Abstract
Whether the intranasal (i.n.) route of Mycobacterium bovis BCG vaccination provides better protection against pulmonary tuberculosis than subcutaneous (s.c.) vaccination remains an incompletely solved issue. In the present study, we compared both immune responses and protection elicited by single BCG vaccinations via the i.n. or s.c. route in BALB/c mice. While both i.n. and s.c. vaccination triggered comparable levels of primary immune activation in the spleen and draining lymph nodes, i.n. vaccination led to a greater antigen-specific gamma interferon recall response in splenocytes than s.c. vaccination upon secondary respiratory mycobacterial challenge, accompanied by an increased frequency of antigen-specific lymphocytes. There was also a quicker cellular response in the lungs of i.n. vaccinated mice upon mycobacterial challenge. Mice vaccinated i.n. were found to be much better protected, particularly in the lung, than s.c. vaccinated counterparts against pulmonary tuberculosis at both 3 and 6 months postvaccination. These results suggest that the i.n. route of vaccination improves the protective effect of the current BCG vaccine.
Tuberculosis (TB) is primarily a pulmonary infectious disease and remains one of the leading infectious causes of death worldwide, and the human immunodeficiency virus epidemic has further worsened its morbidity and mortality (8, 15, 36, 37). The attenuated Mycobacterium bovis bacillus Calmette-Guérin (BCG) has been widely used for 80 years as the only available vaccine against TB. However, its protective efficacy varies from 0 to 80% according to several clinical and field trials, and the current TB epidemic indicates that in general BCG vaccine has failed to confer effective immune protection against TB, particularly adult TB (2, 4, 7). Among several factors that are suspected of underlying the failure of BCG is the route of vaccination. BCG has been administered intradermally or percutaneously to humans (15, 25, 36). Such a route of vaccination was chosen based on the earlier belief that the extent of skin delayed-type hypersensitivity (DTH) positively correlated with immune protection and that percutaneous BCG immunization led to stronger DTH than oral immunization. Since TB is primarily a respiratory tract infectious disease and since DTH is no longer believed to correlate with immune protection, the question of whether respiratory mucosal BCG vaccination provides better protection than percutaneous vaccination remains.
Vaccination at the mucosal site has been believed to be superior to vaccination at other sites for eliciting protective immune responses against mucosal infectious diseases (5). Although the mechanisms remain to be fully understood, mucosal vaccination via intranasal (i.n.), intragastric, and intrarectal routes was found to be effective in conferring protection against several nontuberculous infectious diseases (9, 13, 38). While relatively little has been investigated in the context of TB, both intrarectal and intragastric BCG vaccination has been explored (1, 19). In these studies, it was found that an enormous dose of BCG was required to achieve a level of protection comparable to that by subcutaneous (s.c.) BCG vaccination. Since TB is primarily a respiratory airway infectious disease, it is believed that mucosal vaccination directed to the respiratory system may provide the best protection against pulmonary TB (5). While respiratory mucosal TB vaccination has previously been explored by delivering aerosolized BCG, conflicting results were reported (17, 21, 24, 26, 28), and it is unlikely that aerosol delivery of BCG will eventually become a mode of BCG vaccination for humans. In this regard, i.n. vaccination offers desirable vaccination advantages, such as ease, feasibility, and the ability to trigger both mucosal and systemic immune activation (5). Indeed, both in humans and rodents, organized lymphoid aggregates and tissues are present both in the nasal and bronchial mucosa and play an important role in the initiation of mucosa-associated immunity against infectious agents (11, 16). Falero-Diaz et al. reported the first study involving i.n. BCG vaccination which conferred potent protection against pulmonary TB in mice (6). However, such protection involved two sequential i.n. BCG vaccinations and was not compared in parallel with that by s.c. BCG vaccination. Lyadova et al. have recently demonstrated that i.n. BCG vaccination confers slightly better protection than s.c. vaccination against systemic M. bovis challenge (22).
In our present study, we have compared the immune responses and protection in BALB/c mice elicited by a single parenteral immunization (s.c.) or a single i.n. immunization with a moderate dose of BCG. Our results demonstrate that the i.n. route of BCG vaccination is superior to the s.c. route for protection from pulmonary tuberculosis (i.n. vaccination doubles the protection by s.c. vaccination) and thus support the concept of this route of vaccination with BCG or other new TB vaccines under development for potential human applications.
MATERIALS AND METHODS
Mice.
Female BALB/c mice 6 to 8 weeks old were used (Harlan, Indianapolis, Ind.). All mice were housed in a specific-pathogen-free level B facility. Experiments performed were in accordance with the guidelines of the Animal Research Ethics Board of McMaster University.
Preparation of M. bovis BCG and Mycobacterium tuberculosis.
The original stock of M. bovis BCG (Connaught strain) was obtained from Connaught Laboratories (North York, Ontario, Canada) (31). The stock was maintained by collecting mycobacterial colonies from agar plates cultured out of BCG-infected C57BL/6 mouse lungs. To amplify, BCG was inoculated into a cell culture flask with 50 ml of 7H9 broth (Difco, Detroit, Mich.) containing 0.05% Tween 80, 0.002% glycerol, and Middlebrook oleic acid-albumin-dextrose-catalase enrichment (Gibco-BRL, Gaithersburg, Md.). The culture was incubated at 37°C for 10 to 12 days with gentle aeration. A 1/2 volume of medium was then discarded, and glycerol was added to a final concentration of 10%. Aliquoted stock was stored at −70°C. To prepare working stock, mycobacteria were spun down, washed twice with phosphate-buffered saline (PBS)-Tween 80 (0.05%), resuspended in freezing media, and stored in 50-μl aliquots at −70°C until vaccination.
