Abstract
Objective
This quasi-experimental exploratory study investigated neuropsychological effects of exercise among older adults with diabetes mellitus (DM) compared with adults without diabetes (non-DM), and it examined the feasibility of using a stationary bike exergame as a form of exercise for older adults with and without diabetes. It is a secondary analysis that uses a small dataset from a larger randomized clinical trial (RCT) called the Cybercycle Study, which compared cognitive and physiological effects of traditional stationary cycling versus cybercycling.
Methods
In the RCT and the secondary analysis, older adults living in eight independent living retirement facilities in the state of New York were enrolled in the study and assigned to exercise five times per week for 45 min per session (two times per week was considered acceptable for retention in the study) by using a stationary bicycle over the course of 3 months. They were randomly assigned to use either a standard stationary bicycle or a “cybercycle” with a video screen that displayed virtual terrains, virtual tours, and racing games with virtual competitors. For this secondary analysis, participants in the RCT who had type 2 DM (n = 10) were compared with age-matched non-DM exercisers (n = 10). The relationship between exercise and executive function (i.e., Color Trials 2, Digit Span Backwards, and Stroop C tests) was examined for DM and non-DM patients.
Results
Older adults with and without diabetes were able to use cybercycles successfully and complete the study, so the feasibility of this form of exercise for this population was supported. However, in contrast with the larger RCT, this small subset did not demonstrate statistically significant differences in executive function between the participants who used cybercycles and those who used stationary bikes with no games or virtual content on a video screen. Therefore, the study combined the two groups and called them “exercisers” and compared cognitive outcomes for DM versus non-DM patients. As predicted, exercisers with DM exhibited significant gains in executive function as measured by the Color Trails 2 test, controlling for age and education, while non-DM exercisers did not significantly gain in this measure [group × time interaction, F(1,16]) = 9.75; p = .007].
Conclusions
These preliminary results support the growing literature that finds that exercise may improve cognition among older adult with DM. Additional research is needed to clarify why certain aspects of executive function might be differentially affected. The current findings may encourage physicians to prescribe exercise for diabetes management and may help motivate DM patients’ compliance for engaging in physical activity.
Keywords: aging, cognition, diabetes, exercise, videogame
Introduction
Given the increasing proportion of older adults in the global population and the concomitant rise in dementia,1 exercise interventions to promote brain health are becoming increasingly salient.2 Similarly, there are concerns about the worldwide increase in obesity and diabetes and their relationship with dementia.3 Calls have been made to identify interventions to address diabetes mellitus (DM), which has been linked to frontal-subcortical dysfunction and subsequent dementia.4 Elderly patients with DM are at increased risk for dementia and have been shown to exhibit a pattern of cognitive deficits, especially in executive functions.5–8 The research on exercise as an intervention to promote cognitive health in older adults has been encouraging, with significant benefits found for normative and cognitively compromised aging adults,9–12 particularly in the realm of executive function.13,14
Despite the known cognitive benefits of exercise in later life, as well as the general benefits of exercise for diabetes control,15–17 only a tiny fraction of older adults exercise at recommended levels.18 Some research has begun to examine the utility of novel exergaming technologies, which are designed to enhance motivation to exercise by integrating videogame features with exercise.19 However, no prior research has examined the impact of exergames on cognitive function among patients with DM. What follows is a summary of the research that has documented the utility of traditional exercise.
Research has shown an association between DM (as well as glycemic control) and cognitive function in older adults;20–23 this association (more severe DM or poorer glycemic control is associated with lower cognitive functioning) tends to remain even after controlling for a variety of confounding variables such as vascular disease and body weight.24 Data suggest a biological link between DM and Alzheimer’s,25 and some have even proposed a separate “type 3” diagnosis for Alzheimer’s as a specific form of DM that affects the brain.26 This literature points to the need for interventions to enhance cognitive health among older patients with DM who bear multiple risk factors for decline into dementia.
Exercise is an intervention with many known positive physiological and cognitive effects. Reviews of the literature on exercise and DM16,27 conclude that aerobic exercise and combined aerobic and strengthening exercise lead to clear physiological benefits. In one study, improved glycemic control was achieved with only 10 days of exercise training.28 Cross-sectional research tends to support a link between physical activity and better cognitive function among patients with DM.29,30 One trial found glucose-intolerant exercisers improved significantly more on executive function than controls,31 but there is a paucity of controlled research addressing cognitive benefits of exercise in DM.
