Abstract
Fonsecaea pedrosoi is a fungal pathogen that produces melanin. The functions of melanin and its possible influence in the protective immunological response during infection by F. pedrosoi are not known. In this work, treatment of F. pedrosoi mycelia with proteases and glycosidases followed by a denaturing agent and hot concentrated acid left a black residue. Scanning electron microscopy demonstrated that this processed melanized residue resembled very closely the intact mycelium in shape and size. Melanin particles were also isolated from culture fluids of conidia or sclerotic forms of F. pedrosoi. Secreted melanins were reactive with sera from infected human patients, suggesting that F. pedrosoi synthesizes melanin in vivo. The antibodies against melanin were purified from patients’ sera and analyzed by indirect immunofluorescence. They reacted with sclerotic cells from patients’ lesions as well as with sclerotic bodies cultivated in vitro, conidia, mycelia, and digested residues. Treatment of F. pedrosoi with purified antibodies against melanin inhibited fungal growth in vitro. The interaction of F. pedrosoi with phagocytes in the presence of melanin resulted in higher levels of fungal internalization and destruction by host cells, which was accompanied by greater degrees of oxidative burst. Taken together, these results indicate that melanin from F. pedrosoi is an immunologically active fungal structure that activates humoral and cellular responses that could help the control of chromoblastomycosis by host defenses.
Fonsecaea pedrosoi is the major causative agent of chromoblastomycosis, a subcutaneous fungal disease occurring most frequently in tropical and subtropical areas (13, 32). Infection by F. pedrosoi begins with the traumatic implantation of conidia or fragments of hyphae on subcutaneous tissues, producing initial lesions consisting of papules or nodules that become verrucous (6). Inside the host, conidial cells differentiate into mycelial forms, which finally produce spherical, brownish yellow cells with thick, deeply pigmented walls, known as sclerotic cells (17, 33).
Melanins are negatively charged hydrophobic pigments of high molecular weight that are formed by the oxidative polymerization of phenolic and/or indolic compounds (20). The ability of pathogenic microorganisms to produce melanin has been linked with virulence in several models (12). In the fungal pathogen Cryptococcus neoformans, melanin appears to function in virulence by protecting fungal cells against microbicidal oxidants (37) and by impairing the development of cell-mediated responses (14). Melanin can also interfere with complement activation (28) and reduce the susceptibility of pigmented cells to antifungal agents (36). Melanin production has also been linked with virulence in the fungal pathogen Wangiella dermatitidis (31) and members of the genus Aspergillus (20). The fungal pathogens Histoplasma capsulatum (19), Paracoccidioides brasiliensis (11), and Sporothrix schenckii (25) have also been demonstrated to produce melanin or melanin-like compounds in vitro and in vivo, but their effective roles in fungal infections are still unknown.
Melanization of C. neoformans results in the deposition of the polymer in the cell wall (18, 26). Treatment of melanized cells with enzymes, detergents, and hot acid results in the recovery of melanin “ghosts” that retain the size and shape of the original fungal cells (26). Melanin ghost-like particles have also been detected in P. brasiliensis (11), S. schenckii (25), and H. capsulatum (19).
The ability of F. pedrosoi to produce secreted or cell wall-associated melanin-like components has been widely reported (1, 9, 10, 13). Pigmentation protects F. pedrosoi against destruction by host immune cells in vitro (9). In addition, ingestion of F. pedrosoi conidia by mouse macrophages results in the release of melanin granules into the cytosol of animal cells (9). We demonstrate here that F. pedrosoi, besides producing soluble extracellular melanin-like pigments, also forms melanin ghosts. Sera from individuals with chromoblastomycosis reacted with F. pedrosoi melanin, indicating that anti-melanin antibodies are produced during human infections. Melanin-binding antibodies, which were isolated from patients’ sera, recognized melanin ghosts and also conidia, mycelia, and sclerotic cells from in vitro and in vivo sources, confirming that F. pedrosoi becomes melanized during infection. In addition, antibodies against melanin inhibited the in vitro growth of conidial and sclerotic cells. The effect of soluble melanin on the phagocytosis of F. pedrosoi by human neutrophils was also investigated. Our data indicate that soluble melanin enhance the antifungal efficacy of human neutrophils by enhancing phagocytosis and oxidative burst.
MATERIALS AND METHODS
Chemicals.
Reagents and organic solvents were purchased from Merck (Rio de Janeiro, Brazil). Enzyme-linked immunosorbent assay (ELISA) plates, secondary antibodies, and other reagents used for immunofluorescence and flow cytometry were obtained from Sigma Chemical Co. (St. Louis, Mo.). 2′,7′-Bis-(carboxyethyl)-5(6′)-carboxyfluorescein acetoxymethyl ester (BCECF/AM) and dihydrorhodamine (DHR) were purchased from Molecular Probes. Sera from patients with chromoblastomycosis were kindly provided by Claudio Guedes Salgado, Laboratório de Dermato-Imunologia, and Jorge P. Da Silva, Departamento de Farmácia, Universidade Federal do Pará, Belém, Pará, Brazil.
Microorganism.
A human isolate of F. pedrosoi (strain 5VLP) (21) was used for the present work. Stock cultures have been maintained in our laboratory, with 6-month transfers to Sabouraud-dextrose-agar (SDA), and kept at 4°C under mineral oil. Sclerotic cells were obtained in vitro, as described below, or directly from superficial skin scrapings of human patients. Epidermal scraps were washed several times in deionized distilled water and incubated sequentially for 1 h at 37°C in the presence of collagenase type IA (1 mg ml−1) and then DNase I (50 μg ml−1). After the enzymatic treatments, the samples were washed twice in distilled water and the sclerotic cells were separated by centrifugation (5,000 × g, 5 min) (33). For interaction with neutrophils, Candida albicans (strain ATCC 18804) was also used. Candida organisms were cultivated for 24 h at 37°C in Sabouraud broth, collected by centrifugation, washed in phosphate-buffered saline (PBS), counted in a Neubauer chamber, and incubated with human cells as described below.
Growth conditions and fungal morphology.
F. pedrosoi from stock cultures was inoculated in Butterfield and Jong medium (BFJ), pH 6.5 (4). After 15 days at room temperature (28°C), the mycelium form was obtained by filtration. To induce conidium formation, the stock culture was inoculated in BFJ medium, pH 5.5, at room temperature (28°C) for 5 days, with shaking. Conidia were isolated by filtration through gauze, while the culture fluids were separated from remaining cells by centrifugation (5,000 × g, 5 min) and lyophilized. Cultures containing the sclerotic cells were obtained after inoculation of the 15-day mycelium in BFJ medium supplemented with 800 μM dl-propranolol, pH 2.7. Propranolol-containing cultures were grown for 45 days at 37°C, with shaking, as described by Alviano et al. (2).
Isolation and purification of melanin.
