Abstract
The absorption profile of rapid-acting insulin analogs delivered subcutaneously is slow compared with physiological insulin. Shorter time to peak and shorter duration of insulin action are important steps toward reducing high postprandial blood glucose concentrations in diabetes therapy and are critical for the development of a closed-loop insulin delivery system. Many attempts have been made to develop more rapid-acting insulins. Since the 1950s, different approaches, such as jet injectors and sprinkler needles, which try to increase the absorption areas of injected insulin, have been developed; however, none of them are commonly used in diabetes therapy. Massage and heat increase tissue blood perfusion and, thereby, the absorption of subcutaneously applied insulin. The main focus of this article is a novel device that allows local application of heat to human skin. The device can be connected to a regular insulin pump. This device could demonstrate a significant effect on insulin absorption and postprandial glucose excursions in multiple clinical trials.
Keywords: continuous subcutaneous insulin infusion, insulin absorption, postprandial excursion, prandial insulin, skin heating, skin temperature
Reducing postprandial hyperglycemia is a major challenge in the treatment of patients with diabetes. Despite the availability of rapid-acting insulin analogs,1 postprandial hyperglycemia still occurs. Hyperglycemic postprandial glucose excursions were found to reach glucose levels > 300 mg/dl in 50% and over 180 mg/dl in almost 90% of children with type 1 diabetes with relatively good metabolic control.2 Similar findings were made for adult and adolescent subjects with type 1 diabetes receiving multiple insulin injection therapy.3,4
The incidence of postprandial hyperglycemia may be partially related to the time delay between fast excursions in blood glucose levels following meals and, in comparison, the slow absorption of subcutaneously injected/infused insulin. Indeed, when insulin is given 20 min ahead of the meal,5 a significant reduction in postprandial glucose is observed. However, this method bears a potential hypoglycemic risk when meals are delayed or forgotten and increases patient inconvenience.
Many factors influence the absorption rate of sub-cutaneously infused/injected insulin into the bloodstream.6 Among them are insulin formulation, insulin delivery site, insulin dose, insulin delivery method, and local blood perfusion at the insulin delivery site. These factors can cause unwanted variability in the metabolic effect of insulin, but they can be tuned to stabilize the insulin metabolic effect and to accelerate insulin action. Many studies have been accomplished to investigate the limiting factors in order to accelerate the overall process of insulin absorption from the delivery site to the blood system.
Some approaches for accelerating insulin action are using additional substances in combination with insulin.7,8 Physical interventions such as exercise, temperature, and other physical factors can lead to changes in the pharmaco-logical profile of a drug.9 The following discussion focuses on stimulation of local blood flow, especially by warming the insulin application site, and its effect on postprandial glycemic excursions.
Local Blood Circulation
Subcutaneous blood circulation is very important for the absorption of subcutaneously delivered insulin. Local blood flow can be measured with several invasive or noninvasive techniques, e.g., radioisotope clearance, laser Doppler flowmetry, pulse oximetry, and photo-plethysmography. Circulation can be influenced by many parameters, including mechanical stimulation, chemical stimulation, ambient temperature, and even emotional state.
Local Skin Massage
In the early 1980s, a study by Dillon10 reported improved insulin absorption following local skin stimulation. They used local massage at the area of the injection site for 3 min post-insulin injection in a study with 26 diabetes subjects. In that study, a significant decrease in mean glycosylated hemoglobin (HbA1c) level was observed, from 10.6% to 8.6% at 3–6 months. These results indicate that local skin blood flow stimulation that improves insulin absorption may also improve overall metabolic control.
Local Skin Warming
During heat stress, elevated skin temperature leads to cutaneous vasodilatation.11,12 Laser Doppler measurements of local skin blood flow were found to increase from baseline when skin temperature was over 37 °C and reach a maximum when skin temperature was held at 42 °C.13 The local effect of heat on skin blood vessels is biphasic and appears to be mediated by two independent pathways: the initial phase is mediated by local activation of afferent cutaneous sensory nerves, while a prolonged plateau phase is mediated by local generation of nitric oxide.11,12
In 1980, Koivisto14 published a study that investigated the effect of sauna (twice for 25 min at 85 °C) on the disappearance rate of 125I-labelled rapid-acting insulin and found that sauna accelerated insulin absorption by 110% as compared with room temperature (p < .01). Additionally, sauna conditions were found to reduce the postprandial glucose rise 2 h after breakfast to 3.2 mmol/liter compared with a rise of 5 mmol/liter observed at room temperature (p < .05).