The original stock of a standard stain of virulent M. tuberculosis (H37Rv) was provided by Fiona Smaill (Microbiology Division, McMaster University Medical Centre). The M. tuberculosis stock was maintained by collecting colonies from tissue homogenates of lungs of M. tuberculosis-infected BALB/c mice. M. tuberculosis bacilli were cultured in 7H9 broth for 10 to 12 days and harvested by removing 1/2 volume of culture medium and adding glycerol to a final concentration of 10%. The culture was stored at −70°C. Before infection, an aliquot of M. tuberculosis was spun down, washed twice with PBS-Tween 80 (0.05%), resuspended in PBS, and dispersed by being passed through a 27-gauge needle 10 times. The titer of stock was verified on a regular basis.
s.c. and i.n. BCG vaccination.
Before use, a brief sonication was performed to disperse BCG clumps. Mice were immunized with BCG (5 × 104, 1 × 105, and 5 × 105 CFU/mouse for dose-response experiments; 5 × 105 CFU/mouse for all challenge experiments) s.c. or i.n. s.c. injection was performed with a 26-gauge needle on both sides of the subiliac area of the mouse in a volume of 50 μl for each side. i.n. administration was carried out by inoculation of a total volume of 30 μl of BCG suspension to the nostril by using a pipette and tip, and the mouse was allowed to breathe the suspension into the lung naturally (33). For either route of vaccination, PBS was used as the control.
Pulmonary BCG and M. tuberculosis challenge.
At selected time points postvaccination, mice were challenged intratracheally with 5 × 106 CFU of live BCG by following a previously described procedure (31-34). For observation of protection, mice were challenged i.n. with virulent M. tuberculosis (10,000 CFU/mouse; diluted in 20 μl) and housed in our biohazard level III TB vaccine research facility.
BAL and cytologic analysis.
Bronchoalveolar lavage (BAL) was carried out as previously described (31-34). Briefly, lungs were removed along with the heart and a portion of the trachea. A segment of polyethylene tube (Becton Dickinson, Sparks, Md.) was attached to a 23-gauge needle with syringe. The tube was inserted into the trachea portion, lungs were lavaged twice with PBS (0.25 and 0.2 ml), and approximately 0.4 ml of BAL fluid was recovered. BAL supernatants were removed and stored at −20°C for cytokine analysis. Cell pellets were resuspended in PBS, and the total cell number was determined. Cytospins were made with about 0.8 × 105 to 1 × 105 cells per spin in a cytospin machine (Shandon Inc., Pittsburgh, Pa.), and cells were stained by Diff-Quick stain (Baxter, McGaw Park, Ill.) for differential cell counting. Normally, 400 to 500 cells/cytospin were counted and differentiated under a microscope.
Isolation of lymphocytes and in vitro antigen stimulation.
Following vaccination or challenge, mice were sacrificed at different time points, and spleens and the local lymph nodes draining the site of vaccination (subiliac and mediastinal thoracic lymph nodes for skin and i.n. vaccination, respectively) were aseptically removed. Cells were isolated and cultured as previously described (32, 33, 39). Approximately 0.5 × 106 cells/well were seeded into 96-well plates and cultured with RPMI 1640 medium (supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine) in the presence or absence of mycobacterial antigens including crude BCG (cBCG; UV-inactivated BCG bacilli; 1 μl/well), M. tuberculosis culture filtrate protein (CFP) (4 μg/ml), or the irrelevant antigen keyhole limpet hemocyanin (Sigma, St. Louis, Mo.; 10 μg/ml). Supernatant was collected at 72 h and stored at −20°C until cytokine analysis by enzyme-linked immunosorbent assay (ELISA).
Mycobacterial colony enumeration assay.
At different time points after BCG or M. tuberculosis challenge, lungs and spleens were removed and placed in 4.5 ml of PBS/organ with 0.05% Tween 80 and homogenized with a tissue homogenizer (Kinematica, Littau, Switzerland) for about 30 s or until no tissue chunks remained. Two hundred microliters of serially diluted homogenates was plated onto Middlebrook 7H10 agar plates containing 10% oleic acid-albumin-dextrose-catalase enrichment and 0.5% glycerol (Difco) (31, 32, 39). Plates were semisealed in a plastic bag to prevent overdrying and incubated at 37°C for 13 to 18 days. Colonies were enumerated with a dissection microscope.
Measurement of cytokines in BAL fluid and cell culture supernatant.
The level of gamma interferon (IFN-γ) was measured in BAL fluid, sera, and cell culture supernatant by using a mouse-specific ELISA kit (R&D Systems Inc., Minneapolis, Minn.). The sensitivity of the assay is ≤5 pg/ml.
ELISpot assay.
The number of mycobacterial-antigen-specific IFN-γ-producing cells was determined by enzyme-linked immunospot (ELISpot) assay (35). Ninety-six-well filtration plates (IPVH membrane with 0.45-μm pores; Millipore Corporation, Bedford, Mass.) were coated with 1:60-diluted anti-mouse IFN-γ capture antibody (R&D Systems Inc.) at 4°C overnight and then washed with 0.05% Tween 20-PBS. Membranes were blocked by adding blocking buffer (1% bovine serum albumin-5% sucrose in PBS), incubated at room temperature for 2 h, and then washed again and rinsed with culture medium. Splenocytes or lymph node cells (0.5 × 106) were added to each well with or without stimulation by M. tuberculosis CFP (1 μl/well). Each condition was set up in duplicate. After 24 h of incubation at 37°C in a 5% CO2 incubator, cells were washed away, and 1:60-diluted detection antibody (R&D Systems Inc.) was added and incubated at 4°C overnight. Nonbound antibody was washed away with 0.5% Tween 20-PBS, and 1:60-diluted streptavidin-conjugated alkaline phosphatase was added and incubated for 2 h at room temperature. Substrates BCIP (5-bromo-4-chloro-3-indolylphosphate) and nitroblue tetrazolium (R&D Systems Inc.) were added, cells were incubated for 30 min at room temperature, and then plates were rinsed with distilled water. Dark blue spots in each well were quantified with a dissection microscope.