Extracellular melanin was obtained from lyophilized culture fluids of conidia and sclerotic cells, as previously described (2). A standard preparation of cell-associated mycelial melanin, characterized in a previous work (1), was also used. The purified extracellular melanins from F. pedrosoi are not soluble in aqueous solutions at neutral pHs, but they can be solubilized in strong alkali and remain soluble after neutralization. Cell-associated melanin particles were isolated from mycelia by a modification of the methodology previously described by Rosas and coworkers (26). Briefly, mycelia were collected by centrifugation at 1,200 × g for 30 min, washed with PBS, and suspended in 1.0 M sorbitol containing 0.1 M sodium citrate (pH 5.5). Cell wall-lysing enzymes from Trichoderma harzianum (Sigma) were added at 10 mg/ml, and the suspensions were incubated overnight at 30°C to generate protoplasts. The protoplasts were collected by centrifugation, washed with PBS, and incubated in 4.0 M guanidine thiocyanate overnight at room temperature. Cell debris were collected by centrifugation, washed three times with PBS, and treated overnight at 37°C with proteinase K (1.0 mg/ml), made up in a reaction buffer containing 10.0 mM Tris, 1.0 mM CaCl2, and 0.5% sodium dodecyl sulfate (SDS), pH 7.8. The resultant material was washed three times with PBS and then boiled in 6.0 M HCl for 1 h. The latter treatment was also applied to the secreted melanin from conidia and sclerotic cells. The materials remaining after acid digestion were collected by centrifugation, washed extensively with PBS, and dialyzed against distilled water for 10 days. Melanin samples were analyzed by light microscopy and infrared spectroscopy. Infrared spectra were obtained by use of a double-beam Perkin-Elmer spectrometer (model 467) and KBr pellets. Precautions were taken for elimination of moisture interference (34).
Scanning electron microscopy (SEM).
Melanin particles from mycelia of F. pedrosoi were fixed for 2 h at room temperature with 2.5% glutaraldehyde, 4% paraformaldehyde, and 5 mM CaCl2 in 0.1 M cacodylate buffer, pH 7.2, washed, and then attached to a glass coverslip covered with poly-l-lysine. They were postfixed, dehydrated in an ethanol graded series, and critical point dried with CO2. The specimen was coated with a 2-nm thickness of chromium in a Gatan (model 681) high-resolution ion beam coater and examined in a JEOL JMS 6340 F scanning electron microscope.
Human sera.
Sera from five male individuals infected with F. pedrosoi were used in this study. They were all immunocompetent workers from rural areas of Brazil (state of Pará) presenting typical lesions of chromoblastomycosis for periods between 10 and 20 years. Lesions in these patients were 5 to 10 cm in diameter and were restricted to the lower limbs. The serological reactivity of each patient's serum against protein extracts from F. pedrosoi was very similar, as evaluated by ELISA (33). Sera from five healthy individuals with no previous laboratory exposure to F. pedrosoi were also collected and tested against the same extracts by ELISA. All of these normal sera presented negligible reactivities with fungal protein extracts. Groups of sera from healthy or infected individuals were then pooled and used in ELISA, with purified melanin as antigen.
Reactivity of melanin with human sera.
The reactivity of F. pedrosoi melanin with the pool of sera from uninfected individuals or patients with chromoblastomycosis was evaluated by ELISA. Extracellular melanin preparations (100 μg) from mycelia and culture fluids of conidia or sclerotic cells were dissolved in 0.1 N NaOH and neutralized to pH 7.0 with 0.1 N HCl to a final volume of 1 ml. The soluble melanin was diluted 1:10 in carbonate buffer (pH 9.6), and 100 μl of this solution per well was added to a flat-bottomed polystyrene microtiter plate. After incubation for 1 h at 37°C, the plate was rinsed with PBS and then blocked with PBS containing 10% bovine serum albumin for 1 h at 37°C. After washing with PBS, 100 μl of normal human sera or sera from infected patients, diluted 1:500 in PBS, was added to each of the coated wells and incubated at 28°C for 1 h. The plate was washed three times prior to incubation with peroxidase-labeled goat anti-human immunoglobulin (1:5,000) for 1 h at 28°C. The plate was again washed three times with PBS, and 100 μl of o-phenylenediamine in citrate-phosphate buffer containing H2O2 was added to each well, followed by incubation in the dark and spectrophotometric reading at 492 nm.
Purification of antibodies against melanin.
The purified melanin residue from conidial culture fluids (40 mg) was incubated overnight at 4°C in the presence of a pool of five sera obtained from patients with chromoblastomycosis. After repeated washing to remove unbound proteins, bound antibodies were eluted with 2 ml of 100 mM glycine acid buffer (pH 3.0) and immediately neutralized with 1 M Tris-HCl (pH 9.0). This process was repeated three times, and the unbound depleted fraction contained antibodies that gave absorbance readings by ELISA similar to those with normal human serum at the same low dilution. Fractions containing antibodies against melanin were concentrated by vacuum centrifugation and analyzed by SDS-polyacrylamide gel electrophoresis under denaturing conditions, which demonstrated the occurrence of two bands, with molecular masses corresponding to 50 and 25 kDa. These preparations were also analyzed by ELISA with antibodies against human immunoglobulin M (IgM) or IgG. Purified preparations were exclusively reactive with antibodies against IgG (data not shown).
Antibody binding.
For fluorescence microscopy and flow cytometry analysis, F. pedrosoi conidia, mycelia, and sclerotic cells obtained from in vitro and in vivo sources, as well as ghost-like preparations, were fixed in 4% paraformaldehyde-cacodylate buffer (0.1 M, pH 7.2) for 1 h at room temperature. Systems were rinsed in PBS and then in 1% bovine serum albumin in PBS for 1 h for further incubation with antibodies against melanin (1:10 dilution) and fluorescein isothiocyanate (FITC)-labeled anti-human IgG (1:100 dilution). Control cells, which were incubated with the fluorescein conjugate but not with antibodies against melanin, were also prepared. Fluorescent reactions were observed under a Zeiss epifluorescence microscope (Axioplan 2). Sclerotic cells and conidia were also screened by flow cytometry analysis (n = 5,000) by a FACScalibur flow cytometer (Becton Dickinson) equipped with a 15 mW argon laser emitting at 488 nm. The data obtained were run in list mode, which makes further analysis possible. Control cells were first analyzed in order to determine their auto-fluorescence and relative size.
Cell growth.
For evaluation of the influence of antibodies against melanin on the growth of F. pedrosoi, conidia or sclerotic cells (104/ml) were incubated in the presence of these antibodies (1:100) for 24 h at 37°C. Viable CFU were evaluated by plating 20 μl of the samples, diluted with 180 μl of PBS, onto SDA plates, followed by incubation for 15 days at 28°C. Control systems were similarly treated with irrelevant human IgG (100 μg/ml) and plated onto SDA. All the experiments were repeated at least three times, and results are expressed as means of these assays ± standard deviations.
Interaction with human cells.
