Abstract
Glutathione peroxidases are widespread among eukaryotic organisms and function as a major defense against hydrogen peroxide and organic peroxides. However, glutathione peroxidases are not well studied among prokaryotic organisms and have not previously been shown to promote bacterial virulence. Recently, a gene with homology to glutathione peroxidase was shown to contribute to the antioxidant defenses of Streptococcus pyogenes (group A streptococcus). Since this bacterium causes numerous suppurative diseases that require it to thrive in highly inflamed tissue, it was of interest to determine if glutathione peroxidase is important for virulence. In this study, we report that GpoA glutathione peroxidase is the major glutathione peroxidase in S. pyogenes and is essential for S. pyogenes pathogenesis in several murine models that mimic different aspects of streptococcal suppurative disease. In contrast, glutathione peroxidase is not essential for virulence in a zebrafish model of streptococcal myositis, a disease characterized by the absence of an inflammatory cell infiltrate. Taken together, these data suggest that S. pyogenes requires glutathione peroxidase to adapt to oxidative stress that accompanies an inflammatory response, and the data provide the first demonstration of a role for glutathione peroxidase in bacterial virulence. The fact that genes encoding putative glutathione peroxidases are found in the genomes of many pathogenic bacterial species suggests that glutathione peroxidase may have a general role in bacterial pathogenesis.
The generation and release of toxic reactive oxygen species by phagocytic cells is thought to be an important component of the host's immunity against bacterial infections. In response, successful pathogens have evolved effective systems for defense against oxidative stress that include combinations of reducing enzymes, molecular scavengers, and protein and DNA repair enzymes (for reviews, see references 11 and 23). Not surprisingly, mutants defective for resistance to oxidative stress are often avirulent in animal models of infection (8, 17, 21, 32).
For some bacteria, the mechanisms used to defend themselves from oxidative stress and the possible contribution that these defense pathways may make to virulence is much less clear. For example, as a member of the lactic acid family of bacteria, Streptococcus pyogenes (group A streptococcus) does not produce heme and therefore lacks many of the reducing enzymes required for resistance to oxidative stress in other bacterial species (5). Despite this apparent deficiency, this gram-positive bacterium causes numerous suppurative diseases that require it to thrive in the presence of the oxidative stresses caused by the intense inflammatory response that is characteristic of streptococcal infection (7). In addition, hydrogen peroxide is a by-product of the fermentative metabolism of S. pyogenes, and the bacterium can survive the millimolar concentrations of peroxide it can produce endogenously (14). These observations have suggested that S. pyogenes must have a robust ability to defend itself from oxidative stress.
A previous study used a functional genomics approach to identify possible nonheme oxoreductases that could contribute to the oxidative defenses of S. pyogenes (18). One of the genes examined encoded a putative protein with homology to glutathione (GSH) peroxidase (GpoA) (genomic locus Spy0605; GenBank accession no. AE006515), a selenoprotein oxoreductase that has been well studied in eukaryotic cells and that plays important roles in the protection of cells against oxidation of DNA and maintenance of cellular redox balance (2). GSH peroxidase activity has been studied previously for only one prokaryote, Neisseria meningitidis (24, 25). Inactivation of the gene for GpoA in N. meningitidis increases the sensitivity of the resulting mutants to oxidative stress induced by the redox-cycling agent paraquat (methyl viologen) (24). A similar phenotype has also been observed in eukaryotic cells depleted of GSH peroxidase activity (2). Examination of the plethora of recently completed prokaryotic genome sequences, including those of Staphylococcus aureus, Bacillus subtilis, Lactococcus lactis, Listeria monocytogenes, Pseudomonas aeruginosa, Clostridium acetobutylicum, Caulobacter crescentus, Salmonella enterica, and Yersinia pestis, has revealed the presence of genes with homology to GSH peroxidase (K. King and M. Caparon, unpublished data). This broad distribution among diverse bacterial species suggests that GSH peroxidase also makes an important contribution to stress resistance in prokaryotes and contributes to bacterial virulence. However, its importance to virulence remains to be established.