Data analysis.
Wherever applicable, the difference comparison was made by using a Student t test within the Microsoft Excel data analysis program. The difference was considered statistically significant when P was ≤0.05.
RESULTS
Dose-dependent immune responses triggered by s.c. and i.n. BCG vaccination.
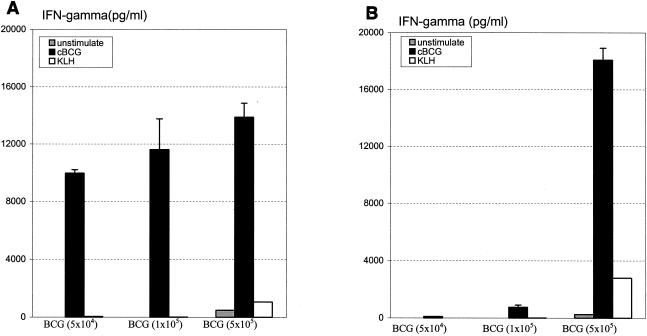
To assess the impact of BCG dose on the level of immune responses, three different doses of BCG (5 × 104, 1 × 105, and 5 × 105 CFU) were administered s.c. or i.n. to BALB/c mice. At 3 weeks, cells from the spleen were cultured with mycobacterial antigen in vitro, and the level of antigen recall IFN-γ response was used as a readout of type 1 immune activation. After s.c. vaccination, high levels of immune activation were detected with the three BCG doses used, resulting in IFN-γ levels that ranged from 9,969.11 ± 242.95 to 13,883 ± 969.80 pg/ml (Fig. 1A). In comparison, following i.n. vaccination, while there was also a BCG dose-dependent IFN-γ response, a dramatically enhanced response was seen only in mice i.n. vaccinated with the dose of 5 × 105 CFU of BCG (18,081.31 ± 838.54 pg/ml) (Fig. 1B). Thus, for all of the following experiments, this dose of BCG vaccine was used for the comparison between s.c. and i.n. vaccinations.
FIG. 1.
BCG dose-dependent immune activation. Three doses of BCG were administered s.c. (A) or i.n. (B) to BALB/c mice. After 3 weeks, splenocytes were pooled from two mice per treatment and were stimulated with mycobacterial antigens (cBCG) or unrelated antigen (keyhole limpet hemocyanin [KLH]). IFN-γ in the culture supernatant was measured. Results are expressed as means ± standard errors of the means from triplicate determinations per condition.
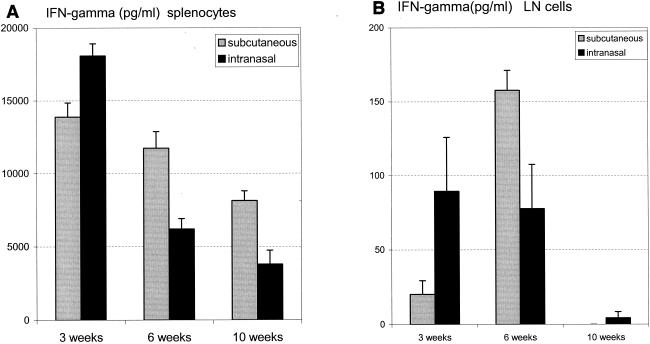
Kinetics of primary immune responses triggered by s.c. and i.n. BCG vaccination.
The kinetics of immune responses in the spleen and draining lymph nodes was determined at weeks 3, 6, and 10 after BCG vaccination through s.c. and i.n. routes. In general, with both s.c. and i.n. vaccinations, immune activation in the spleen peaked at week 3 and gradually declined thereafter (Fig. 2). In comparison, the responses in the lymph nodes of s.c. and i.n. immunized mice appeared to peak around week 6 and were drastically reduced by week 10 (Fig. 2). The potentially different kinetics of lymph node responses due to i.n. and s.c. vaccinations perhaps reflect different migratory properties of lymphocytes assumed as a result of different sites of vaccination.
FIG. 2.
Kinetics of immune responses triggered by s.c. or i.n. BCG vaccination. A dose of 5 × 105 CFU of BCG was administrated s.c. or i.n. to BALB/c mice. At weeks 3, 6, and 9, splenocytes (A) and thoracic lymph node (LN) cells (B) were isolated and pooled from two or three mice per group and cultured with or without mycobacterial antigen cBCG. IFN-γ in the culture supernatant was measured. Results are presented as means ± standard errors of the means from triplicate determinations.
Secondary immune responses upon reexposure to M. bovis BCG challenge in mice after s.c. and i.n. vaccination.
