Abstract
This review summarizes the clinical development of a family of ultra-rapid-acting recombinant human insulin formulations. These formulations use ethylenediaminetetraacetic acid (EDTA) to chelate zinc and thereby destabilize insulin hexamers. In addition, insulin monomer surface charges are chemically masked with citrate to prevent reaggregation. The first phase 1 trials were performed using BIOD-090, an acidic 25 unit U/ml insulin formulation, which contained disodium-EDTA (NaEDTA). When compared with regular human insulin (RHI) and/or insulin lispro in multiple phase 1 studies, BIOD-090 consistently showed more rapid absorption and/or onset of action. A standard meal challenge study also demonstrated improved postprandial glucose profiles associated with BIOD-090. However, increased patient exposure in larger phase 3 trials showed that this formulation was associated with an increased incidence of local injection site reactions, most commonly pain. A next generation formulation, BIOD-100, contained the same excipients as a standard insulin concentration of 100 U/ml. BIOD-100 maintained an ultra-rapid action profile and was associated with modest but significantly improved toleration when compared with BIOD-090. In order to further improve toleration, the hypothesis that NaEDTA contributed to discomfort by chelating endogenous calcium was tested by either substituting calcium-EDTA for NaEDTA or by adding calcium chloride to the NaEDTA formulation. These calcium formulations essentially eliminated the excess discomfort associated with BIOD-090 but were associated with less optimal pharmacokinetic profiles in humans. Recent efforts have succeeded in developing ultra-rapid-acting human insulin formulations with acceptable injection site toleration by optimizing concentrations of calcium (BIOD-125) and with the use of magnesium sulfate to mitigate discomfort (BIOD-123). Similar formulation technology has also been shown to accelerate absorption of insulin analogs in animal models.
Keywords: insulin monomers, insulin therapy, Linjeta, prandial insulin, ultra-rapid insulin
Introduction
Ultra-rapid-acting insulins are intended to better match the time-action profile of prandial insulins to cover the rapid increase in insulin needs after meals, thereby reducing postprandial glycemic excursions. The rate of insulin absorption from the subcutaneous (SC) space is governed by several factors, including the properties of the tissue through which it must pass, such as capillary pore size and capillary membrane surface area, as well as the properties of the insulin molecule complex. Because human insulin and insulin analogs generally exist in solution as stable hexamers, the delay in absorption is largely accounted for by the time it takes for hexamers to dissociate into monomers and dimers, which is the form of insulin required for absorption after SC injection.1 One approach to creating ultra-rapid-acting insulin is use of a novel combination of excipients to modify the insulin hexamer complex resulting in more rapid dissociation of the hexamers into monomers and dimers following SC injection.
The key formulation excipients utilized are citrate and ethylenediaminetetraacetic acid (EDTA). This combination of excipients has been shown to result in large, loosely packed insulin multimers in solution which dissociate more rapidly into monomers/dimers after dilution than those of regular human insulin (RHI) or rapid-acting insulin analogs.2 The increased speed of insulin complex dissociation correlates with increased speed of absorption demonstrated in clinical trials.
During the clinical development of the BIOD formulations, it was found that disodium-EDTA was associated with local injection site discomfort. This article summarizes the evolution of a family of formulations that have progressed to product candidates with both ultra-rapid absorption and acceptable injection site toleration.
BIOD-090
The initial formulation of ultra-rapid-acting human insulin, BIOD-090 (formerly referred to as Viaject®, Viaject® 25, or Linjeta™) consisted of a two-part presentation, i.e., a lyophilized cake of recombinant human insulin (Organon-Merck; Oss, Netherlands), which was reconstituted with a diluent containing citric acid, disodium-EDTA, m-cresol as a preservative, sodium chloride, sodium phosphate, and sodium hydroxide and/or hydrochloric acid to adjust the pH to approximately 4. The final concentration of insulin was 25 U/ml (U-25). This presentation allowed room temperature storage for at least 18 months and at refrigerated temperatures for up to 30 days after reconstitution. In addition, it was found that after reconstitution, this formulation remained stable through multiple freeze-thaw cycles.
BIOD-090: Pharmacokinetic, Pharmacodynamic, and Dose Response Profiles
The pharmacokinetic (PK), pharmacodynamic (PD), and dose response profiles of BIOD-090 were assessed in a 5-period crossover study in 10 healthy volunteers.3 In this study, the test injection treatments were fixed in the following order: 12 U of regular human insulin, 12 U of lispro (LIS), 12 U of BIOD-090, 6 U of BIOD-090, and 3 U of BIOD-090. All injections were administered subcutaneously in the abdomen using standardized technique. Pharmacokinetics were assessed using specific assays for the measurement of either human insulin or LIS. Pharmacodynamics were assessed using the euglycemic glucose clamp method. Key PK timing parameters are summarized in Table 1.
Table 1.
Comparison of the PK Timing Parameters of BIOD-090, LIS, and RHI following SC Injections of Each Insulin. Mean ± SD.
| Variable (min) | BIOD-090 3 U | BIOD-090 6 U | BIOD-090 12 U | LIS 12 U | RHI 12 U |
|---|---|---|---|---|---|
| T50%-early | 12 ± 13 | 7 ± 3 | 18 ± 18 | 26 ± 10 | 37 ± 22 |
| Tmax | 54 ± 44 | 51 ± 48 | 60 ± 43 | 66 ± 34 | 120 ± 70 |
| T50%-late | 126 ± 44 | 145 ± 59 | 181 ± 76 | 170 ± 39 | 260 ± 105 |
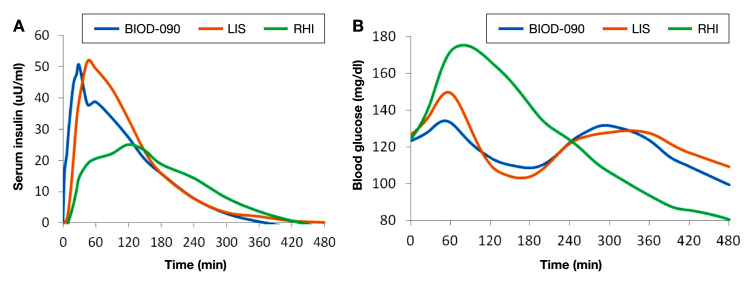
A comparison of the PD [glucose infusion rate (GIR)] curves of BIOD-090, LIS, and RHI at comparable doses of 12 U is shown in Figure 1.