In S. pyogenes, GpoA− mutants are aerotolerant and grow at rates identical to that of the wild type under aerobic and anaerobic conditions (18). However, the mutants do demonstrate the characteristic enhanced sensitivity to paraquat observed in N. meningitidis and eukaryotic cells (18), suggesting that GpoA does contribute to stress resistance. In addition, S. pyogenes contains a gene with homology to the gene encoding glutathione reductase (GSSG reductase) (genomic locus Spy0813; GenBank accession no. AE006532) and is one of the few streptococcal species that accumulates significant amounts of GSH (29). Taken together, these data suggest that GSH and GSH peroxidase are important for stress resistance and virulence of S. pyogenes.
In the present study, we examined the ability of GpoA-deficient S. pyogenes to cause disease in several different animal models of infection. The data indicate that the GpoA GSH peroxidase is the major GSH peroxidase in S. pyogenes and makes a significant contribution to the severity of disease in those infections that are characterized by high levels of inflammation. This study provides the first direct demonstration of a role for GSH peroxidase in bacterial pathogenesis.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The construction of a mutant (G8.7) containing an in-frame deletion mutation of gpoA in a wild-type S. pyogenes background (HSC5) (16) was performed as described in a previous study (18). Routine culture utilized Todd-Hewitt broth (BBL) supplemented with 0.2% yeast extract (Difco) (THY medium). Culturing of streptococci for infection of animals followed established protocols, including growth in THY for infection of mice and zebrafish (26). For determination of the number of CFU, appropriate dilutions of cultures were prepared in saline and plated on solid THY medium containing Bacto agar (Difco) at a final concentration of 1.4%. Incubation of all bacterial cultures was conducted at 37°C, and cultures on solid media were incubated under the anaerobic conditions produced using a commercial gas generator (GasPack, catalogue no. 70304; BBL).
Determination of GSH peroxidase activity.
Wild-type and GpoA− S. pyogenes were grown with agitation in THY medium for 16 h at 37°C. The cells were washed twice with 20 mM Tris-HCl buffer, pH 8.2, and a soluble protein extract was prepared by breaking the cells using sonication followed by centrifugation at 27,200 × g for 15 min at 4°C to collect the supernatant fluids. To measure GSH peroxidase activity, a coupled spectrophotometric assay was utilized in which NADPH oxidation by GSSG reductase was monitored at 340 nm (ɛ = 6.2 mM−1 cm−1). Reaction mixtures initially contained the followings: 10 mM Na phosphate buffer (pH 7.0), 1 mM diethylenetriaminepentaacetic acid, 250 μM NADPH, 2 mM GSH, and 0.5 U of GSSG reductase in a final volume of 1 ml. The reaction mixture was equilibrated at 25°C for 5 min, and the background rate of NADPH oxidation was determined following the addition of soluble protein extract containing up to 6 μg of total soluble protein. For calculation of GSH peroxidase activity, H2O2 was added to a final concentration of 50 μM and the rate of NADPH oxidation was measured. Activity reported was calculated by subtracting the background rate from the rate in the presence of H2O2. To confirm the accuracy of the assay, known concentrations of bovine erythrocyte GSH peroxidase (Sigma) were added to selected reactions, and in all cases the rate increased by the amount expected based on the amount of bovine GSH peroxidase added. To confirm that observed NADPH oxidation reflected GSH peroxidase and not NADPH-dependent alkylhydroperoxidase, the entire set of experiments was repeated leaving out GSH, GSSG reductase, or both, and NADPH oxidation was found to be negligible.
Murine subcutaneous infection model.
The method of Bunce et al. (4) as modified by others (20, 28) was used to establish an infection of S. pyogenes in the subcutaneous tissue of mice. Briefly, streptococcal strains were prepared for injection into mice by culturing them in 40 ml of THY medium to the mid-logarithmic phase of growth (optical density at 600 nm = 0.300). Bacteria were collected by centrifugation, washed once with 10 ml of sterile saline, and resuspended in 1 ml of sterile saline. To disrupt streptococcal chains, this suspension was subjected to brief sonication (four pulses of 10 s each), using a cup horn probe with the sonic disruptor (Branson Model 185) at a power setting of 8. The number of CFU in the suspension was determined as described above. For infection of soft tissue, 6- to 8-week-old female SKH1 hairless mice (Charles River Labs), commonly used in the subcutaneous model of S. pyogenes infection, received subcutaneous injections into the left flank of 107 CFU in a 100-μl volume that was delivered using a 1-ml insulin syringe (Becton-Dickinson). Control mice were injected with saline alone, and each study group consisted of 10 mice that were housed together. Mice were observed and weighed daily, and the appearance of the cutaneous tissue at the site of injection was documented every 24 h by digital photography. Any visible ulceration, as defined by the loss of the overlaying stratum corneum and epidermis, was analyzed from the photorecord using MetaMorph image analysis software (version 4.6; Universal Imaging Corp.) to precisely determine the area contained by the irregular border of each ulcer. Data presented were representative of three independent experiments.
