Abstract
Barriers to the use of prandial insulin regimens include inadequate synchronization of insulin action to postprandial plasma glucose excursions as well as a significant risk of hypoglycemia and weight gain. Technosphere® insulin (TI) is an inhaled ultra-rapid-acting human insulin that is quickly absorbed in the alveoli. With a time to maximum plasma drug concentration of approximately 14 min and a time to maximum effect of 35 to 40 min, TI more closely matches the postprandial insulin concentrations seen in nondiabetic individuals. Studies have shown that long-term administration of prandial TI in combination with long-acting basal insulin results in reductions in hemoglobin A1c comparable to conventional subcutaneously injected prandial insulins but with improved control of early postprandial BG. Furthermore, TI has been associated with less weight gain and a lower incidence of hypoglycemia, which may enhance patient satisfaction and acceptability of insulin therapy. This review discusses the clinical properties of TI and proposes strategies for optimal use.
Keywords: diabetes mellitus, hypoglycemia, insulin, postprandial hyperglycemia, weight gain
Introduction
Ultra-rapid-acting insulin was developed to improve synchronization between the postprandial action of prandial insulin and the postprandial glucose (PPG) dynamics and to reduce the incidence of hyperglycemia and hypoglycemia. Table 3 of Heinemann and Muchmore1 provides the pharmacokinetic (PK)/pharmacodynamic (PD) characteristics of various prandial insulins.
Current prandial insulin, administered as a subcutaneous bolus, does not meet the challenge of mimicking physiologic postprandial insulin action.2 A strategy for enhancing postprandial insulin synchronization is the absorption of insulin via the pulmonary route. This is a rational option because the lungs have a large absorption surface area and a thin epithelium and are richly perfused with blood.3–6 Optimal absorption from the lung can be achieved when insulin is deposited deep in the alveoli, which requires a particle size between 1 and 5 µm.5,7
Technosphere® insulin (TI), or AFREZZA®, is an inhaled insulin product currently in clinical development (MannKind Corporation, Valencia, CA). With this formu-lation, insulin is adsorbed to fumaryl diketopiperazine (small-diameter microparticle; average size 2.5 µm).7,8 The microparticles are freeze dried, forming a dry powder suitable for inhalation via a small inhaler device (Figure 1).9 Upon inhalation, the microparticles dissolve, releasing insulin, which is rapidly absorbed into the systemic circulation.8,10,11
Figure 1.
Technosphere inhalation insulin device. Images used with permission of MannKind Corporation.
In patients with type 1 or type 2 diabetes, TI exhibits a linear, dose-related insulin PK profile.12,13 Maximum plasma drug concentration occurs sooner with TI (10 to 15 min) than with regular human insulin or rapid-acting insulin analog (RAA), as does the peak glucose-lowering effect (45 min after dosing; Figure 2).12–15 These results more closely mimic the normal physiological plasma profile of prandial insulin seen in nondiabetic individuals.16 In patients with type 2 diabetes mellitus, maximal suppression of postprandial endogenous glucose production occurred more rapidly with 60 U of TI (45 min) than with 10 U of subcutaneous RAA (105 min).16
Figure 2.