Phagocytosis assays were analyzed by using BCECF/AM, as described previously for similar purposes (31). BCECF/AM is a nonfluorescent membrane-permeative ester that is converted to the trapped fluorescent indicator BCECF by nonspecific prokaryotic and eukaryotic intracellular esterases. Briefly, conidia and sclerotic cells were incubated in saline solution containing BCECF/AM (final concentration, 1 μM) for 30 min at 37°C. Approximately 5 × 106 of these labeled fungal cells were incubated at 37°C, with rotation, in 1 ml of heparinized blood (10 IU ml−1) obtained from healthy donors. For evaluation of the influence of melanin in the interaction of phagocytes with F. pedrosoi, the medium of interaction of human cells and fungi was supplemented with the F. pedrosoi extracellular melanin (5 μg/ml) and/or anti-melanin antibodies (1:100). These were the minimum concentrations required for the inhibition of antibody binding, as determined by ELISA (data not shown). After 60 min, 100-μl aliquots were taken and mixed with 0.9 ml of a lysis buffer (NH4Cl, 9 g; KHCO3, 1 g; EDTA, 37 mg in 1 liter volume, to pH 7.3), which resulted in the elimination of erythrocytes. Washed sediments were resuspended in 500 μl of PBS and analyzed immediately by flow cytometry using Cellquest software (Becton Dickinson, version 3.1.f), with settings as described previously (31). Neutrophils were selected by the examination of two distinct parameter plots, using measurements of forward and side scattering. To ensure the intracellular location of the fungal cells associated with the neutrophils, representative samples used in the flow cytometry analyses were examined by epifluorescence interference contrast microscopy (Zeiss Axioplan 2 microscope). For evaluation of the influence of melanin on the phagocytosis of different fungal cells, the procedure described above was entirely reproduced, using C. albicans instead of F. pedrosoi cells.
Oxidative burst.
For assessment of the level of oxidative burst, unlabeled fungal cells were incubated under the same conditions as those used for the phagocytosis assay, but in the presence of DHR instead of BCECF, at a final concentration of 10 μg/ml of blood. As detailed by Schnitzler and coworkers (31), DHR is freely permeative and localizes to the mitochondria of neutrophils, where it is oxidized to rhodamine by H2O2 and O2 during the respiratory burst, causing an emission of bright green fluorescence under excitation by blue light (488 nm). Washed sediments were analyzed after 10, 30, and 60 min of incubation and analyzed by flow cytometry. Neutrophils were gated according to their relative size and granularity. The oxidative burst of the neutrophils, induced by unlabeled fungal cells in the presence of DHR, is expressed as an increase in green fluorescence emission by the neutrophils. The percentage of neutrophils exhibiting green fluorescence was determined with the quadrant statistics option in Cellquest software. The fluorescence cutoff was set before the fungal cells were added by gating 99% of the neutrophils in a nonfluorescent quadrant.
Killing assay.
For quantification of killing of conidia and sclerotic bodies after incubation with human cells, fungal forms were cultured as described above, harvested, and then diluted in PBS to 104 cells/ml. Aliquots of 100 μl were mixed with 900 μl of fresh heparinized blood at 37°C for 4 h under constant rotation. Numbers of viable CFU were estimated by plating 20 μl of the samples, diluted with 180 μl of PBS, onto SDA plates, followed by incubation for 15 days at 28°C.
Interaction with peritoneal murine macrophages.
Resident peritoneal cells were collected from male BALB/c mice by washing of the peritoneal cavities with cold RPMI 1640 medium. Approximately 18 to 24 h before the assay, the macrophages were plated at a density of 1 × 105 to 3 × 105 cells to each well of a 24-well plate with tissue culture slides in RPMI 1640 medium containing 10% fetal calf serum. The cells were allowed to settle and adhere at 37°C in a moist chamber of 5% CO2 and air. After this incubation, the medium was removed from each well and nonadherent cells were eliminated. The viability of the cells was over 95%, as determined by trypan blue dye exclusion. F. pedrosoi conidia (1.5 × 107 cells/ml) were mixed with murine macrophages at a ratio of five fungal cells per macrophage. Phagocytosis was measured in the presence or absence of soluble melanin (10 μg per well) after incubation at 37°C for 2 h. The medium from the wells was carefully removed, and the remaining macrophages were washed three times with 0.5 ml of sterile PBS to remove fungal cells that were not attached to or engulfed by phagocytes. Slides were fixed in absolute methanol for 1 min and stained with a 1:20 solution of Giemsa stain for 10 min. After the slides were air dried, the cells were observed microscopically at a magnification of ×1,000. The phagocytic index is defined as the ratio of phagocytes with attached and internalized conidia to total phagocytes per field. For each experiment, an average of 200 macrophages were counted.
Statistical analysis.
All the experiments were performed in triplicate sets. Serum reactivity data, phagocytosis of C. albicans by neutrophils, ingestion of F. pedrosoi by mouse macrophages, and levels of oxidative burst were statistically analyzed by the t test, with the approximation of Satterthwaite. Data obtained from experiments of phagocytosis and killing of F. pedrosoi by human neutrophils were analyzed by analysis of variance followed by the post hoc Fisher's protected least significant difference test. All of the statistical tests were reviewed by A. Nobrega, an expert in statistics at our institution.
RESULTS
Comparative analysis of F. pedrosoi melanin.
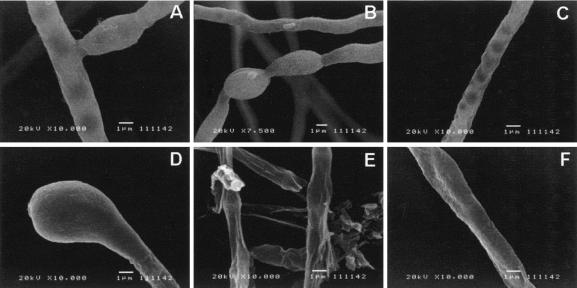
Cell-associated and extracellular melanins were prepared as previously described (1, 26) and analyzed by infrared spectroscopy. Very similar spectra were obtained by comparing the melanin preparations obtained for this work and a similar sample from mycelial cultures (1; data not shown). The infrared spectra of melanins from all of the fungal forms showed hydroxyl and carboxyl (1,715 cm−1) groups and a band at 1,615 cm−1 attributable to either a carboxylate anion or an aromatic structure. A large band at 2,920 cm−1, consistent with the aliphatic structure, was also observed. The analysis of the melanin preparations under the light microscope (Fig. 1) revealed the extracellular pigment from fungal culture fluids forms amorphous aggregates. As described for several other fungal species (11, 19, 20, 25, 26), treatment of mycelia with proteases and glycosidases followed by a denaturing agent and hot concentrated acid left a black residue. Under the light microscope, this residue had a morphological aspect very similar to those observed for intact mycelia. Very similar results were obtained when F. pedrosoi conidia were digested (data not shown). SEM demonstrated that, as described for conidia or yeast cells of S. schenckii (25), P. brasiliensis (11), H. capsulatum (19), and C. neoformans (18, 26), the processed melanin residue resembles the intact mycelium very closely in shape and size (Fig. 2). These structures are therefore comparable to the ghost-like structures proposed in previous reports (11, 19, 20, 25, 26).
FIG. 1.