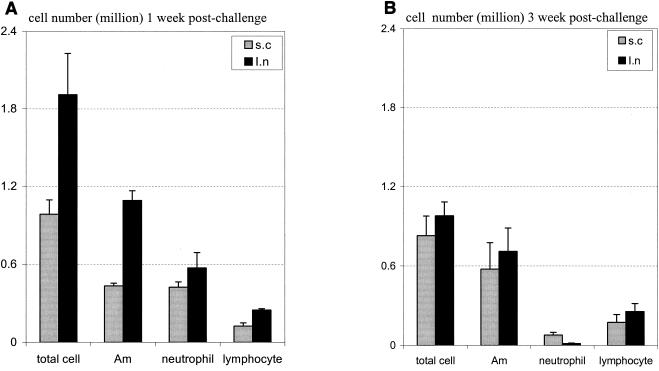
Having examined the primary immune response to s.c. and i.n. BCG vaccination, we compared the secondary immune responses upon airway challenge with a high dose of BCG bacilli (5 × 106 CFU/mouse) in mice at 10 weeks after s.c. or i.n. vaccination. This time point was chosen because we found that, by 10 weeks after i.n. immunization, the inflammation in the lung was largely resolved and there were a very small number of mycobacteria remaining in the lung and spleen (on average, 2,800 and 45 CFU in the lung and spleen, respectively). In addition, the primary immune response also largely subsided (32). Hence, week 10 postvaccination represents a good time point where the secondary immune response and mycobacterial burden upon reexposure to mycobacterial infection, could be appropriately evaluated. We first examined the cellular responses in the lung at 1 and 3 weeks postchallenge. Examination at 1 week allowed an assessment of the early immune response to secondary challenge. At 1 week postchallenge, there was a greater number of BAL inflammatory cells in i.n. vaccinated mice than in s.c. vaccinated counterparts (Fig. 3A). There were a total of (1.91 ± 0.32) × 106 leukocytes/lung recovered from i.n. vaccinated mice, with significantly more macrophages and lymphocytes. In comparison, a total of (0.99 ± 0.11) × 106 leukocytes/lung were recovered from the lungs of s.c. vaccinated mice. At 3 weeks postchallenge, the overall magnitude of cellular responses in the lungs of i.n. vaccinated mice declined and became similar to that in the lungs of s.c. vaccinated mice (Fig. 3B). Thus, i.n. vaccinated hosts were able to mount a more vigorous secondary cellular response than s.c. vaccinated hosts upon reexposure to mycobacteria in the lung.
FIG. 3.
Inflammatory cell composition in the lung upon secondary pulmonary BCG challenge. BALB/c mice were challenged intratracheally with BCG at 10 weeks after s.c. or i.n. vaccination, and BAL fluid was obtained at 1 week (A) and 3 weeks (B) postchallenge. The numbers of total leukocytes and differential immune cell subsets (alveolar macrophages [Am]) were determined by differential cell counting performed on stained BAL fluid cytospins. Results are expressed as means ± standard errors of the means from four (1 week) or five (3 weeks) mice per time point. The differences in Am and lymphocytes between s.c. and i.n. vaccinated mice shown in panel A are statistically significant (P < 0.05).
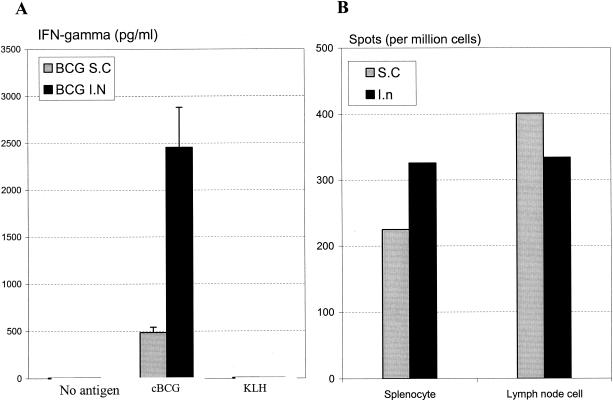
Also, the in vitro lymphocyte recall response upon mycobacterial antigen stimulation was evaluated ex vivo postchallenge. While s.c. and i.n. vaccination elicited comparable levels of IFN-γ response in antigen-stimulated lymph node-derived lymphocytes (data not shown), splenocytes isolated from i.n. vaccinated mice released much more IFN-γ than those from s.c. vaccinated mice (Fig. 4A). To examine whether such heightened IFN-γ production by lymphocytes of i.n. vaccinated mice was attributed at least in part to an increased frequency of antigen-specific lymphocytes, an ELIspot assay was carried out. Indeed, there were approximately one-third more antigen-specific, IFN-γ-producing cells in the spleens of i.n. vaccinated mice than in those of s.c. vaccinated mice (Fig. 4B).
FIG. 4.
Mycobacterial antigen-stimulated IFN-γ recall release by lymphocytes. BALB/c mice were challenged intratracheally with BCG at 10 weeks after s.c. or i.n. vaccination, and splenocytes from four mice per group were isolated and pooled at 3 weeks postchallenge. Cells were then stimulated with or without mycobacterial antigen cBCG for 72 h. Culture supernatant was assayed for the amount of IFN-γ (A). Results are expressed as means ±standard errors of the means from triplicate determinations. Also, splenocytes isolated at the same time point postchallenge were cultured with mycobacterial antigen M. tuberculosis CFP for 24 h, and the numbers of antigen-specific IFN-γ-producing cells both in spleen and thoracic lymph nodes were determined by ELISpot assay (B). Results are expressed as averages from duplicate determinations.
Protection against M. bovis BCG challenge in mice after s.c. or i.n. vaccination.
