Abstract
Background
Intradermal (ID) delivery has been shown to accelerate insulin pharmacokinetics (PK). We compared the PK and pharmacodynamic (PD) effects of insulin lispro administered before two daily standardized solid mixed meals (breakfast and lunch), using microneedle-based ID or traditional subcutaneous (SC) delivery.
Method
The study included 22 subjects with type 1 diabetes in an eight-arm full crossover block design. One arm established each subject’s optimal meal dose. In six additional arms, the optimal, higher, and lower doses (+30%, -30%) were each given ID and SC delivery, in random order. The final arm assessed earlier timing for the ID optimal dose (-12 versus -2 min). The PK/PD data were collected for 6 h following meals. Intravenous basal regular insulin was given throughout, and premeal blood glucose (BG) adjusted to 115 mg/dl.
Results
The primary end point, postprandial time in range (70–180 mg/dl), showed no route-based differences with a high level of overall BG control for both SC and ID delivery. Secondary insulin PK end points showed more rapid ID availability versus SC across doses and meals (∆Tmax -16 min, ∆T50rising -7 min, ∆T50falling -30 min, all p < .05). Both intrasubject and intersubject variability for ID Tmax were significantly lower. Intradermal delivery showed modest, statistically significant secondary PD differences across doses and meals, generally within 90–120 min postprandially (∆12 mg/dl BG at 90 min, ∆7 mg/dl BGmax, ∆7 mg/dl mean BG 0–2 h, all p < .05).
Conclusions
This study indicates that ID insulin delivery is superior to SC delivery in speed of systemic availability and PK consistency and may improve postprandial glucose control.
Keywords: clinical research, clinical trial, insulin pharmacokinetics and pharmacodynamics, intradermal, microneedle, ultra-rapid insulin
Introduction
Flexible or intensive insulin therapy today is widely used in type 1 diabetes mellitus (T1DM) patients and in some insulin-requiring patients with type 2 diabetes mellitus to achieve lower overall glycemic exposure (without undue risk of hypoglycemia) and to prevent or delay microvascular complications.1,2 Such therapy requires both appropriate basal or background insulini-zation and patient-managed preprandial insulin dose titration to optimize postprandial glycemic excursions (PPG). While the “gold standard” for diabetes clinical management remains the level of glycated hemoglobin (HbA1c), observational and biochemical studies suggest a role for glycemic variability (GV) in both microvascular and macrovascular complication development.3–8 Epidemiological studies have also associated PPG with increased macrovascular disease and mortality risk, suggesting the importance of controlling not only fasting, but also post-meal glucose.9–11 However, it is by no means proven that GV or PPG independently contribute to adverse diabetic outcomes.12–15 Studies to date have been inadequate to separate out mean glycemic exposure from intraday variability, including PPG.16 At HbA1c levels of ~10%, the relative contributions of fasting and postprandial glucose to HbA1c are ~70% and 30%, respectively, versus nearly the opposite relative contributions with HbA1c levels near 7%.17 Better control of PPG is needed to attain near-normal HbA1c and reduce complications. Faster-acting and more reproducible insulin kinetic profiles would be valuable in this regard to achieve a better match of prandial insulin use to meal consumption and glucose uptake patterns.
Insulin delivery by continuous subcutaneous insulin infusion (CSII) is considered the most advanced method to administer insulin and may be supplemented by continuous glucose monitoring (CGM). However, current sensor-augmented pump therapy18 still relies on patient or caregiver actions and is considered “open loop.” “Closed-loop” insulin delivery algorithms to apply CGM data for CSII control, i.e., the artificial pancreas (AP), have been greatly facilitated by the development of glucose metabolism computer models in T1DM patients.19 Regulatory agency acceptance of such in silico modeling has led to an upsurge of work by investigators across the United States, Europe, and Israel, funded by the National Institutes of Health, the Juvenile Diabetes Research Foundation (JDRF), the AP@Home Project in Europe, and others.20 However, to date, these studies of control algorithms are almost entirely in tightly controlled clinical research center settings. There are major challenges in these investigations, including performance of today’s CGM sensors, the relatively slow (and inconsistent) kinetics of today’s “rapid” insulin analogs,21 and the peripheral (versus intraportal) route of insulin administration. It is questionable whether control algorithms can reproduce physiological metabolic control when subcutaneous (SC) analog insulin averages 1 h to reach maximum concentration in the blood (Tmax) and 90–120 min for maximum glucose-lowering effect (GIRTmax). There is a clear need for faster-acting insulin, for which the JDRF has funded its Ultra-Fast-Acting Insulin Project. How can insulin be made faster?