Intraperitoneal infection of mice.
A lethal systemic infection of mice was established by intraperitoneal injection of S. pyogenes as follows: streptococcal strains were cultured and prepared as described above for infection of the subcutaneous tissues. A dose of 108 CFU in a volume of 100 μl was injected into 4- to 6-week-old C57BL/6J mice (Jackson Laboratories) at a location approximately 0.5 cm to the left of the midline at the lower abdomen. This species of mice is susceptible to lethal infection by S. pyogenes, and its immune system is well characterized. A control group of mice received an injection of sterile saline alone. Mice were housed in groups of five with free access to food and water throughout the entire experiment. Infected mice were observed daily for a period of 9 days. Data presented are representative of two independent experiments, each conducted with 10 mice per experimental group, and each with similar results.
Infection of zebrafish.
Intramuscular infection of zebrafish was conducted as previously described (26) with the addition of a brief sonication to disrupt streptococcal chains as described above. Each zebrafish was injected in the dorsal muscle with 106 CFU in a volume of 10 μl. Following infection, zebrafish were monitored daily for a period of 5 days. Each experimental group consisted of at least 16 zebrafish, and a mock-infected control group was included in each experiment which consisted of a group of eight zebrafish injected with sterile medium alone. Data presented are representative of at least two independent experiments.
Analysis of bacteria in infected tissues.
To determine the number of CFU in cutaneous tissue, a tissue block was removed from infected animals that included the skin surrounding the lesion, the underlying adipose tissue, and muscle. The tissue block was mixed with 1 ml of saline solution and homogenized using a motorized homogenizer (PCR Homogenizer; Cole-Parmer Instrument Co.), and the number of CFU determined as described above. The number of viable bacteria present in the peritoneal cavity was sampled by washing the peritoneal cavity with 5 ml of ice-cold saline according to the method of Dunn et al. (10). Aseptic removal of the spleen, followed by homogenization and plating as described above, was done to determine the number of CFU in the spleens of infected mice.
Statistical analyses.
Kaplan-Meier product limit estimates of survival curves were used to compare infection by wild-type and mutant streptococci, and differences were tested for significance by the log rank test (15). The difference between the numbers of mice developing an ulcer following subcutaneous challenge with wild-type bacteria and with mutant bacteria was tested for significance by the Chi-square test with Yates' correction (15), and differences in the areas of the resulting ulcers were tested by the Mann-Whitney U test (15). For all test statistics, the null hypothesis was rejected when P values were <0.05.
RESULTS
GpoA− mutants are deficient in GSH peroxidase activity.
Prior examination of the genome of S. pyogenes revealed a gene (gpoA) that is highly homologous to a family of genes that encode GSH peroxidases (18). Consistent with a function as a peroxidase, mutation of gpoA was associated with an increased sensitivity to peroxide stress (18). However, it has not been shown that S. pyogenes has the capacity to express GSH peroxidase activity and that this activity is missing in GpoA− mutants. Upon analysis, it was found that the wild-type strain expressed a GSH peroxidase activity corresponding to 1.32 ± 0.22 nmol of NADPH oxidized min−1 μg of total soluble protein−1 (n = 12). This level of activity is approximately fivefold higher than the GSH peroxidase activity expressed by a human endothelial cell line (12). In contrast, it was not possible to detect GSH peroxidase activity in the GpoA− mutant of S. pyogenes at any rate consistently higher than background (n = 24). Taken together, these data indicate that S. pyogenes can express a robust GSH peroxidase activity and that this activity is negligible in a GpoA− mutant.
Mutants deficient in GpoA are attenuated in a murine model of subcutaneous infection.