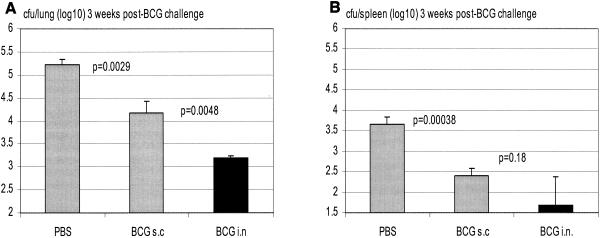
To examine whether i.n. vaccinated hosts could be better protected, we challenged, via the airway, s.c. and i.n. vaccinated mice first with BCG bacilli at 2.5 and 6 months postvaccination. We found that, at 2.5 months, i.n. vaccination conferred improved protection against secondary BCG challenge over s.c. vaccination (13,753 ± 3,012 versus 26,212 ± 1,527 CFU; P = 0.013; data not shown), whereas in the spleen, the levels of infection were small and were comparable between two groups. At 6 months postvaccination, s.c. BCG vaccination markedly reduced the level of infection in the lung compared to the level in nonvaccinated control mice (P = 0.0029); the level of infection was further remarkably reduced by i.n. mucosal vaccination (further reduction of 1 log unit; P = 0.0048 compared with s.c. vaccination) (Fig. 5A). In comparison, the levels of infection in the spleen were very small and were comparably reduced both in s.c. and i.n. vaccinated groups (Fig. 5B). These findings suggest that i.n. mucosal BCG vaccination confers better protection against secondary challenge by attenuated mycobacteria than parenteral s.c. vaccination.
FIG. 5.
Protection from secondary airway challenge by attenuated mycobacteria in s.c. or i.n. BCG-vaccinated hosts. BALB/c mice were challenged intratracheally with BCG at 3 months after s.c. or i.n. vaccination, and mycobacterial burden in the lung and spleen was assayed at 3 weeks postchallenge. Lungs and spleens were homogenized and subjected to a mycobacterial colony assay. Results are expressed as means ± standard errors of the means from five mice per group.
Protection against M. tuberculosis challenge in mice after s.c. or i.n. vaccination.
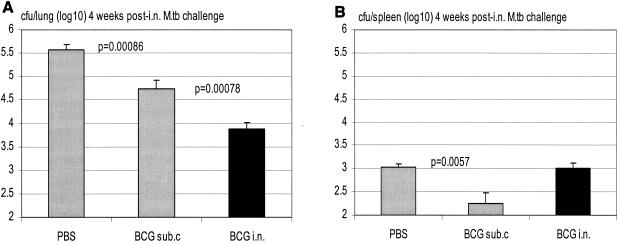
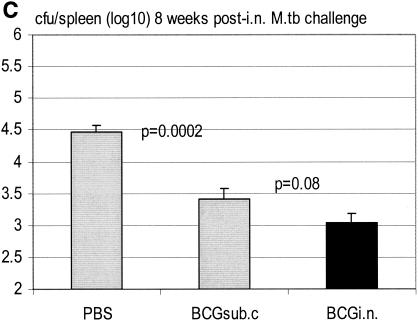
To further compare the efficacies of protection by s.c. and i.n. BCG vaccination, mice were vaccinated s.c. or i.n. with BCG, challenged via the airway with a virulent strain of M. tuberculosis bacilli at 3 months postvaccination, and sacrificed for colony assay 4 weeks postchallenge. We found that, while s.c. BCG vaccination reduced the level of M. tuberculosis infection by close to 1 log unit (Fig. 6A; P = 0.00086 compared to the nonvaccinated group receiving), i.n. vaccination doubled the level of protection conferred by s.c. vaccination, reducing the level of infection by more than 1.5 log units (Fig. 6A; P = 0.00078 compared to s.c. vaccinated group). While the overall level of M. tuberculosis infection in the spleen was much lower than that in the lung, i.n. vaccination did not reduce the level of infection in the spleen as much as s.c. vaccination (Fig. 6B). To examine whether such a pattern of protection in the spleen may change over time post-M. tuberculosis challenge, we assessed M. tuberculosis infection in the spleen 8 weeks postchallenge. We found that, by 8 weeks, the levels of infection in the spleens of control PBS and s.c. vaccinated groups increased and eventually exceeded that in the spleens of i.n. vaccinated mice (Fig. 6C; PBS versus s.c, P = 0.0002; s.c. versus i.n., P = 0.08). This suggests that i.n. mucosal vaccination significantly improves protection primarily in the lung.
FIG. 6.
Protection from secondary airway challenge by virulent M. tuberculosis (M.tb) in s.c. or i.n. BCG-vaccinated hosts at 3 months postvaccination. BALB/c mice were challenged i.n. with M. tuberculosis at 3 months after s.c. or i.n. vaccination, and mycobacterial burden in the lung and spleen was assayed at 4 or 8 weeks postchallenge. Lungs and spleens collected at 4 weeks postchallenge (A and B) and spleens collected at 8 weeks postchallenge (C) were homogenized and subjected to a mycobacterial colony assay. Results are expressed as means ± standard errors of the means from seven or eight mice per group. Statistical analysis was carried out by using log-transformed data.
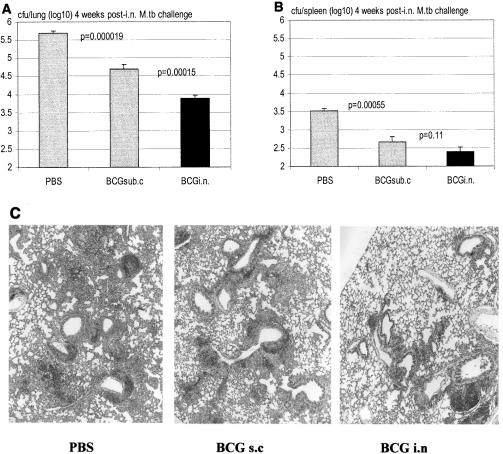
To investigate whether improved protection against M. tuberculosis infection by i.n. mucosal vaccination would last beyond 3 months, mice were vaccinated s.c. or i.n. and then challenged with M. tuberculosis at 6 months postvaccination. We found that, indeed, while s.c. vaccinated mice were protected from M. tuberculosis infection in the lung compared to PBS controls (Fig. 7A; P = 0.000019 compared to PBS), i.n. vaccination almost doubled such protection (Fig. 7A; P = 0.00015 compared to s.c vaccination). In the spleen, while the level of infection in the s.c. vaccinated group was significantly lower than that in the PBS control group (Fig. 7B; P = 0.00055), it was even lower in the spleens of i.n. vaccinated mice although the difference was not statistically significant. Histologically, there was also correspondingly less tissue granulomatous pathology in the lungs of i.n. vaccinated mice than in s.c. vaccinated mice (Fig. 7C). This suggests that significantly improved protection against pulmonary TB by i.n. mucosal BCG vaccination could last at least up to 1/2 year postvaccination.