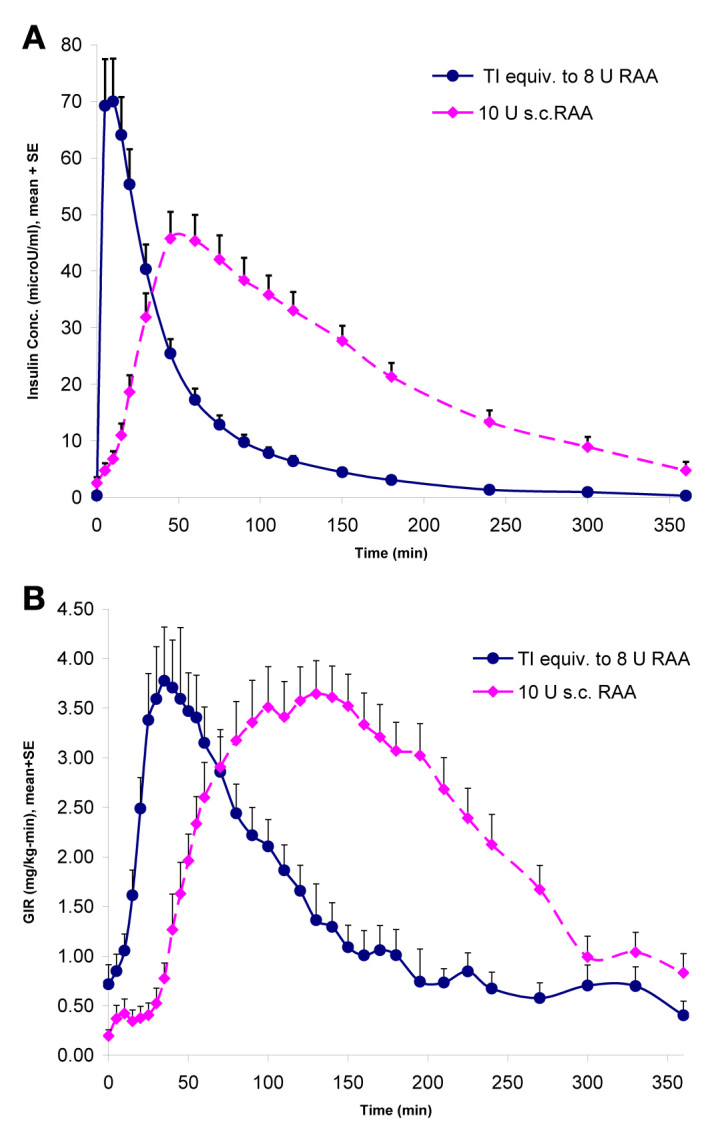
PK/PD profile of inhaled TI versus a RAA presented by Cassidy and colleagues15 at the 2009 Annual Scientific Sessions of the American Diabetes Association. SE, standard error; conc., concentration; s.c., subcutaneous; GIR, glucose infusion rate.
Clinical Experience with Technosphere Insulin as an Ultra-Rapid-Acting Insulin
Clinical studies of prandial TI combined with long-acting basal insulin have consistently shown improved early postprandial control and less hypoglycemia compared with subcutaneous insulin regimens incorporating long-acting and short-acting insulins in patients with type 1 or type 2 diabetes.17–21
A 52-week, randomized, open-label, parallel-group study evaluated the efficacy and safety of basal insulin (glargine) plus prandial TI (n = 211) versus a twice-daily premixed insulin, biaspart insulin 30 (n = 237) in patients with type 2 diabetes.17 Change in hemoglobin A1c (A1C) with TI plus insulin glargine [-0.68% ± 0.077%; 95% confidence intervals (CIs), -0.83 to -0.53] was similar and noninferior to that with biaspart insulin (-0.76% ± 0.071%; 95% CI, -0.90 to -0.62). The mean insulin dose for the last 12 weeks of the study was 81 (±48) IU of biaspart insulin and 198 (±74) U of TI (approximately equivalent to 52 IU subcutaneous insulin) together with 43 (±22) IU of insulin glargine.
The TI group had significantly less risk of hypoglycemia (0.41 versus 0.61 events/patient month; p < .01), and the incidence rate for severe hypoglycemic events was lower (4% versus 10%; p = .0066) with TI plus insulin glargine than with biaspart insulin. A standardized meal test was performed as part of the study. Somewhat surprisingly, the 2 h PPG was similar between treatment groups (212.4 ± 73.8 mg/dl versus 212.4 ± 77.4 mg/dl), possibly because the 2 h PPG was regularly measured and used as a secondary titration time point. The PPG area under the curve (AUC) from 0 to 360 min was similar between treatment groups (1076.4 mg/h/dl for TI plus insulin glargine versus 1020.6 mg/h/dl for biaspart insulin), but each group reached these AUC values differently (Figure 3). Consistent with the PK/PD properties of the treatments, AUC from 0 to 120 min was lower with inhaled insulin plus insulin glargine, whereas AUC from 120 to 360 min was lower and dropped below baseline with biaspart insulin. More than twice as many events of confirmed hypoglycemia (≤63 mg/dl) occurred in the biaspart group, of which 83% occurred between 120 and 360 min (data on file, MannKind Corporation). This corresponds with the longer duration of action for biaspart insulin, which may exceed the duration of meal absorption.
Figure 3.