Figure 1.
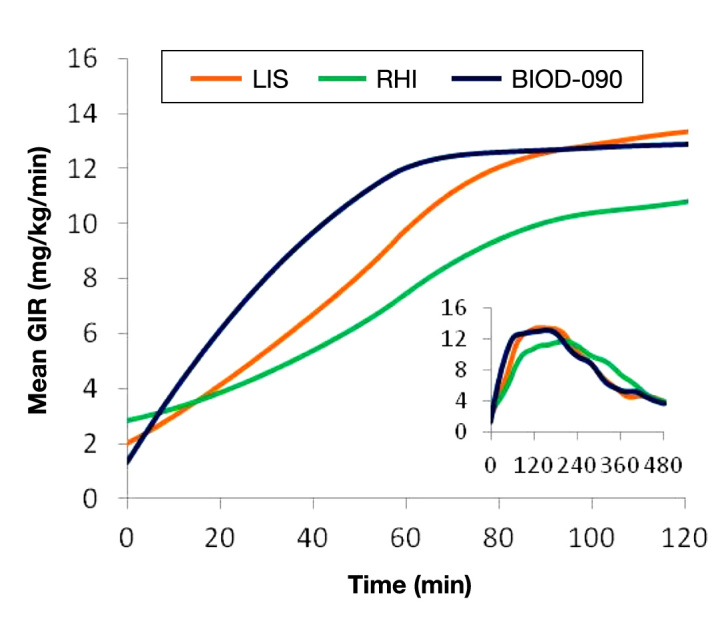
Mean PD curves for 12 U SC doses of LIS, RHI, and BIOD-090 in 10 healthy volunteers. The large figure shows the mean GIRs for the first 120 min after each injection. The inset shows the mean GIR curves for the entire 480-min observation period after each injection.
BIOD-090 was characterized by a more rapid onset of metabolic action than LIS or RHI. The time to early half-maximal activity was 33 ± 17 min for BIOD-090 [mean ± standard deviation (SD)] vs LIS 51 ± 13 min vs RHI 66 ± 15 min (p < .05, BIOD-090 < LIS < RHI). The time to maximal activity was 136 ± 56 min for BIOD-090 vs LIS (152 ± 30 min) vs RHI (193 ± 57 min) (p < .05, BIOD-090 and LIS < RHI). The metabolic activity in the first 2 h after injection was higher with BIOD-090 and LIS than with RHI [area under the curve (AUC) GIR 0–120 min: 915 ± 301 and 781 ± 174 vs 580 ± 164 mg/kg, p < .05].
The PD responses to 3-, 6-, and 12-U injections of BIOD-090 are shown in Figure 2.
Figure 2.
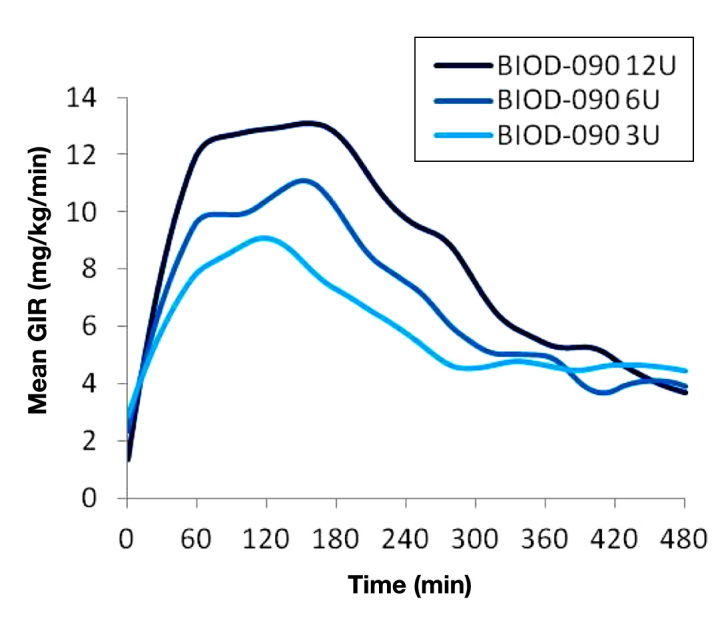
Mean PD responses to 3, 6, and 12 U SC injections of BIOD-090 in 10 healthy volunteers.
A dose–response relationship was demonstrated for BIOD-090 as evidenced by the dose related increases in the area under the glucose infusion rate curves (AUCGIR) for the first 120 min (AUCGIR 0–120 min; mean ± SD): 3 U, 524 ± 262 vs 6 U, 718 ± 255 vs 12 U, 915 ± 301 mg/kg.
BIOD-090 and Postprandial Glycemia
To evaluate the potential clinical significance of the unique PK/PD properties of BIOD-090, a standardized meal study was performed.4 After stabilization of preprandial glucose concentrations, 18 patients with type 1 diabetes received individualized SC injections of BIOD-090, LIS, or RHI immediately prior to a standardized solid meal.
Injection of BIOD-090 resulted in more rapid insulin absorption than with either LIS or RHI (time to half-maximal insulin levels: 13.1 ± 5.2, 25.4 ± 7.6, and 38.4 ± 19.5 min for BIOD-090, LIS, and RHI, respectively; p = .001, BIOD-090 vs LIS; p < .001, BIOD-090 vs RHI, and p < .001, LIS vs RHI). Maximal postprandial glycemia was lower with BIOD-090 (0–180 min; 157 ± 30 mg/dl; p = .002 vs RHI) and LIS (170 ± 42 mg/dl; p = .668 vs RHI) than after RHI (191 ± 46 mg/dl; RHI vs LIS, p = .008). The difference between maximum and minimum glycemia was smaller with BIOD-090 (70 ± 17 mg/dl) than with either RHI (91 ± 33 mg/dl; p = .007 vs BIOD-090) or LIS (89 ± 18 mg/dl; p = .011 vs BIOD-090). In addition, the area under the blood glucose profile was lower with BIOD-090 than with RHI (0–180 min; 21.8 ± 5.8 vs 28.4 ± 7.6 g·min/dl; p < .001). Figure 3 shows mean PK (A) and postprandial glucose curves (B).
Figure 3.
