Abstract
Background: The organochlorine dichlorodiphenyltrichloroethane (DDT), a known estrogen mimic and endocrine disruptor, has been linked to animal and human disorders. However, the detailed mechanism(s) by which DDT affects cellular physiology remains incompletely defined.
Objectives: We and others have shown that DDT activates cell-signaling cascades, culminating in the activation of estrogen receptor-dependent and -independent gene expression. Here, we identify a mechanism by which DDT alters cellular signaling and gene expression, independent of the estrogen receptor.
Methods: We performed quantitative polymerase chain reaction array analysis of gene expression in MCF-7 breast cancer cells using either estradiol (E2) or o,p´-DDT to identify distinct cellular gene expression responses. To elucidate the mechanisms by which DDT regulates cell signaling, we used molecular and pharmacological techniques.
Results: E2 and DDT treatment both altered the expression of many of the genes assayed, but up-regulation of vascular endothelial growth factor A (VEGFA) was observed only after DDT treatment, and this increase was not affected by the pure estrogen receptor α antagonist ICI 182780. Furthermore, DDT increased activation of the HIF-1 response element (HRE), a known enhancer of the VEGFA gene. This DDT-mediated increase in HRE activity was augmented by the coactivator CBP (CREB-binding protein) and was dependent on the p38 pathway.
Conclusions: DDT up-regulated the expression of several genes in MCF-7 breast cancer cells that were not altered by treatment with E2, including VEGFA. We propose that this DDT-initiated, ER-independent stimulation of gene expression is due to DDT’s ability to initiate crosstalk between MAPK (mitogen-activated protein kinase) signaling pathways and transcriptional coactivators.
Keywords: breast cancer, CBP, coactivator, crosstalk, DDT, dichlorodiphenyltrichloroethane, endocrine-disrupting chemical, HIF-1α, MAPK, organochlorine, p38 kinase, vascular endothelial growth factor
Endocrine-disrupting chemicals (EDCs), such as polychlorinated biphenyls (PCBs), phthalates, phenolics, and other organochlorines, can affect the endocrine system by altering steroid receptor function, resulting in apparent estrogen-like activity and possible reproductive dysfunction (McLachlan 2001; McLachlan et al. 2006; Tilghman et al. 2010). The estrogen-like activity of the organochlorine pesticide dichlorodiphenyltrichloroethane (DDT) and its congeners was first shown > 50 years ago (Tullner 1961), yet the mechanism of action of DDT as a hormone remains an enigma (see McLachlan 2001 for review). Although its use has been restricted to use for mosquito control in developing countries with tropical climates, DDT remains active in the environment worldwide and bioaccumulates in the fat stores of animals and humans because of its lipophilic nature and chemical stability (Kelly et al. 2004). The DDT metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) continues to be detected in human serum with a high frequency at concentrations up to and exceeding 1,000 μg/kg lipids (Cole et al. 2006). DDT and its metabolites have been associated with human diseases including type 2 diabetes (Codru et al. 2007; Rignell-Hydbom et al. 2007), testicular tumors (McGlynn et al. 2008), pancreatic cancer (Porta et al. 2008), endometrial cancer (Hardell et al. 2004), and breast cancer (Cocco et al. 2000; Rier and Foster 2002; Safe and Zacharewski 1997; Sasco 2003; Wolff et al. 1993), but mechanisms to explain these associations remain elusive.
DDT mimics the natural hormone estradiol (E2) and can bind to estrogen receptor α (ERα) (Ahlborg et al. 1995; Gulledge et al. 2001; Klotz et al. 1996; Kuiper et al. 1998). In addition, DDT exerts cellular effects independently of ERα. For example, we previously demonstrated that DDT and its active metabolites are capable of inducing AP-1 mediated transcription, in both ERα-positive and ERα-negative cells (Frigo et al. 2002). We have also shown that DDT activates transcription at multiple DNA response elements through p38-mediated phosphorylation and activation of the coactivators p300 (Bratton et al. 2009) and GRIP1 (Frigo et al. 2006). Using endometrial cells, we have shown that DDT can activate both the p38 and ERK1/2 (extracellular signal-regulated kinases 1/2) pathways, again independently of the ER (Frigo et al. 2004). Therefore, we hypothesized that treatment of MCF-7 breast cancer cells with DDT would result in an altered gene expression profile compared with cells treated with E2, and that this altered phenotype could provide clues regarding the molecular mechanism of DDT’s distinct effects on cell physiology.
Materials and Methods
Chemicals. We purchased o,p´-DDT, p,p´-DDT, o,p´- and p,p´-dichlorodiphenyldichloroethane (DDD), p,p´-dichlorodiphenyl acetic acid (DDA), and o,p´- and p,p´-DDE from AccuStandard (New Haven, CT); 17β-estradiol (E2); all protease inhibitors; and porcine insulin from Sigma Chemical Company (St. Louis, MO); UO126 (an ERK inhibitor) from Promega (Madison, WI); SP600125 (a JNK inhibitor) from BIOMOL Research Laboratories Inc. (Plymouth Meeting, PA); and SB203580 (a p38α/β inhibitor) from EMD Biosciences (Billerica, MA). Dulbecco’s modified Eagle medium (DMEM), phenol-red free DMEM, fetal bovine serum (FBS), BME (basal medium Eagle) amino acids, MEM (minimum essential medium) amino acids, l-glutamine, penicillin, streptomycin, and sodium pyruvate were obtained from GibcoBRL (Gaitherburg, MD). We purchased charcoal-stripped FBS from HyClone (Logan, UT), Effectene from QIAGEN (Valencia, CA), and MPER (mammalian protein extraction reagent) from Pierce (Thermo Scientific, Rockford, IL).