The relation between temperature and medication kinetics was tested in several studies. In 1986, Danon and associates15 investigated the effect of exercise and heat exposure on absorption of methyl salicylate. The systemic availability with both heat exposure and exercise was increased threefold compared with resting at room temperature, with heat exposure having a slightly larger effect. Ashburn and colleagues16 found significantly increased serum fentanyl concentrations during the first 4 h after application of a transdermal fentanyl patch when heat was applied.16
The relation between kinetics and temperature was also tested for insulin. In one study, Sindelka and coworkers17 demonstrated a positive correlation between local skin temperatures at the insulin injection site and serum insulin levels 45 min after injection for skin temperatures ranging between 30 and 37 °C for U40 and U100 regular human insulin.
Local Skin-Warming Device
In the following, we focus on an innovative approach developed in order to accelerate insulin absorption into the bloodstream using a device for warming the skin locally. The InsuPatchTM device (InsuLine Medical, Petach-Tikva, Israel) is an add-on to insulin pumps that applies local, controlled heat to the skin in the vicinity of the insulin delivery site (Figure 1). It consists of a ring-shaped heating pad attached to the bottom of the insulin infusion set catheter and a controller that monitors the temperature of the heating pad. The control unit is housed in the infusion pump case, and it monitors the activity of the insulin pump. Once the controller detects an insulin bolus infusion, it activates the heating pad to warm the tissue surrounding the injection site to 38.5 °C for 30 min after insulin delivery, without overheating the insulin itself to avoid temperature-driven degradation of the insulin. The effect of the local warming device on the pharmacokinetic and pharmacodynamic profiles of rapid-acting insulin analogs was tested in a series of clinical studies.
Figure 1.
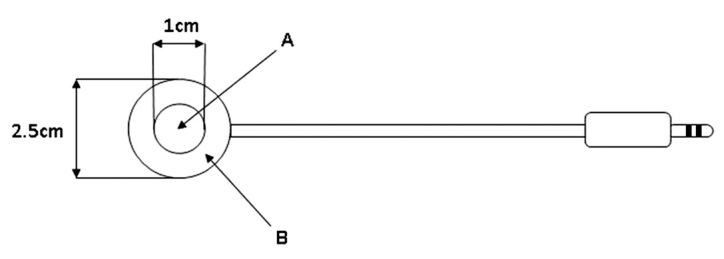
Schematic description of the InsuPatch device heating pad. The heating pad is attached to the bottom of the infusion set before applying the infusion set to the body. Ring B is the heating element, and circle A is open and allows for the infusion set needle to penetrate into the skin.
Meal Tolerance Test Study
Raz and colleagues18 presented results of a meal tolerance test glucose clamp study in 2009 in which the device for application-site warming was used. Study population was 17 subjects with type 1 diabetes aged 18 to 65 years (mean 31.8 years) with HbA1c of 6% to 12% (mean 7.1%) who were using continuous subcutaneous insulin infusion therapy. The meal tolerance test protocol was performed twice in 3 weeks, once with the device and once without it, in randomized order.
Subjects’ fasting glucose levels were stabilized to 100–150 mg/dl (mean blood glucose was 126 ± 21 mg/dl) using insulin or a glucose (20% concentration of dextrose solution) drip if needed. Insulin and glucose infusions were stopped 30 min before time 0, when an insulin bolus of 0.15 U/kg was delivered using the subject’s own insulin pump, and then the pump was stopped. Immediately afterward, the subject drank a standardized liquid meal (480 ml Boost®, Novartis Medical Nutrition, Munich, Germany; 480 kcal, 8 g fat, 81 g total carbohydrate, and 20 g protein).
The average postprandial glucose change from baseline with and without the local warming device is shown in Figure 2. Using the device was associated with reduced postprandial glucose levels until almost 3 h after meal. The area under the curve (AUC) of glucose change for the total 4 h study was also reduced when the device was applied. The glucose excursion was significantly reduced at 60 min post-meal when using the device in comparison with not using the device (59 ± 42 versus 93 ± 40 mg/dl; p < .005), with maximum reduction at 90 min post-meal (74 ± 48 versus 121 ± 43 mg/dl; p < .005). Glucose excursion AUC was significantly reduced when using the device compared with not using it at 120 min post-meal (33% reduction, 104 ± 65 versus 155 ± 56 mg/dl/h; p < .005) and at 180 min post-meal (26% reduction, 192 ± 109 versus 261 ± 95 mg/dl/h; p < .005).