Mean plasma insulin profiles (A) with baseline correction (mean of the last three samples prior to injection), and mean blood glucose profiles (B) measured by the Biostator, obtained after SC injection of RHI, LIS, and BIOD-090 in 18 patients with type 1 diabetes.
BIOD-090 Dose Reproducibility and Microvascular Properties
In a study designed to assess within subject variability of the PK and PD profiles following repeated injections of BIOD-090 vs RHI in 14 patients with type 1 diabetes, BIOD-090 was associated with significantly less variability of the t50 early, time to maximal insulin concentration (tmax), and tGIRmax (time to maximal GIR) parameters.5 In this study, BIOD-090 was again found to have a more rapid absorption and onset of action profile compared with RHI.
A standardized meal crossover study in 14 patients with type 2 diabetes assessed the potential microvascular effects of insulins with different absorption and action kinetics.6 In this study, BIOD-090 was shown to be associated with lower levels of peak postprandial asymmetric dimethylarginine (a marker of endothelial dysfunction) compared with LIS and RHI (Figure 4). Likewise, postprandial rise in nitrotyrosine, a marker of oxidative stress, was significantly less after BIOD-090 compared with RHI (BIOD-090 -0.22 ± 0.17 vs RHI 0.25 ± 0.15 µg/ml; p < .05), whereas nitrotyrosine after LIS was intermediate (LIS 0.09 ± 0.07 µg/ml; not significant). Furthermore, earlier and more pronounced increases in postprandial microvascular blood flow (measured by simultaneous microlightguide spectrophotometry) and skin oxygen-ation (measured with laser Doppler fluximetry) were obtained after BIOD-090 injections.
Figure 4.
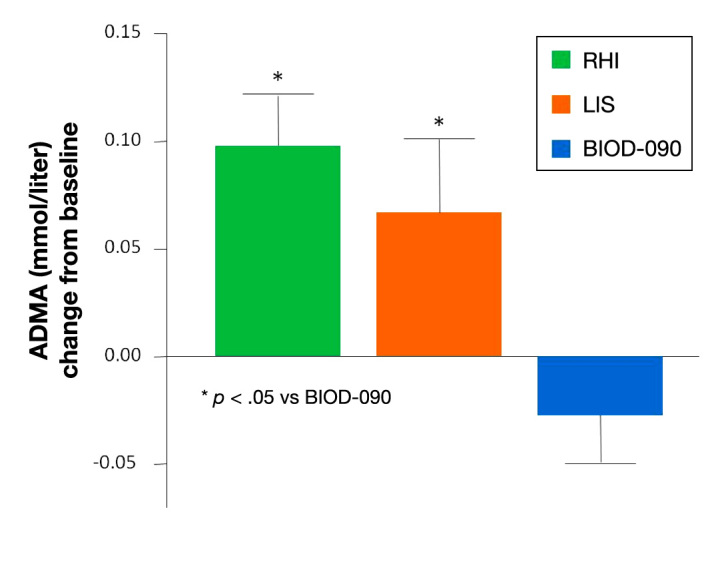
Peak postprandial change in asymmetric dimethylarginine (ADMA) levels measured in 14 patients with type 2 diabetes given each insulin immediately before a standardized meal.
Long-term multiple dosing experience with BIOD-090
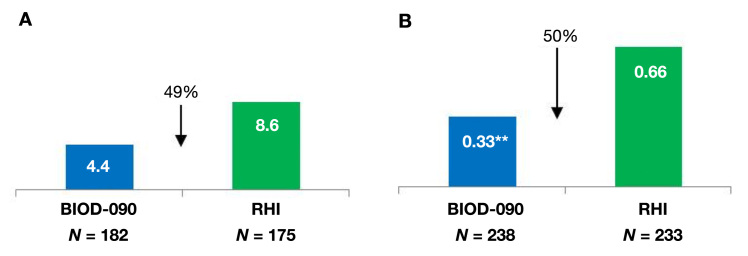
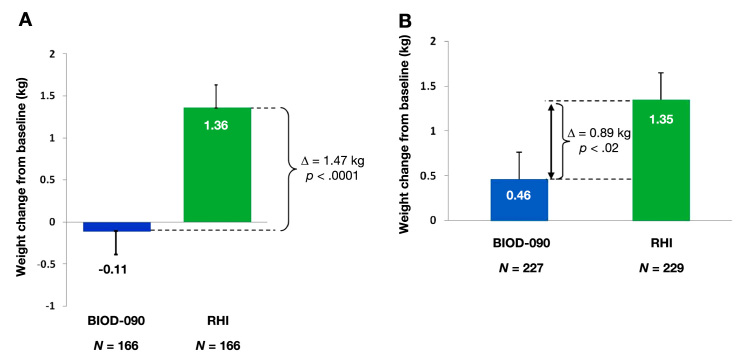
BIOD-090 was assessed in two large phase 3 safety and efficacy trials in patients with type 1 (n = 463)7 and type 2 (n = 471)8 diabetes. These were 6-month open-label trials designed to demonstrate noninferiority of hemoglobin A1c (HbA1c) control compared with RHI. Demonstration of HbA1c noninferiority was narrowly missed, likely due to differences in dosing instructions between treatment arms and by geographic heterogeneity of HbA1c response in the type 1 trial.7 Despite these issues, there was some evidence for downstream benefit that would be expected from a more rapidly absorbed insulin, namely less hypoglycemia and less weight gain (Figure 5 and Figure 6). Post hoc analyses did not show that these differences in weight and hypoglycemia were correlated with treatment group differences in HbA1c or insulin dosing. The observed weight and hypoglycemia differences await confirmation in future clinical trials.
Figure 5.
(A) Proportion of patients with at least one severe hypoglycemic event in phase 3 study in patients with type 1 diabetes, p = .1324. Severe hypoglycemia was defined as requiring the assistance of a third party. (B) Median hypoglycemic event rates in phase 3 study in patients with type 2 diabetes. Double asterisks indicate p = .018. Hypoglycemia was defined as home glucose readings <70 mg/dl or symptomatic episodes resolving with treatment.
Figure 6.
Weight change in phase 3 study of (A) type 1 diabetes and (B) type 2 diabetes.