Plasmids. Hypoxia-inducible factor 1 (HIF-1)-luciferase (HRE-luc) was donated by B.S. Beckman (Tulane University); CMV-GAL4 (negative control) was a gift from E. Flemington (Tulane University); and GAL4-CBP was donated by R. Goodman (Oregon Health Sciences University, Portland, OR). We purchased pFR-Luc [GAL4-luciferase (GAL4-luc) reporter] and pFC-MEK1 [CA-MKK1; constitutively active MAPK kinase (MKK) 1] from Stratagene (La Jolla, CA), and pcDNA3.1 from Invitrogen (Carlsbad, CA). pcDNA3-CA-MKK5 [CA-MKK5; constitutively active MAPK kinase (MKK) 5] and dominant-negative (DN) ERK2 (DN-ERK2) were gifts from J.-D. Lee (Scripps Research Institute, La Jolla, CA). pcDNA3-CA-MKK6 [CA-MKK6; constitutively active MAPK kinase (MKK) 6] and pcDNA3-CA-MKK7 [CA-MKK7; constitutively active MAPK kinase (MKK) 7] were gifts from J. Han (Scripps Research Institute). JNK1 and p38α MAPK DN mutants (DN-JNK1, DN-p38α) were provided by R. Davis (University of Massachusetts Medical School, Worcester, MA). GST (glutathione S-transferase) expression vector was purchased from Amersham Biosciences (Piscataway, NJ). pGEX-CBP1 (aa: 390-790) and pGEX-CBP3 (aa: 1990-2441) were gifts from R.G. Roeder (Rockefeller University, New York, NY). pGEX-CBP2 (aa:1680-1892) was generated by polymerase chain reaction (PCR) using HA-CBP (histone acetyltransferase–CREB-binding protein) full length (gift from R. Goodman, Oregon Health Sciences University) as a template. Resultant DNA was subcloned into the EcoR1/Sal1 site of pGEX-5X-1 (Amersham Pharmacia Biotech, Arlington Heights, IL).
Cell culture. ER-positive MCF-7 human breast carcinoma cells (Burow et al. 2000) and ER-negative human embryonic kidney (HEK) 293 cells (Kuiper et al. 1998) were maintained as previously described (Bratton et al. 2009; Rhodes et al. 2010). MCF-7 cells were grown for 48 hr in phenol red–free DMEM supplemented with 5% charcoal-stripped FBS and supplements but without insulin (5% charcoal-stripped DMEM), as previously described (Burow et al. 1999). Fulvestrant resistant MCF-7F cells were grown as previously described (Fan et al. 2006).
Quantitative PCR (qPCR) array analysis. MCF-7 cells were seeded in 6-well plates, and drug treatment was initiated after 24 hr. Cells were lysed 48 hr later, and total RNA was harvested using the RNeasy Mini Kit (QIAGEN). We used the RT2 First Strand cDNA kit (SABiosciences, Frederick, MD) to perform cDNA synthesis from total RNA according to the manufacturer’s protocol. qPCR was then performed on a BioRad IQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using a 96-well RT2 Profiler PCR Array (Breast Cancer and Estrogen Receptor Signaling PCR Array; PAHS-005; QIAGEN). Generation and analysis of cycle threshold (Ct) values were performed according to manufacturer’s instructions for the array. Three independent arrays were run for each treatment; values are presented as fold change relative to several housekeeping genes (18S rRNA, HPRT1, RPL13A, GAPDH, and ACTB). qPCR of VEGFA mRNA was performed on samples of MCF-7 cells treated with either vehicle (i.e., DMSO), DDT, or DDT plus ICI 182780 (ICI) as previously described (Bratton et al. 2009). qPCR arrays of MCF-7F cells were run on samples isolated from three independent experiments using triplicate Breast Cancer and Estrogen Receptor Signaling PCR Arrays as previously described (Tilghman et al. 2012).
Luciferase assays. MCF-7 and HEK 293 cells were transfected as previously described (Bratton et al. 2010). A GAL4-luc reporter, along with an empty expression vector or a GAL4-CBP fusion, was transfected into HEK 293 cells. The cells were then treated with vehicle or different MAPK inhibitors for 1 hr, followed by addition of vehicle or 50 μM o,p´-DDT for 18 hr. Luciferase activity was measured in 100 μL of the lysed sample using a Berthold luminometer (Titertek Instruments Inc., Huntsville, AL) and 100 μL Bright Glo luciferase assay reagent (Promega, Madison, WI).
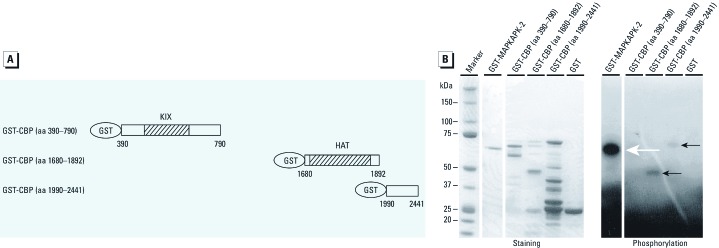
GST-fusion protein purification and in vitro kinase assay. The GST and GST-CBP fusion proteins were generated as previously described (Bratton et al. 2009). Roughly, 3–5 μg of eluted purified GST-fusion protein or 200 ng of purified mitogen-activated protein kinase (MAPK)-activated protein kinase-2 (Upstate Biotechnology, Lake Placid, NY) was phosphorylated by activated p38α as previously described (Bratton et al. 2009). Samples were analyzed by 4–12% SDS-PAGE (Invitrogen), stained with coomassie blue to monitor expression, and subjected to autoradiography as described by Bratton et al. (2010).