Figure 2.
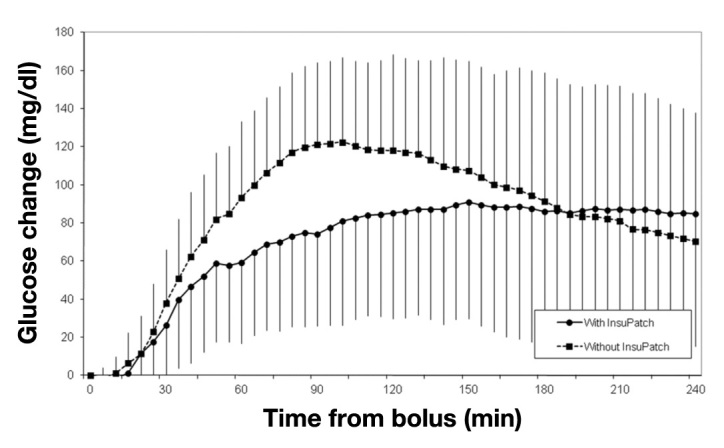
Postprandial glucose change from baseline in the meal tolerance test protocol with and without the application of the InsuPatch device in subjects with type 1 diabetes mellitus using continuous subcutaneous insulin infusion therapy (N = 17).
In the same study, plasma insulin levels were measured randomly in nine subjects during the meal tolerance test procedure. The subjects’ demographic, anthropometric, and baseline data were not different from those of the entire sample. The mean plasma insulin profile during the meal tolerance test is shown in Figure 3. Heating with the device was associated with a significant reduction in time to maximum insulin concentration (45 ± 28 versus 78 ± 35 min; p < .05). Use of the device was also associated with an increase in maximum insulin concentration (118 ± 35 versus 86 ± 16 mU/liter; p < .05). The area under the insulin concentration curve during the first hour was also increased (80 ± 28 versus 55 ± 12 mU/liter/h; p < .05).
Figure 3.
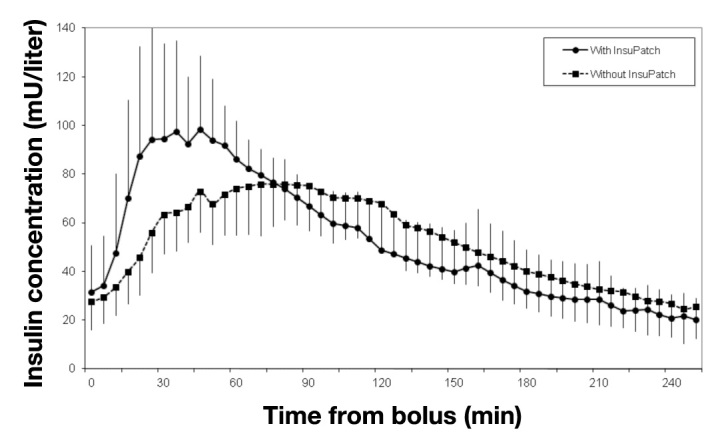
Mean insulin concentration in the blood after bolus injection with and without the use of the InsuPatch device in subjects with type 1 diabetes mellitus using continuous subcutaneous insulin infusion therapy (n = 9). The error bars indicate one standard deviation.
Clamp Study 1
The effect of the local warming device on rapid-acting insulin analog pharmacodynamics was also studied using the euglycemic glucose clamp technique19 after an overnight fast.20 The euglycemic glucose clamps were performed in nine subjects with type 1 and type 2 diabetes [gender, male/female 6/3; age 21–62 years (mean 39 years); weight 56–124 kg (mean 75.2 kg); body mass index 21.4–43.6 kg/m2 (mean 26.2 kg/m2); and HbA1c 5.7–8.0% (mean 6.5%)]. The clamp procedure was performed on two separate visits under identical conditions except that the local heating device was used only during one visit in randomized order. After glucose stabilization at 100 ± 10 mg/dl, a standardized bolus of 0.15 U/kg was delivered and glucose was clamped using dextrose infusion. The end point of the euglycemic clamp study was the time to maximum insulin concentration. The glucose infusion rate as function of time is shown in Figure 4. Time to maximum insulin concentration was significantly decreased (51 ± 10 versus 90 ± 21 min; p < .002). A reduction was also observed for the time until glucose infusion rate declined below 2 mg/kg/min (183 ± 75 versus 219 ± 82 min, mean ± standard deviation; p < .05).