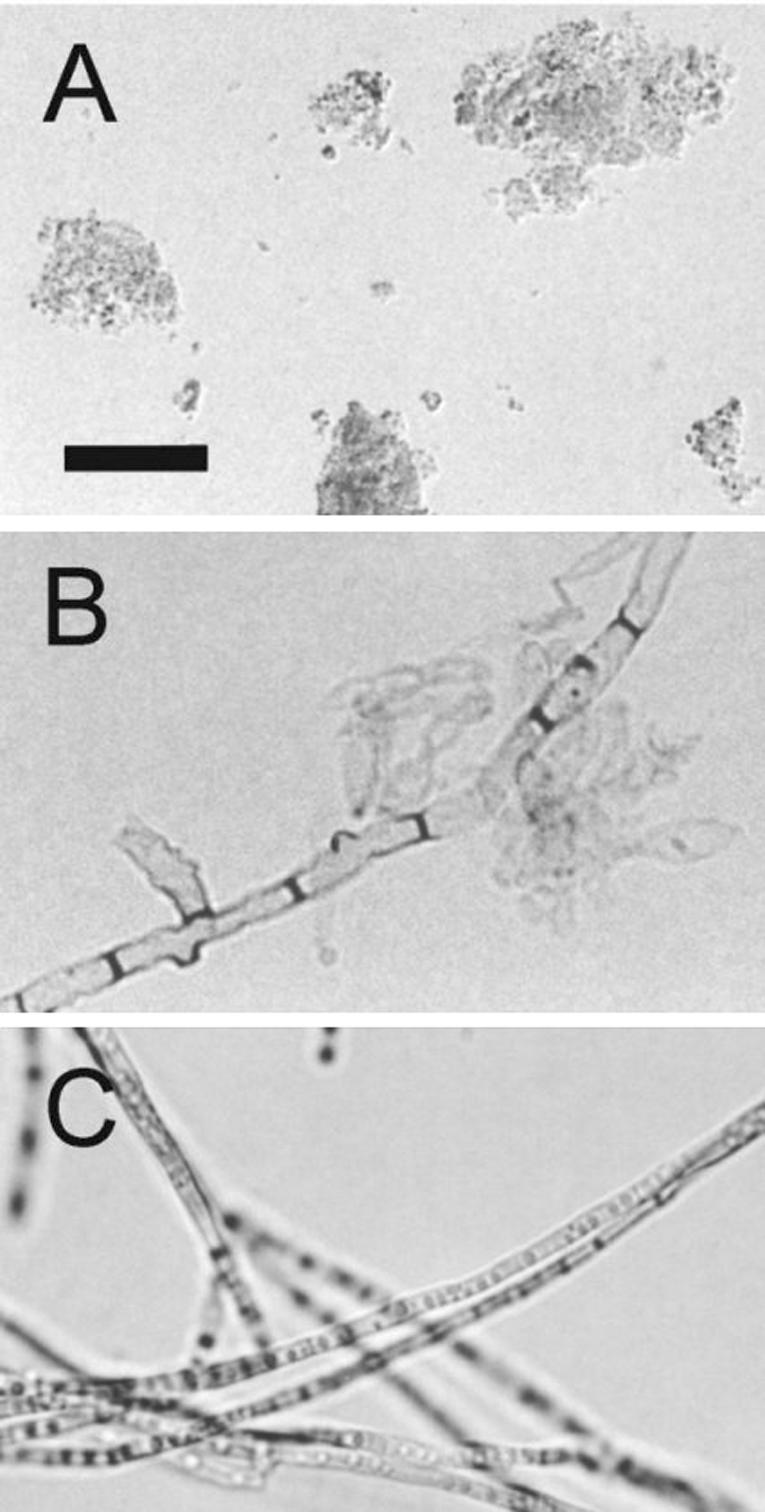
Light microscopy of F. pedrosoi melanins. Isolated melanin from conidial culture fluids forms amorphous aggregates (A). Treatment of mycelia with lytic enzymes and denaturing agents results in the production of black residues (B) that closely resemble intact mycelia (C). Bar, 10 μm.
FIG. 2.
SEM of F. pedrosoi viable mycelia (A to C) or digested preparations (D to F) (see Materials and Methods for details). As suggested by light microscopy, treatment of mycelia with lytic enzymes and denaturing agents results in the production of sediments that are very similar to intact cells in shape and size.
F. pedrosoi melanin induces the production of human antibodies during chromoblastomycosis.
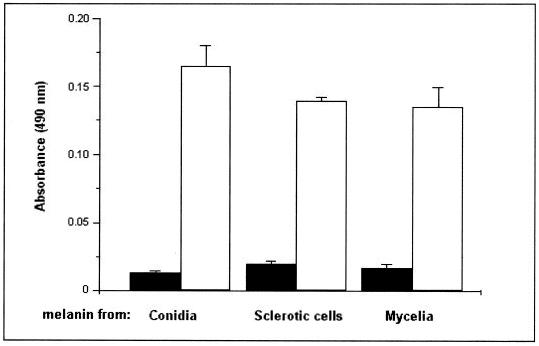
The reactivity of sera from healthy individuals or chromoblastomycosis patients with melanin was evaluated by ELISA. Melanin samples obtained from mycelia and culture fluids of conidia or sclerotic cells were significantly more reactive with a pool of patients’ sera than with sera from healthy individuals (Fig. 3) (P < 0.001), suggesting that F. pedrosoi synthesizes melanin in vivo as an immunologically active fungal structure. For further experiments, the antibodies against melanin were purified from patient sera and characterized as IgG by ELISA and SDS-polyacrylamide gel electrophoresis.
FIG. 3.
Antibodies against fungal melanin are produced during chromoblastomycosis. The reactivity of sera from healthy individuals (black bars) or patients with chromoblastomycosis (white bars) with melanin particles from culture fluids of conidia or sclerotic cells, as well as with cell-associated mycelial melanin, was tested by ELISA. The reactivity of patients’ sera was significantly higher than that of uninfected sera (P < 0.001). Results are expressed as means of three independent experiments ± standard deviations.
Human antibodies against F. pedrosoi melanin bind fungal forms from in vivo or in vitro sources.
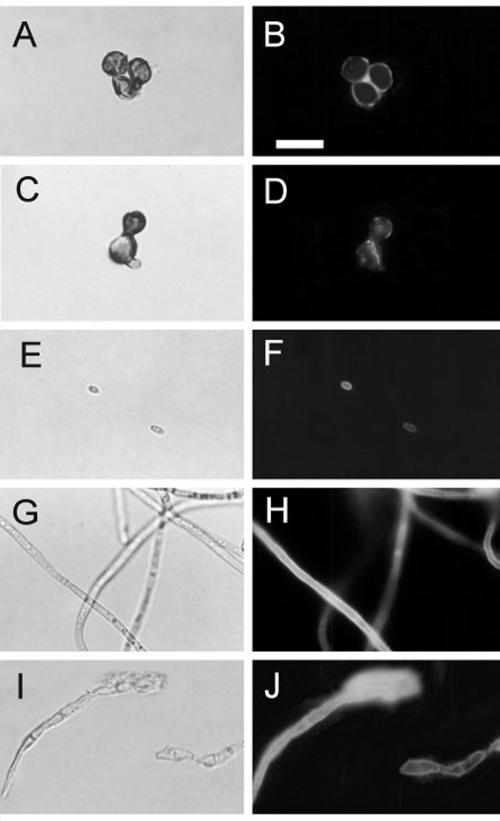
The purified antibodies against melanin were incubated with F. pedrosoi mycelia, conidia, sclerotic forms, or melanin ghosts and analyzed by flow cytometry or fluorescence microscopy. As demonstrated in Fig. 4, conidia and sclerotic cells are strongly recognized by these antibodies. Fluorescence microscopy (Fig. 5) demonstrated that the cell wall is the major site of reactivity of F. pedrosoi with antibodies against melanin. Sclerotic cells from patient lesions were strongly recognized by the antibodies, strongly supporting the hypothesis that F. pedrosoi is melanized during human infection. Sclerotic cells cultivated in vitro, as well as conidia, mycelia, and digested residues, reacted with anti-melanin antibodies in a similar fashion. No fluorescent reactions were observed for fungal preparations that had not been incubated with antibodies against melanin (data not shown).