Results
DDT- and E2-induced gene expression. We used a qPCR-based human breast cancer pathway array to compare gene expression in MCF-7 breast cancer cells after treatment with vehicle, 1 nM E2, or 10 μM o,p´-DDT for 18 hr. E2 and DDT both significantly altered the expression of 13 genes known to be involved in breast cancer signaling. Interestingly, several genes were differentially up-regulated by DDT compared with E2, including Fas ligand (FASLG), integrin alpha 6 (ITGA6), and vascular endothelial growth factor A [VEGFA; an important factor in cellular angiogenic control mechanisms and differentiation (Zhang et al. 1995)] [Table 1; see also Supplemental Material, Table S2 (http://dx.doi.org/10.1289/ehp.1104296)]. To address whether the effect of DDT on VEGFA expression in MCF-7 cells is dependent on E2 or ERα, we assayed VEGFA expression by qPCR in MCF-7 cells incubated in the presence of the ERα inhibitor ICI. Because ICI had no effect on the DDT-mediated increase in VEGFA expression in MCF-7 cells, we concluded that the effect of DDT was ERα independent (Figure 1A). Consistent with this hypothesis, we observed a statistically significant increase in VEGFA expression in ERα-negative MCF-7F cells in response to DDT (Figure 1B; see also Supplemental Material, Table S1).
Table 1.
qPCR array analysis of MCF-7 cells.
| Gene | Description | o,p’-DDT | p-Value (DDT/veh) | E2 | p-Value (E2/veh) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2 | B-cell CLL/lymphoma 2 | 3.00 | 0.0011 | 2.65 | 0.0006 | |||||
| CCNA1 | Cyclin A1 | 1.97 | 0.0444 | 1.94 | 0.0057 | |||||
| CTSD | Cathepsin D | 2.96 | 0.0228 | 2.64 | 0.0431 | |||||
| FASLG | Fas ligand | 2.61 | 0.0156 | 0.98 | 0.9500 | |||||
| FOSL1 | FOS-like antigen 1 | 2.81 | 0.0002 | 2.72 | 0.0000 | |||||
| HMGB1 | High-mobility group box 1 | 1.70 | 0.0172 | 1.44 | 0.0013 | |||||
| IL6R | Interleukin 6 receptor | 2.11 | 0.0161 | 1.7 | 0.0548 | |||||
| ITGA6 | Integrin, alpha 6 | 2.28 | 0.0376 | 1.47 | 0.1152 | |||||
| NGFR | Nerve growth factor receptor | 1.49 | 0.0486 | 1.33 | 0.2321 | |||||
| NME1 | Non-metastatic cells 1 | 2.46 | 0.0006 | 2.96 | 0.0000 | |||||
| PGR | Progesterone receptor | 229 | 0.0000 | 152 | 0.0000 | |||||
| SCGB1D2 | Secretoglobin, family 1D, member 2 | 6.88 | 0.0035 | 2.43 | 0.0511 | |||||
| SERPINA3 | Serpin peptidase inhibitor, clade a, member 3 | 2.72 | 0.0139 | 2.62 | 0.0042 | |||||
| SERPINB5 | Serpin peptidase inhibitor, clade b, member 5 | 4.70 | 0.0004 | 4.70 | 0.0004 | |||||
| SLC7A5 | Solute carrier family 7, member 5 | 13.7 | 0.0002 | 11.62 | 0.0003 | |||||
| STC2 | Stanniocalcin 2 | 5.46 | 0.0001 | 3.94 | 0.0000 | |||||
| TFF1 | Trefoil factor 1 | 23.3 | 0.0000 | 28.93 | 0.0000 | |||||
| VEGFA | Vascular endothelial growth factor A | 1.97 | 0.0474 | 1.63 | 0.1023 | |||||
| veh, vehicle. Significantly up‑regulated genes are shown with their corresponding p‑values (n = 3 separate arrays). | ||||||||||
Figure 1.
VEGFA expression in ERα-positive MCF‑7 cells incubated for 18 hr with vehicle, 10 μM DDT, or DDT + ICI (100 nM) (A) and ERα-negative MCF‑7F cells incubated for 18 hr with vehicle or 10 μM DDT (B). qPCR results are presented as fold change relative to housekeeping genes. *p < 0.05 compared with vehicle control (n = 3).
DDT and its metabolites activate the HIF-1 response element (HRE). The VEGFA gene contains an estrogen responsive element (Kazi et al. 2005; Stoner et al. 2000, 2004) and is regulated by estrogens in mammary and uterine cells (Hyder et al. 1996; Nakamura et al. 1996, 1999). However, VEGFA expression is down-regulated by E2 in human breast cancer cells (Hyder et al. 1998). We previously showed that DDT stimulated transcription in ERα-negative human embryonic kidney cells by activating the HRE (Bratton et al. 2009). Because VEGFA contains an HRE within its promoter (Liu et al. 1995), we tested the effects of DDT and DDT metabolites on transcription of an HRE-luc reporter construct in MCF-7 breast cancer cells. Transcription was more than doubled in response to 10 μM o,p´-DDT (Figure 2A). HRE activity also increased significantly in response to the active metabolites p,p´-DDT, p,p´-DDD, o,p´-DDE, and p,p´-DDE, but not in response to the inactive metabolite p,p´-DDA (Figure 2A). E2 also activated the HRE-luc reporter in MCF-7 cells, but this effect was blocked by ICI (Figure 2B). This suggests that E2 can activate HREs; this is not surprising considering the general nature of the HRE reporter and the possibility that HREs are located within genes mediated by ERα–E2. Our cumulative results suggest that DDT alters VEGFA expression in MCF-7 cells in part by activating an HRE within the VEGFA promoter, in a manner independent of the ERα or E2. However, the fact that E2 stimulates an HRE reporter in MCF-7 cells leaves open the possibility that the DDT effect on VEGFA expression could be mediated, at least in part, through the ERα-E2 pathway.