The phase 3 studies also showed an increased incidence of local injection site reactions, most commonly injection site pain in BIOD-090 treated patients compared with those treated with RHI. The injection site pain was usually described as mild to moderate, the prevalence of which declined with time. Study discontinuation resulting from injection site reactions occurred in approximately 5% of subjects. Open label single arm long-term safety extension studies showed stability of glycemic control with BIOD-090 treatment, and no long-term safety or immunogenicity issues arose.9–10 These studies also showed declining prevalence of injection site pain with continued treatment (Table 2). In Table 2, all patients who completed a parent randomized controlled trial of BIOD-090 vs RHI were eligible to enroll in this noncomparative open-label safety extension trial in which all patients used BIOD-090 as their prandial insulin. Patients who continued BIOD-090 from the parent trial are tabulated separately from those who switched from control RHI to BIOD-090 in the extension study. The presence or absence of injection site pain was elicited systematically at each study visit.
Table 2.
Prevalence of Injection Site Pain in Patients with Type 1 Diabetes during Long-Term Treatment with BIOD-090.
| Interval | Continuing BIOD-090 from prior study n = 100 | Starting BIOD-090 in extension study n = 123 | Overall n = 223 |
|---|---|---|---|
| Week 0 to 4 | 5.2% (5/97) | 22.8% (28/123) | 15.0% (33/220) |
| Week 4 to 9 | N/A (0/0) | 13.4% (15/112) | 13.4% (15/112) |
| Week 9 to 18 | 3.2% (3/95) | 5.7% (6/106) | 4.5% (9/201) |
| Week 18 to 31 | 3.2% (3/93) | 4.2% (4/95) | 3.7% (7/188) |
| Week 31 to 45 | 1.2% (1/84) | 1.1% (1/87) | 1.2% (2/171) |
| Week 45 to 58 | 2.5% (2/81) | 6.1% (5/82) | 4.3% 7/163) |
| Week 58 to 72 | 1.5% (1/65) | 1.4% (1/69) | 1.5% (2/134) |
U-100 Formulations
A major goal of the formulation development program was to bridge BIOD-090 to a U-100 formulation, which would be more familiar to patients and health care providers. Furthermore, the fourfold increased injection volumes necessary to achieve comparable doses of the BIOD-090 formulation (25 U/ml) and/or its acidic pH likely contributed to the increased local discomfort observed in the previous studies.
Development of a liquid recombinant human insulin-based ultra-rapid-acting formulation at a concentration of 100 U/ml and neutral pH with identical excipients (BIOD-100, formerly referred to as Viaject® 7 or Linjeta™) was successfully accomplished. This formulation was used to evaluate the effect of injection volume and pH on BIOD-090 toleration.
The PKs and PDs of BIOD-100 were evaluated in a double-blind random sequence 3-period crossover trial performed in 40 patients with type 1 diabetes.11 BIOD-090 and LIS were used as controls. This study demonstrated PK bioequivalence between the BIOD-090 and BIOD-100 and confirmed that both were associated with a significantly more rapid rate of absorption compared with LIS. Pharmacokinetic and PD timing parameters are shown in Table 3. BIOD-100 showed a faster absorption compared with LIS [time to maximal insulin concentration 23 vs 60 min; difference [confidence interval (CI)] -30 (-35 to -23)] and faster onset of action [time to early half-maximal glucose infusion rate (GIR) 25 vs 44 min; -18 (-26 to -10)], and a higher AUCGIR in the first 60 min after injection [176 vs 107 mg/kg; ratio 1.65 (1.27 to 2.14)]. Maximum GIR was similar between BIOD-100 and LIS [6.1 vs 6.6 mg/kg/min; ratio 0.93 (0.86 to 1.01)], whereas the duration of action (tGIR 50%–late) was longer with BIOD-100 [274 vs 228 min; 50 (25 to 73)]. Figure 7 shows mean PD curves from this study.
Table 3.
Pharmacokinetic and PD Characteristics of BIOD-090, BIOD-100, and LIS.
| Variables | BIOD-100 | BIOD-090 | LIS | BIOD-100 vs LIS median difference / ratio (90% CI) |
|---|---|---|---|---|
| PK | ||||
| T50%-early (min)a | 7.7 / 7.9 (3.0) | 9.7 / 9.8 (3.8) | 21.6 / 23.3 (6.3) | -15.5c(-17.3; -13.8) |
| Tmax (min)a | 20.0 / 29.4 (29.3) | 27.5 / 31.6 (15.7) | 60.0 / 57.1 (21.8) | -30.0c(-35.0; -22.5) |
| T50%-late (min)a | 135.6 / 142.9 (78.5) | 131.4 / 141.6 (62.1) | 130.2 / 136.0 (41.3) | 6.9 (-14.9; 26.8) |
| PD | ||||
| tGIR50%-early (min)a | 24.2 / 27.8 (18.2) | 23.5 / 28.4 (17.5) | 44.3 / 45.4 (20.1) | -17.9c(-25.6; -10.4) |
| tGIRmax (min)a | 132.0 / 137.8 (74.7) | 75.0 / 99.3 (53.9) | 114.0 / 118.8 (49.5) | 20.0 (-3.0; 43.0) |
| tGIR50%-late (min)a | 276.1 / 285.3 (80.6) | 252.0 / 249.8 (66.0) | 227.9 / 234.2 (56.7) | 49.6c(24.6; 72.7) |
| tGIR10%AUC (min)a | 46.1 / 47.0 (14.2) | 42.2 / 45.3 (13.6) | 52.0 / 52.7 (15.6) | -5.3c(-10.5; -0.8) |
| GIRmax (mg/kg/min)b | 6.0 / 6.1 (0.37) | 6.0 / 6.3 (0.38) | 6.7 / 6.6 (0.38) | 0.93 (0.86; 1.01) |
| AUCGIR0-60 (mg/kg)b | 210 / 176 (0.77) | 239 / 191 (0.68) | 150 / 111 (1.24) | 1.65c(1.27; 2.14) |
Pharmacokinetic:
Median and arithmetic mean (SD) plus median differences with 90% CI, or
Median and geometric mean (SD of log) plus ratios with 90% CI.
p < .10 BIOD-100 vs LIS.
Figure 7.
