusion during the pump start and then continuously following the insulin treatment over time until the end point after 1 year. We found a decrease in TDD in all age groups, in contrast to Conrad and colleagues19 who found this only in the adolescent group. The decrease in TDD in our study was mainly due to lower bolus doses.
Conrad and colleagues19 also found the highest basal rate to be in the hours before midnight. As in our study, DiMeglio and associates20 and Shashaj and Sulli21 both found the lowest basal rates during the night and early morning in the youngest patient group, in contrast both to the German–Austrian registry study14 and Scheiner and Boyer.17 Our results with an increase in the basal rate in the evening for the young age group are similar to those of a large European pediatric study that downloaded the pump memory pump from 377 children and adolescents.22 However, the results from the European study in the adolescent group is more in line with the German-Austrian registry study. Scheiner and Boyer17 found a true dawn phenomenon only in the 13–20-year-old group, which is similar to our results in the 15–21-year-old group.
All patients were followed by the same doctor (Ragnar Hanas). Notably, there are some limitations with this study regarding the relatively few children included. However, despite the small number of included children, our results were significant. The study is retrospective, with data taken from patient records, which may have affected its reproducibility in a prospective study. The higher decrease in patients with high HbA1c may be partly due to missed insulin boluses while on MDI therapy. Since the insulin pens did not have a memory for recording given doses, this was impossible to address within the study. The higher basal rate in the afternoon for the high HbA1c group may also be caused by an attempt to correct for missed lunch doses at school. A higher risk for ketoacidosis after pump start has previously been recorded in Sweden during the same time period.23 Seventy-seven percent of the episodes in that study occurred during the first year in this 5-year follow-up, indicating that it is, in part, a beginner's problem.
The reason for the reversed dawn phenomenon in the younger age group is unclear. An emptying of the gastric contents after falling asleep could be one explanation. Gastric emptying is slow during sleep, but rapid eye movement (REM) sleep is associated with faster gastric emptying.24 Children who take “afternoon naps” reach REM sleep faster after sleep onset than preadolescents who have discontinued their afternoon naps. In pre-adolescents and adolescents, the REM sleep is dominantly in the last two-thirds of the night. The REM sleep period is longer than in adults.25
Growth hormone secretion increases after onset of sleep in children. However, the insulin resistance effect of this hormone usually has a lag time of 3–5 h,26 which makes it unlikely that growth hormone is responsible for the increased insulin requirements before midnight. With insufficient insulin levels in the early morning, insulin-like growth factor binding protein-1 was increased in one study,27 leading to a decrease in insulin-like growth factor, which may cause insulin resistance and a true dawn phenomenon.
Low doses of melatonin improve gastrointestinal transit.28 Melatonin is produced in cells of the gastrointestinal mucosa and seems to be related to periodicity of food intake.28 Melatonin levels seem to be higher after midnight29 and peak at 4:00 AM.30 However, if already low concentrations affect gastric emptying, this could be a part of the explanation for a reversed dawn phenomenon, as the rise in melatonin comes after 8:00 PM according to one study of children aged 6–11 years.30
In conclusion, we found that the basal insulin dose when starting with an insulin pump in children and adolescents can be reduced by approximately 20%. The bolus doses were reduced by 25–30% when the indication was high HbA1c and by approximately 15% in the other cases. The younger children needed higher basal rates late in the evening and early in the night (reversed dawn phenomenon), while older teenagers seem to need an increase in the early morning, corresponding to a true dawn phenomenon.
Acknowledgments
We highly appreciate the help of our diabetes nurses Catarina Andreasson and Lena Windell.
Glossary
Abbreviations
- (HbA1c)
hemoglobin A1c
- (MDI)
multiple daily injections
- (PG)
plasma glucose
- (REM)
rapid eye movement
- (TDD)
total daily dose
Funding
Unrestricted financial support was obtained from Fyrbodal Research Foundation.
Disclosures
Ragnar Hanas has received lecture honoraria from Medtronic, Infucare (Swedish distributor of the Cozmo pump), and Roche.