Once it was established that the GpoA− mutant lacked GSH peroxidase activity, it was of interest to determine the contribution of GpoA to streptococcal pathogenesis. The range of different infections that can be caused by S. pyogenes is extensive and includes both suppurative and nonsuppurative diseases (7). As a consequence, no one animal model can accurately represent the features of all S. pyogenes diseases. For example, the suppurative diseases range from localized infections of the soft tissues of the pharynx and skin (pharyngitis, impetigo), to involvement of the deeper cutaneous tissues (erysipelas, cellulitis) to highly destructive infections characterized by extensive necrosis (necrotizing fasciitis, myositis) (7). Thus, to most thoroughly examine the role of GSH peroxidase in streptococcal pathogenesis, the behavior of GpoA− mutants was evaluated in animal models that reproduce different elements of a number of streptococcal diseases. The initial studies utilized the murine subcutaneous infection model (4, 20, 28), which produces a highly inflammatory localized lesion in soft tissue characterized by extensive recruitment of inflammatory cells to the site of bacterial proliferation (4, 28).
The wild-type S. pyogenes strain HSC5 and an isogenic mutant (G8.7) containing an in-frame deletion mutation in GpoA (18) were used to infect SKH1 hairless mice. At a dose of 107 CFU, the wild-type strain typically produces a well-defined area of induration by between 8 to 12 h postinfection that is characterized by the recruitment of large numbers of inflammatory cells, of which the majority are neutrophils (data not shown). By 18 to 24 h, the region of induration ulcerates and develops an eschar followed by the gradual expansion of the margins of the ulcer to reach a maximum area by about day 3 (data not shown). Beginning around day 8 the lesion begins to resolve, and by day 14 it is typically healed. The streptococci remain localized to the lesion, and only low numbers of bacteria (<10 CFU/g of tissue) can be recovered from the spleen at any time point.
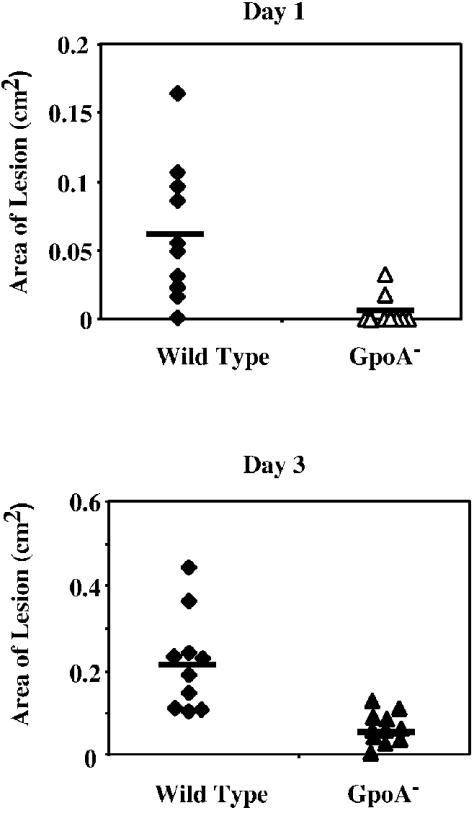
When the mutant and the wild type were compared at the point when lesions typically first appear, very few mice infected by the GpoA mutant had developed lesions (day 1, Fig. 1), and the few mice that had developed lesions showed small lesions. Over the course of the infection, mice infected by the GpoA mutant developed significantly smaller ulcerations than mice infected by the wild-type strain (Fig. 1, day 3).
FIG. 1.
Skin lesion development in mice following subcutaneous infection. Groups of at least 10 female SKHI hairless mice received a subcutaneous injection of 107 CFU of wild-type or GpoA mutant streptococci and were observed after 1 and 3 days (top and bottom panels, respectively) for the development of a visible ulcer, whose area was determined as described in Materials and Methods. At the time of maximal lesion development for the wild-type strain (3 days), the wild-type strain produced a significantly more severe disease in that the areas of the resulting ulcers were significantly larger (P < 0.01). Each symbol represents the area of ulcer observed in an individual mouse, and the horizontal bar indicates the mean value obtained for each group of 10 mice.
GpoA− mutant bacteria are abundant in infected skin.