A number of investigators and companies are attempting to accelerate insulin pharmacokinetics (PK) and action [pharmacodynamics (PD)]. Various approaches include modifying the insulin molecule itself22 or altering the insulin formulation to increase the formation of insulin monomers,23–25 adding new excipients to accelerate insulin uptake from the SC space,26–30 warming the SC injection/infusion site,31 jet spray injection,32 or administering the insulin via new routes—either inhaled,33–36 intranasal,37,38 or intradermal.39–41
Intradermal (ID) insulin injection and infusion has been investigated by Gupta and colleagues42 and by BD (Becton Dickinson Inc.). Gupta and colleagues42 reported the use of borosilicate glass microneedles 900 µm in length to administer insulin into the dermis. In five subjects with T1DM, they found significantly faster insulin absorption (Tmax 27 min) than with a 9 mm SC catheter (Tmax 57 min). Peak insulin concentration was nonsignificantly higher with ID delivery (Cmax 32 µU/ml versus 25 µU/ml), and insulin area under the curve (AUC) did not differ. Postprandial changes in blood glucose (BG) levels were lower with ID than SC delivery but of unclear statistical significance.42
During initial preclinical studies in swine using 1.0 mm length cannulae, BD has evaluated steel microneedles for the ID delivery of a variety of protein drugs, showing consistently faster uptake and distribution and, in some cases, higher bioavailability.43 Subsequent clinical trials of microneedle insulin delivery have utilized microneedles from 1.25 to 1.75 mm in length and 31 to 34 G in diameter, bolus injection or pump bolus infusion, and both regular human insulin (RHI) and insulin lispro (IL) in both normal and T1DM subjects.41,44,45 Insulin uptake and clearance have been shown to be consistently faster for ID versus SC insulin dosing but with similar overall bioavailability. Tmax after bolus injection of IL in normal subjects with 31 G 1.25, 1.5, and 1.75 mm length microneedles was 36, 41, and 46 min, respectively—all significantly shorter than SC lispro (64 min). The Cmax was higher, and the GIRTmax was also significantly shorter—ranging from 106 to 112 min versus 130 min for SC lispro.44 Further studies using bolus infusion with 1.5 mm 34 G microneedle catheters showed significantly faster insulin uptake for both regular and lispro insulin in T1DMs, following weight-based dosing prior to a liquid meal. Postprandial glycemic excursion was significantly improved with ID versus SC regular insulin and trended positively for ID versus SC IL. Interestingly, intradermal regular insulin provided similar control of PPG as did SC lispro.45
The acceleration of insulin uptake when administered intradermally suggests a different anatomic distribution pathway from traditional SC dosing. This has been studied extensively in vivo, in swine46,47 as well as in humans, using radiolabeled insulin48 and near-infrared dyes.49 These studies show direct flow of insulin or dye to regional lymph nodes and then through the lymph to the thoracic duct and the systemic circulation, with lymph flow substantially faster than previously measured intrinsic flow.
The current study was undertaken to evaluate both PK and PD for IL administered via ID or SC infusion from standard insulin pump in patients with T1DM across a range of doses given 2 min before two types of solid meals. In addition, the optimal insulin dose was repeated intradermally, given an additional 10 min before the meals.
Materials and Methods
Subjects
Male and female patients with T1DM on CSII or multiple daily injection for at least 1 year, using carbohydrate (CHO) counting for at least 6 months, aged 18 to 55 years, with body mass index ≤ 32 kg/m², with HbA1c ≤ 8.0%, and negative for hepatitis B/C and HIV were eligible for participation. Females of childbearing potential had to use adequate contraceptive methods. Exclusion criteria included gastroparesis; impaired hepatic, renal, or cardiac functions; uncontrolled hypertension, retinopathy, or maculopathy; recurrent major hypoglycemia or hypoglycemic unawareness; plaster/adhesive allergy; pregnancy; other concomitant interfering conditions judged by the investigator; and lipodystrophy or other delivery-site abnormalities that might interfere with insulin absorption.
Study Design and Procedures
This was a single-center, randomized, open-label, eight-period crossover study using a five-block design (ClinicalTrials.gov; NCT01120444; EudraCT, 2010-019161-28). The study design was completed over 10 clinic visits in a multiweek period as shown in Table 1. The protocol was approved by an ethics committee and the appropriate regulatory body (Bundesinstitut für Arzneimittel und Medizinprodukte) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practices. All patients provided written, informed consent.
Table 1.
Clinical Study Visit, Block, and Dosing Schedule
| Visit # | 1 | 2 | 3–4 | 5–6 | 7–8 | 9 | 10 |
|---|---|---|---|---|---|---|---|
| Purpose | Screening | “Optimal” individual dose determination via insulin sensitivity testing and test meal | ID and SC dosing at “optimal” insulin dose | ID and SC dosing at “optimal” dose -30% | ID and SC dosing at “optimal” dose +30% | “Optimal” ID dosing with earlier premeal timing (-12 min) | Follow-up |
| Block # | 1 | 2 | 3 | 4 | 5 | ||
| Allowable timing within blocks | — | — | Both doses complete within 3–10 days | Both doses complete within 3–10 days | Both doses complete within 3–10 days | — | — |
| Allowable timing between blocks | — | Within 21 days post-screening | 3–14 days post-dose determination | Within 3–14 days of previous block | Within 3–14 days of previous block | Within 3–14 days of previous block | Immediately after visit 9 study procedures |
| Order of completion | 1 | 2 | 3 | 4–5 (randomized between blocks) | 6 | 7 | |
Prior to each dosing intervention, subjects were fasted for 8 h and discontinued short-, intermediate-, and long-acting insulin therapy 6, 12, or 24 h prior, respectively, from either injection or CSII. Basal insulin requirements were covered using intravenous (IV) infusion of recombinant RHI (Humulin®, Eli Lilly and Company, Indianapolis, IN). Dosing routes were randomized within blocks.
On visit 2, subject insulin sensitivity was determined using an isoglycemic manual clamp with a 2 IU IV RHI challenge. During a subsequent test meal (60 g CHO mixed meal) covered with a single SC IL injection (Humalog®, Eli Lilly and Company, Indianapolis, IN), an “optimal” dose was determined for each subject. These doses were targeted to achieve a 4 h postprandial glucose level within 15% of the preprandial level and were set by the investigator based on the test meal results. This dose served as basis for all subsequent IL preprandial bolus administrations.
Visits 3–8 comprised a randomized full crossover design in which each subject was given each combination of two delivery routes (SC or ID) and three individualized dose levels (optimal, -30%, and +30%) for two meals per day.
Continuous glucose monitors (Seven Plus®, DexCom Inc., San Diego, CA) were applied 24 h prior to the breakfast meal and calibrated per manufacturer’s recommendations. Blood glucose was stabilized overnight to a target concentration of 115 ± 15 mg/dl using a background IV basal infusion of RHI that was fixed 3 h premeal, while minor adjustments using small IV RHI and glucose boluses could be made up to 15 min premeal.