FIG. 7.
Protection from secondary airway challenge by virulent M. tuberculosis in s.c. or i.n. BCG-vaccinated hosts at 6 months postvaccination. BALB/c mice were challenged i.n. with virulent M. tuberculosis (M.tb) at 6 months after s.c. or i.n. vaccination, and M. tuberculosis burden in the lung and spleen was assayed at 4 weeks postchallenge. Lungs (A) and spleens (B) were homogenized and subjected to a mycobacterial colony assay. Results are expressed as means ± standard errors of the means from eight mice per group. Statistical analysis was carried out by using log-transformed data. (C) Lung sections were stained by hematoxylin and eosin. Magnification, ×4.
DISCUSSION
In this study, we have demonstrated that a single i.n. BCG vaccination confers better protection, particularly in the lung, against pulmonary M. tuberculosis infection than s.c. BCG vaccination. Such superior protection by i.n. BCG vaccination could last at least for 1/2 year after the initial vaccination. Our findings thus lend experimental support to the concept of airway mucosal vaccination against pulmonary TB.
Mucosal vaccination has received increasing attention due to its potency in inducing mucosa-associated protection from mucosal infectious diseases (16, 23, 30). In this regard, both intragastric and intrarectal routes of TB vaccination have been explored, but it was found that not only were larger doses of vaccines required but also the protection level did not exceed that by percutaneous BCG vaccination (1, 19). In comparison, i.n. vaccination, aiming to target the lymphoid tissues present both in the nasal and bronchial mucosa (5, 11, 16), represents an attractive way to elicit mucosa-associated immunity against TB of pulmonary mucosal origin due to its ease and feasibility in humans. However, this route of vaccination has not been widely explored, and, in particular, the commonalities and differences between conventional parenteral percutaneous and i.n. vaccinations against pulmonary TB have not been appreciated. Falero-Diaz and colleagues recently reported that two sequential i.n. BCG vaccinations in mice induced a robust protection from airway M. tuberculosis challenge (6). While our results lend further support to their findings, our study is the first to compare side-by-side s.c. and i.n. vaccinations and, importantly, to demonstrate the superior efficacy in protection from pulmonary TB conferred by a single i.n. vaccination with a moderate dose of BCG. Recently, Lyadova and colleagues have also reported that i.n. BCG vaccination provided slightly better protection than s.c. vaccination from intravenous wild-type M. bovis infection (22). The difference in the extent of improved protection by i.n. vaccination between this and our present study may be because, first, M. bovis infection differs from M. tuberculosis infection and, second, airway mucosal vaccination may not necessarily confer the best protection from an infection of systemic origin. Our finding that i.n. BCG vaccination most dramatically improved protection locally in the lung following airway infection, but not systemically in the spleen, supports such a view. We found that lungs of i.n. vaccinated hosts were significantly better protected from M. tuberculosis challenge than those of s.c. vaccinated hosts whereas the levels of infection in the spleen for the i.n. and s.c. vaccinated groups were similar. This could be because the greater systemic dissemination of M. tuberculosis bacilli from the lungs of s.c. vaccinated mice is balanced by a more sustained immune activation in the spleens in these mice (Fig. 2A). It is noteworthy that, in contrast to our present findings and those by others (6, 22), Palendira and colleagues have recently reported that aerosol BCG vaccination does not differ from s.c. vaccination in protection against pulmonary TB (26). There were several potentially important differences between their studies and our present studies. (i) Palendira et al. used C57BL/6 mice, whereas we used BALB/c mice. Since BALB/c mice are weaker responders to BCG than C57BL/6 mice (32, 35), it is likely that the optimal protection for BALB/c mice is more dependent on the route of vaccination than that for C57BL/6 mice. (ii) Palendira et al. delivered BCG by aerosol, whereas we performed i.n. delivery. It is possible that i.n. delivery targets both the nasal and bronchial lymphoid organs better than aerosol delivery. (iii) Palendira delivered only 1,000 CFU of BCG into the lung, whereas we delivered 0.5 × 106 CFU. We found that, compared to s.c. vaccination, i.n. vaccination required a much larger dose for the optimal immune activation.