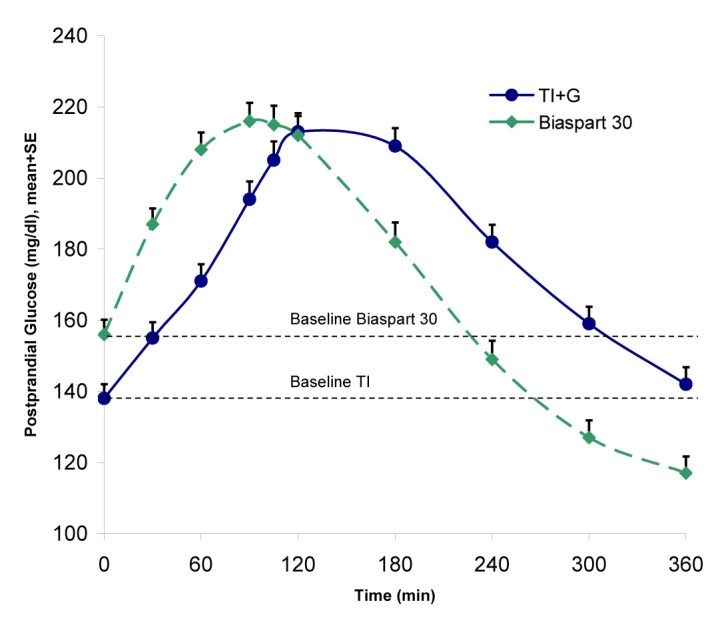
Postprandial glucose excursions following a standardized meal test (12 oz. Boost Plus™) for TI versus biaspart insulin 30. Reprinted with permission from Rosenstock and associates.17 SE, standard error; G, glargine.
One mechanism contributing to the lower early PPG level seen with TI may be a greater suppression of endogenous glucose production. This explanation is consistent with a study by Potocka and associates18 that showed that TI suppresses endogenous glucose production earlier and more completely when compared with both insulin lispro and a different inhaled insulin (Exubera). Thus, although the PPG and AUC data suggest that TI is associated with improved control of early postprandial control of glucose, TI may, in some cases, exert suboptimal control of late PPG levels (Figure 3).
Fasting plasma glucose values were also lower with TI than with biaspart insulin.17 Changes from baseline were 36.0 ± 5.4 mg/dl (95% CI, -45.0 to -27.0) for TI plus insulin glargine versus 18.0 ± 3.6 mg/dl (95% CI, -27 to -9) for biaspart insulin. Patients on TI plus glargine also had significantly less weight gain (0.9 ± 0.3 kg versus 2.5 ± 0.3 kg; p = .0002). The lower weight gain with TI plus insulin glargine compared with biaspart insulin was not attributable to metformin use, which was equal in both treatment groups.
Another randomized study evaluated the efficacy and safety of prandial TI (n = 293) versus a subcutaneous RAA (insulin aspart, n = 272), both administered with daily insulin glargine for 52 weeks in patients with type 1 diabetes and A1C >7.0% and ≤11.0%.19 The change in A1C was -0.11% for TI and -0.36% for RAA, making TI noninferior to RAA for a 0.4% noninferiority margin (mixed model-repeated measure, difference = 0.25% CI, 0.11 to 0.38). The PPG values followed a similar pattern as the type 2 study (discussed earlier). Interestingly, as in the type 2 study, fasting plasma glucose levels were significantly lower with TI than with RAA (-44.9 ± 104.7 versus -23.4 ± 103.1 mg/dl; p = .0052). The mechanism underlying this phenomenon is unclear.22 Patients who administered TI reported weight loss while those who administered RAA reported weight gain (-0.5 ± 0.1 versus +1.4 ± 3.9 kg; p < .0001). Finally, the TI group had a statistically significant reduction in the incidence of mild/moderate hypoglycemia [odds ratio (OR), 0.474; 95% CI, 0.0271 to 0.831; p = .0091] and total hypoglycemia (OR, 0.488; 95% CI, 0.278 to 0.856; p = .0124) compared with the RAA group.