The breakfast meal contained predominantly rapidly absorbed CHOs (standard 60 g CHO; approximately 70:15:15 CHO:protein:fat ratio). At 2 min before eating, each insulin bolus infusion was administered into the periumbilical abdominal wall via an insulin pump (OneTouch® Ping®, Animas Corporation, West Chester, PA) using a freshly preprimed and inserted ID catheter (34 G × 1.5 mm length stainless steel, BD Research Catheter Set, BD Technologies, Research Triangle Park, NC, previously described45) or SC infusion catheter (28 G × 6 mm stainless steel, ACCU-CHEK® Rapid-D, Roche Insulin Delivery Systems Inc., Fishers, IN) set. Meal consumption occurred over 10–15 min.
Samples for serum insulin analysis and BG monitoring (Super-GL Ambulance glucose analyzer, Ruhrtal Labor- technik, Delecke-Möhnesee, Germany) were taken at pre-determined time points from 15 min premeal through 360 min postmeal. During the time period from 360–420 min, BG was again adjusted to 115 ± 15 mg/dl by IV insulin and glucose, if required. If the target BG level could not be reached during this 1 h stabilization period, the lunch meal start could be postponed up to 1 h. A second equivalent insulin infusion was given with a new catheter set at 2 min prior to the lunch meal (standard 60 g CHO; 35:25:40 ratio), followed by an additional 6 h sampling period.
At visit 9, to investigate the effect of dose timing, lispro was administered intradermally at the optimal dose but 12 min before each meal challenge. Otherwise, procedures at this visit were the same as described for visits 3 to 8.
In addition, to evaluate longer-term ID catheter flow functionality and performance, at visits 5 to 9, subjects were maintained on a second Animas pump delivering a combined ID bolus and basal regimen using a placebo solution (5% dextrose for injection) from the start of the breakfast challenge until the end of the observation period after the lunch challenge (i.e., over approximately 12–14 h).
Study End Points
The primary PD end point was total and percentage postprandial BG “time in range” (70–180 mg/dl) over several postmeal periods (0–1, 0–1.5, 0–2, 0–4, and 0–6 h). Additional secondary postprandial PD end points included absolute BG, maximum BG (BGmax), average BG (BGavg), glycemic range (BGmax–BGmin), area under the BG versus time curve (AUC BG), and total and percentage BG “time out of range” for moderate and severe hypoglycemia (<70, <60 mg/dl) and hyperglycemia (>180 and >220 mg/dl). The PK end points included maximum plasma insulin concentration (Cmax), time to maximum plasma insulin concentration (Tmax), time to 50% Cmax during insulin onset (T50%max rising) and offset (T50%max falling), area under the plasma insulin versus time curve (AUC insulin), and intrasubject and intersubject PK variability at equivalent dose. Where appropriate, PK concentration effects were examined both unadjusted and normalized for dose and subject mass to compensate for individualized dose variations due to insulin sensitivity and to compare the route effects across all administered dose ranges. Due to their time-dependent nature, all PK and PD end points were examined at specific predetermined postprandial intervals similar to the primary end point to provide a complete analysis of the insulin uptake and effect. Device functional performance was also monitored for leakage, adhesion, and occurrence of pump occlusion alarms.
Perception and Safety
Patients completed a survey in which they electronically rated the perceived discomfort of the ID or SC insulin administration on a standard 10 cm pain visual analog scale (VAS) ranging between no pain (0 cm) and severe pain (10 cm). Ratings were scored at two time points: after ID or SC catheter insertion and after infusion delivery but before device removal for each administration. The placebo ID device was also rated by VAS at placement and after each bolus.
The study physician checked the site of insulin adminis-tration upon removal of the infusion device as well as 1 and 4 h later and scored any local reactions according to the four-point Draize erythema and edema scale.
Other safety end points included collection of the number and seriousness of adverse events, including hypoglycemia. Interventional treatment for hypoglycemia events occurred at BG below 50 mg/dl, with or without symptoms, or a BG < 70 mg/dl with clinical symptoms (e.g., dizziness, anxiety, sweating, hunger, weakness, or nausea). In such cases, IV glucose was administered to increase BG to 65 mg/dl.
Insulin Analysis
To differentiate administered IL from the IV basal RHI, a specific non-cross-reactive IL radioimmunoassay (LisPro RIA, LPI-16K, Linco Research, St. Charles, MO) was performed at a central laboratory (IKFE, Mainz, Germany). The assay has negligible cross-reactivity with human insulin and proinsulin (<0.05%) and a typical lower detection limit of 5 µIU/ml.
Statistical Analysis
The primary PD end points were analyzed (both ID versus SC and ID 2 versus 12 min premeal) non-parametrically using a two-sided Wilcoxon signed rank test. The secondary PD end points were analyzed using an analysis of variance (ANOVA) with block sequence, block, sequence of application route, application route, meal, dose, and interactions as fixed factors and subject within block sequence as a random factor. The dose-adjusted insulin AUCs and Cmax PK end points were analyzed in the same way as the secondary PD end points, while Tmax, T50%max rising, and T50%max falling were analyzed nonparametrically in the same way as the primary PD end points. Intersubject and intrasubject variability of PK and PD end points by application route was assessed using a permutation test combining data across doses and meals. Log-transformed pain scores were analyzed with an ANOVA model, including application route, meal, assessment stage, dose, and subject as factors. For ANOVA models used to assess PK end points, secondary PD end points, and pain scores, interaction effects were assessed, and nonsignificant main effects of delivery route are reported. Lack of significant interactions between delivery route and other factors such as dose and meal suggest that the route effect does not differ significantly based on dose or meal, although these factors may have independent effects on the analyzed responses. Graphical data are shown combined across route, when no interaction effects were found, and also as individual dose/meal combinations for perspective.