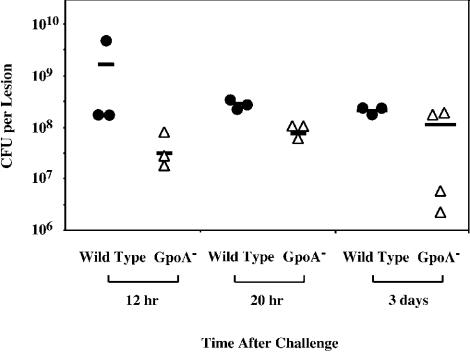
Most mice infected with the GpoA− mutant showed no symptoms of infection, including visible induration or ulceration of tissue and weight loss, by day 1 after infection. However, since mice infected by the wild-type strain accumulate a large number of inflammatory cells at the injection site by 8 h after infection, and ulceration begins to develop about 20 h after infection, it was of interest to determine if the mutant was attenuated, because it was rapidly cleared from the tissue by this time point. To test this, the cutaneous tissue and underlying muscle at the site of injection was excised from groups of mice at 12 and 20 h and at 3 days after infection. Mice infected with 107 CFU of the wild-type strain demonstrated high numbers of bacteria in tissue at all time points (Fig. 2). Contrary to expectations, the GpoA− mutant was not rapidly cleared from all mice over the first 20 h of infection and was present in a recoverable form at a time when the mice infected by the wild-type strain were beginning to demonstrate extensive tissue pathology (Fig. 2). In fact, high numbers of GpoA− mutant streptococci could still be recovered from mice at 3 days postchallenge. These data suggest that the development of pathology in cutaneous tissue is not simply dependent on the ability of the streptococcus to persist in tissue.
FIG. 2.
Persistence of S. pyogenes in skin. Mice were challenged with wild-type and GpoA− mutant S. pyogenes as described for Fig. 1. At the time points indicated, the skin including underlying adipose tissue and abdominal muscle was excised at an additional radius extending at least 2 mm from the margin of induration present at the site of infection. Each symbol represents the number of CFU of the indicated strain recovered from an individual mouse, and the bar represents the mean value obtained for each group of mice.
GpoA− mutants are attenuated in a murine model of systemic infection.
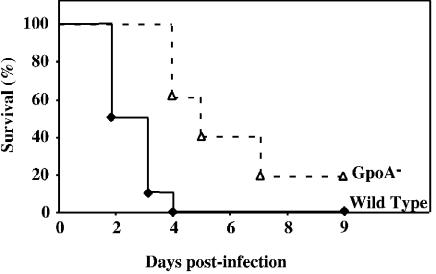
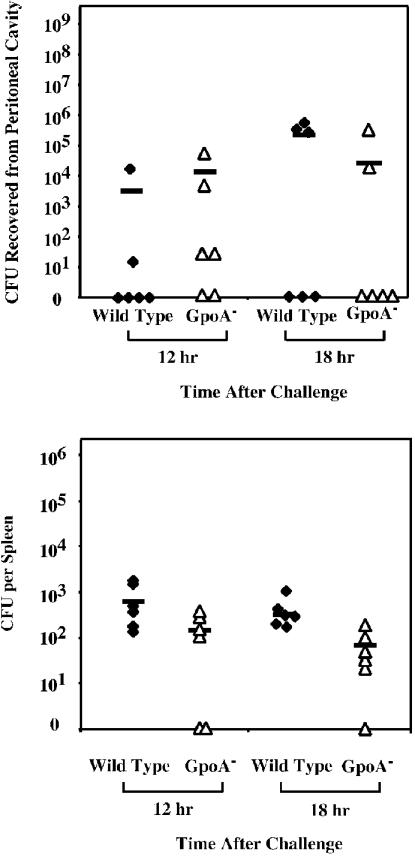
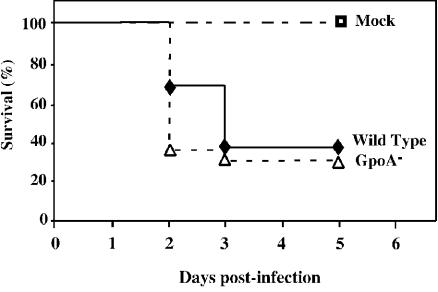
Unlike some strains (28, 20), HSC5 does not produce a systemic infection following its introduction into the subcutaneous tissues. However, it will produce lethal systemic disease subsequent to infection of the peritoneal cavity. Thus, in order to determine the contribution of GSH peroxidase to the pathogenesis of systemic disease, the abilities of the wild type and mutant to cause death following intraperitoneal challenge were compared. At a dose of 108 CFU, 100% of mice injected with the wild-type strain were dead 4 days after challenge (Fig. 3). At that same time point a majority of the mice injected with the GpoA− mutant survived. Additional mortality was observed in the group infected by the GpoA− mutant over the next several days. Overall, the time to death was significantly reduced for animals infected by the GpoA− mutant (Fig. 3), indicating that the loss of GSH peroxidase activity is associated with a reduced ability to cause lethal systemic infection. Examination of the number of viable streptococci that could be recovered from the peritoneal cavity over the first 18 h of infection revealed no large difference between wild type-infected and GpoA− mutant-infected mice (Fig. 4). However, when the numbers of bacteria recovered from the spleen were evaluated, smaller numbers were consistently observed in mice infected by the GpoA− mutant (Fig. 4). These data suggest that the slower kinetics of death observed with the mutant may be associated with a reduced ability to disseminate from the spleen.