Figure 4.
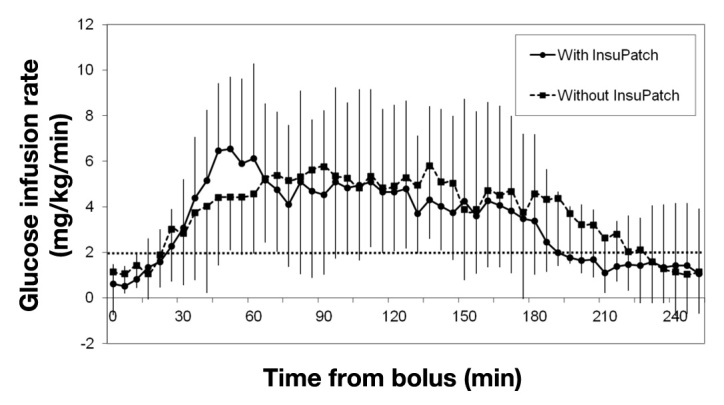
Insulin action expressed as mean glucose infusion rate required to maintain euglycemia after standard bolus of 0.15 U/kg rapid-acting insulin.
Clamp Study 2
Preliminary results from another glucose clamp study were presented in early 2011.21 The warming device was used to investigate the effect on pharmacokinetics and pharmacodynamics in eight young patients with type 1 diabetes. Basal infusion was suspended after breakfast bolus delivery and glucose levels were maintained. When the warming device was used, time to peak insulin action occurred earlier than without the device. The AUC during the first 90 min was significantly increased.
Standard Meal Study
Another study used standardized meals and was composed of two parts: an in-house part where the effect of the device on insulin pharmacokinetics and postprandial glucose levels was tested22 and an outpatient part to test the safety of the device under home-use conditions. All 24 type 1 diabetes subjects in this study were on continuous subcutaneous insulin infusion therapy and aged 43.5 ± 11.3 years, with a mean HbA1c of 7.4% ± 0.8%.
The in-house part of the study included a 4-day clinical meal test repeating the same meal and insulin therapy, 2 days with and 2 days without the device. Breakfast meals were composed of 65% carbohydrates, 15% protein, and 20% fat. Dinner meals were composed of 40% carbohydrates, 20% protein, and 40% fat. Meal insulin doses were identical for the experiment days with and without the heating device. The primary end point of this study was the AUC of capillary blood glucose above baseline between 0 and 120 min after meals divided by integration time (AUC/t120).
The AUC/t120 was significantly reduced when the local warming device was used (breakfast not heated 66.4 ± 32.8 mg/dl versus heated 56.8 ± 34.0 mg/dl, 42 meal pairs, p = .0170; dinner not heated 30.8 ± 31.0 mg/dl versus heated 18.4 ± 23.9 mg/dl, 38 meal pairs, p = .0028).
Interestingly, the effect of the device on the AUC120 after the slowly absorbed dinner was approximately three times stronger than after the fast-absorbed breakfast (14% reduction in AUC120 for breakfast and 40% reduction in AUC120 for dinner). This difference might be due to the difference in meal composition. Postprandial glucose excursions are affected by several factors such as meal composition23 and diurnal variations of the reaction to meal intake.24 Both are likely to have contributed to the different effects observed in the studies reviewed here.
Insulin concentration measurements during 2 h after insulin bolus in 23 breakfast meals with and without warming during the first two days were analyzed. The maximum venous insulin concentration above baseline was significantly (p < .001) increased, and it was reached earlier when the local warming device was used (not heated, 63.3 ± 34.8 mU/liter after 52.1 ± 24.8 min; heated, 79.3 ± 41.6 mU/liter after 43.3 ± 13.5 min). The amount of insulin during the first hour as measured by the AUC of insulin concentration above baseline divided by integration time was increased by 21% when the local warming device was used (not heated, 44.1 ± 23.2 mU/liter; heated, 53.4 ± 30.6 mU/liter; p = .007).