FIG. 4.
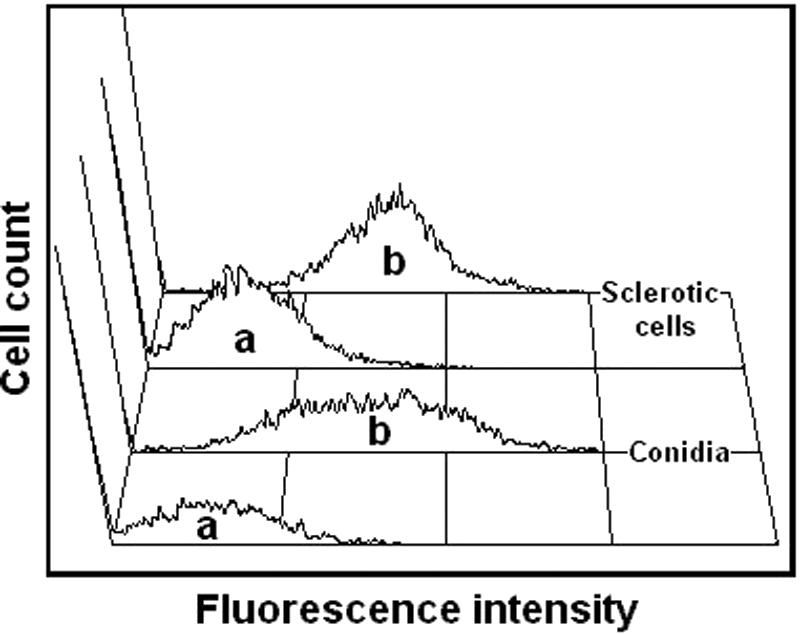
Purified antibodies against melanin bind conidia and sclerotic forms of F. pedrosoi. Fungal cells were incubated in the presence of human antibodies against melanin followed by FITC-labeled anti-human antibodies and were analyzed by flow cytometry. Fungal cells that had not been incubated with antibodies against melanin (a) showed basal levels of fluorescence, while fungal forms that were incubated with melanin antibodies (b) became fluorescent.
FIG. 5.
Cell wall distribution of melanin in F. pedrosoi. Sclerotic cells from patient lesions (A and B) or from in vitro cultivation (C and D), conidia (E and F), mycelia (G and H), or melanin ghosts (I and J) were incubated with antibodies against melanin followed by FITC-labeled anti-human antibodies and were analyzed by fluorescence microscopy. Left panels show fungal cells under differential interference contrast, while right panels show the same images under fluorescence. Bar, 10 μm.
Antibodies against melanin inhibit the growth of F. pedrosoi.
Rosas and coworkers (27) described that monoclonal anti-melanin antibodies inhibit the growth of pigmented C. neoformans. Using the method described by Polonelli and coworkers (22), we compared the growth of F. pedrosoi conidia and sclerotic cells after the treatment of fungal cells with antibodies against melanin or irrelevant IgG. Taking the growth of fungal forms treated with irrelevant IgG as a reference, it was possible to state that purified antibodies against melanin almost completely inhibited (P < 0.0001) the growth of F. pedrosoi conidia (Table 1). Sclerotic cells were also inhibited, but to a lesser extent (P < 0.005). This observation may be a consequence of the fact that sclerotic bodies typically form large aggregates (2, 33), which would impair the access of external ligands to inner cells.
TABLE 1.
Antibodies against melanin inhibit the growth of F. pedrosoia
| Cell type | No. of CFU (mean ± SD) after antibody treatment
|
P | |
|---|---|---|---|
| Irrelevant IgG | Antibodies against melanin | ||
| Conidia | 388 ± 99 | 11 ± 8 | <0.0001 |
| Sclerotic cells | 184 ± 24 | 122 ± 14 | <0.005 |
Inhibition of fungal growth was determined after the treatment of F. pedrosoi with human antibodies against melanin or with irrelevant IgG, followed by plating onto SDA. After incubation of SDA plates for 15 days at 28°C, means and standard deviations were obtained by the estimation of the number of CFU for three different experiments. P, t test significance of differences between the results obtained after treatment with irrelevant IgG or anti-melanin antibodies.
Soluble melanin and melanin antibodies enhance the antifungal functions of phagocytes.
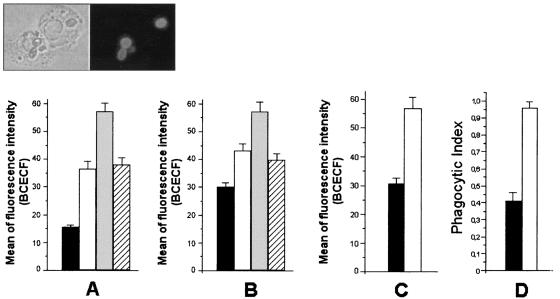
The association between neutrophils and fungal cells was analyzed by flow cytometry (Fig. 6). To ensure the intracellular location of fungi, representative samples used in the flow cytometry analyses were examined by fluorescence microscopy (Fig. 6, inset). Different forms of F. pedrosoi were ingested by human neutrophils after 60 min of interaction. The addition of soluble melanin to the medium of interaction enhanced this association (P < 0.0001, for both conidia and sclerotic cells). These results suggested that human neutrophils could be activated in the presence of melanin, which could result in higher levels of phagocytosis. This hypothesis was supported by the use of different experimental conditions. The phagocytosis of BCECF-stained C. albicans by human neutrophils was similarly enhanced (P < 0.01) in the presence of melanin (Fig. 6C). Alternatively, the use of mouse peritoneal macrophages followed by estimation of the phagocytic indices by counting the number of attached or internalized F. pedrosoi conidia in Giemsa-stained phagocytes revealed that melanin also stimulated the phagocytosis (P < 0.005) of these fungal forms by peritoneal macrophages (Fig. 6D).
FIG. 6.
Soluble melanin influences the ingestion of fungal cells by human or animal phagocytes. The indices of phagocytosis for F. pedrosoi conidia (A), sclerotic forms (B), or C. albicans organisms (C) by human neutrophils were expressed as a function of BCECF staining, as measured by flow cytometry. The interaction of F. pedrosoi conidia with mouse macrophages was evaluated microscopically after staining with Giemsa stain (D). Black bars, phagocytosis of fungal forms after interaction with phagocytes. The addition of soluble melanin (white bars) caused an increase in the phagocytosis levels in all systems. The influence of melanin antibodies alone (gray bars) or in association with melanin (hatched bars) on the phagocytosis of conidia (A) or sclerotic cells (B) was also evaluated. Values of P and statistical significance are described in Results. Data are expressed as means of three independent experiments ± standard deviations. Inset, representative sample of the interaction of BCECF-labeled conidia and human neutrophils.