Results
Subjects and Demographics
In total, 34 subjects were screened and 22 subjects (n = 17 men, 5 women) were enrolled and randomized to treatment; all completed all protocol treatments. Subject demographics were as follows [mean (± standard deviation), median, range] : age (years) 39.7 (9.6), 41, 23–55; height (cm) 179.0 (7.1), 179.5, 163–193; weight (kg) 82.6 (11.2), 79.7, 64.6–104.2; body mass index (kg/m2) 25.8 (2.8), 25.2, 21.1–31.8; HbA1c (%) 7.1 (0.60), 7.3, 5.8–7.9; and T1DM duration (years) 15.5 (7.4), 14.5, 4–31. Twenty-one subjects were white, and one subject was African.
Primary Pharmacodynamic End Point of Time in Range (70–180 mg/dl)
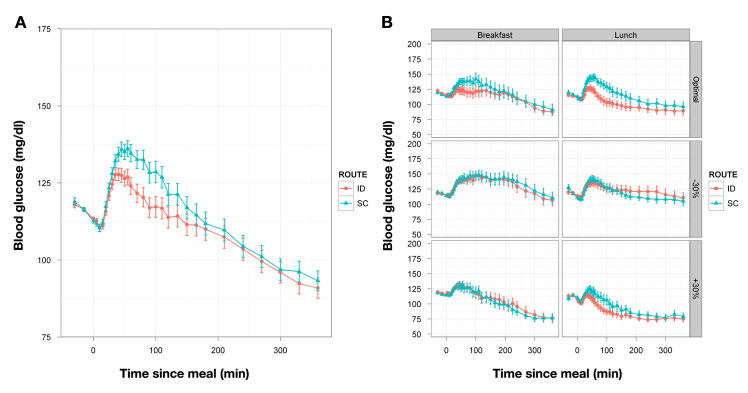
No significant differences were observed in the primary end point, BG time in range (70–180 mg/dl), during any of the postprandial periods examined. In the 6 h postmeal, the median percentage of time in range across doses and meals was 86% for both SC and ID delivery (Table 2, Figure 1). Additionally, comparison of the earlier dose timing (-12 min) to the -2 min timing for ID insulin at the optimal dose was statistically inconclusive for this outcome (data not shown).
Table 2.
Postprandial Percentage of Time in Blood Glucose Range (70–180 mg/dl)a
| Postprandial period (h) | Route | Mean | Median |
|---|---|---|---|
| 0–1 | ID | 98 | 100 |
| 0–1 | SC | 98 | 100 |
| 0–1.5 | ID | 95 | 100 |
| 0–1.5 | SC | 94 | 100 |
| 0–2 | ID | 92 | 100 |
| 0–2 | SC | 91 | 100 |
| 0–4 | ID | 83 | 96 |
| 0–4 | SC | 84 | 100 |
| 0–6 | ID | 76 | 86 |
| 0–6 | SC | 78 | 86 |
Route differences were not significant at all time points.
Figure 1.
(A) Mean PD curves for BG (±standard error) versus time across all meals and insulin doses (n = 132) for delivery at 2 min prior to the meal. (B) Mean PD curves (n = 22) broken out for each meal and dose combination. SE, standard error.
Secondary Pharmacokinetic/Pharmacodynamic End Points
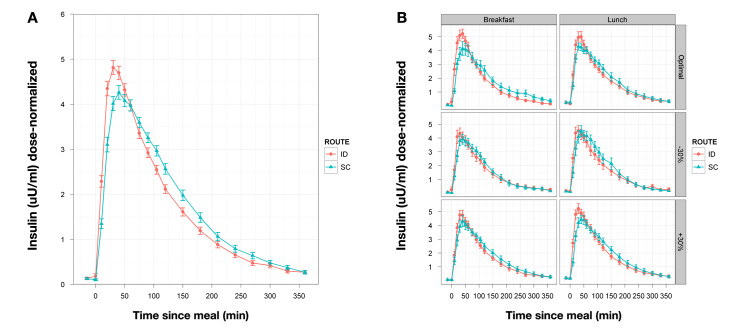
Secondary PK end points (Table 3, Figure 2) showed more rapid systemic insulin availability for ID versus SC delivery across doses and meals (∆Tmax, 16 min; ∆T50% rising, 7 min; ∆T50% falling, 30 min). Other than some differences in Tmax, ∆T50 rising, and ∆T50 falling between the -12 and -2 min optimal ID conditions, no other PK parameters were affected by dose timing. Both intrasubject and intersubject variability for Tmax were significantly lower for ID delivery (Table 4).
Table 3.
Secondary Pharmacokinetic and Pharmacodynamic End Points
| End point | Time (h) | p < .05 | ID (mean) | SC (mean) | Difference (ID - SC) |
|---|---|---|---|---|---|
| PK | |||||
| Cmax (dose normal) | x | 5.2 | 4.9 | 0.3 | |
| Tmax (min) | x | 36.0 | 51.6 | -15.6 | |
| T50%max rising (min) | x | 11.6 | 18.3 | -6.7 | |
| T50%max falling (min) | x | 112.8 | 142.3 | -29.5 | |
| PD | |||||
| AUC BG | 0–1 | x | 120.8 | 125.0 | -4.2 |
| 0–1.5 | x | 181.2 | 191.1 | -9.9 | |
| 0–2 | x | 239.5 | 254.5 | -15.0 | |
| 0–4 | 459.3 | 481.1 | -21.8 | ||
| 0–6 | 651.9 | 677.7 | -25.8 | ||
| BG (mg/dl) | 1 | x | 124.0 | 134.7 | -10.7 |
| 1.5 | x | 116.9 | 128.4 | -11.5 | |
| 2 | 113.8 | 121.3 | -7.5 | ||
| 4 | 103.6 | 104.5 | -0.9 | ||
| 6 | 90.9 | 93.3 | -2.4 | ||
| BG mean (mg/dl) | 0–1 | x | 120.9 | 125.0 | -4.1 |
| 0–1.5 | x | 120.9 | 127.3 | -6.4 | |
| 0–2 | x | 119.8 | 127.2 | -7.4 | |
| 0–4 | 114.8 | 120.2 | -5.4 | ||
| 0–6 | 108.6 | 112.9 | -4.3 | ||
| BG max (mg/dl) | 0–1 | x | 139.8 | 145.4 | -5.6 |
| 0–1.5 | x | 141.8 | 148.7 | -6.9 | |
| 0–2 | x | 143.9 | 151.1 | -7.2 | |
| 0–4 | x | 148.0 | 154.3 | -6.3 | |
| 0–6 | 149.7 | 155.7 | -6.0 | ||
Figure 2.