Figure 2.
Organochlorines augment CBP activation of transcription from an HRE. (A) MCF‑7 cells transfected with an HRE-luc reporter and incubated with DDT metabolites (10 μM; n = 3). (B) MCF‑7 cells transfected with an HRE as in A, followed by incubation with vehicle, E2, or E2 + ICI (n = 3). (C) MCF‑7 cells transfected with an empty vector or a CBP expression vector plus an HRE-containing luciferase reporter and incubated overnight with vehicle or 10 μM o,p’-DDT (n = 3). (D) MCF‑7 cells treated as in C with metabolites 10 μM DDT (n = 4–6). Luminescence values are shown as the mean ± SE percentage of vehicle control, with the vehicle control set to 100%. *p < 0.05, **p < 0.01, and #p < 0.001, compared with vehicle control (A and B), the metabolite without CBP (D), or as indicated (C).
DDT potentiates CBP-induced transcriptional activation of the HRE. CBP is a general transcriptional coactivator that functions to regulate gene expression through interaction with various transcription factors, including CREB (Giordano and Avantaggiati 1999), Elk 1 (Janknecht and Nordheim 1996), c-Jun (Giordano and Avantaggiati 1999), and TBP (TATA box binding protein) (Goodman and Smolik 2000). Based on previously published data showing a direct interaction between HIF-1 and CBP (Dames et al. 2002), we hypothesized that DDT activation of CBP may potentiate the activation of HRE-mediated transcription. HRE-luc activity was unchanged in MCF-7 cells transfected with CBP, but activity increased approximately 3.3 times following the addition of 10 μM DDT to CBP-transfected cells, compared with only a 2× increase in cells transfected with an empty vector (p < 0.001 for CBP-positive versus CBP-negative cells) (Figure 2C). Other DDT metabolites also enhanced activation of the HRE-luc construct in cells expressing CBP, with the exception of the negative metabolite control p,p-DDA (Figure 2D).
DDT and its active metabolites potentiate CBP activity. HIF-1 forms a complex with CBP that increases CBP’s transactivation potential (Arany et al. 1996; Dames et al. 2002; Ema et al. 1999). We tested effects of DDT on CBP activity using a mammalian one-hybrid assay in which the full-length CBP is tethered to GAL4-DBD in conjunction with a GAL4 responsive luciferase reporter. Because our results suggested that the effect of DDT on VEGFA expression was ERα-independent, we used ERα-negative HEK 293 cells for this and subsequent experiments. The active DDT metabolites o,p´-DDT, p,p´-DDT, and o,p´-DDD potentiated CBP transactivation in a dose-dependent manner, whereas the inactive DDT metabolite, p,p´-DDA, had no effect (Figure 3A).
Figure 3.
DDT and its metabolites stimulate the coactivator CBP through activation of the p38 MAPK pathway. (A) ER-negative HEK 293 cells transfected with GAL4-CBP and a GAL4-luc reporter and incubated overnight with organochlorines; n = 4. (B) HEK 293 cells transfected overnight with GAL4‑CBP, a GAL4-luc reporter, and either an empty vector or a vector expressing constitutively active MKK1 (ERK1/2), MKK5 (ERK5), MKK6 (p38), or MKK7 (JNK); n = 3. (C) HEK 293 cells transfected with GAL4-CBP, GAL4-luc, and increasing amounts of dominant negative (DN) mutants and then treated with 50 μM o,p’‑DDT for 24 hr; n = 4–6. (D) HEK 293 cells transfected with either empty vector or GAL4-CBP and GAL4-luc; after 6 hr, MAPK inhibitors were added (1 μM UO126, 1 μM SP600125, 6 μM SB203580), followed 1 hr later by 50 μM o,p’‑DDT for 18 hr (n = 4). Values are mean ± SE luciferase activity, with control values set to 100%. *p < 0.05, **p < 0.01, and #p < 0.001, compared with vehicle control (A) or empty vector control (B,C,D).
DDT activation of CBP is dependent on the p38α MAPK pathway. We previously demonstrated that AP-1 stimulation by DDT is dependent upon the p38α MAPK cascade (Frigo et al. 2004). Therefore, we tested the role of individual MAPK signaling pathways on DDT’s activation of CBP. HEK 293 cells were transfected with GAL4-CBP and either empty vector or vectors overexpressing constitutively active MKK1, MKK5, MKK6, or MKK7 mutants that selectively activate ERK1/2, ERK5, p38α, and JNK (respectively). MKK6, and to a lesser extent MKK1, potentiated CBP activity (Figure 3B). We next tested whether p38α was necessary for DDT-induced activation of CBP in HEK 293 cells transfected with GAL4-CBP (a GAL4-luc reporter) and increasing concentrations of DN-p38α, DN-ERK1/2, or DN-JNK1 in the presence of 50 μM o,p´-DDT. DDT-mediated activation of CBP was significantly inhibited in the absence of p38α-DN expression and to a lesser extent by ERK1/2-DN (Figure 3C). To confirm our molecular findings, we blocked DDT-induced coactivator activity with pharmacological inhibitors of the MAPK pathways. A GAL4-luc reporter, along with an empty expression vector or a GAL4-CBP fusion, was transfected into HEK 293 cells. The cells were then treated with vehicle or different MAPK inhibitors for 1 hr, followed by addition of vehicle or 50 μM o,p´-DDT for 18 hr. The p38α/β inhibitor SB203580 significantly blocked (p < 0.01) o,p´-DDT induction of CBP activity (Figure 3D), whereas neither the ERK inhibitor UO126 nor the JNK inhibitor SP600125 had a significant effect (Figure 3D). Collectively, these data confirm that DDT activates the transcriptional coactivator CBP via the p38 MAPK pathway.