The increase in the levels of phagocytosis of F. pedrosoi by human neutrophils was even more pronounced when antibodies against melanin were added (P < 0.0001, for both conidia and sclerotic cells), probably because F. pedrosoi became opsonized by the antibodies. When the medium of interaction was supplemented with melanin and the corresponding antibody in association, the enhancement of phagocytosis was partially reverted.
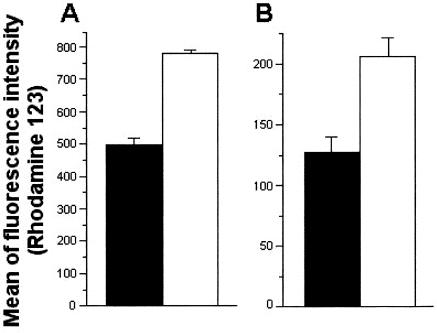
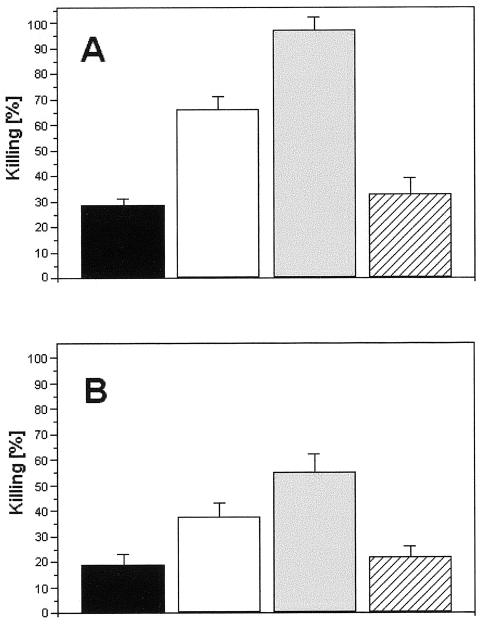
The activation of human neutrophils by soluble melanin was confirmed by the higher degrees of oxidative burst in the presence of the pigment (Fig. 7). For this assay, the enhanced production of oxidant compounds was evaluated by the levels of conversion of DHR to rhodamine and consequent emission of green fluorescence under excitation at 488 nm (31). After 60 min of interaction between fungal cells and human blood in the presence of DHR, populations corresponding to neutrophils became more fluorescent when soluble melanin was added to the medium than when the incubations were performed without melanin (P < 0.005 for interaction with conidia; P < 0.01 for interaction with sclerotic forms). In this context, higher indices of F. pedrosoi killing by neutrophils would be expected in the presence of soluble melanin, which was in fact observed (Fig. 8). The determination of numbers of viable cells of F. pedrosoi after the interaction with human cells demonstrated that conidia or sclerotic forms recovered from systems from which melanin was absent proliferated more intensively than fungal cells obtained from melanin-activated human phagocytes (P < 0.0001 for interaction with conidia; P < 0.003 for interaction with sclerotic forms). When antibodies against melanin were used, the percentages of killing were even higher (P < 0.0001 for both conidia and sclerotic forms), probably because of the additional antifungal effect promoted by the antibodies, as demonstrated in Table 1. The association of melanin and the corresponding antibodies resulted in levels of fungal survival similar to those observed when no compounds were added (P = 0.2958 for interaction with conidia; P = 0.4851 for interaction with sclerotic forms), which was probably due to an in vitro neutralization of melanin by the human antibodies.
FIG. 7.
Influence of soluble melanin in the oxidative burst of human neutrophils during incubation with F. pedrosoi conidia (A) or sclerotic bodies (B). The degrees of oxidative burst were measured by the emission of fluorescence after the oxidation of DHR to rhodamine. Bars represent the intensity of rhodamine fluorescence after interaction of F. pedrosoi with neutrophils in the absence (black bars) or presence (white bars) of soluble melanin. In the presence of soluble melanin, oxidative burst was significantly enhanced after interaction of neutrophils with conidia (P < 0.005) or sclerotic forms (P < 0.01). Results are expressed as means of three independent experiments ± standard deviations.
FIG. 8.
Killing of F. pedrosoi conidia (A) or sclerotic cells (B) after interaction with human neutrophils. Black bars, percentage of killing of fungal cells after interaction with neutrophils. Supplementation of the medium of interaction with melanin (white bars) or melanin antibodies (gray bars) significantly enhanced the antifungal efficacy of neutrophils, while the association between these components (hatched bars) resulted in levels of fungal death similar to those observed in the absence of antibodies or soluble melanin. Values of P and statistical significance are described in Results. Data are expressed as means of three independent experiments ± standard deviations.
DISCUSSION
Synthesis of melanins by pathogenic fungi has been the focus of several studies in past years (12, 20). The fungal species F. pedrosoi (1), W. dermatitidis (31), C. neoformans (18), H. capsulatum (19), S. schenckii (25), and P. brasiliensis (11) produce melanin-like pigments which seem to contribute to microbial pathogenesis. In this regard, studies on the mechanisms by which fungal melanins are synthesized or how they influence the interaction with the host can contribute to the development of new antifungal therapies as well as to the understanding of how fungal cells escape host defenses.
F. pedrosoi has previously been demonstrated to produce extracellular (9) and cell-associated (10) melanin. For this pathogen, a close association between the increase of cytoplasmic electron-dense granules, acidic organelles, and pigmentation of fungal cultures has been demonstrated (10). In addition, transmission electron microscopy revealed that melanin synthesis in F. pedrosoi involves the formation of melanosome-like compartments, as described for mammalian cells (16, 23). The biosynthesis of melanin in many fungal pathogens leads to the accumulation of the pigment as a highly resistant structure underlying the cell wall (12, 20, 26). Because of this combination of cellular distribution and high resistance against different chemicals, the digestion of melanized fungi with proteases, glycanases, denaturant, and hot concentrated acid yields melanin particles that retain the shape of fungal cells, which were therefore called melanin ghosts (26).
We demonstrate here that the digestion of pigmented F. pedrosoi also yields particles that are of the same size and shape as intact cells, as are the previously described melanin ghosts. To our knowledge, this is the first demonstration of melanin ghosts in the mycelial form of a fungal pathogen. These results provided evidence that melanin synthesis in this pathogen follows the previously described pattern of wall-associated pigment accumulation in fungal cells. Secreted particles of melanin were also isolated. These extracellular molecules were recognized by sera from individuals with chromoblastomycosis, which agreed with previous studies demonstrating that fungal melanins are immunogenically active compounds (18, 26). A purified melanin from C. neoformans induced a strong antibody response in mice (18). Furthermore, melanin from C. neoformans activated the alternative complement cascade (28).