(A) Mean PK curves for dose-normalized insulin versus time profiles (±standard error) across all meals and insulin doses (n = 132) for delivery at 2 min prior to the meal. (B) Mean PK curves (n = 22) broken out for each meal and dose combination. SE, standard error.
Table 4.
Intrasubject Variability Due to Route of Administrationa
| End point | Time (h) | p value | ID %CV | SC %CV |
|---|---|---|---|---|
| PK | ||||
| Cmax | NS | |||
| Tmax (min) | p < .0001 | 30.2 | 40.9 | |
| T50% max rising (min) | p < .001 | 45.5 | 50.2 | |
| T50% max falling (min) | p < .05 | 20.6 | 23.2 | |
| PD | ||||
| AUC BG | 0–1 | p < .05 | 12.0 | 14.1 |
| BG max (mg/dl) | 0–1 | p < .05 | 13.0 | 15.6 |
NS indicates lack of statistical significance (alpha = 0.05); NS variability PD end points and time periods have been omitted.
Intradermal delivery also showed statistically significant but modest differences in secondary PD end points across doses and meals (∆12 mg/dl BG 90 min postmeal, ∆7 mg/dl BGmax, ∆7 mg/dl mean BG 0–2 h, Table 3). Improved glycemic response was also evident with the earlier (12 min premeal) optimal ID dose administration only within the first 90 min (data not shown). Pharmacodynamic end points, including AUC, BGmax, and BGmean, did not show significantly different intrasubject or intersubject variability beyond the first hour postmeal.
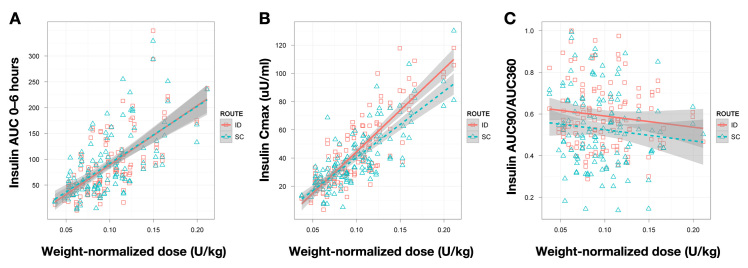
To examine the dose ranging linearity of ID delivery, insulin AUC 0–6 h [with standard error of the mean (SEM)] was plotted versus patient-weight-normalized dose levels (see Figure 3). Intradermal Cmax and fractional insulin AUC in the first 1.5 h (AUC 0–90 min/AUC 0–6 h) data were examined in a similar fashion. Both ID and SC dosing show essentially identical insulin AUC dose response linearity and similar SEM values across the study dose range (2.8–18.2 IU).
Figure 3.
Comparison of dose response relationships for various PK factors between ID and SC delivery. (A) Insulin AUC 0–6 h. (B) Insulin Cmax. (C) Insulin fractional AUC 0–90 all plotted versus weight-normalized dose. Grey bars indicate standard errors in the fitted curve.
Perception and Safety
Mean pain scores for all conditions were low overall. Small route-based differences in pain perception via VAS were observed. After application, ID delivery was significantly less painful than SC delivery (0.3 versus 0.6, p = .01). After delivery (before device removal), SC insulin was significantly less painful than ID insulin (1.1 versus 2.4, p < .0001). However, ID placebo delivery was significantly less painful than either, with a mean VAS score of 0.3 after each bolus (p < .0001). There was also no detectable relationship between dose volume and VAS score.
There were no significant differences between delivery routes in the number of hypoglycemic events or time reported in the hypoglycemic and hyperglycemic ranges. Hypoglycemic events increased significantly with increasing insulin dose, but no differences were seen between ID and SC administrations when affected subject numbers (16 ID versus 17 SC) and number of affected meals (41 ID versus 39 SC) are compared (Table 5).
Table 5.
Number of Meal Doses Requiring Hypoglycemic Rescue as a Function of Route (Includes All Meals, All Doses) and Dose (Includes Both Routes)a
| Number of meal doses requiring hypoglycemic rescue | |||||
|---|---|---|---|---|---|
| No rescue | At least 1 | More than 1 | More than 2 | ||
| Route | ID | 91 | 41 | 23 | 15 |
| SC | 93 | 39 | 20 | 12 | |
| Dose | -30% | 79 | 9 | 3 | 2 |
| Optimal | 65 | 23 | 9 | 3 | |
| +30% | 40 | 48 | 31 | 22 | |
The first two colums reflect the total 132 meals/route and 88 meals/dose (p < .05 for dose-relationship).
No serious adverse events were recorded during the study, and the totals and severity of mild and moderate adverse events was similar between SC and ID routes. The most commonly recorded adverse event was “headache” for both routes. The relationship to the investigational product was assessed as unrelated in all cases.
Draize skin effect scoring at device removal was similar between routes for erythema, with a maximum recorded score of Draize 2, and the vast majority of scores were ≤1 (undetectable–minimal). Intradermal delivery exhibited both slightly higher frequency and severity for edema scoring, with the highest edema grade of 3 across all dose levels, while, for the SC application, the highest grade was 2 (at the +30% dose level).