The outpatient part of the study compared 14 days using the device with 14 days without the device. There was no increase in incidence of hyperglycemic and hypoglycemic events when the local warming device was used compared with when the device was not used. At the end of each period, patients were asked to have the same meals on 2 days with additional postprandial blood glucose measurements. A reduction of postprandial excursion could be observed, but it was not statistically significant.
The insulin dose has an effect on insulin action profile25 and should therefore be taken into account when comparing the aforementioned studies. A euglycemic clamp study conducted with 18 subjects with type 1 diabetes evaluated the dose response of insulin glulisine.25 Time to maximum insulin concentration was reported to increase with increasing glulisine dose; its values were 47, 57, and 72 min for insulin doses of 0.075, 0.15, and 0.3 U/kg, respectively.
The difference in dosing could also explain the different effect magnitude of local skin warming. It is expected that increasing local blood perfusion would have more effect on insulin pharmacokinetics when the absorption rate is slow, e.g., with a high insulin dose.
As already mentioned, the observed effect of accelerating insulin absorption and reduced postprandial glucose excursion by local heating of the injection site is most probably due to improved local perfusion. The effect of the local warming device can potentially be further increased by applying a higher heating temperature to the skin. In the current device version, a heating pad is kept at 38.5 °C. It was shown that the maximum effect of heating on local blood perfusion starts to increase at temperatures above 37 °C and is maximal at 42 °C.13 Increasing the temperature from 38.5 °C will possibly result in an increased insulin absorption rate, but the temperature will have to be low enough so that the subject will be comfortable and not be physically injured, and the insulin will not be affected.
Local blood flow is affected by the skin area, age, and diabetes,26 and the importance of these factors for the use of a local heating device has to be studied in future. The local warming device can be combined with an insulin pump, but the higher power consumption might lead to a more frequent battery change. This issue will be addressed in newer models of the device. First studies in patients have been performed successfully, but larger daily life studies have to be done. These studies could provide helpful insight into the intra- and inter-individual variability in the response to local heat application.
Conclusions
The results reported here have shown that using devices like a local warming device have a beneficial effect on postprandial blood glucose excursion by enhancing insulin absorption. This effect should be further studied in different types of populations and different experimental settings to specify indications for everyday use.
The improvement in insulin pharmacokinetics profile demonstrated by the local warming device, which can be combined with an insulin pump, may be beneficial for the development of closed-loop systems. The pharmaco-kinetic parameters of current insulin are presently a barrier in the design of completely automated closed-loop insulin delivery systems around meal times. A faster insulin profile may enable an adaptive model-based algorithm to compensate for postprandial blood glucose excursions in a much more flexible way.
Glossary
- (AUC)
area under the curve
- (HbA1c)
glycosylated hemoglobin
Disclosures
Guido Freckmann is general manager of the Institut fuer Diabetes-Technologie GmbH, Ulm, Germany. This institute performs clinical trials in cooperation with different medical device companies. Gabriel Bitton and Ron Nagar are employees of Insuline Medical Ltd., Petach Tikva, Israel, the developer of the InsuPatch device.