FIG. 3.
Survival of mice following intraperitoneal challenge. Groups of at least 10 C57BL/6 mice were challenged intraperitoneally with 108 CFU of wild-type or GpoA− mutant S. pyogenes. Survival of mice was monitored daily for 9 days, and the data were presented as a Kaplan-Meier plot. The data indicate that the wild-type strain was significantly more lethal than the GpoA− mutant (P < 0.01).
FIG. 4.
Recovery of wild-type and mutant S. pyogenes following intraperitoneal challenge. Groups of six C57BL/6J mice were challenged intraperitoneally as described for Fig. 3. At the indicated time points, the numbers of CFU that could be recovered from the peritoneal cavity and the spleen were determined (top and bottom panels, respectively). Each symbol represents the number of CFU of the indicated strain recovered from an individual mouse, and the bar represents the mean value obtained for each group of six mice.
GpoA− mutants are not attenuated for virulence in a zebrafish model of intramuscular infection.
Among the most severe forms of invasive group A streptococcal infection is myositis, which may have fatality rates as high as 80% (30). In an experimental baboon model of S. pyogenes myositis, a key difference between fatal and nonfatal infection was the intensity of the inflammatory response. Surviving animals exhibited an intense neutrophilic infiltrate at the site of infection, while nonsurvivors had virtually no influx of neutrophils and extensive bacterial growth in muscle (31). A more tractable model of myositis has recently been developed using zebrafish (26). In this model, injection of S. pyogenes into the dorsal muscle of zebrafish results in a fatal myositis with pathology that closely represents that of fatal disease in baboons, including a dramatic absence of an inflammatory infiltrate (26). This lack of inflammatory cells produces a tissue environment that contrasts with the large numbers of inflammatory cells that are observed at the site of infection in mouse subcutaneous tissue (4, 28). In order to determine the contribution of GSH peroxidase to survival in tissue in the absence of an inflammatory infiltrate, the wild type and the GpoA− mutant were injected into zebrafish muscle at a dose of 106 CFU and survival was observed for 5 days. While all fish that had received an injection of sterile medium alone survived, there was no significant difference in the rate of death between fish challenged with the wild-type or with GpoA− mutant bacteria (Fig. 5).
FIG. 5.
The GpoA− mutant is virulent in a zebrafish model of myositis. Groups of at least 16 zebrafish were challenged intramuscularly with 106 CFU of wild-type and GpoA− mutant S. pyogenes. Survival was monitored over a period of 5 days. Data indicate that there was no significant difference in virulence between the wild-type and GpoA− mutant strains (P > 0.1).
DISCUSSION
These data provide the first direct evidence that GSH peroxidase makes a contribution to bacterial pathogenesis. Consistent with a role in defense against oxidative stress, GpoA was important in those infections that required S. pyogenes to interact with host cells in an inflammatory exudate. These data, combined with the distribution of genes with homology to GpoA in other pathogenic bacterial species, suggests that GSH peroxidase may have a general role in bacterial virulence.
The specific contribution of GpoA to S. pyogenes virulence appears to be complex. While a previous study found that the GpoA− mutant was more sensitive to the oxidative stress generated by paraquat, the mutant grew normally under aerobic conditions and was not more sensitive to hydrogen peroxide (18). Furthermore, the wild type and the GpoA− mutant could both be induced to high-level peroxide resistance by a prior exposure to a sublethal dose of peroxide (18). These data suggest that S. pyogenes has a complex defense against oxidative stress that involves several overlapping antioxidant systems. This same phenomenon has been well studied with many organisms, including Escherichia coli and Salmonella (for a review, see reference 11), although in the case of S. pyogenes, the identities of the major antioxidant systems have not been established (18). However, the fact that GpoA− mutants are attenuated in vivo indicates that the gpoA gene product makes an essential contribution to stress resistance under highly inflammatory conditions.