Device Performance
Use of the insulin infusion pump catheter for ID bolus insulin administration was effective with no recorded occlusion alarms, and all devices adhered securely during delivery. Furthermore, combined basal/bolus ID placebo delivery also performed well over the 12–13 h of use. The only recorded leakage events occurred in four SC control catheters, while all ID devices remained leak-free.
Discussion
In this extended crossover trial, we examined PK and postprandial glucodynamics of insulin administered preprandially by two routes—SC and ID—across a range of insulin doses and with two different types of solid meals. The time of all insulin dosing was 2 min prior to meal ingestion (e.g., immediate) but was also tested at -12 min for ID administration of the optimal dose.
The primary objective was to demonstrate improvements in PPG with ID dosing, expressed as percentage of time in range out to 6 h. For the first 2 h, 91–98% of glucose values were in range with SC insulin, and these numbers declined modestly to 84% and 78% in range through 4 and 6 h, respectively. With ID dosing, time in range was almost identical to SC (Table 2), and no significant differences could be demonstrated between the two routes, reflecting the very tight glycemic control obtained from individualized insulin sensitivity testing and the meal dose optimization. Even under conditions of intentional overdosage and underdosage and different meal types, the majority of subjects stayed well within target. More “out-of-range” excursions were seen after 4 h; however, no route or dose level effects were found. This degree of postprandial control is quite unusual for T1DM patients outside the intensive control found in the clinical research environment.50 The mean PD profiles in Figure 1 also show a declining BG baseline both immediately premeal and through the sampling duration, indicating that background insulin basal rates used for BG titration to the 115 mg/dl starting value may have resulted in slight overinsulinization with reduced hepatic glucose output, decreasing the ability to discern PD differences between delivery routes.
A number of secondary measures, including additional PD metrics, insulin PK, and PK variability, were statistically improved with ID dosing (Tables 3 and 4). Although ID and SC insulin dosing were compared with two meal types and across a three-dose range, there was no evidence of statistically significant interaction between route and either dose or meal—suggesting that the route effect is similar across meals and doses tested. Therefore, BG and insulin data were analyzed across all meal/dose combinations for the main effects of dosing route, providing many replicates (n = 132/route) for each time point over the 6 h test period. The mean BG with SC dosing was ~129 mg/dl at 1.5 h, and ID BG was modestly but significantly lowered ~12 mg/dl (p < .05). Other PD factors, including BGmax, BGmean, and BG AUC also showed modest but statistically different (p < .05) changes with ID delivery. Further improvement in PPG would be difficult to achieve and likely associated with increased hypoglycemia.
The changes in insulin PK observed are consistent with prior studies of ID insulin by microneedle bolus injection or infusion.44,45 Tmax was accelerated by ~16 min from 52 to 36 min, with ~7 and 30 min shortening, respectively, of T50%max rising and T50%max falling. Cmax was statistically increased for dose-normalized data, although the difference is relatively small (5.2 versus 4.9). Insulin AUC 0–90 was significantly increased, reflecting a relative shifting of insulin uptake to the earlier time period, but overall insulin AUC at 6 h was not significant versus SC dosing. The Tmax with SC administration—51.6 min—is at the lower end of the range usually found with SC lispro (55–65 min). In the two earlier studies with ID IL, Tmax was observed at ~58 and 64 min.44,45 These differences with ID delivery remain potentially valuable for closed-loop algorithm development. Another microneedle study has found similar acceleration of insulin PK.42
The essentially identical insulin AUC dose response linearity and similar SEM values for ID and SC dosing across the study dose range indicate good predictability of ID insulin exposure as a function of dose (Figure 3). Intradermal Cmax versus dose had a higher slope than the SC graph. To examine whether this altered Cmax dose relationship could affect required insulin dosing predictions, ID fractional insulin AUC in the first 1.5 h (AUC 0–90 min/AUC 0–6 h) was plotted and was higher than SC (as expected due to the time shift in insulin uptake) but parallel as a function of dose. Taken together, these data imply that predicting required ID insulin dosing and the total resulting insulin exposure should be comparable to current SC delivery.
An additional potentially important observation of this trial was reduced intrasubject and intersubject variation in ID insulin PK. Intrasubject variability in insulin Tmax across all meals and doses, expressed as percentage coefficient of variation (%CV) was reduced from 40% to 30% overall, an ~25% relative decrease (p < .0001); T50%max rising and T50%max falling %CV were also significantly reduced, as was intersubject variability for Tmax. Intrasubject BG variability measured by two factors (BG AUC and BGmax) was also reduced but by a lower amount (Δ ~3%CV) and only in the first hour. Variability in insulin absorption is a well-known barrier to intensive insulin therapy. In a well-controlled clinical research center setting, uptake of SC IL under closed-loop control varied from 56 to 72 min in six subjects who did not experience hypoglycemia and from 71 to 191 min in five subjects who did.21 This indicates a more-than-three-fold variation in the availability of a hormone with a narrow therapeutic index. Any improvement in consistency of insulin uptake would be advantageous to patients and closed-loop AP investigators.
Intradermal insulin delivery was well tolerated in this study. On a noncomparative 10 cm VAS, mean ratings for pain at needle insertion were similarly low; at the end of the infusion bolus, infusion pain was reported as increased with the ID needle but still quite small overall (mean 2.4 cm). Dermal reactions from ID delivery, especially localized edema due to shallow injection, were higher but resolved rapidly (typically within an hour). Recorded adverse events were similar between the two routes in this study. Intradermal device functional performance was as good as SC and had no recorded leakage or occlusion alarms during either bolus or extended-duration basal/bolus use.