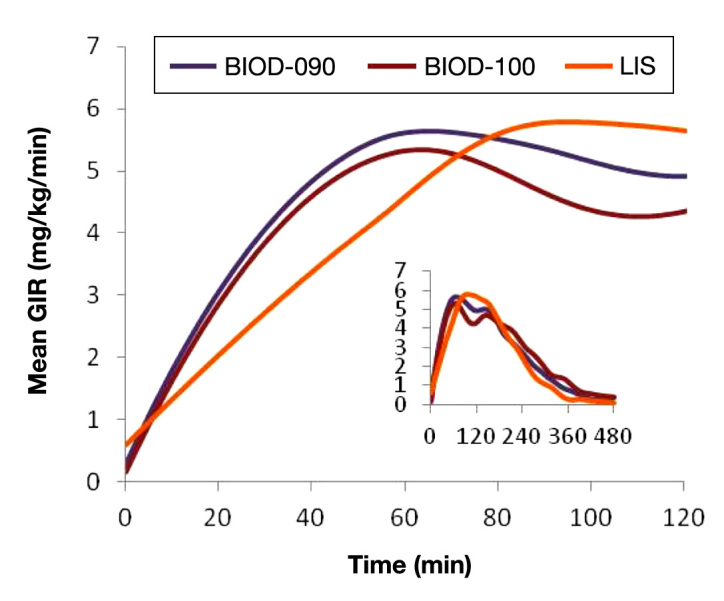
Mean PD curves for 12 U SC doses of BIOD-090, BIOD-100, and LIS in 40 patients with type 1 diabetes. The large figure shows the mean GIRs for the first 120 min after each injection. The inset shows the mean GIR curves for the entire 480-min observation period after each injection.
BIOD-100 Toleration
Toleration of BIOD-100 was formally compared with BIOD-090 and LIS in a double-blind randomized 3-period crossover study of 54 insulin-treated patients. Eligible subjects were randomly assigned to 1 of 6 sequences of 9 study medication administrations (3 formulations, each tested in triplicate). The primary measure was a 100 mm visual analog scale (VAS). Visual analog scale is measured in millimeters: 0 indicates no discomfort, and 100 indicates the worst possible discomfort. The severity scale is scored as follows: 0 = no discomfort, 1 = mild discomfort, 2 = moderate discomfort, and 3 = severe discomfort. The relative scale is scored as follows: 1 = much less discomfort than usual mealtime insulin injections at home, 2 = less discomfort than usual mealtime insulin injections at home, 3 = equal discomfort compared with usual mealtime insulin injections at home, 4 = increased discomfort compared with usual mealtime insulin injections at home, and 5 = greatly increased discomfort compared with usual mealtime injections at home. Secondary measures included absolute and relative severity scores. A summary of the results from this study is provided in Table 4 in arithmetic means ± standard error of the mean (SEM).
Table 4.
Toleration Assessments of BIOD-090, BIOD-100, and LIS (mean ± SEM).
| Variable | BIOD-100 | BIOD-090 | LIS | P value BIOD-100 vs BIOD-090 |
|---|---|---|---|---|
| VAS | 17.3 ± 2.5 | 22.0 ± 2.8 | 5.3 ± 1.0 | .041a |
| Absolute severity | 0.85 ± 0.10 | 1.07 ± 0.10 | 0.19 ± 0.05 | .017a |
| Relative severity | 3.30 ± 0.08 | 3.49 ± 0.08 | 2.73 ± 0.07 | .019a |
Statistically significant difference.
A significantly lower mean VAS score was observed for BIOD-100 compared with BIOD-090; however, it was still higher than that of LIS. The severity scores for both BIOD-090 and BIOD-100 were in the mild range. For each test injection, a majority of subjects treated with BIOD-100, BIOD-090, and LIS experienced mild or no discomfort. The average discomfort associated with either BIOD formulation was slightly greater than that experienced with their usual mealtime injections, and BIOD-100 was significantly improved when compared with BIOD-090 on this scale. For each injection, the majority of subjects treated with BIOD-100 and LIS described an intensity of discomfort equal to or less than usual insulin injections at home.
Excipient Modifications and Toleration
While reducing injection volumes and neutralization of pH-improved BIOD-100 toleration, it remained inferior to that of commercial LIS. It was hypothesized that disodium-EDTA (NaEDTA) in these formulations might directly contribute to the discomfort potentially induced by chelation of endogenous calcium in the SC space.12 This hypothesis was tested by developing formulations BIOD-102 and BIOD-103, which contain calcium so as to reduce endogenous SC calcium binding post-injection. BIOD-102 was formulated with calcium ethylene-diaminetetraacetic acid (CaEDTA) instead of NaEDTA. BIOD-103 contained a mixture of CaEDTA and NaEDTA. The toleration and PK properties of these formulations were compared with those of BIOD-100 in a double-blind 3-period crossover study in 13 subjects with type 1 diabetes.13 Toleration data are summarized in Figure 8.
Figure 8.
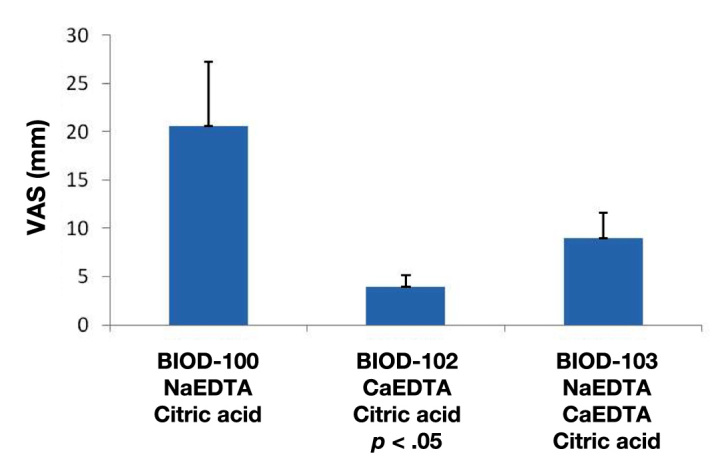
Toleration as measured on a 100 mm VAS from 0 (no discomfort) to 100 (worst possible discomfort) (mm). Mean ± standard error of the mean.
These results were consistent with the hypothesis that complete substitution of CaEDTA (BIOD-102) for NaEDTA resulted in a significantly improved toleration compared with the BIOD-100. The mean VAS score for BIOD-102 was similar to that associated with LIS in a different study (Table 4). BIOD-103, which contained a mixture of NaEDTA and CaEDTA, was associated with a VAS score intermediate to those of BIOD-102 and BIOD-100.