In our present study, we have chosen to evaluate the efficacy of s.c. and i.n. BCG vaccination in BALB/c mice since we have reported that, compared to C57BL/6 hosts, BALB/c mice are a much weaker responders to BCG vaccination (35) or local lung infection (32). Thus this fact further demonstrates the potency of the i.n. route of BCG vaccination in conferring immune protection against pulmonary TB. Of note, we found that effective i.n. vaccination required a dose of BCG different from that required by s.c. vaccination. In particular, i.n. vaccination requires a larger dose when the immune activation was calibrated as the mycobacterial-antigen-stimulated IFN-γ response in lymphocytes. However, a comparison with the study by Falero-Diaz and colleagues (6) showed that our effective i.n. dose was only one-half of the one that they used. Relatively lower doses of BCG are believed to preferentially trigger an immune response of type 1 nature, regardless of the route of administration, whereas extremely large doses may skew toward a type 1/type 2 phenotype, which is less desirable for anti-TB immunity (27). Large doses of BCG vaccine, when delivered i.n., may also cause unnecessary lung tissue immunopathology (31, 32; data not shown). In this regard, our present results warrant further investigation by using auxotrophic BCG which remains viable but is unable to replicate in vivo. Since some auxotrophic mycobacterial strains have been shown to confer a level of protection similar to that offered by the wild-type BCG (3, 10, 14, 29), conceivably i.n. vaccination with auxotrophic BCG vaccine, perhaps in conjunction with a form of immune adjuvant such as the granulocyte-macrophage colony-stimulating factor transgene (35), will be not only safer but also effective, particularly in immunocompromised hosts. Furthermore, our present findings also lend support to using BCG i.n. to vaccinate hosts against infectious diseases other than TB. Indeed, recombinant BCG has been used i.n. to induce potent protective immune responses against such infectious agents as Borrelia burgdorferi and simian immunodeficiency virus (18, 20).
The mechanisms by which i.n. mucosal vaccination confers potent protection from pulmonary TB remain to be completely understood at this point. We found that, except for the 3-week time point, antigen recall IFN-γ responses both in the spleens and local lymph nodes of i.n. vaccinated hosts at weeks 6 and 10 postvaccination were in fact similar to or even smaller than those of s.c. vaccinated hosts. The fact that i.n. vaccinated hosts were indeed better protected from pulmonary M. tuberculosis challenge suggests that the level of immune responses detected in lymphoid organs outside the lung may not necessarily correlate with protection in the lung. In support of this view, by using a different model of virally mediated i.n. and parenteral TB vaccinations, we have recently found that high levels of antigen recall immune responses in the spleens and lymph nodes of parenterally vaccinated hosts fail to translate into airway mucosal protection from TB; instead, such protection is much more dependent on the level of antigen-specific T cells within the lung prior to airway M. tuberculosis challenge (J. Wang, A. Zganiacz, L. Thorson, R. W. Stokes, M. Hitt, and Z. Xing, submitted for publication). Although whether this might be the case in our present study remains to be determined, we found that splenocytes, but not lung draining lymph node cells, isolated from i.n. vaccinated mice at 3 weeks after mycobacterial challenge, released more IFN-γ upon antigen stimulation than those from s.c. vaccinated mice. Thus, it is possible that the number and the quality of antigen-specific T cells residing within the lung of i.n. vaccinated hosts are different from those of T cells in s.c. vaccinated hosts and that these cells of i.n. vaccinated hosts may more quickly undergo activation and expansion in the lung upon reexposure to mycobacteria and circulate out of the lung through distant lymphoid organs such as the spleen. In this regard, we found a much greater number of immunocytes, including lymphocytes, in the BAL fluid of i.n. vaccinated mice by 1 week in response to mycobacterial challenge. A recent clinical study has demonstrated that, when BCG was administered mucosally to human volunteers, it preferentially induced a T-cell population expressing mucosal homing molecule α4β7, which was accompanied by reduced purified protein derivative skin reactivity (12). Collectively, our present study supports the notion that airway mucosal immunization provides better immune protection in the lung upon secondary infection (16, 22, 23, 30).
Acknowledgments
This study is supported by research grants from the Canadian Institutes of Health Research.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abolhassani, M., M. Lagranderie, P. Chavarot, A.-M. Balazuc, and G. Marchal. 2000. Mycobacterial bovis BCG induces similar immune responses and protection by rectal and parenteral immunization routes. Infect. Immun. 68:5657-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer, T. F. 2000. Preventing tuberculosis with BCG vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31(Suppl. 3):S64-S67. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, M. A., A. Williams, D. Gavier-Widen, A. Whelan, G. Hall, P. D. Marsh, B. R. Bloom, W. R. Jacob, and R. G. Hewinson. 2000. Identification of a Mycobacterium bovis BCG auxotrophic mutant that protects guinea pigs against M. bovis and hematogenous spread of Mycobacterium tuberculosis without sensitization to tuberculin. Infect. Immun. 68:7094-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 5.Davis, S. S. 2001. Nasal vaccines. Adv. Drug Deliv. Rev. 51:21-42. [DOI] [PubMed] [Google Scholar]
- 6.Falero-Diaz, G., S. Challacombe, D. Banerjee, G. Douce, A. Boyd, and J. Ivanyi. 2000. Intranasal vaccination of mice against infection with Mycobacterium tuberculosis. Vaccine 18:3223-3229. [DOI] [PubMed] [Google Scholar]