Conclusions
This study provides new information on microneedle-based ID infusion of IL in patients with T1DM, compared with SC delivery. Intradermal lispro is absorbed significantly faster than SC and with greater consistency between doses and between subjects. Glycemic control expressed as percentage of time-in-range over 6 h post-prandially was not significantly improved, but this lack of difference may reflect the unusually tight control of postmeal glycemia with SC lispro dosing after individual dose optimization or potential basal overinsulinization. A number of secondary PD measures were significantly lower with ID delivery, particularly in the first 90 to 120 min. Further studies with ID insulin therapy are warranted in efforts to maximize postprandial glycemic control outside the clinical research environment and for development of closed-loop AP systems.
Acknowledgments
We thank the patients who participated in this study as well as the JDRF, which provided funding via an Insulin Infusion Industry Development Partnership Proposal. We also thank the numerous extended team members from BD and Profil who contributed to the successful planning, organization, execution, and analysis of this study.
Glossary
- (%CV)
percentage coefficient of variation
- (ANOVA)
analysis of variance
- (AP)
artificial pancreas
- (AUC)
area under the curve
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (CHO)
carbohydrate
- (CSII)
continuous subcutaneous insulin infusion
- (GV)
glycemic variability
- (HbA1c)
glycated hemoglobin
- (ID)
intradermal
- (IL)
insulin lispro
- (JDRF)
Juvenile Diabetes Research Foundation
- (PD)
pharmacodynamics
- (PK)
pharmacokinetics
- (PPG)
postprandial glycemic excursion
- (RHI)
regular human insulin
- (SC)
subcutaneous
- (SEM)
standard error of the mean
- (T1DM)
type 1 diabetes mellitus
- (VAS)
visual analog scale
Funding
This work was supported by Becton Dickinson, Inc. and the JDRF.
Disclosures
Elaine McVey, Diane E. Sutter, Kerstin Rebrin, Laurence Hirsch, and Ronald J. Pettis are employees of BD, which sponsored this work. Christoph Kapitza is shareholder of Profil Institut für Stoffwechselforschung GmbH, the clinical research organization that performed the study.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008;31(11):2198–2202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 7.Weber C, Schnell O. The assessment of glycemic variability and its impact on diabetes-related complications: an overview. Diabetes Technol Ther. 2009;11(10):623–633. doi: 10.1089/dia.2009.0043. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA. 2010;303(22):2291–2292. doi: 10.1001/jama.2010.785. [DOI] [PubMed] [Google Scholar]
- 9.De Vegt F, Dekker JM, Ruhé HG, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42(8):926–931. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 10.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23(12):1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 11.Davidson J. Should postprandial glucose be measured and treated to a particular target? Yes. Diabetes Care. 2003;26(6):1919–1921. doi: 10.2337/diacare.26.6.1919. [DOI] [PubMed] [Google Scholar]
- 12.McCarter RJ, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care. 2006;29(2):352–355. doi: 10.2337/diacare.29.02.06.dc05-1594. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29(7):1486–1890. doi: 10.2337/dc06-0293. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009;32(10):1901–1903. doi: 10.2337/dc09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolli GB. Glucose variability and complications. Diabetes Care. 2006;29(7):1707–1709. doi: 10.2337/dc06-0716. [DOI] [PubMed] [Google Scholar]
- 16.Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, Jacober SJ. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32(3):381–386. doi: 10.2337/dc08-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26(3):881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 18.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA, STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 19.Kovatchev BP, Breton M, Man CD, Cobelli C. In silico preclinical trials: A proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamborlane WV. Closed-loop insulin delivery: we’re “virtually” there. Diabetes Technol Ther. 2012;14(3):203–204. doi: 10.1089/dia.2011.0261. [DOI] [PubMed] [Google Scholar]
- 21.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips NB, Whittaker J, Ismail-Beigi F, Weiss MA. Insulin fibrillation and protein design: Topological resistance of single-chain analogs to thermal degradation with application to a pump reservoir. J Diabetes Sci Technol. 2012;6(2):277–288. doi: 10.1177/193229681200600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner S, Hompesch M, Pohl R, Simms P, Flacke F, Mohr T, Pfützner A, Heinemann L. A novel insulin formulation with a more rapid onset of action. Diabetologia. 2008;51(9):1602–1606. doi: 10.1007/s00125-008-1095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hompesch M, McManus L, Pohl R, Simms P, Pfützner A, Bülow E, Flacke F, Heinemann L, Steiner SS. Intra-individual variability of the metabolic effect of a novel rapid-acting insulin (VIAject) in comparison to regular human insulin. J Diabetes Sci Technol. 2008;2(4):568–571. doi: 10.1177/193229680800200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinemann L, Nosek L, Flacke F, Albus K, Krasner A, Pichotta P, Heise T, Steiner S. U-100, pH-Neutral formulation of VIAject(®) : faster onset of action than insulin lispro in patients with type 1 diabetes. Diabetes Obes Metab. 2012;14(3):222–227. doi: 10.1111/j.1463-1326.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- 26.Vaughn DE, Yocum RC, Muchmore DB, Sugarman BJ, Vick AM, Bilinsky IP, Frost GI. Accelerated pharmacokinetics and gluco-dynamics of prandial insulins injected with recombinant human hyaluronidase. Diabetes Technol Ther. 2009;11(6):345–352. doi: 10.1089/dia.2009.0013. [DOI] [PubMed] [Google Scholar]