In a randomized study of prandial TI versus prandial lispro insulin, both given with insulin glargine, in 130 patients with type 1 diabetes mellitus, change in A1C was similar between treatment groups at 16 weeks, but PPG levels were lower with TI than with lispro insulin.20 Technosphere insulin significantly reduced mild/moderate hypoglycemic event rates (5.97 versus 8.01 events/patient month; p = .0269) and overall total hypoglycemic events (6.17 versus 8.19 events/patient month; p = .0345) compared with lispro insulin. Similarly, in an open label study of patients with type 2 diabetes mellitus treated with diet and exercise alone, oral antidiabetes drugs, or insulin, the addition of prandial TI resulted in a comparable reduction between the groups in A1C (-0.70% for TI plus usual diabetes care versus -0.59% for usual diabetes care alone; p = .30). In addition, total hypoglycemic event rates were reduced in those receiving insulin (0.15 per patient month for TI versus 0.24 for subcutaneous insulin; p = .03).21
Safety of Technosphere Insulin as an Ultra-Rapid-Acting Prandial Insulin
The safety of TI has been studied extensively, both preclinically and in a clinical program involving more than 5600 subjects. The most common treatment-emergent adverse events were hypoglycemia, cough, and upper respiratory tract infection.17 Cough occurred in approxi-mately 32% of patients administering TI but tended to be mild and transient, occurring within minutes of inhalation.17 Furthermore, cough diminished over time and rarely led to study discontinuation.
A pooled analysis of cardiovascular events from nine clinical studies comprising 4467 patients who administered TI or usual diabetes care showed that the incidence of cardiovascular or cerebrovascular events was similar between the TI and usual diabetes care groups (relative risk, 1.01; 95% CI, 0.84 to 1.20).23
Lung function was examined in a 2-year prospective, multicenter, randomized, open-label study in patients with type 1 or type 2 diabetes administering TI (n = 730) or usual diabetes care (n = 824) and a cohort without diabetes who did not receive any specific therapy (n = 145).24 Lung function declined from baseline in all treatment groups, in line with normal age-related changes and as seen in diabetes in general. Compared with usual diabetes care, TI showed a small reduction in forced expiratory volume in 1 s (FEV1) from baseline to month 24 (-0.037 ± 0.0119 liter; 95% CI, 0.014 to 0.060). After a greater initial decline by month 3 with TI, the rate of change (slope) in FEV1, forced vital capacity, and diffusing capacity of the lung for carbon monoxide over months 3 to 24 was not statistically different between treatment groups. This observed treatment group difference was small, occurred early after therapy initiation, did not progress over 2 years, and resolved after discontinuation of therapy.
Two cases of cancer involving the lung have been reported during clinical trials, both in ex-smokers. The incidence does not exceed what would be expected in a similar, nontreated population.25 A 2-year carcinogenicity study in rats26 and a 6-month study in transgenic mice (data on file, MannKind Corporation) did not indicate a carcinogenic potential.
Acceptability of Technosphere Insulin as an Ultra-Rapid-Acting Prandial Insulin
Treatment outcomes may be substantially impacted by patients’ acceptance of their prescribed antidiabetes regimen, as well as their ability to overcome barriers to therapy (i.e., concerns about injections, hypoglycemia, or weight gain).27–29 Because of these factors, not all patients who would benefit from basal–bolus insulin therapy are receptive to initiating this therapy.
Findings from several studies support enhanced patient acceptance and satisfaction with TI therapy. A 52-week, randomized, open-label, parallel-group study of adult patients with type 2 diabetes included an assessment of patient satisfaction with TI plus bedtime insulin glargine versus twice-daily biphasic aspart insulin.30 As assessed in the diabetes worries section of the inhaled insulin treatment questionnaire, patient satisfaction significantly improved (p = .008) with TI plus long-acting basal insulin compared with twice-daily biaspart insulin. Diabetes worries decreased significantly from baseline in the TI plus insulin glargine group (p = .008) but not in the biaspart insulin group, according to the 36-item short-form health survey quality-of-life and insulin treatment questionnaires.17 In another study, health-related quality of life and treatment satisfaction showed greater improvement among those administering TI (with or without oral antidiabetes drugs) compared with those taking oral antidiabetes drugs alone.31
Using an Ultra-Rapid-Acting Insulin
Using TI as an ultra-rapid-acting prandial insulin, with fast onset and short duration, results in comparable A1C reductions to existing, currently available insulin treatment. There is improved early control of postprandial blood glucose (BG), as assessed by 1 h PPG measurements, compared with subcutaneously administered RAA,17 and a reduction in late postprandial hypoglycemia. However, TI is not associated with improved late (i.e., 2 h) PPG control,17 as in some cases, the short duration of action provides insufficient insulin effect in the late postprandial period (beyond 2 to 3 h). The length of the period for which prandial insulin is required is very variable, depending on multiple factors, such as meal composition and gastric emptying. Thus, where existing injected insulins are too long acting, an ultra-rapid insulin may, in some cases, not be long acting enough.32 The next studies explore whether a treatment strategy that includes a second, postprandial dose of ultra-rapid insulin can address this and provide better late PPG control.