The absorption rates of BIOD-102 and BIOD-103 were modestly slower than that of BIOD-100. Mean maximal concentrations of both BIOD-102 and BIOD-103 were lower than that of the BIOD-100 (56.7 ± 11.4 and 56.4 ± 9.5 vs 73.8 ± 10.0, respectively, with p values = .06 and .05 compared with BIOD-100). Correspondingly, the washout times of BIOD-102 and BIOD-103 were longer. Pharmacodynamics were not assessed in this trial.
Two additional calcium-containing formulations (BIOD-105 and BIOD-107) were compared with LIS in a double-blind 3-period crossover trial evaluating PK, PD (euglycemic clamps), and toleration (data on file). Pharmacokinetic and toleration data are shown in Figure 9.
Figure 9.
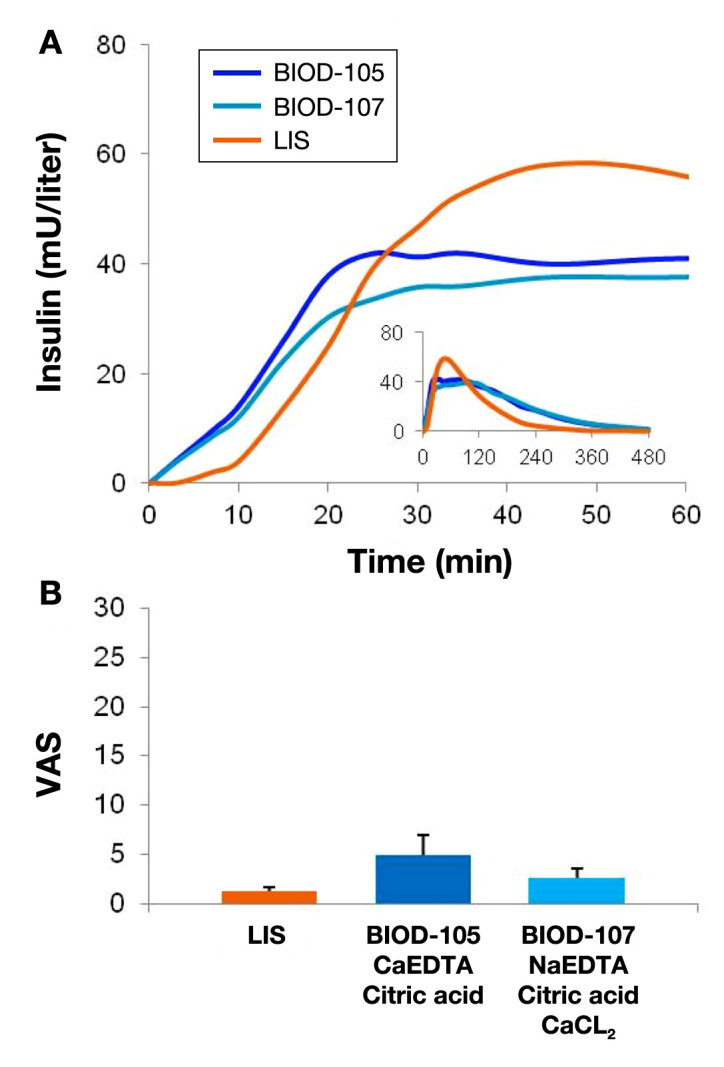
(A) Mean PK curves associated with 0.15 U/kg SC doses of BIOD-105, BIOD-107, and LIS in 18 patients with type 1 diabetes. (B) Toleration as measured on a 100 mm VAS from 0 (no discomfort) to 100 (worst possible discomfort) (mm). Mean ± SEM.
Recent Formulation Development
Newer generation RHI formulations have utilized revised excipient compositions and new excipients known to have pain-mitigating properties. Clinical data suggests that RHI-based formulations can be optimized by reducing calcium concentrations (BIOD-125) or by using magnesium sulfate to modify the pain response to NaEDTA (BIOD-123). Pharmacokinetic data for BIOD-123 and BIOD-125 in patients with type 1 diabetes are shown in Figure 10.
Figure 10.
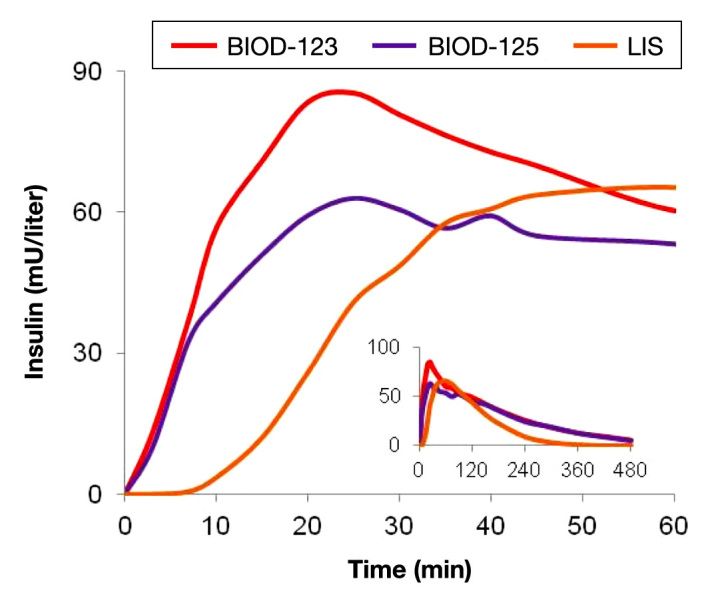
Pharmacokinetic profile of BIOD-123 and BIOD-125 vs LIS following SC administration to 12 patients with type 1 diabetes. Dose 0.20 U/kg.
Both BIOD-125 and BIOD-123 are associated with improved local toleration similar to that of LIS (see Table 5).
Table 5.
Toleration Assessments of BIOD-123, BIOD-125, and LIS (mean ± SEM).
| Variable | BIOD-123 | BIOD-125 | LIS |
|---|---|---|---|
| VAS | 3.6 ± 2.1 | 6.8 ± 2.9a | 1.8 ± 1.1 |
| Absolute severity | 0.36 ± 0.15 | 0.50 ± 0.19 | 0.17 ± 0.11 |
| Relative severity | 2.91 ± 0.25 | 3.08 ± 0.26 | 2.92 ± 0.08 |
p < .05 vs LIS.