References
- 1.Garg SK. New insulin analogues. Diabetes Technol Ther. 2005;7(5):813–817. doi: 10.1089/dia.2005.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24(11):1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 3.Heptulla RA, Allen HF, Gross TM, Reiter EO. Continuous glucose monitoring in children with type 1 diabetes: before and after insulin pump therapy. Pediatr Diabetes. 2004;5(1):10–15. doi: 10.1111/j.1399-543X.2004.00035.x. [DOI] [PubMed] [Google Scholar]
- 4.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a trans-cutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 5.Cobry E, McFann K, Messer L, Gage V, VanderWel B, Horton L, Chase HP. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther. 2010;12(3):173–177. doi: 10.1089/dia.2009.0112. [DOI] [PubMed] [Google Scholar]
- 6.Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673–682. doi: 10.1089/152091502320798312. [DOI] [PubMed] [Google Scholar]
- 7.Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114(2):230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Steiner S, Hompesch M, Pohl R, Simms P, Flacke F, Mohr T, Pfutzner A, Heinemann L. A novel insulin formulation with a more rapid onset of action. Diabetologia. 2008;51(9):1602–1606. doi: 10.1007/s00125-008-1095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccone CD. Basic pharmacokinetics and the potential effect of physical therapy interventions on pharmacokinetic variables. Phys Ther. 1995;75(5):343–351. doi: 10.1093/ptj/75.5.343. [DOI] [PubMed] [Google Scholar]
- 10.Dillon RS. Improved serum insulin profiles in diabetic individuals who massaged their insulin injection sites. Diabetes Care. 1983;6(4):399–401. doi: 10.2337/diacare.6.4.399. [DOI] [PubMed] [Google Scholar]
- 11.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91(4):1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 12.Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100(5):1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 13.Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol. 1996;497(Pt 3):837–848. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koivisto VA. Sauna-induced acceleration in insulin absorption from subcutaneous injection site. Br Med J. 1980;280(6229):1411–1413. doi: 10.1136/bmj.280.6229.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danon A, Ben-Shimon S, Ben-Zvi Z. Effect of exercise and heat exposure on percutaneous absorption of methyl salicylate. Eur J Clin Pharmacol. 1986;31(1):49–52. doi: 10.1007/BF00870985. [DOI] [PubMed] [Google Scholar]
- 16.Ashburn MA, Ogden LL, Zhang J, Love G, Basta SV. The pharmaco-kinetics of transdermal fentanyl delivered with and without controlled heat. J Pain. 2003;4(6):291–297. doi: 10.1016/s1526-5900(03)00618-7. [DOI] [PubMed] [Google Scholar]
- 17.Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E. Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologia. 1994;37(4):377–380. doi: 10.1007/BF00408474. [DOI] [PubMed] [Google Scholar]
- 18.Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach B. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31(5):980–987. doi: 10.1016/j.clinthera.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 20.Raz I, Yegorchikov Y, Bitton G, Pesach B, Nagar R, Weiss R. Improving pharmacokinetic and pharmacodynamic profiles of rapid-acting insulin analogs using the InsuPatch device. J Diabetes Sci Technol. 2009;3(2):384. A138. [Google Scholar]
- 21.Cengiz E, Tamborlane W, Sherr J, Martin M, Carria L, Sikes K, Urban A, Bitton G, Weinzimer S. O-11 Effect of a novel warming device on pharmacodynamics (PD) of rapid acting insulin in youth with type 1 diabetes (T1D). Abstracts. Diabetes Technol Ther. 2011;13(2):173–293. [Google Scholar]
- 22.Freckmann G, Pleus S, Westhoff A, Krinelke LG, Buhr A, Jendrike N, Haug C. Clinical performance of a device that applies local heat to the insulin infusion site: a crossover study. J Diabetes Sci Technol. 2012;6(2):320–327. doi: 10.1177/193229681200600215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters AL, Davidson MB. Protein and fat effects on glucose responses and insulin requirements in subjects with insulin-dependent diabetes mellitus. Am J Clin Nutr. 1993;58(4):555–560. doi: 10.1093/ajcn/58.4.555. [DOI] [PubMed] [Google Scholar]
- 24.Service FJ, Rizza RA, Hall LD, Westland RE, O’Brien PC, Clemens AH, Haymond MW, Gerich JE. Prandial insulin requirements in insulin-dependent diabetics: effects of size, time of day, and sequence of meals. J Clin Endocrinol Metab. 1983;57(5):931–936. doi: 10.1210/jcem-57-5-931. [DOI] [PubMed] [Google Scholar]
- 25.Becker RH, Frick AD, Nosek L, Heinemann L, Rave K. Dose-response relationship of insulin glulisine in subjects with type 1 diabetes. Diabetes Care. 2007;30(10):2506–2507. doi: 10.2337/dc06-2114. [DOI] [PubMed] [Google Scholar]
- 26.Petrofsky J, Paluso D, Anderson D, Swan K, Alshammari F, Katrak V, Murugesan V, Hudlikar AN, Chindam T, Trivedi M, Lee H, Goraksh N, Yim JE. The ability of different areas of the skin to absorb heat from a locally applied heat source: the impact of diabetes. Diabetes Technol Ther. 2011;13(3):365–372. doi: 10.1089/dia.2010.0161. [DOI] [PubMed] [Google Scholar]