References
- 1.Maniatis AK, Klingensmith GJ, Slover RH, Mowry CJ, Chase HP. Continuous subcutaneous insulin infusion therapy for children and adolescents: an option for routine diabetes care. Pediatrics. 2001;107(2):351–356. doi: 10.1542/peds.107.2.351. [DOI] [PubMed] [Google Scholar]
- 2.McMahon SK, Airey FL, Marangou DA, McElwee KJ, Carne CL, Clarey AJ, Davis EA, Jones TW. Insulin pump therapy in children and adolescents: improvements in key parameters of diabetes management including quality of life. Diabet Med. 2005;22(1):92–96. doi: 10.1111/j.1464-5491.2004.01359.x. [DOI] [PubMed] [Google Scholar]
- 3.Sulli N, Shashaj B. Long-term benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes: a 4-year follow-up. Diabet Med. 2006;23(8):900–906. doi: 10.1111/j.1464-5491.2006.01935.x. [DOI] [PubMed] [Google Scholar]
- 4.Ahern JA, Boland EA, Doane R, Ahern JJ, Rose P, Vincent M, Tamborlane WV. Insulin pump therapy in pediatrics: a therapeutic alternative to safely lower HbA1c levels across all age groups. Pediatr Diabetes. 2002;3(1):10–15. doi: 10.1034/j.1399-5448.2002.30103.x. [DOI] [PubMed] [Google Scholar]
- 5.Samuelsson U. Data from the Swedish National Pediatric Diabetes Register (SWEDIABKIDS) 2010. Diabetolognytt. 2011;24(Suppl):1–77. [Google Scholar]
- 6.Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes. 2009;10(1):52–58. doi: 10.1111/j.1399-5448.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 7.Danne T, von Schutz W, Lange K, Nestoris C, Datz N, Kordonouri O. Current practice of insulin pump therapy in children and adolescents - the Hannover recipe. Pediatr Diabetes. 2006;7(Suppl 4):25–31. doi: 10.1111/j.1399-543X.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 8.Boland EA, Grey M, Oesterle A, Fredrickson L, Tamborlane WV. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes. Diabetes Care. 1999;22(11):1779–1784. doi: 10.2337/diacare.22.11.1779. [DOI] [PubMed] [Google Scholar]
- 9.Litton J, Rice A, Friedman N, Oden J, Lee MM, Freemark M. Insulin pump therapy in toddlers and preschool children with type 1 diabetes mellitus. J Pediatr. 2002;141(4):490–495. doi: 10.1067/mpd.2002.127500. [DOI] [PubMed] [Google Scholar]
- 10.Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27(7):1554–1558. doi: 10.2337/diacare.27.7.1554. [DOI] [PubMed] [Google Scholar]
- 11.Carroll MF, Schade DS. The dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract. 2005;11(1):55–64. doi: 10.4158/EP.11.1.55. [DOI] [PubMed] [Google Scholar]
- 12.Edge JA, Matthews DR, Dunger DB. The dawn phenomenon is related to overnight growth hormone release in adolescent diabetics. Clin Endocrinol (Oxf) 1990;33(6):729–737. doi: 10.1111/j.1365-2265.1990.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 13.Perriello G, De Feo P, Torlone E, Fanelli C, Santeusanio F, Brunetti P, Bolli GB. Nocturnal spikes of growth hormone secretion cause the dawn phenomenon in type 1 (insulin-dependent) diabetes mellitus by decreasing hepatic (and extrahepatic) sensitivity to insulin in the absence of insulin waning. Diabetologia. 1990;33(1):52–59. doi: 10.1007/BF00586461. [DOI] [PubMed] [Google Scholar]
- 14.Bachran R, Beyer P, Klinkert C, Heidtmann B, Rosenbauer J, Holl RW, German/Austrian DPV Initiative. German Pediatric CSII Working Group. BMBF Competence Network Diabetes Basal rates and circadian profiles in continuous subcutaneous insulin infusion (CSII) differ for preschool children, prepubertal children, adolescents and young adults. Pediatr Diabetes. 2012;13(1):1–5. doi: 10.1111/j.1399-5448.2011.00777.x. [DOI] [PubMed] [Google Scholar]
- 15.Klinkert C, Bachran R, Heidtmann B, Grabert M, Holl RW, DPV-Initiative Age-specific characteristics of the basal insulin-rate for pediatric patients on CSII. Exp Clin Endocrinol Diabetes. 2008;116(2):118–122. doi: 10.1055/s-2007-990296. [DOI] [PubMed] [Google Scholar]