DDT induces the p38α-mediated phosphorylation and transcriptional activation of CBP. Various kinases have been shown to potentiate CBP by phosphorylation (Ait-Si-Ali et al. 1999; Constantinescu et al. 2004). We hypothesized that p38α MAPK directly phosphorylates CBP, leading to its potentiation. To test this, we bacterially expressed recombinant CBP fused to GST for purification (Figure 4A) and subjected the purified proteins to an in vitro kinase assay in the presence of 32P (phosphorus-32) and activated p38α MAPK. The C-terminal fragments of CBP containing amino acids 1680–1892 and, to a lesser extent, 1990–2441 were phosphorylated by activated p38α, whereas the N-terminal fragment (amino acids 390–790) was not (Figure 4B). Activation of the C-terminal of CBP by DDT was tested using a deletion mutant of CBP containing amino acids 1300–2441 in a GAL4 fusion vector (Figure 5A). We overexpressed either the empty vector or a constitutively active MKK6 mutant in HEK 293 cells, in the presence or absence of o,p´-DDT. MKK6 activated the C-terminal of CBP in the absence of o,p´-DDT, but CBP activity was further augmented with the addition of 50 μM o,p´-DDT (Figure 5B). Taken together, these results suggest that DDT augments p38 activity, which in turn phosphorylates CBP within its C-terminal, resulting in increased CBP transcriptional activation.
Figure 4.
Activated p38α phosphorylates CBP in vitro. (A) Schematic of GST fusion proteins used for the in vitro kinase assays; aa, amino acids. (B) GST fusion proteins were purified and standardized according to protein concentration. Purified activated p38α was used to phosphorylate GST-CBP fragments in the presence of 32P-ATP followed by SDS‑PAGE and coomassie staining (left). Gels were dried and autoradiographed (right). GST-MAPKAPK2 was used as a positive control for p38α phosphorylation. The white arrow indicates phosphorylated GST-MAPKAPK2, and black arrows indicate phosphorylated GST-CBP fragments. Similar results were obtained in three independent experiments.
Figure 5.
DDT and p38α target the C‑terminal of CBP. (A) Schematic of GAL4-CBP fusion constructs used for mammalian one-hybrid analysis; aa, amino acids. (B) HEK 293 cells transfected for 6 hr with full-length GAL4-CBP (FL) or the C‑terminal (C‑term) fragment GAL4-CBP (aa 1300-2441) plus GAL4‑luc. Some cells were also transfected with the CA-MKK6 mutant; other cells were transfected with MKK6-CA and incubated overnight with 50 μM o,p’‑DDT. Values represent the percent change (mean ± SE; n = 4) in CBP activity, with the vehicle set to 100%. **p < 0.01, and #p < 0.001 compared with vehicle control.
Discussion
Although estrogenic activity of DDT has been reported (Ahlborg et al. 1995; Gulledge et al. 2001; Klotz et al. 1996; Kuiper et al. 1998), the mechanism underlying the hormone activity of the organochlorine pesticide remains unclear. We have previously shown that DDT and its metabolites activate transcription factors such as AP-1 independently of ERα (Bratton et al. 2009; Frigo et al. 2004). In the present study, we further investigated the molecular differences in hormone action between DDT and E2 and characterized the qualities of DDT compared with other compounds that display estrogen-like properties. Although DDT and E2 both stimulated the transcription of a subset of ERα-regulated genes, including Bcl-2, PgR, and trefoil factor 1 (TFF1), DDT also up-regulated genes that were not affected by E2, including FASLG, ITGA6, and VEGFA (Table 1).
Differential gene expression induced by “estrogenic” environmental contaminants has been reported. For example, Goodson and colleagues treated nonmalignant high-risk donor breast epithelial cells (HRBECs) with E2 and BPA; using global gene expression analysis, they determined that BPA produced a distinct gene expression pattern compared with E2 (Dairkee et al. 2008; Goodson et al. 2011). Han et al. (2010) recently reported that DDT up-regulated aromatase gene expression in MCF-7 cells independently of ER function. Results of the present study also suggest that DDT is capable of altering gene expression in breast cancer cells in a manner different from that of E2.
Our gene expression analysis revealed that DDT up-regulated VEGFA, an important factor in angiogenic cell response and regulation, as well as cell differentiation (Zhang et al. 1995). DDT increased VEGFA expression in MCF-7 cells, even in the presence of the pure antiestrogen ICI, suggesting that the DDT effect is ERα independent. In addition, DDT increased VEGFA expression in the ERα-negative MCF-7F cell line. Although crosstalk can occur between DDT signaling estrogen response elements, as previously shown (Bratton et al. 2009), the results presented here strongly suggest that DDT-altered VEGFA expression in MCF-7 breast cancer cells is ERα independent.