While exercise is widely known to have beneficial effects, only 14% of adults 65–74 years old and 7% over 75 years of age report regular vigorous exercise (20 min three or more days per week).18 Given poor exercise participation among the older cohort that is most at risk for cognitive decline, research has examined ways to increase exercise participation. Among DM patients, interventions that use regular consultation strategies to increase physical activity have mixed results, showing both potential and problems.32,33 It has been speculated that the engaging and motivating features of exergames and exergame technologies, which require physical exertion in order to interface with the game, may help increase DM patients’ exercise participation and effort, as has been found in other populations.19
The purpose of this exploratory quasi-experimental study is to compare the potential motivating effects of a game-based form of exercise with a non-game-based form of the same exercise and to compare the potential influence of exercise on the cognitive functioning of older adults who either have diabetes or do not have diabetes (non-DM). Hypotheses include (1) exercise with a game-based form of exercise will be associated with greater increases in executive function than a traditional form of the same exercise that is not game based and (2) exercise (including both game-based and non-game-based) will be associated with improved executive function for older adults who have diabetes more than for older adults who do not have diabetes.
Methods
This exploratory quasi-experimental study is a secondary analysis that uses the complete DM patient subset (n = 10) of a larger randomized clinical trial (RCT), the Cybercycle Study9 (described later). The neuro-psychological performance of the DM patient subsample before and after 3 months of exercise was compared with age-matched non-DM patients who also participated in the larger trial. In particular, the impact of exercise on executive function among the DM patient subsample was compared with non-DM exercisers, controlling for age and education. Furthermore, because the larger RCT compared cybercycling (a form of exergaming) with traditional stationary biking, the feasibility of using exergames with DM patients was also examined.
Cybercycle Study
This larger RCT9 for older adults compared effects of (1) traditional stationary cycling that had no virtual game components with (2) cybercycling, which provided stationary cycling plus a video display of virtual environments and games, including interactive virtual terrain the cyclist was traversing, virtual-reality tours while cycling, and racing games with on-screen racing competitors. The Cybercycle Study examined cognitive and physiological effects for older adults after 3 months of either traditional stationary cycling or cybercycling.
In the study, older adults ages 60 to 88 and living in independent living retirement facilities in the state of New York were enrolled and assigned to exercise five times per week for 45 min per session (two times per week was considered acceptable for retention in the study) by using a stationary bicycle over the course of 3 months. They were randomly assigned to use either a standard stationary bicycle or a cybercycle.
The Cybercycle Study was approved by the institutional review board at both Union and Skidmore Colleges and registered with www.clinicaltrials.gov (NCT01167400); participants provided written informed consent. Participants were from eight retirement communities chosen for their proximity, size, and availability of space for placing a bike within the facility to minimize barriers to participation. Participants were recruited by way of fliers and informational sessions and were included in the study if they had no known neurological conditions, were in good physical health, and were not currently participating in regular exercise but were physically able to do so. Physician approval was required (including cardio-logist approval if applicable). Participants (102 enrolled, 79 randomized, and 63 completers) were trained and encouraged to strive gradually toward recommended exercise levels for older adults [five times a week for 45 min of moderate to vigorous exercise, per the American College of Sports Medicine (ACSM) and the American Heart Association (AHA)].18 Compliance was defined as an average of at least two times per week over the course of the 3 months.
Participants were evaluated on cognitive measures at three time points using alternate forms to minimize the impact of learning effects known to play a role in serial assessment. The first evaluation (T1a) was at enrollment and was used as a “run-in” evaluation to familiarize the participants with the testing procedures and to minimize the impact of practice and learning effects by concentrating those effects in T1a and excluding that time period from analyses.
The study found greater cognitive gains in executive function for older adults who rode a cybercycle compared with those who rode a traditional stationary bike.9 There was no significant difference in the exercise effort of these two groups (e.g., minutes, mileage, or power/watts). The findings suggest that the interactive combination of mental and physical exercise experienced on a cybercycle may enhance cognitive benefits more than physical exercise alone, all for the same amount of physical effort (see the published findings9).