The unique contribution of GpoA could result from its exclusive expression in a critical host compartment that lacks cues for expression of other streptococcal antioxidant enzymes. Alternatively, since GSH peroxidases often have activity against a broad range of substrates, including hydrogen peroxide, organic peroxides, peroxynitrites, and others (1, 2), it is also possible that GpoA provides protection from a specific reactive species that it encounters only in vivo and that is not detoxified by any other antioxidant system. Instead of a unique reactive species, it could also be the case that the concentration of one particular toxic species plays a role. For example, E. coli has several enzymes with an ability to degrade hydrogen peroxide, but these demonstrate different kinetic efficiencies (6). It has been suggested that the importance of any one enzyme to peroxide homeostasis is linked to the concentration of peroxide the organism encounters (6). In this model, an enzyme like catalase is important at high peroxide concentrations that saturate a more kinetically efficient scavenger like alkyl hydroperoxide reductase, which is the predominant scavenger at low peroxide concentrations (6).
It is also possible that the effects of the loss of GpoA activity are due to secondary effects on streptococcal gene regulation caused by an alteration in the redox balance within the bacterium. For example, the expression of the adhesin protein F is altered in mutants lacking superoxide dismutase (13). Similarly, eukaryotic GSH peroxidases can alter the redox activation of transcription factors such as NFκB (reviewed in reference 2). This phenomenon could explain why the attenuation of the GpoA− mutant in subcutaneous infection was not the result of rapid clearance of the streptococci. In this scenario, an altered redox balance leads to changes in expression of a streptococcal gene product that is involved in promoting an inflammatory response. A precedent for this model has been established for Yersinia enterocolitica, where mutation of a gene for a transcription regulator known as RovA attenuates disease, even though the mutant has a normal ability to proliferate in regional lymph nodes (9). There is a marked lack of inflammation in lymph nodes during infection produced by the mutant that correlates with the inability of the mutant to induce interleukin 1 alpha during infection, suggesting that a RovA-regulated gene product is required to actively induce inflammation to support pathogenesis (9). Interestingly, RovA is highly homologous to SlyA, a regulator of oxidative stress resistance in Salmonella (3).
Mutants defective in various stress response pathways can be used to probe the host's response against a pathogen in specific tissues. For example, while GpoA− mutants were abundant in the subcutaneous tissue during infection, they produced significantly less damage to tissue than the wild-type strain. This suggests that GpoA modulates how S. pyogenes adapts to the host's response.
Another tissue-specific difference between the wild type and the mutant was the observation that the GpoA mutant was not attenuated for infection of zebrafish, despite the fact that teleost fish have a complex immune system that resembles that of mammals (for a review, see references 22 and 27). In fact, zebrafish granulocytes have a complement of enzymes for the generation of reactive oxygen species that is similar to that of mammalian cells (19). Thus, it is unlikely that the lack of attenuation of the mutant is due to the failure of zebrafish leukocytes to produce any particular reactive species. Rather, the virulence of the mutant is likely due to the fact that S. pyogenes infection of zebrafish muscle is associated with a profound absence of an infiltration of inflammatory cells into the tissue (26). These data suggest that in the absence of inflammation GpoA is not required for virulence and that determinants of streptococcal virulence differ according to the tissue site of infection.
Assessment of the role of other bacterial GSH peroxidases will provide further knowledge about the importance of this enzyme for virulence in diverse host environments. Additional study of GpoA in infection by S. pyogenes should prove valuable for further understanding the interplay of host and pathogen in the pathogenesis of the diverse diseases that this bacterium can cause.
Acknowledgments
We thank Katie Tripp, Shari O'Brien, and Melody Neely for sharing their expertise in animal models of infection and Jeremy Ortiz for maintaining the zebrafish stocks.
This work was supported by Public Health Service grant AI38273 from the National Institutes of Health.