To study melanization of F. pedrosoi and its possible relevance in the interaction with mammalian cells, we therefore purified antibodies against melanin from sera of patients with chromoblastomycosis. Immunofluorescence and flow cytometry analyses demonstrated that melanin-binding human antibodies reacted with the pigmented conidia, mycelia, and sclerotic cells, as well as with the ghost particles. In addition, antibodies against melanin similarly recognized sclerotic bodies obtained from patient lesions, strongly indicating that F. pedrosoi produces melanin during human infection. The pattern of reactivity was similar to that described for C. neoformans (18), H. capsulatum (19), S. schenckii (25), and P. brasiliensis (11).
In C. neoformans, therapy of lethally infected mice with monoclonal antibodies (MAbs) against melanin significantly improved survival (27). Mice treated with the melanin-binding MAbs had an approximately 100-fold lower fungal burden than mice given an irrelevant MAb. In the same study, it was demonstrated that melanin-binding MAbs completely eliminated cell growth, whereas there was no effect on the replication of nonmelanized cells. Due to the similarities between the profiles of reactivity of C. neoformans and F. pedrosoi with antibodies against melanin, we evaluated the effects of these antibodies on the cell growth of conidia and sclerotic bodies of Fonsecaea. Treatment of these fungal forms with melanin-binding human antibodies completely inhibited the proliferation of conidia and, to a lesser extent, sclerotic cells. The greater resistance of the latter may be explained by the natural formation of large cellular aggregates in culture (2), which could diminish the access of these antifungal ligands to internally located cells. The mechanisms by which antibodies against melanin inhibit fungal growth are still not known, but binding to the cell surface could impair the biological functions of molecules involved in wall assembly. Likewise, the recently described anti-glucosylceramide antibodies also impair fungal growth after binding to the cell wall (24).
The influence of melanin particles and their corresponding antibodies in the interaction of F. pedrosoi conidia and sclerotic forms with mammalian cells was also evaluated in the present work. The phagocytosis levels for conidia or sclerotic cells in neutrophils were enhanced in the presence of fungal melanin, suggesting that melanin particles could activate phagocytic cells. This hypothesis was supported by the facts that (i) melanin increased the oxidative burst of neutrophils in the presence of F. pedrosoi cells, (ii) the phagocytosis of viable C. albicans organisms by neutrophils was augmented in the presence of F. pedrosoi melanin, and (iii) melanin enhanced the ingestion of F. pedrosoi conidia by different phagocytic cells (peritoneal mouse macrophages) for different experimental approaches. In addition, fungal killing by host phagocytic cells was increased when the cells were incubated in the presence of melanin. These observations agree with previous reports demonstrating that melanins or their precursors influence the activation of phagocytes (5) and the proliferation and differentiation of human keratinocytes and fibroblasts (3). Killing and phagocytosis of fungal cells by neutrophils were even higher in the presence of antibodies against melanin, probably because of the direct antifungal effects of the antibodies and opsonization of fungi, respectively. Interestingly, the effects of soluble melanin or the corresponding antibodies were neutralized when these components were used in association, raising the possibility of in vivo neutralization. It is still impossible to predict if this in fact occurs during infection by F. pedrosoi, but previous results by our group (9) indicate that the production of soluble pigment is dependent on the contact of F. pedrosoi with host cells, which results in the secretion of melanin inside phagocytes. Based on our present results, it would be reasonable to expect phagocyte activation and also the subsequent production of antibodies against soluble or cell-associated melanin in this case. If this model is true, the extracellular melanin particles would not be susceptible to neutralization by external ligands and the produced antibodies against melanin could therefore react with fungal cells, exerting their antifungal action. Therefore, if soluble melanin were in fact produced during infection by F. pedrosoi, it would be interesting to analyze the microenvironment into which the pigment is secreted and also to estimate its in vivo concentration, because of its possible neutralization by host antibodies.
Infection by F. pedrosoi generally results from traumatic implantation of conidia or fragments of mycelia into host tissues. Inside the host, infectious propagules adhere to epithelial cells and differentiate into sclerotic forms, which effectively resist destruction by host effector cells and allow the establishment of a chronic disease. The currently used therapies against chromoblastomycosis involve use of antifungal agents and/or surgical excision but, as for other subcutaneous mycoses, treatment is not very effective, producing relapses during therapy and lack of tolerability of antifungal drugs (7, 15).
The mechanisms by which F. pedrosoi escapes host defenses and persists in human tissues are not known, but cell-associated melanins seem to protect fungal cells against the antimicrobial action of macrophages and neutrophils (8, 9, 29, 30). We demonstrated here, however, that antifungal antibodies against melanin are produced during human chromoblastomycosis and that secreted melanin activates phagocytic cells in vitro. It remains unclear whether F. pedrosoi produces extracellular melanin-like pigments in vivo, but this possibility is supported by the fact that conidia interacting with mouse macrophages abundantly secrete melanin granules into the cytosol of animal cells (9). The present results raise the possibility that secreted melanin could induce humoral and cellular responses, protecting the host against chromoblastomycosis. In this regard, mimic peptides reproducing the immunological properties of melanins could be considered promising candidates for the design of vaccines for chromoblastomycosis. In this context, the recently described methodology for the structural elucidation of fungal melanins by use of nuclear magnetic resonance (35) will be of enormous applicability.
Acknowledgments
We thank Fátima Regina de Vasconcelos Goulart for technical assistance and Alberto Da Nobrega for help with statistical analyses.