Our results also suggest that DDT and its metabolites potentiate the activity of HIF-1α, which is known to bind the VEGFA promoter (Liu et al. 1995). However, because E2 activated the HRE reporter, ERα-independent effects of E2 on HRE activation and VEGFA expression remain a possibility. We have previously shown that DDT can regulate gene expression through the phosphorylation of coregulatory proteins such as SRC-2/GRIP1 (glucocorticoid receptor-interacting protein 1, steroid receptor coactivator-2), a member of the NCoA family of coregualtors (Frigo et al. 2006). Here, we demonstrated that active DDT compounds increased CBP activity and CBP-mediated transactivation of an HRE-linked reporter gene. DDT concentrations used in our experiments (10–50 μM) may appear high, but DDT metabolite levels > 20 ng/mL in blood (equivalent to 63 μM) have been reported (Longnecker et al. 2002; López-Carrillo et al. 2001; Martin et al. 2002), as well as levels > 4 mM in soils throughout North America (Aigner et al. 1998; Falconer et al. 1997; U.S. Geological Survey 2001). These results, taken together, support a role for DDT in activation of the CBP–HIF-1 complex and suggest a mechanism by which DDT increases VEGFA expression.
We used both molecular and pharmacological tools to investigate the role of MAPK pathways in the DDT–CBP–HIF-1 signaling cascade. We showed that activation of the p38 pathway potentited CBP activity and that DDT’s effect on CBP activation was inhibited by blocking p38α. Finally, we showed that p38α directly phosphorylated the C-terminal of CBP,and that p38 activated CBP via its C-terminal region. These data, in conjunction with published reports of a direct interaction between the coactivator CBP and HIF-1α (Dames et al. 2002) suggest a mechanism for the expression of VEGFA in MCF-7 cells following DDT exposure: DDT activates p38, which leads to phosphorylation of CBP and enhanced binding to HIF-1α; the resulting HIF-1α–CBP complex binds to VEGFA promoter, increasing its transcription (Figure 6).
Figure 6.
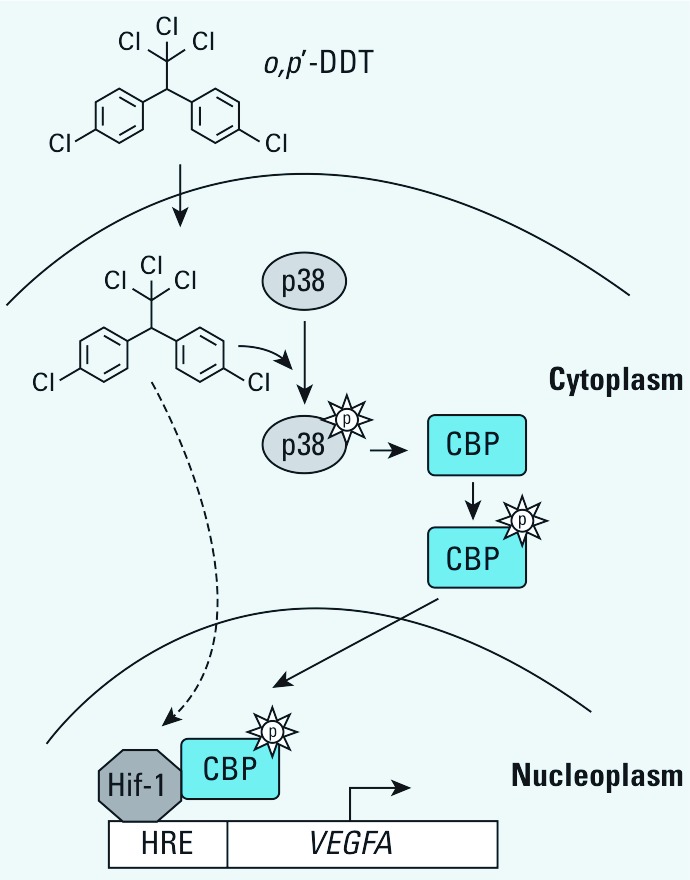
Proposed mechanism of organochlorine-mediated up‑regulation of VEGFA expression. We propose a mechanism whereby o,p’-DDT stimulates phosphorylation of p38 kinase, which in turn phosphorylates CBP. The activated CBP binds HIF-1, and this complex binds to the VEGFA promoter at the HRE, thereby increasing transcription of VEGFA.
Conclusions
Overall, our data demonstrate a link between organochlorine-mediated cell signaling through a MAPK pathway and the direct phosphorylation and regulation of coactivator function. These data suggest that coactivator phosphorylation might serve as a cellular sensor of environmental stress and lead to the modulation of key sets of adaptive genes. Moreover, these results suggest a possible mechanism by which environmental compounds may exert more, or less, E2-like potency than their ERα affinity implies.
Supplemental Material
Footnotes
This work was supported by the Office of Naval Research (N00014-11-1-0177 to J.A.M. and M.E.B.), the National Institutes of Health (DK059389 to M.E.B., 5G12RR026260-02 to T.E.W., K01DK084205 to D.E.F., and National Cancer Institute U54 CA113001-07 to K.P.N.), and the Department of Defense (W81XWH-04-1-0557 BC030300 to T.E.W.).
The authors declare they have no actual or potential competing financial interests.