BIOD-200 and BIOD-300
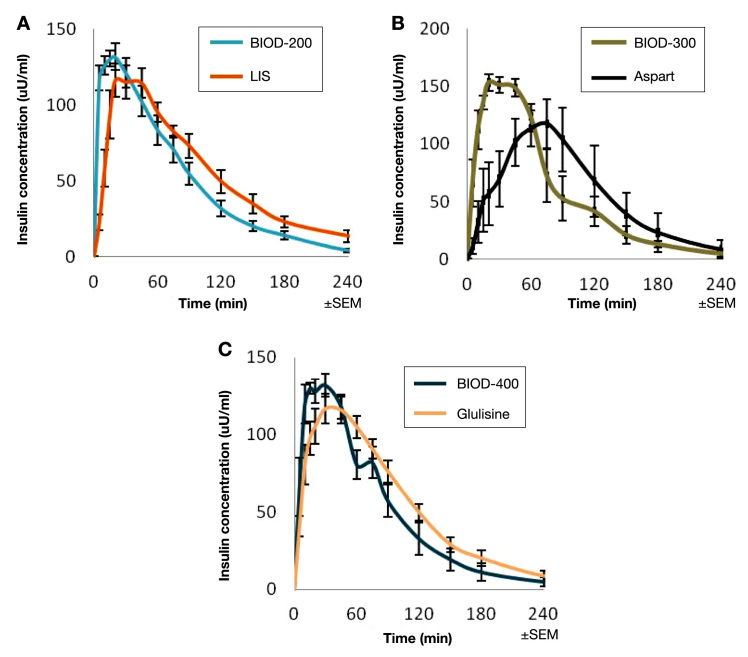
A second approach to creating an ultra-rapid-acting prandial insulin is to use a rapid-acting insulin analog as an active pharmaceutical ingredient. By virtue of modified amino acid sequences, such insulin analogs tend to dissociate from multimeric stable storage forms more rapidly than RHI. The combination of disodium-EDTA and citrate has been shown to accelerate the absorption of all three currently approved rapid-acting insulin analogs in diabetic swine (Figure 11), although somewhat less so with glulisine than with LIS and aspart. The commercial formulation of glulisine does not contain zinc and therefore may be less affected by the addition of the chelator to the formulation.
Figure 11.
Mean PK profiles of ultra-rapid analog insulin formulations following SC administration to diabetic miniature swine (dose = 0.25 U/kg). (A) BIOD-200 (n = 10) vs lispro (n = 8), (B) BIOD-300 (n = 4) vs aspart (n = 4), and (C) BIOD-400 (n = 5) vs glulisine (n = 11).
Discussion
This review summarizes a body of animal model and human studies that reproducibly demonstrates that insulin formulations containing EDTA and citrate result in more rapid insulin absorption from the SC space than is seen with either regular human insulin or LIS. The original formulation tested in clinical trials was a U-25, pH 4 two-part formulation for reconstitution (BIOD-090). In phase 1 trials of BIOD-090, the following characteristics were observed: (1) more rapid absorption compared with LIS, with comparable peak action and offset periods, (2) improved or comparable dose reproducibility compared with regular insulin, (3) improved postprandial glycemia compared with LIS or regular insulin, and (4) evidence for improved postprandial microvascular dynamics in patients with type 2 diabetes. In phase 3 studies, despite study design challenges, trends towards improvement in important secondary variables including hypoglycemia and weight change were observed, as might be expected of a mealtime insulin better suited to match postprandial glucose excursions. Importantly, long-term safety was demonstrated in extension studies of up to 18 months. However, acceptance of BIOD-090 in clinical practice could be significantly impaired by the fact that a U-25 formulation requiring reconstitution is unfamiliar to patients and that this formulation was associated with an increased incidence of local injection site reactions, most commonly pain, compared with regular insulin.
Three potential sources of the local injection site discomfort associated with BIOD-090 were considered: (1) increased injection volumes necessitated by a formulation with a fourfold lower concentration, (2) acidic pH, or (3) a direct effect of one of the excipients in the formulation. Efforts were therefore undertaken to create a U-100 liquid neutral pH version with improved local toleration. As the formulation was advanced in this way, measurable improvements in toleration were demonstrated, however, VAS discomfort scores were still not comparable to the commercial LIS formulation. Disodium-EDTA has previously been associated with SC injection related discomfort.14 The mechanism for this local irritation phenomenon is unknown but is likely related to the ability of EDTA to bind calcium in the SC space, promoting abnormal depolarizations in small sensory nerves. The hypothesis that the NaEDTA in BIOD-090 and BIOD-100 was also a cause of local discomfort was tested by adding calcium to several variants of the formulation. All CaEDTA formulations tested in clinical trials had improved toleration compared with the NaEDTA-based formulations, and most were associated with VACs comparable to those of LIS. Although calcium largely resolves the remaining source of excess local discomfort, at certain concentrations it also appears to be capable of altering the PK/PD profile, resulting in slower absorption than the NaEDTA formulations, lower peak activities, and consequent longer “tails” than seen with the NaEDTA formulations. It is possible that in this context, calcium at certain concentrations is able to promote reaggregation of insulin monomers. BIOD-125, a candidate with a modified calcium concentration, has been demonstrated in patients to allow for BIOD-100-like absorption profiles with similar toleration to LIS. Another successful approach was the development of BIOD-123, in which magnesium sulfate is added to a NaEDTA-based formulation similar to BIOD-100. Magnesium sulfate has been shown to have pain-mitigating properties in other preclinical and clinical settings.15–17
Now that target recombinant human insulin formulations have been identified in patients, attention is being focused on similar formulations of rapid-acting insulin analogs, which have been shown to have attractive PK profiles in diabetic swine. Evaluation of human PK, PD, toleration, and anticipated downstream effects of ultra-rapid insulin absorption, such as better postprandial glucose control and favorable effects on hypoglycemia rates and weight changes await clinical trials.
In conclusion, insulin chelation and charge masking result in ultra-rapid absorption of human insulin and insulin analogs. Formulation optimization for recombinant human insulin is completed with BIOD-123 and BIOD-125, and rapid-acting insulin analog formulation development is underway.