This work was supported by Programa de Apoio a Núcleos de Excelência (PRONEX), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação Universitária José Bonifácio (FUJB), and Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Editor: T. R. Kozel
REFERENCES
- 1.Alviano, C. S., S. R. Farbiarz, W. De Souza, J. Angluster, and L. R. Travassos. 1991. Characterization of Fonsecaea pedrosoi melanin. J. Gen. Microbiol. 137:837-844. [DOI] [PubMed] [Google Scholar]
- 2.Alviano, C. S., L. R. Travassos, J. Angluster, and W. De Souza. 1992. Effect of environmental factors on Fonsecaea pedrosoi morphogenesis with emphasis on sclerotic cells induced by propranolol. Mycopathologia 119:17-23. [DOI] [PubMed] [Google Scholar]
- 3.Blinova, M. I., N. M. Yudintseva, N. V. Kalmykova, E. V. Kuzminykh, N. A. Yurlova, O. A. Ovchinnikova, and I. L. Potokin. 2003. Effect of melanins from black yeast fungi on proliferation and differentiation of cultivated human keratinocytes and fibroblasts. Cell. Biol. Int. 27:135-146. [DOI] [PubMed] [Google Scholar]
- 4.Butterfield, W., and S. C. Jong. 1987. Effect of carbon source on conidiogenesis in Fonsecaea dermatitidis, agent of chromomycosis. Mycopathologia 58:59-62. [DOI] [PubMed] [Google Scholar]
- 5.D'Acquisto, F., R. Carnuccio, M. D'Ischia, and G. Misuraca. 1995. 5,6-Dihydroxyindole-2-carboxylic acid, a diffusible melanin precursor, is a potent stimulator of lipopolysaccharide-induced production of nitric oxide by J774 macrophages. Life Sci. 57:401-406. [DOI] [PubMed] [Google Scholar]
- 6.De Hoog, G. S., F. Queiroz-Telles, G. Haase, G. Fernandez-Zeppenfeldt, D. Attili Angelis, A. H. G. Gerrits Van Den Ende, T. Matos, H. Peltroche-Llacsahuanga, A. A. Pizzirani-Kleiner, J. Rainer, N. Richard-Yegres, V. Vicente, and F. Yegres. 2000. Black fungi: clinical and pathogenic approaches. Med. Mycol. 38:243-250. [PubMed] [Google Scholar]
- 7.Esterre, P., M. Jahevitra, and A. Andriantsimahavandy. 2000. Humoral immune response in chromoblastomycosis during and after therapy. Clin. Diagn. Lab. Immunol. 7:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farbiarz, S. R., T. U. De Carvalho, C. S. Alviano, and W. de Souza. 1990. Fine structure and cytochemistry of the interaction between Fonsecaea pedrosoi and mouse resident macrophages. J. Med. Vet. Mycol. 28:373-383. [PubMed] [Google Scholar]
- 9.Farbiarz, S. R., T. U. De Carvalho, C. S. Alviano, and W. de Souza. 1992. Inhibitory effect of melanin on the interaction of Fonsecaea pedrosoi with mammalian cells in vitro. J. Med. Vet. Mycol. 30:265-273. [PubMed] [Google Scholar]
- 10.Franzen, A. J., W. de Souza, M. Farina, C. S. Alviano, and S. Rozental. 1999. Morphometric and densitometric study of the biogenesis of electron-dense granules in Fonsecaea pedrosoi. FEMS Microbiol. Lett. 173:395-402. [DOI] [PubMed] [Google Scholar]
- 11.Gómez, B. L., J. D. Nosanchuk, S. Diez, S. Youngchim, P. Aisen, L. E. Cano, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2001. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect. Immun. 69:5760-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez, B. L., and J. D. Nosanchuk. 2003. Melanin and fungi. Curr. Opin. Infect. Dis. 16:91-96. [DOI] [PubMed] [Google Scholar]
- 13.Hamza, S. H., P. J. Mercado, H. G. Skelton, and K. J. Smith. 2003. An unusual dematiaceous fungal infection of the skin caused by Fonsecaea pedrosoi: a case report and review of the literature. J. Cutan. Pathol. 30:340-343. [DOI] [PubMed] [Google Scholar]
- 14.Huffnagle, G. B., G. H. Chen, J. L. Curtis, R. A. McDonald, R. M. Strieter, and G. B. Toews. 1995. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 155:3507-3516. [PubMed] [Google Scholar]
- 15.Koga, T., T. Matsuda, T. Matsumoto, and M. Furue. 2003. Therapeutic approaches to subcutaneous mycoses. Am. J. Clin. Dermatol. 4:537-543. [DOI] [PubMed] [Google Scholar]
- 16.Marks, M. S., and M. C. Seabra. 2001. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell. Biol. 2:738-748. [DOI] [PubMed] [Google Scholar]
- 17.McGinnis, M. R., and E. A. Hilger. 1987. Infections caused by black fungi. Arch. Dermatol. 123:1300-1302. [PubMed] [Google Scholar]
- 18.Nosanchuk, J. D., A. L. Rosas, and A. Casadevall. 1998. The antibody response to fungal melanin in mice. J. Immunol. 160:6026-6031. [PubMed] [Google Scholar]
- 19.Nosanchuk, J. D., B. L. Gomez, S. Youngchim, S. Diez, P. Aisen, R. M. Zancope-Oliveira, et al. 2002. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect. Immun. 70:5124-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosanchuk, J. D., and A. Casadevall. 2003. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5:203-223. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira, L. G., M. A. Resende, C. F. Lopes, and E. O. Cisalpino. 1973. Isolamento e identificação dos agentes da cromomicose em Belo Horizonte. Rev. Soc. Bras. Med. Trop. 7:1. [Google Scholar]
- 22.Polonelli, L., N. Seguy, S. Conti, M. Gerloni, D. Bertolotti, C. Cantelli, W. Magliani, and J. C. Cailliez. 1997. Monoclonal yeast killer toxin-like candidacidal anti-idiotypic antibodies. Clin. Diagn. Lab. Immunol. 4:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raposo, G., and M. S. Marks. 2002. The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic 3:237-248. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues, M. L., L. R. Travassos, K. R. Miranda, A. J. Franzen, S. Rozental, W. De Souza, C. S. Alviano, and E. Barreto-Bergter. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 68:7049-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero-Martinez, R., M. Wheeler, A. Guerrero-Plata, G. Rico, and H. Torres-Guerrero. 2000. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 68:3696-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosas, A. L., J. D. Nosanchuk, B. L. Gomez, W. A. Edens, J. M. Henson, and A. Casadevall. 2000. Isolation and serological analyses of fungal melanins. J. Immunol. Methods 244:69-80. [DOI] [PubMed] [Google Scholar]
- 27.Rosas, A. L., J. D. Nosanchuk, and A. Casadevall. 2001. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun. 69:3410-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosas, A. L., R. S. MacGill, J. D. Nosanchuk, T. R. Kozel, and A. Casadevall. 2002. Activation of the alternative complement pathway by fungal melanins. Clin. Diagn. Lab. Immunol. 9:144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozental, S., W. De Souza, and C. S. Alviano. 1994. The in vitro susceptibility of Fonsecaea pedrosoi to activated macrophages. Mycopathologia 126:85-91. [DOI] [PubMed] [Google Scholar]
- 30.Rozental, S., C. S. Alviano, and W. De Souza. 1996. Fine structure and cytochemical study of the interaction between Fonsecaea pedrosoi and rat polymorphonuclear leukocyte. J. Med. Vet. Mycol. 34:323-330. [PubMed] [Google Scholar]
- 31.Schnitzler, N., H. Peltroche-Llacsahuanga, N. Bestier, J. Zuendorf, R. Lluetticken, and G. Haase. 1999. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect. Immun. 65:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva, J. P., W. De Souza, and S. Rozental. 1999. Chromoblastomycosis: a retrospective study of 325 cases in Amazonic region (Brazil). Mycopathologia 143:171-175. [DOI] [PubMed] [Google Scholar]
- 33.Silva, J. P., D. S. Alviano, C. S. Alviano, W. De Souza, L. R. Travassos, J. A. P. Diniz, and S. Rozental. 2002. Comparison of Fonsecaea pedrosoi sclerotic cells obtained in vivo and in vitro: ultrastructure and antigenicity. FEMS Immunol. Med. Microbiol. 33:63-69. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson, F. J., and K. M. Goh. 1972. Infrared spectra of humic and fulvic acids and their methylated derivatives: evidence for non-specificity of analytical methods for oxygen containing functional groups. Soil Sci. 113:334-345. [Google Scholar]
- 35.Tian, S., J. Garcia-Rivera, B. Yan, A. Casadevall, and R. E. Stark. 2003. Unlocking the molecular structure of fungal melanin using 13C biosynthetic labeling and solid-state NMR. Biochemistry 42:8105-8109. [DOI] [PubMed] [Google Scholar]
- 36.Van Duin, D., A. Casadevall, and J. D. Nosanchuk. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibility to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 46:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Y., and A. Casadevall. 1994. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect. Immun. 62:3004-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]