Editor: V. J. DiRita
REFERENCES
- 1.Arteel, G. E., K. Brivida, and H. Sies. 1999. Protection against peroxynitrite. FEBS Lett. 445:226-230. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, J. R. 2000. The glutathione peroxidases. Cell. Mol. Life Sci. 57:1825-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchmeier, N., S. Bossie, C. Y. Chen, F. C. Fang, D. G. Guiney, and S. J. Libby. 1997. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect. Immun. 65:3725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condon, S. 1987. Responses of the lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 46:269-280. [Google Scholar]
- 6.Costa Seaver, L., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhandayuthapani, S., M. W. Blaylock, C. M. Bebear, W. G. Rasmussen, and J. B. Baseman. 2001. Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J. Bacteriol. 183:5645-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dube, P. H., P. A. Revell, D. D. Chaplin, R. G. Lorenz, and V. L. Miller. 2001. A role of Il-1 alpha in inducing pathologic inflammation during bacterial infection. Proc. Natl. Acad. Sci. USA 98:10880-10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn, D. L., A. Barke, N. B. Knight, E. W. Humphrey, and R. L. Simmons. 1985. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect. Immun. 49:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faucher, K., H. Rabinovitch-Chable, G. Barriere, J. Cook-Moreau, and M. Rigaud. 2003. Overexpression of cytosolic glutathione peroxidase (GPX1) delays endothelial cell growth and increases resistance to toxic challenges. Biochimie 85:611-617. [DOI] [PubMed] [Google Scholar]
- 13.Gibson, C., and M. Caparon. 1996. Insertional inactivation of Streptococcus pyogenes sod suggests that prtF is regulated in response to a superoxide signal. J. Bacteriol. 178:4688-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson, C. M., C. Mallet, A. Clairborne, and M. G. Caparon. 2000. The contribution of NADH oxidase to the aerobic metabolism of Streptococcus pyogenes. J. Bacteriol. 182:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glantz, S. 2002. Primer of biostatistics, 5th ed. McGraw-Hill Co., New York, N.Y.
- 16.Hanski, E., P. A. Horwitz, and M. G. Caparon. 1992. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect. Immun. 60:5119-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King, K. Y., J. A. Horenstein, and M. G. Caparon. 2000. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J. Bacteriol. 182:5290-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieschke, G. J., A. C. Oates, M. O. Crowhurst, A. C. Ward, and J. E. Layton. 2001. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98:3087-3096. [DOI] [PubMed] [Google Scholar]
- 20.Limbago, B., V. Penumalli, B. Weinrick, and J. R. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68:6384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg, B. E., J. Wolf, R. E., M. C. Dinauer, Y. Xu, and F. C. Fang. 1999. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect. Immun. 67:436-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, N., M. Wilson, E. Bengten, T. Stuge, G. Warr, and W. Clem. 1998. Functional and molecular characterization of teleost leukocytes. Immunol. Rev. 166:187-197. [DOI] [PubMed] [Google Scholar]
- 23.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore, T. D. E., and P. F. Sparling. 1996. Interruption of the gpxA gene increases the sensitivity of Neisseria meningitidis to paraquat. J. Bacteriol. 178:4301-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore, T. D. E., and P. F. Sparling. 1995. Isolation and identification of a glutathione peroxidase homolog gene, gpxA present in Neisseria meningitidis but absent in Neisseria gonorrhoeae. Infect. Immun. 63:1603-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neely, M. N., J. D. Pfeifer, and M. Caparon. 2002. Streptococcus-zebrafish model of bacterial pathogenesis. Infect. Immun. 70:3904-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann, N. F., J. L. Stafford, D. Barreda, A. J. Ainsworth, and M. Belosevic. 2001. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev. Comp. Immunol. 25:807-825. [DOI] [PubMed] [Google Scholar]
- 28.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherrill, C., and R. C. Fahey. 1998. Import and metabolism of glutathione by Streptococcus mutans. J. Bacteriol. 180:1454-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens, D. L. 2000. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu. Rev. Med. 51:271-288. [DOI] [PubMed] [Google Scholar]
- 31.Taylor,, F. B., Jr., A. E. Bryant, K. E. Blick, E. Hack, P. M. Jansen, S. D. Kosanke, and D. L. Stevens. 1999. Staging of the baboon response to group A streptococci administered intramuscularly: a descriptive study of the clinical symptoms and clinical chemical response patterns. Clin. Infect. Dis. 29:167-177. [DOI] [PubMed] [Google Scholar]
- 32.van Diepen, A., T. van der Straaten, S. M. Holland, R. Janssen, and J. T. van Dissel. 2002. A superoxide-hypersusceptible Salmonella enterica serovar Typhimurium mutant is attenuated but regains virulence in p47phox−/− mice. Infect. Immun. 70:2614-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]