The Secondary Analysis
Thirteen Cybercycle Study participants carried a diagnosis of type 2 diabetes mellitus (T2DM); three were noncompleters (one moved, two had medical compli-cations). Ten T2DM older adult completers (8 female) were the focus of this study, and they were compared with 10 age-matched non-DM older adults (10 female), all of whom were exercising as part of the larger RCT (see Table 1 for demographics). Matching was accomplished by sorting non-DM participants by age and, if more than one participant of the same age was available for matching, the decision was narrowed down by matching on additional variable(s) in this order of priority: cycling condition, sex, and education. At pretest, prior to exercise, there were no significant differences between the DM patient and non-DM groups on age, education, exercise behavior, or measures of executive function (see Table 1).
Table 1.
Descriptives and Outcomes for T2DM and non-DM Older Adult Exercisers
| T2DM | Nondiabetic controls | t-test | Analysis of covariance | |||||
|---|---|---|---|---|---|---|---|---|
| Average | Standard deviation | n | Average | Standard deviation | n | p | p | |
| Demographics | ||||||||
| Age | 72.8 | 8.6 | 10 | 73.7 | 9.1 | 10 | 0.82 | |
| Education (years) | 12.2 | 1.5 | 10 | 13.6 | 1.9 | 10 | 0.09 | |
| Physical activity during the study period (3 months; kcal) | 307.1 | 174.9 | 10 | 298.0 | 232.6 | 8 | 0.93 | |
| Exercise behaviors | ||||||||
| Frequency (rides/3 months) | 60.2 | 17.8 | 9 | 48.3 | 13.1 | 10 | 0.11 | |
| Energy (kcal) | 105.5 | 38.4 | 9 | 115.0 | 58.2 | 10 | 0.68 | |
| Minutes per session | 36.0 | 8.1 | 9 | 33.3 | 13.5 | 10 | 0.61 | |
| Miles per session | 4.7 | 1.6 | 9 | 5.6 | 2.1 | 10 | 0.34 | |
| Effort per session (watts) | 27.6 | 12.1 | 9 | 31.7 | 14.9 | 10 | 0.52 | |
| Miles per hour average | 7.3 | 1.1 | 5 | 8.6 | 2.0 | 4 | 0.25 | |
| Miles per hour max | 10.5 | 1.1 | 5 | 11.0 | 2.4 | 4 | 0.66 | |
| Cognitive outcomes | ||||||||
| Color Trails 2 pre | 116.8 | 24.4 | 10 | 103.3 | 49.0 | 10 | 0.45 | |
| Color Trails 2 post | 92.4 | 15.9 | 10 | 98.6 | 44.9 | 10 | 0.69 | 0.007 |
| Stroop C pre | 57.9 | 17.2 | 10 | 55.2 | 16.6 | 10 | 0.72 | |
| Stroop C post | 49.6 | 12.3 | 10 | 50.8 | 23.2 | 10 | 0.89 | 0.56 |
| Digit Span Backwards pre | 6.7 | 2.2 | 10 | 6.4 | 1.4 | 10 | 0.72 | |
| Digit Span Backwards post | 5.1 | 0.7 | 10 | 6.3 | 1.9 | 10 | 0.08 | 0.10 |
| Pegboard pre | 96.8 | 21.1 | 10 | 107.1 | 36.4 | 10 | 0.45 | |
| Pegboard post | 102.4 | 30.8 | 10 | 120.0 | 53.4 | 10 | 0.38 | 0.42 |
Exercise Equipment
Our term “cybercycle” denotes a stationary bike with a video screen that displays interactive virtual game components. In year 1, a recumbent Tunturi stationary bike (e60r) was used, equipped with NetAthlon 2.0 software on an Acer laptop. In year 2, the recumbent Expresso bike (S3R) was used instead, and it had a touchpad that was easier for this population to operate. The cybercycle computers recorded heart rate, mileage, time, and effort (in calories or watts) and saved it in an electronic database while participants traveled along a virtual bike path and altered their pedaling resistance via gear shifts. In the larger RCT, participants assigned to traditional stationary cycling used the same equipment, but the virtual reality enhancement was not available to them.