A predictive study concluded that a subcutaneously administered RAA does not have a PK/PD profile that adapts proficiently to a closed-loop system.33 The investigators performed an in silico study of an autonomous artificial pancreas, consisting of a subcutaneous continuous glucose monitor with a continuous subcutaneous insulin infusion (CSII) pump. The study simulated 100 subjects from the University of Virginia/Padova metabolic simulator, each receiving a single meal with 100 g glucose, with an observation time of 15 h. When the mealtime bolus dose of infused insulin was replaced by a TI dose (approximately equivalent to 4 IU RAA) at the initiation of the meal, the time spent with a BG >180 mg/dl was reduced from 3.1 ± 0.9 to 1.7 ± 1.4 h and peak BG from 267 ± 54 to 207 ± 47 mg/dl, in both cases without any hypoglycemia, with a BG < 50 mg/dl. A TI dose equivalent to 8 IU RAA reduced the time of BG >180 mg/dl to 0.9 ± 1.2 h and the peak BG to 181 mg/dl, but 4 simulated subjects had a BG <50 mg/dl.33
A pilot study of seven patients with type 1 diabetes evaluated PPG excursions after a standard, large meal (data on file, MannKind Corporation). Seven subjects were enrolled, of which, one discontinued before receiving study medication. Patients (two male, five female) had a mean age of 50 (±10.5) years, had a mean body mass index of 28 (±2.9) kg/m2, and had used CSII with RAA for at least 3 months. During the entire study, CSII was used to provide basal insulin (mean 0.68 IU/h). Three prandial treatment strategies were evaluated: (1) bolus insulin through CSII, dosed in advance of the meal; (2) a single TI dose at the start of the meal, equivalent to the CSII bolus; and (3) an initial TI dose at the start of the meal, followed by a second dose (4 to 8 U) after 75 min. Each patient served as their own control. Prandial treatment was optimized based on continuous glucose monitoring through a 1-week period between tests. At the end of each period, patients underwent a standardized mixed meal test, providing an evening meal of approximately 700 kcal. When subjects were treated with a single TI dose (test 2), all patients experienced a 180 min PPG higher than baseline values, as did four of six patients when prandial RAA was administered via CSII (test 1). When a second dose of TI was given at 75 min (test 3), all patients had BG levels at 180 min that were within 20 mg/dl of baseline (Figure 4; data on file, MannKind Corporation). The addition of a second dose of TI after a large meal provided better PPG control than a single dose at meal initiation and/or than a RAA bolus provided by CSII.
Figure 4.
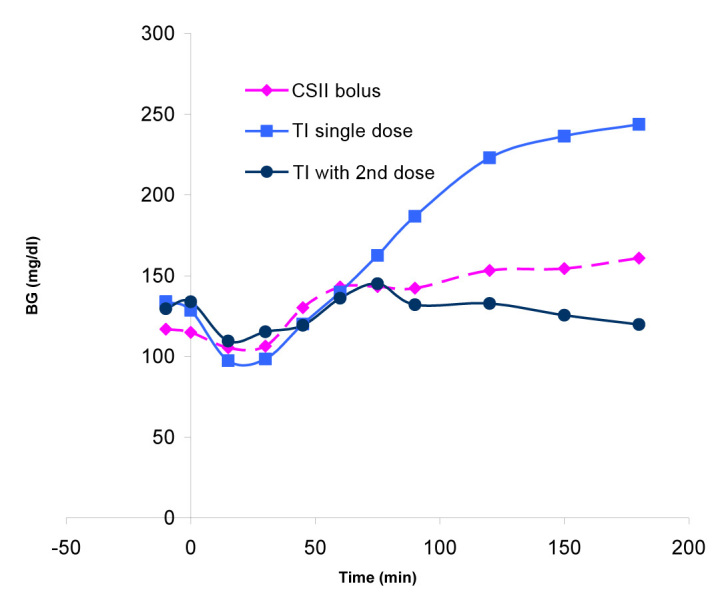
Postprandial glucose excursions after a standard, large meal (data on file, MannKind Corporation).