- 16.Wizemann E, Renner R, Hepp K. Prospective evaluation of a standardized basal rate distribution for CSII in type 1 diabetes over 6 months. Diabetologie Stoffwechsel. 2001;10:57. [Google Scholar]
- 17.Scheiner G, Boyer BA. Characteristics of basal insulin requirements by age and gender in Type-1 diabetes patients using insulin pump therapy. Diabetes Res Clin Pract. 2005;69(1):14–21. doi: 10.1016/j.diabres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, Hoshino T, John WG, Kobold U, Little R, Mosca A, Mauri P, Paroni R, Susanto F, Takei I, Thienpont L, Umemoto M, Wiedmeyer HM, IFCC Working Group on HbA1c Standardization IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50(1):166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 19.Conrad SC, McGrath MT, Gitelman SE. Transition from multiple daily injections to continuous subcutaneous insulin infusion in type 1 diabetes mellitus. J Pediatr. 2002;140(2):235–240. doi: 10.1067/mpd.2002.120509. [DOI] [PubMed] [Google Scholar]
- 20.DiMeglio LA, Boyd SR, Pottorff TM, Cleveland JL, Fineberg N, Eugster EA. Preschoolers are not miniature adolescents: a comparison of insulin pump doses in two groups of children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17(6):865–870. doi: 10.1515/jpem.2004.17.6.865. [DOI] [PubMed] [Google Scholar]
- 21.Shashaj B, Sulli N. Difference in insulin usage patterns with pubertal development in children with type 1 diabetes during transition from multiple daily injections to continuous subcutaneous insulin infusion (CSII) and through the CSII treatment. Diabetes Technol Ther. 2009;11(12):767–774. doi: 10.1089/dia.2009.0049. [DOI] [PubMed] [Google Scholar]
- 22.Danne T, Battelino T, Kordonouri O, Hanas R, Klinkert C, Ludvigsson J, Barrio R, Aebi C, Gschwend S, Mullis PE, Schumacher U, Zumsteg U, Morandi A, Rabbone I, Cherubini V, Toni S, de Beaufort C, Hindmarsh P, Sumner A, van Waarde WM, van den Berg N, Phillip M. A cross-sectional international survey of continuous subcutaneous insulin infusion in 377 children and adolescents with type 1 diabetes mellitus from 10 countries. Pediatr Diabetes. 2005;6(4):193–198. doi: 10.1111/j.1399-543X.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanas R, Lindgren F, Lindblad B. A 2-yr national population study of pediatric ketoacidosis in Sweden: predisposing conditions and insulin pump use. Pediatr Diabetes. 2009;10(1):33–37. doi: 10.1111/j.1399-5448.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 24.Dantas RO, Aben-Athar CG. [Aspects of sleep effects on the digestive tract] Arq Gastroenterol. 2002;39(1):55–59. doi: 10.1590/s0004-28032002000100010. [DOI] [PubMed] [Google Scholar]
- 25.Ross JJ, Agnew HW, Jr, Williams RL, Webb WB. Sleep patterns in pre-adolescent children: an EEG-EOG study. Pediatrics. 1968;42(2):324–335. [PubMed] [Google Scholar]
- 26.Bolli GB, Perriello G, Fanelli CG, De Feo P. Nocturnal blood glucose control in type I diabetes mellitus. Diabetes Care. 1993;16(Suppl 3):71–89. doi: 10.2337/diacare.16.3.71. [DOI] [PubMed] [Google Scholar]
- 27.Yagasaki H, Kobayashi K, Saitou T, Nagamine K, Mitsui Y, Mochizuki M, Kobayashi K, Cho H, Ohyama K, Amemiya S, Nakazawa S. Nocturnal blood glucose and IGFBP-1 changes in type 1 diabetes: Differences in the dawn phenomenon between insulin regimens. Exp Clin Endocrinol Diabetes. 2010;118(3):195–199. doi: 10.1055/s-0029-1239518. [DOI] [PubMed] [Google Scholar]
- 28.Thor PJ, Krolczyk G, Gil K, Zurowski D, Nowak L. Melatonin and serotonin effects on gastrointestinal motility. J Physiol Pharmacol. 2007;58(Suppl 6):97–103. [PubMed] [Google Scholar]
- 29.Ardura J, Gutierrez R, Andres J, Agapito T. Emergence and evolution of the circadian rhythm of melatonin in children. Horm Res. 2003;59(2):66–72. doi: 10.1159/000068571. [DOI] [PubMed] [Google Scholar]
- 30.Ardura-Fernandez J, Andres De Llano JM, Garmendia-Leiza JR, Agapito T. Melatonin rhythm in children with enuresis. BJU Int. 2007;99(2):413–415. doi: 10.1111/j.1464-410X.2006.06531.x. [DOI] [PubMed] [Google Scholar]