References
- Ahlborg UG, Lipworth L, Titus-Ernstoff L, Hsieh CC, Hanberg A, Baron J, et al. Organochlorine compounds in relation to breast cancer, endometrial cancer, and endometriosis: an assessment of the biological and epidemiological evidence. Crit Rev Toxicol. 1995;25(6):463–531. doi: 10.3109/10408449509017924. [DOI] [PubMed] [Google Scholar]
- Aigner EJ, Leone AD, Falconer RL. Concentrations and enantiomeric ratios of organochlorine pesticides in soils from the U.S. Corn Belt. Environ Sci Technol. 1998;32(9):1162–1168. [Google Scholar]
- Ait-Si-Ali S, Carlisi D, Ramirez S, Upegui-Gonzalez LC, Duquet A, Robin P, et al. Phosphorylation by p44 MAP kinase/ERK1 stimulates CBP histone acetyl transferase activity in vitro. Biochem Biophys Res Commun. 1999;262(1):157–162. doi: 10.1006/bbrc.1999.1132. [DOI] [PubMed] [Google Scholar]
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93(23):12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton MR, Duong BN, Elliott S, Weldon CB, Beckman BS, McLachlan JA, et al. Regulation of ERα-mediated transcription of Bcl-2 by PI3K-AKT crosstalk: implications for breast cancer cell survival. Int J Oncol. 2010;37(3):541–550. doi: 10.3892/ijo_00000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton MR, Frigo DE, Vigh-Conrad KA, Fan D, Wadsworth S, McLachlan JA, et al. Organochlorine-mediated potentiation of the general coactivator p300 through p38 mitogen-activated protein kinase. Carcinogenesis. 2009;30(1):106–113. doi: 10.1093/carcin/bgn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow ME, Tang Y, Collins-Burow BM, Krajewski S, Reed JC, McLachlan JA, et al. Effects of environmental estrogens on tumor necrosis factor α-mediated apoptosis in MCF-7 cells. Carcinogenesis. 1999;20(11):2057–2061. doi: 10.1093/carcin/20.11.2057. [DOI] [PubMed] [Google Scholar]
- Burow ME, Weldon CB, Chiang TC, Tang Y, Collins-Burow BM, Rolfe K, et al. Differences in protein kinase C and estrogen receptor alpha, beta expression and signaling correlate with apoptotic sensitivity of MCF-7 breast cancer cell variants. Int J Oncol. 2000;16(6):1179–1187. doi: 10.3892/ijo.16.6.1179. [DOI] [PubMed] [Google Scholar]
- Cocco P, Kazerouni N, Zahm SH. Cancer mortality and environmental exposure to DDE in the United States. Environ Health Perspect. 2000;108:1–4. doi: 10.1289/ehp.001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect. 2007;115:1442–1447. doi: 10.1289/ehp.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DC, Wainman B, Sanin LH, Weber JP, Muggah H, Ibrahim S. Environmental contaminant levels and fecundability among non-smoking couples. Reprod Toxicol. 2006;22(1):13–19. doi: 10.1016/j.reprotox.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Constantinescu A, Wu M, Asher O, Diamond I. cAMP-dependent protein kinase type I regulates ethanol-induced cAMP response element-mediated gene expression via activation of CREB-binding protein and inhibition of MAPK. J Biol Chem. 2004;279(41):43321–43329. doi: 10.1074/jbc.M406994200. [DOI] [PubMed] [Google Scholar]
- Dairkee SH, Seok J, Champion S, Sayeed A, Mindrinos M, Xiao W, et al. Bisphenol A induces a profile of tumor aggressiveness in high-risk cells from breast cancer patients. Cancer Res. 2008;68(7):2076–2080. doi: 10.1158/0008-5472.CAN-07-6526. [DOI] [PubMed] [Google Scholar]
- Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Structural basis for HIF-1α/CBP recognition in the cellular hypoxic response. Proc Natl Acad Sci USA. 2002;99(8):5271–5276. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, et al. Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18(7):1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer RL, Bidleman TF, Szeto SY. Chiral pesticides in soils of the Fraser Valley, British Columbia. J Agric Food Chem. 1997;45(5):1946–1951. [Google Scholar]
- Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66(24):11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- Frigo DE, Basu A, Nierth-Simpson EN, Weldon CB, Dugan CM, Elliott S, et al. p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol. 2006;20(5):971–983. doi: 10.1210/me.2004-0075. [DOI] [PubMed] [Google Scholar]
- Frigo DE, Burow ME, Mitchell KA, Chiang TC, McLachlan JA. DDT and its metabolites alter gene expression in human uterine cell lines through estrogen receptor-independent mechanisms. Environ Health Perspect. 2002;110:1239–1245. doi: 10.1289/ehp.021101239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigo DE, Tang Y, Beckman BS, Scandurro AB, Alam J, Burow ME, et al. Mechanism of AP-1-mediated gene expression by select organochlorines through the p38 MAPK pathway. Carcinogenesis. 2004;25(2):249–261. doi: 10.1093/carcin/bgh009. [DOI] [PubMed] [Google Scholar]
- Giordano A, Avantaggiati ML. p300 and CBP: partners for life and death. J Cell Physiol. 1999;181(2):218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14(13):1553–1577. [PubMed] [Google Scholar]
- Goodson WH, III, Luciani MG, Sayeed SA, Jaffee IM, Moore DH, II, Dairkee SH. Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women. Carcinogenesis. 2011;32(11):1724–1733. doi: 10.1093/carcin/bgr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge CC, Burow ME, McLachlan JA. Endocrine disruption in sexual differentiation and puberty. What do pseudohermaphroditic polar bears have to do with the practice of pediatrics? Pediatr Clin North Am. 2001;48(5):1223–1240. doi: 10.1016/s0031-3955(05)70371-0. [DOI] [PubMed] [Google Scholar]