The clinical use of a second postprandial dose of TI was studied for 45 days in a single-arm, open-label, treat-to-target pilot study of 15 patients administering prandial TI for type 1 diabetes. At baseline, patients had a mean age of 38.3 (±9.6) years, mean body mass index of 26.4 (±3.7) kg/m2, and mean total daily insulin dose of 52.1 (±14.3) IU [basal insulin 31.1 IU, prandial insulin 21.0 IU (5.5, 6.3, and 6.7 IU at breakfast, lunch, and dinner, respectively)]; 67% of patients used insulin pumps for basal insulin delivery, and 33% were on multiple dose injection therapy. Patients were instructed to take a second dose of TI if the 2 h PPG level was ≥180 mg/dl.34 Continuous blood glucose measurement (CBGM; Dexcom SEVEN PLUS™) was conducted throughout the trial. A second supplemental dose of TI was used 38% of the time. The A1C values decreased from 7.86% to 7.47% over the 45-day study period, while the time spent with BG < 60 mg/dl remained unchanged at 3.4% of measurements by CBGM.
In a second pilot study, 39 patients with type 2 diabetes receiving insulin glargine and oral antidiabetes drugs were randomized to either TI (n = 19) or RAA (n = 20) as prandial insulin. Oral antidiabetes drug doses were unchanged, and basal insulin was optimized over the initial 6 weeks, after which patients were randomized to add either TI or RAA for another 16 weeks. Patients were instructed to take a second TI dose if 90 to 120 min PPG was >140 mg/dl. In total, 21% of patients took a supplemental dose, most frequently with the larger meals (usually dinner). After 16 weeks of treatment, A1C levels fell similarly in both groups (1.21% in the TI group, 1.27% in the RAA group; differences = 0.06%, p = not significant). Hypoglycemia was similar between groups, as was the incidence of adverse events (15 in both groups; data on file, MannKind Corporation).
Thus the use of an initial prandial and potentially an added supplemental postprandial dose of TI may result in an improvement in both early and late PPG control without an increase in hypoglycemia. Predicting in advance the insulin requirement for a meal is difficult, as the PPG response is influenced by multiple factors (e.g., meal composition, variability in gastric emptying, duration of the meal). Being able to react to the actual glycemic response to a meal may provide patients with additional freedom in their diet. The administration by inhalation, using a simple, breath-operated device (Figure 1) may overcome the difficulties of introducing multiple prandial doses.
Summary
Currently available, subcutaneously delivered RAAs have PK/PD profiles that are still poorly synchronized with PPG excursions. Ultra-rapid-acting insulins, such as inhaled TI, result in better control of early PPG with less weight gain and less frequent hypoglycemia, but control of late PPG remains suboptimal. Preliminary evidence suggests that, when needed, a second dose of ultra-rapid-acting insulin may result in more prolonged control of late PPG without further adverse effects. Future study is needed to explore the utility of this strategy.
Acknowledgments
Editorial assistance was provided by Peloton Advantage LLC and supported by MannKind Corporation. Technosphere and AFREZZA are registered trademarks of MannKind Corporation.
Glossary
- (A1C)
hemoglobin A1c
- (AUC)
area under the curve
- (BG)
blood glucose
- (CBGM)
continuous blood glucose measurement
- (CI)
confidence interval
- (CSII)
continuous subcutaneous insulin infusion
- (FEV1)
forced expiratory volume in 1 s
- (OR)
odds ratio
- (PD)
pharmaco-dynamic
- (PK)
pharmacokinetic
- (PPG)
postprandial glucose
- (RAA)
rapid-acting insulin analog
- (TI)
Technosphere insulin
Funding
This review was funded by MannKind Corporation.
Disclosures
Anders H. Boss and Richard Petrucci are employees of and hold stock in MannKind Corporation. Daniel Lorber is a prior advisor to MannKind Corporation; he has received research support from MannKind Corporation, Novo Nordisk, Merck & Co., Johnson & Johnson, and Boehringer Ingelheim and was on a speaker panel for Novo Nordisk.