- 7.Fine, P. E. 1995. Variation in protection by BCG: implication of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 8.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 9.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 10.Guleria, I., R. Teitelbaum, R. A. McAdam, G. Kalpana, W. R. Jacobs, Jr., and B. R. Bloom. 1996. Auxotrophic vaccines for tuberculosis. Nat. Med. 2:334-337. [DOI] [PubMed] [Google Scholar]
- 11.Heritage, P. L., B. J. Underdown, A. L. Arsenault, D. P. Snider, and M. R. McDermott. 1997. Comparison of murine nasal-associated lymphoid tissue and Peyer's patches. Am. J. Respir. Crit. Care Med. 156:1256-1262. [DOI] [PubMed] [Google Scholar]
- 12.Hoft, D. F., R. M. Brown, and R. B. Belshe. 2000. Mucosal BCG vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-gamma responses. Clin. Infect. Dis. 30(Suppl. 3):S217-S222. [DOI] [PubMed] [Google Scholar]
- 13.Isaka M, Y. Yasuda, S. Kozuka, T. Taniguchi, K. Matano, J. Maeyama, T. Komiya, K. Ohkuma, N. Goto, and K. Tochikubo. 1999. Induction of systemic and mucosal antibody responses in mice immunized intranasally with aluminium-non-adsorbed diphtheria toxoid together with recombinant cholera toxin B subunit as an adjuvant. Vaccine 18:743-751. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann, S. H. E. 2000. Is the development of a new tuberculosis vaccine possible? Nat. Med. 6:955-960. [DOI] [PubMed] [Google Scholar]
- 16.Kyd, J. M., A. R. Foxwell, and A. W. Cripps. 2001. Mucosal immunity in the lung and upper airway. Vaccine 19:2527-2533. [DOI] [PubMed] [Google Scholar]
- 17.Lagranderie, M., P. Ravisse, G. Marchal, M. Gheorghiu, V. Balasubramanian, E. H. Weigeshaus, and D. W. Smith. 1993. BCG-induced protection in guinea pigs vaccinated and challenged via the respiratory route. Tuber. Lung Dis. 74:38-46. [DOI] [PubMed] [Google Scholar]
- 18.Lagranderie, M., N. Winter, A. M. Balazuc, B. Gicquel, and M. Gheorghiu. 1998. A cocktail of Mycobacterium bovis BCG recombinants expressing the SIV Nef, Env, and Gag antigens induces antibody and cytotoxic responses in mice vaccinated by different mucosal routes. AIDS Res. Hum. Retroviruses 14:1625-1633. [DOI] [PubMed] [Google Scholar]
- 19.Lagranderie, M., P. Chavarot, A. M. Balazuc, and G. Marchal. 2000. Immunogenicity and protective capacity of Mycobacterium bovis BCG after oral and intragastric administration in mice. Vaccine 18:1186-1195. [DOI] [PubMed] [Google Scholar]
- 20.Langermann, S., S. Palaszynski, A. Sadziene, C. K. Stover, and S. Koenig. 1994. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature 372:552-555. [DOI] [PubMed] [Google Scholar]
- 21.Lefford M. J. 1977. Induction and expression of immunity after BCG immunization. Infect. Immun. 18:646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyadova, I. V., H. M. Vordermeier, E. B. Eruslanov, S. V. Khaidukov, A. S. Apt, and R. G. Hewinson. 2001. Intranasal BCG vaccination protects BALB/c mice against virulent Mycobacterium bovis and accelerates production of IFN-γ in their lungs. Clin. Exp. Immunol. 126:274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orme, I. M., and F. M. Collins. 1986. Aerogenic vaccination of mice with M. bovis BCG. Tubercle 67:133-140. [DOI] [PubMed] [Google Scholar]
- 25.Orme, I. M. 2001. The search for new vaccines against tuberculosis. J. Leukoc. Biol. 70:1-10. [PubMed] [Google Scholar]
- 26.Palendira, U., A. G. D. Bean, C. G. Feng, and W. J. Britton. 2002. Lymphocyte recruitment and protective efficacy against pulmonary mycobacterial infection are independent of the route of prior Mycobacterium bovis BCG immunization. Infect. Immun. 70:1410-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Power, C. A., G. J. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independent of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schell, R. F., W. F. Ealey, G. E. Harding, and D. W. Smith. 1974. The influence of vaccination on the course of experimental airborne TB in mice. J. Reticuloendothel. Soc. 16:131-138. [PubMed] [Google Scholar]
- 29.Smith, D. A., T. Parish, N. G. Stoker, and G. J. Bancroft. 2001. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 69:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevceva, L., A. G. Abimiku, and G. Franchini. 2000. Targeting the mucosa: genetically engineered vaccines and mucosal immune responses. Genes Immun. 1:308-315. [DOI] [PubMed] [Google Scholar]
- 31.Wakeham, J., J. Wang, J. Magram, K. Croitoru, R. Harkness, P. Dunn, A. Zganiacz, and Z. Xing. 1998. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis BCG in IL-12-deficient mice. J. Immunol. 160:6101-6111. [PubMed] [Google Scholar]
- 32.Wakeham, J., J. Wang, and Z. Xing. 2000. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BL/6 and BALB/c mice. Infect. Immun. 68:6946-6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, J., K. Palmer, J. Lötvall, S. Milan, X.-F. Lei, K. I. Matthaei, J. Gauldie, M. Inman, M. Jordana, and Z. Xing. 1998. Circulating, but not local lung, IL-5, is required for the development of antigen-induced airway eosinophilia. J. Clin. Investig. 102:1132-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, J., J. Wakeham, R. Harkness, and Z. Xing. 1999. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J. Clin. Investig. 103:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, J., A. Zganiacz, and Z. Xing. 2002. Enhanced immunogenicity of BCG vaccine by using a viral-based GM-CSF transgene adjuvant formulation. Vaccine 20:2887-2898. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., and Z. Xing. 2002. Tuberculosis vaccines: the past, present and future. Expert Rev. Vaccines 1:341-354. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. 2001. Vaccine-preventable diseases—global summary (2001). World Health Organization, Geneva, Switzerland.
- 38.Xin, K. Q., T. Ooki, H. Mizukami, K. Hamajima, K. Okudela, K. Hashimoto, Y. Kojima, N. Jounai, Y. Kumamoto, S. Sasaki, D. Klinman, K. Ozawa, and K. Okuda. 2002. Oral administration of recombinant adeno-associated virus elicits human immunodeficiency virus-specific immune responses. Hum. Gene Ther. 13:1571-1581. [DOI] [PubMed] [Google Scholar]
- 39.Xing, Z., A. Zganiacz, J. Wang, M. Divangahi, and F. Nawaz. 2000. IL-12-independent Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFN-γ release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J. Immunol. 164:2575-2584. [DOI] [PubMed] [Google Scholar]