- Han EH, Kim HG, Hwang YP, Choi JH, Im JH, Park B, et al. The role of cyclooxygenase-2-dependent signaling via cyclic AMP response element activation on aromatase up-regulation by o,p’-DDT in human breast cancer cells. Toxicol Lett. 2010;198(3):331–341. doi: 10.1016/j.toxlet.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Hardell L, van Bavel B, Lindström G, Björnfoth H, Orgum P, Carlberg M, et al. Adipose tissue concentrations of p,p’-DDE and the risk for endometrial cancer. Gynecol Oncol. 2004;95(3):706–711. doi: 10.1016/j.ygyno.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Murthy L, Stancel GM. Progestin regulation of vascular endothelial growth factor in human breast cancer cells. Cancer Res. 1998;58(3):392–395. [PubMed] [Google Scholar]
- Hyder SM, Stancel GM, Chiappetta C, Murthy L, Boettger-Tong HL, Makela S. Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res. 1996;56(17):3954–3960. [PubMed] [Google Scholar]
- Janknecht R, Nordheim A. MAP kinase-dependent transcriptional coactivation by Elk-1 and its cofactor CBP. Biochem Biophys Res Commun. 1996;228(3):831–837. doi: 10.1006/bbrc.1996.1740. [DOI] [PubMed] [Google Scholar]
- Kazi AA, Jones JM, Koos RD. Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor α and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol. 2005;19(8):2006–2019. doi: 10.1210/me.2004-0388. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Gobas FA, McLachlan MS. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ Toxicol Chem. 2004;23(10):2324–2336. doi: 10.1897/03-545. [DOI] [PubMed] [Google Scholar]
- Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 1996;104:1084–1089. doi: 10.1289/ehp.961041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ Res. 1995;77(3):638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, et al. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol. 2002;155(4):313–322. doi: 10.1093/aje/155.4.313. [DOI] [PubMed] [Google Scholar]
- López-Carrillo L, Torres-Sánchez L, Moline J, Ireland K, Wolff MS. Breast-feeding and serum p,p’DDT levels among Mexican women of childbearing age: a pilot study. Environ Res. 2001;87(3):131–135. doi: 10.1006/enrs.2001.4296. [DOI] [PubMed] [Google Scholar]
- Martin SA, Jr, Harlow SD, Sowers MF, Longnecker MP, Garabrant D, Shore DL, et al. DDT metabolite and androgens in African-American farmers. Epidemiology. 2002;13(4):454–458. doi: 10.1097/00001648-200207000-00014. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst. 2008;100(9):663–671. doi: 10.1093/jnci/djn101. [DOI] [PubMed] [Google Scholar]
- McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev. 2001;22(3):319–341. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Simpson E, Martin M. Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab. 2006;20(1):63–75. doi: 10.1016/j.beem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Lu Q, Aberdeen G, Albrecht E, Brodie A. The effect of estrogen on aromatase and vascular endothelial growth factor messenger ribonucleic acid in the normal nonhuman primate mammary gland. J Clin Endocrinol Metab. 1999;84(4):1432–1437. doi: 10.1210/jcem.84.4.5641. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Savinov A, Lu Q, Brodie A. Estrogen regulates vascular endothelial growth/permeability factor expression in 7,12-dimethylbenz(a)anthracene-induced rat mammary tumors. Endocrinology. 1996;137(12):5589–5596. doi: 10.1210/endo.137.12.8940388. [DOI] [PubMed] [Google Scholar]
- Porta M, Bosch de Basea M, Benavides FG, Lopez T, Fernandez E, Marco E, et al. Differences in serum concentrations of organochlorine compounds by occupational social class in pancreatic cancer. Environ Res. 2008;108(3):370–379. doi: 10.1016/j.envres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, et al. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat. 2010;121(2):293–300. doi: 10.1007/s10549-009-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rier S, Foster WG. Environmental dioxins and endometriosis. Toxicol Sci. 2002;70(2):161–170. doi: 10.1093/toxsci/70.2.161. [DOI] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Rylander L, Hagmar L. Exposure to persistent organochlorine pollutants and type 2 diabetes mellitus. Hum Exp Toxicol. 2007;26(5):447–452. doi: 10.1177/0960327107076886. [DOI] [PubMed] [Google Scholar]
- Safe SH, Zacharewski T. Organochlorine exposure and risk for breast cancer. Prog Clin Biol Res. 1997;396:133–145. [PubMed] [Google Scholar]
- Sasco AJ. Breast cancer and the environment. Horm Res. 2003;60(suppl 3):50. doi: 10.1159/000074500. [DOI] [PubMed] [Google Scholar]
- Stoner M, Wang F, Wormke M, Nguyen T, Samudio I, Vyhlidal C, et al. Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor α and Sp3 proteins. J Biol Chem. 2000;275(30):22769–22779. doi: 10.1074/jbc.M002188200. [DOI] [PubMed] [Google Scholar]
- Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, et al. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor α and SP proteins. Oncogene. 2004;23(5):1052–1063. doi: 10.1038/sj.onc.1207201. [DOI] [PubMed] [Google Scholar]
- Tilghman SL, Bratton MR, Segar HC, Martin EC, Rhodes LV, Li M, et al. 2012Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PloS one 73e32754 doi: 10.1371/journal.pone.0032754[Online 5 March 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman SL, Nierth-Simpson EN, Wallace R, Burow ME, McLachlan JA. Environmental hormones: multiple pathways for response may lead to multiple disease outcomes. Steroids. 2010;75(8-9):520–523. doi: 10.1016/j.steroids.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Tullner WW. Uterotrophic action of the insecticide methoxychlor. Science. 1961;133:647–648. doi: 10.1126/science.133.3453.647. [DOI] [PubMed] [Google Scholar]
- U.S. Geological Survey. Reston, VA: U.S. Geological Survey; 2001. Summary Statistics for Organochlorine Compounds in Whole Fish. [Google Scholar]
- Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993;85(8):648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Craft P, Scott PA, Ziche M, Weich HA, Harris AL, et al. Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst. 1995;87(3):213–219. doi: 10.1093/jnci/87.3.213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.