References
- 1.Heinemann L, Muchmore DB. Ultrafast-acting insulins: state of the art. J Diabetes Sci Technol. 2012;6(4):728–742. doi: 10.1177/193229681200600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JW, Christiansen JS, Lauritzen T. Limitations to subcutaneous insulin administration in type 1 diabetes. Diabetes Obes Metab. 2003;5(4):223–233. doi: 10.1046/j.1463-1326.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- 3.Siekmeier R, Scheuch G. Inhaled insulin--does it become reality? J Physiol Pharmacol. 2008;59(Suppl 6):81–113. [PubMed] [Google Scholar]
- 4.Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1(4):338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann L, Heise T. Current status of the development of inhaled insulin. Br J Diabetes Vasc Dis. 2004;4(5):295–301. [Google Scholar]
- 6.Wall DA. Pulmonary absorption of peptides and proteins. Drug Deliv. 1995;2:1–20. [Google Scholar]
- 7.Rathbone M, Hadgraft J, Roberts M, Lane M, Leone-Bay A, Grant M, editors. Modified release drug delivery technology. 2nd ed. New York: Informa Healthcare; 2008. Technosphere®/insulin: mimicking endogenous insulin release. Vol 2. [Google Scholar]
- 8.Potocka E, Cassidy JP, Haworth P, Heuman D, van Marle S, Baughman RA., Jr Pharmacokinetic characterization of the novel pulmonary delivery excipient fumaryl diketopiperazine. J Diabetes Sci Technol. 2010;4(5):1164–1173. doi: 10.1177/193229681000400515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumiller JJ, Campbell RK. Technosphere insulin: an inhaled prandial insulin product. BioDrugs. 2010;24(3):165–172. doi: 10.2165/11536700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Pfützner A, Mann AE, Steiner SS. Technosphere/Insulin--a new approach for effective delivery of human insulin via the pulmonary route. Diabetes Technol Ther. 2002;4(5):589–594. doi: 10.1089/152091502320798204. [DOI] [PubMed] [Google Scholar]
- 11.Pfützner A, Forst T. Pulmonary insulin delivery by means of the Technosphere drug carrier mechanism. Expert Opin Drug Deliv. 2005;2(6):1097–1106. doi: 10.1517/17425247.2.6.1097. [DOI] [PubMed] [Google Scholar]
- 12.Rave K, Potocka E, Heinemann L, Heise T, Boss AH, Marino M, Costello D, Chen R. Pharmacokinetics and linear exposure of AFRESA compared with the subcutaneous injection of regular human insulin. Diabetes Obes Metab. 2009;11(7):715–720. doi: 10.1111/j.1463-1326.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy J, Baughman R, Schwartz S, Haworth PA, Boss AH, Richardson PC. AFRESA (Technosphere insulin) dosage strengths are interchangeable. Diabetes. 2009;59(Suppl 1):A112–A113. [Google Scholar]
- 14.Boss A, Rave K, Cheatham W, Heise T. Inhaled Technosphere/insulin: glucose elimination at the right time? Poster presented at: 65th Annual Scientific Sessions of the American Diabetes Association, June 10-14, 2005, San Diego, CA.
- 15.Cassidy JP, Baughman RA, Schwartz SL, Haworth PC, Boss AH, Richardson PC. AFRESA (Technosphere insulin) dosage strengths are interchangeable. Poster presented at: 69th American Diabetes Association Scientific Sessions, June 5-9, 2009, New Orleans, LA.
- 16.Potocka E, Hovorka R, Baughman R, Klein O, Dellweg S, Umpleby M, Haworth P, Mills R, Boss AH, Richardson PC. Characterization of metabolism parameters following Technosphere insulin and insulin lispro. Diabetes. 2010;59(Suppl 1):A413. [Google Scholar]
- 17.Rosenstock J, Lorber DL, Gnudi L, Howard CP, Bilheimer DW, Chang PC, Petrucci RE, Boss AH, Richardson PC. Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. Lancet. 2010;375(9733):2244–2253. doi: 10.1016/S0140-6736(10)60632-0. [DOI] [PubMed] [Google Scholar]
- 18.Potocka E, Hovorka R, Baughman RA, Umpleby M, Diaz ML, Chen R, Boss AH, Richardson PC. AFRESA™ suppresses endogenous glucose production earlier than a rapid-acting analog (lispro) and inhaled Exubera. Diabetes. 2009;58(Suppl 1):A61. [Google Scholar]
- 19.Bergenstal RM, Kapsner PL, Rendell MS, Boss AH, Howard CP, Chang PC. Comparative efficacy and safety of AFRESA and rapid-acting analog both given with glargine in subjects with T1DM in a 52-week study. Presented at: 69th American Diabetes Association Scientific Sessions, June 5-9, 2009, New Orleans, LA.