Acknowledgments
The authors thank Justine Hager for her excellent assistance in the preparation of the manuscript.
Glossary
- (AUC)
area under the curve
- (AUCGIR)
area under the glucose infusion rate curve
- (CaEDTA)
calcium ethylenediaminetetraacetic acid
- (EDTA)
ethylenediaminetetraacetic acid
- (GIR)
glucose infusion rate
- (HbA1c)
hemoglobin A1c
- (LIS)
insulin lispro
- (NaEDTA)
disodium ethylenediaminetetraacetic acid
- (PD)
pharmacodynamic
- (PK)
pharmacokinetic
- (RHI)
regular human insulin
- (SC)
subcutaneous
- (SD)
standard deviation
- (tGIR)
duration of action
- (tGIRmax)
time to maximum GIR
- (tmax)
time to maximal insulin concentration
- (VAS)
visual analog scale
Funding
This work was funded by Biodel Inc., Danbury, Connecticut.
Disclosures
The authors are employees of Biodel Inc.
References
- 1.Brange J, Langkjoer L. Insulin structure and stability. Pharm Biotechnol. 1993;5:315–350. doi: 10.1007/978-1-4899-1236-7_11. [DOI] [PubMed] [Google Scholar]
- 2.Pohl R, Hauser R, Li M, DeSouza E, Feldstein R, Seibert R, Ozhan K, Kashyap N, Steiner S. Ultra-rapid absorption of recombinant human insulin induced by zinc chelation and surface charge masking. J Diabetes Sci Technol. 2012;6(4):755–763. doi: 10.1177/193229681200600404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner S, Hompesch M, Pohl R, Simms P, Flacke F, Mohr T, Pfützner A, Heinemann L. A novel insulin formulation with a more rapid onset of action. Diabetologia. 2008;51(9):1602–1606. doi: 10.1007/s00125-008-1095-8. Epub 2008 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemann L, Hompesch M, Flacke F, Simms P, Pohl R, Albus K, Pfützner A, Steiner S. Reduction of postprandial glycemic excursions in patients with type 1 diabetes: a novel human insulin formulation versus a rapid-acting insulin analog and regular human insulin. J Diabetes Sci Technol. 2011;5(3):681–686. doi: 10.1177/193229681100500322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hompesch M, McManus L, Pohl R, Simms P, Pfützner A, Bülow E, Flacke F, Heinemann L, Steiner SS. Intra-individual variability of the metabolic effect of a novel rapid-acting insulin (VIAject) in comparison to regular human insulin. J Diabetes Sci Technol. 2008;2(4):568–571. doi: 10.1177/193229680800200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forst T, Pfützner A, Flacke F, Krasner A, Hohberg C, Tarakci E, Pichotta P, Forst S, Steiner S. Postprandial vascular effects of VIAject compared with insulin lispro and regular human insulin in patients with type 2 diabetes. Diabetes Care. 2010;33(1):116–120. doi: 10.2337/dc09-0411. Epub 2009 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollander P, Flacke F, Simms P, Krasner A, Pichotta P, Pfützner A, Steiner S. An Open-Label, Multicenter Trial of VIAject® vs. Regular Human Insulin in Patients with Type 1 Diabetes: Impact of Regional Differences on Efficacy Analyses. Presented at: 2010 ADA 70th Annual Scientific Sessions, Orlando, FL, June 25–29, 2010.
- 8.Rodbard HW, Flacke F, Simms P, Krasner A, Pichotta P, Pfützner A, Steiner S. An Open-Label Multicenter Trial of VIAject® vs. Regular Human Insulin in Patients with Type 2 Diabetes: Final Analyses. Presented at: 2010 ADA 70th Annual Scientific Sessions, Orlando, FL, June 25–29, 2010.
- 9.Hollander P, Flacke F, Simms P, Pichotta P, Steiner S, Krasner A. Assessment of Long-Term Safety and Efficacy of Linjeta™ with 18 Months of Continuous Therapy in Patients with Type 1 Diabetes. Presented at: 2011 ADA 71st Annual Scientific Sessions, San Diego, CA, June 24–28, 2011.
- 10.Rodbard HW, Flacke F, Simms P, Pichotta P, Steiner S, Krasner A. Assessment of Long-Term Safety and Efficacy of Linjeta™ with 18 Months of Continuous Therapy in Patients with Type 2 Diabetes. Presented at: 2011 ADA 71st Annual Scientific Sessions, San Diego, CA, June 24–28, 2011.
- 11.Heinemann L, Nosek L, Flacke F, Albus K, Krasner A, Pichotta P, Heise T, Steiner S. U-100, pH-Neutral formulation of VIAject®: faster onset of action than insulin lispro in patients with type 1 diabetes. Diabetes Obes Metab. 2012;14(3):222–227. doi: 10.1111/j.1463-1326.2011.01516.x. doi: 10.1111/j.1463-1326.2011.01516.x. Epub 2011 Nov 13. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard JI, Jones SF, Landau EM. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flacke F, Blanchfield L, Hem-Lee H, Pichotta P, Krasner A, Steiner S. Characterization of Pharmacokinetics adn Toleration of Three Variant Formulations of Linjeta(TM). Presented at: Diabetes Technology Meeting, Bethesda, MD, November 11–13, 2010.
- 14.Stevens RA, Urmey WF, Urquhart BL, Kao TC. Back pain after epidural anesthesia with chloroprocaine. Anesthesiology. 1993;78(3):492–497. doi: 10.1097/00000542-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Lee C, Song YK, Jeong HM, Park SN. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J Anesthesiol. 2011;61(3):244–250. doi: 10.4097/kjae.2011.61.3.244. Epub 2011 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ushida T, Iwatsu O, Shimo K, Tetsunaga T, Ikeuchi M, Ikemoto T, Arai YC, Suetomi K, Nishihara M. Intradermal administration of magnesium sulphate and magnesium chloride produces hypesthesia to mechanical but hyperalgesia to heat stimuli in humans. J Neuroinflammation. 2009;6:25. doi: 10.1186/1742-2094-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feria M, Abad F, Sánchez A, Abreu P. Magnesium sulphate injected subcutaneously suppresses autotomy in peripherally deafferented rats. Pain. 1993;53(3):287–293. doi: 10.1016/0304-3959(93)90225-E. [DOI] [PubMed] [Google Scholar]