- 27.Morrow L, Muchmore DB, Ludington EA, Vaughn DE, Hompesch M. Reduction in intrasubject variability in the pharmaco-kinetic response to insulin after subcutaneous co-administration with recombinant human hyaluronidase in healthy volunteers. Diabetes Technol Ther. 2011;13(10):1039–1045. doi: 10.1089/dia.2011.0115. [DOI] [PubMed] [Google Scholar]
- 28.Hompesch M, Muchmore DB, Morrow L, Vaughn DE. Accelerated insulin pharmacokinetics and improved postprandial glycemic control in patients with type 1 diabetes after coadministration of prandial insulins with hyaluronidase. Diabetes Care. 2011;34(3):666–668. doi: 10.2337/dc10-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hompesch M, Muchmore DB, Morrow L, Ludington E, Vaughn DE. Improved postprandial glycemic control in patients with type 2 diabetes from subcutaneous injection of insulin lispro with hyaluronidase. Diabetes Technol Ther. 2012;14(3):218–224. doi: 10.1089/dia.2011.0117. [DOI] [PubMed] [Google Scholar]
- 30.Vaughn DE, Muchmore DB. Use of recombinant human hyaluronidase to accelerate rapid insulin analogue absorption: Experience with subcutaneous injection and continuous infusion. Endocr Pract. 2011;17(6):914–921. doi: 10.4158/EP11297.RA. [DOI] [PubMed] [Google Scholar]
- 31.Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach B. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31(5):980–987. doi: 10.1016/j.clinthera.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Engwerda EE, Abbink EJ, Tack CJ, de Galan BE. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care. 2011;34(8):1804–1808. doi: 10.2337/dc11-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rave K, Potocka E, Heinemann L, Heise T, Boss AH, Marino M, Costello D, Chen R. Pharmacokinetics and linear exposure of AFRESA compared with the subcutaneous injection of regular human insulin. Diabetes Obes Metab. 2009;11(7):715–720. doi: 10.1111/j.1463-1326.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 34.Richardson PC, Boss AH. Technosphere insulin technology. Diabetes Technol Ther. 2007;9(Suppl 1):S65–S72. doi: 10.1089/dia.2007.0212. [DOI] [PubMed] [Google Scholar]
- 35.Rave K, Heise T, Pfützner A, Boss AH. Coverage of postprandial blood glucose excursions with inhaled Technosphere insulin in comparison to subcutaneously injected regular human insulin in subjects with type 2 diabetes. Diabetes Care. 2007;30(9):2307–2308. doi: 10.2337/dc07-0478. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstock J, Lorber DL, Gnudi L, Howard CP, Bilheimer DW, Chang PC, Petrucci RE, Boss AH, Richardson PC. Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. Lancet. 2010;375(9733):2244–2253. doi: 10.1016/S0140-6736(10)60632-0. [DOI] [PubMed] [Google Scholar]
- 37.Stote R, Marbury T, Shi L, Miller M, Strange P. Comparison pharmacokinetics of two concentrations (0.7% and 1.0%) of Nasulin, an ultra-rapid-acting intranasal insulin formulation. J Diabetes Sci Technol. 2010;4(3):603–609. doi: 10.1177/193229681000400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stote R, Miller M, Marbury T, Shi L, Strange P. Enhanced absorption of Nasulin™, an ultrarapid-acting intranasal insulin formulation, using single nostril administration in normal subjects. J Diabetes Sci Technol. 2011;5(1):113–119. doi: 10.1177/193229681100500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta J, Felner EI, Prausnitz MR. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol Ther. 2011;13(4):451–456. doi: 10.1089/dia.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28(1):107–116. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 41.Pettis RJ, Ginsberg B, Hirsch L, Sutter D, Keith S, McVey E, Harvey NG, Hompesch M, Nosek L, Kapitza C, Heinemann L. Intradermal microneedle delivery of insulin lispro achieves faster insulin absorption and insulin action than subcutaneous injection. Diabetes Technol Ther. 2011;13(4):435–442. doi: 10.1089/dia.2010.0184. [DOI] [PubMed] [Google Scholar]
- 42.Gupta J, Felner EI, Prausnitz MR. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol Ther. 2011;13(4):451–456. doi: 10.1089/dia.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28(1):107–116. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 44.Pettis RJ, Ginsberg B, Hirsch L, Sutter D, Keith S, McVey E, Harvey NG, Hompesch M, Nosek L, Kapitza C, Heinemann L. Intradermal microneedle delivery of insulin lispro achieves faster insulin absorption and insulin action than subcutaneous injection. Diabetes Technol Ther. 2011;13(4):435–442. doi: 10.1089/dia.2010.0184. [DOI] [PubMed] [Google Scholar]
- 45.Pettis RJ, Hirsch L, Kapitza C, Nosek L, Hövelmann U, Kurth HJ, Sutter DE, Harvey NG, Heinemann L. Microneedle-based intradermal versus subcutaneous administration of regular human insulin or insulin lispro: Pharmacokinetics and postprandial glycemic excursions in patients with type 1 diabetes. Diabetes Technol Ther. 2011;13(4):443–450. doi: 10.1089/dia.2010.0183. [DOI] [PubMed] [Google Scholar]
- 46.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28(1):107–116. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 47.Sharma R, Wang W, Rasmussen JC, Joshi A, Houston JP, Adams KE, Cameron A, Ke S, Kwon S, Mawad ME, Sevick-Muraca EM. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol. 2007;292(6):H3109–H3118. doi: 10.1152/ajpheart.01223.2006. [DOI] [PubMed] [Google Scholar]
- 48.Pettis RJ, Harvey AJ. Microneedle delivery: clinical studies and emerging medical applications. Ther Delivery. 2012;3(3):357–371. doi: 10.4155/tde.12.13. [DOI] [PubMed] [Google Scholar]
- 49.Sevick-Muraca EM, Sharma R, Rasmussen JC, Marshall MV, Wendt JA, Pham HQ, Bonefas E, Houston JP, Sampath L, Adams KE, Blanchard DK, Fisher RE, Chiang SB, Elledge R, Mawad ME. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246(3):734–741. doi: 10.1148/radiol.2463070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care. 2011;34(Suppl 2):S120–S127. doi: 10.2337/dc11-s206. [DOI] [PMC free article] [PubMed] [Google Scholar]