- 20.Garg SK, McGill JB, Rosenstock J, Hirsch IB, Petrucci R, Chang PC. Technosphere insulin vs insulin lispro in patients with type 1 diabetes using multiple daily injections. Presented at: 71st Annual Scientific Sessions of the American Diabetes Association, June 24-28, 2011, San Diego, CA.
- 21.Raskin P, Phillips MD, Rossiter A, Boss AH, Richardson PC. A1C and hypoglycemia in patients with type 2 diabetes mellitus incorporating prandial inhaled Technosphere insulin into their usual antihyperglycemic regimen vs continuing their usual antihyperglycemic regimen. Presented at: 70th Scientific Sessions of the American Diabetes Association, June 25-29, 2010, Orlando, FL.
- 22.DeVries JH. Mealtime inhaled insulin lowers fasting glucose: a look at possible explanations. Diabetologia. 2005;48(12):2682–2683. doi: 10.1007/s00125-005-0027-0. [DOI] [PubMed] [Google Scholar]
- 23.Bilheimer D, Ren Hao, Boss AH. Analysis of cardiovascular adverse events in patients with type 1 or type 2 diabetes enrolled in selected therapeutic trials in the phase 2/3 Technosphere insulin development program. Presented at: 71st Annual Scientific Sessions of the American Diabetes Association, June 24-28, 2011, San Diego, CA.
- 24.Raskin P, Heller S, Honka M, Chang PC, Boss AH, Richardson PC, Amin N. Pulmonary function over 2 years in diabetic patients treated with prandial inhaled Technosphere Insulin or usual antidiabetes treatment: a randomized trial. Diabetes Obes Metab. 2012;14(2):163–173. doi: 10.1111/j.1463-1326.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute Surveillance Epidemiology and End Results. SEER stat fact sheets: lung and bronchus. http://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 18, 2012.
- 26.Greene S, Nikula K, Reynolds J, Poulin D, McInally K, Townson D, Richardson P. Long-term pulmonary safety assessment of Afrezza™ in rats and dogs. Toxicologist. 2011;120(Suppl 2):175. [Google Scholar]
- 27.Korytkowski M, Niskanen L, Asakura T. FlexPen: addressing issues of confidence and convenience in insulin delivery. Clin Ther. 2005;27(Suppl B):S89–S100. doi: 10.1016/j.clinthera.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–S24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- 29.Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy. The basis of patient reluctance. Diabetes Care. 1997;20(3):292–298. doi: 10.2337/diacare.20.3.292. [DOI] [PubMed] [Google Scholar]
- 30.Peyrot M, Rubin RR. Patient-reported outcomes in adults with type 2 diabetes using mealtime inhaled technosphere insulin and basal insulin versus premixed insulin. Diabetes Technol Ther. 2011;13(12):1201–1206. doi: 10.1089/dia.2011.0037. [DOI] [PubMed] [Google Scholar]
- 31.Peyrot M, Rubin RR. Patient reported outcomes in adults with type 2 diabetes using mealtime AFRESA (inhaled Technosphere insulin) or metformin + secretagogue or both. Presented at: 69th American Diabetes Association Scientific Sessions, June 5, 2009, New Orleans, LA.
- 32.Coates PA, Ismail IS, Luzio SD, Griffiths I, Ollerton RL, Vølund A, Owens DR. Intranasal insulin: the effects of three dose regimens on postprandial glycaemic profiles in type II diabetic subjects. Diabet Med. 1995;12(3):235–239. doi: 10.1111/j.1464-5491.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee JJ, Dassau E, Zisser H, Jovanovic L, Doyle FJ., III Semi-automated artificial pancreas using prandial inhaled insulin and zone - model predictive control. Presented at: 12th Annual UC Systemwide Bioengineering Symposium, June 13-15, 2011, Santa Barbara, CA.
- 34.Garg S, Kelly W, Freson B, Petrucci R, Ritchie P. Treat-to-target Technosphere insulin in patients with type 1 diabetes. Presented at: 71st Annual Scientific Sessions of the American Diabetes Association, June 24-28, 2011, San Diego, CA.