Measures
Neuropsychological Evaluation of Executive Function
Three neuropsychological tests were chosen because of their applicability as measures of executive function,34 their ease of administration, availability of alternate forms (to minimize practice effects in repeated adminis-tration), and use in other studies of exercise and cognition. Executive function controls other cognitive domains and encompasses a variety of other subfunctions, including planning, cognitive flexibility, and inhibition. Adequate executive function is essential to maintain independence in later life and is key in multitasking, for example, to safely cook more than one food at a time. Three executive function tests were administered at three time points: T1a (initial pretest/run-in to washout practice effects), T1b (pre-exercise, 1 month after T1a and preceding the start of exercise), and T2 (postexercise, at the end of 3 months of exercise). The focus of analyses herein is on T1b and T2.
Color Trails 2. Participants were first presented with Color Trails 1,35 in which they were instructed to connect numbered dots in numerical order as quickly as possible. Color Trails 2 required alternating the color of the dots while connecting them in numerical order, and this involved using skills such as planning and flexibility for shifting sets. According to the Color Trails 2 test manual,35 test–retest reliability over 2 weeks is adequate (rtt = 0.78), and the test has good construct and criterion-related validity.
Stroop C. Modified versions of Stroop A, B, and C (n = 40 stimuli each) were administered.36 In Stroop A, participants were instructed to say the name of colored blocks as quickly as possible, and in Stroop B, they read the typed color names. For Stroop C, participants had to state the color of the ink of typed words while suppressing the contrasting typed color name. This test assesses cognitive flexibility and inhibition of responses. Good test–retest reliability has been demonstrated (rtt = 0.80),36 and the test has also shown evidence of construct validity.34
Digit Span Backwards. Participants were instructed to listen to strings of numbers of increasing length and repeat them in forward order.34 Then participants were instructed to listen to additional digit strings of increasing length and repeat them in reverse order. This test requires dual tracking of information in working memory. Test–retest reliability for Digit Span is good (rtt = 0.83),34 and adequate construct validity has been reported.34
Behavioral Measures
Exercise frequency, intensity (energy expended in kcal, effort in watts), mileage, and duration (time in minutes) were extracted from the bikes’ recording systems and corroborated with participants’ daily self-report notated in ride logs.
Statistical Analysis
Group (T2DM versus non-DM older adults) × time (pre- versus post-exercise) interaction effects were examined in repeated measures analyses of covariance for the three tests of executive function. Using SPSS v19 statistical software, age and education were entered as covariates, given their significant relationship to tests of cognitive function. Follow-up analyses (paired t-tests) of any significant interaction effect were planned to clarify change over time within groups (pre- to post-exercise) and to examine the contrasts at each time point between groups (T2DM versus non-DM). The alpha level was set at 0.05.
Results
The two forms of exercise (cybercycling and traditional stationary cycling) were compared. No statistically significant differences in executive function were found between the two groups, and so hypothesis 1 was not supported. As a result, the cybercycling and traditional stationary cycling groups were combined for all subsequent analyses focusing on cognitive effects of exercise for DM patients versus non-DM participants (n = 10 in both the T2DM and non-DM groups). This decision was justified not only because there were no differences in cognitive effects of cybercycling versus traditional stationary cycling, but also because the T2DM and non-DM participant groups did not differ significantly in exercise frequency or intensity during the study period.
Hypothesis 2 was partially supported because one cognitive outcome did differ for DM patients compared with non-DM patients. A significant group × time interaction effect was found [F(1,16) = 9.75; p = .007; Figure 1] and follow-up analyses revealed that older adult exercisers with DM exhibited significant improvement on Color Trails 2 (p = .02), while non-DM older adult exercisers were unchanged (p = .83). No significant interaction was found for Digit Span Backwards or Stroop C. Executive function is a broad domain encompassing a variety of cognitive components; one difference between the three tests is that the Color Trails test involves motor function due to speeded drawing components, contrasted with Digit Span and Stroop tasks, which are verbal. Improved motor function has been seen alongside improvement in glycemic control in older adults with DM;22,37 however, no significant improvement was found on a task of motor function administered as part of the larger trial battery (Grooved Pegboard9).
Figure 1.
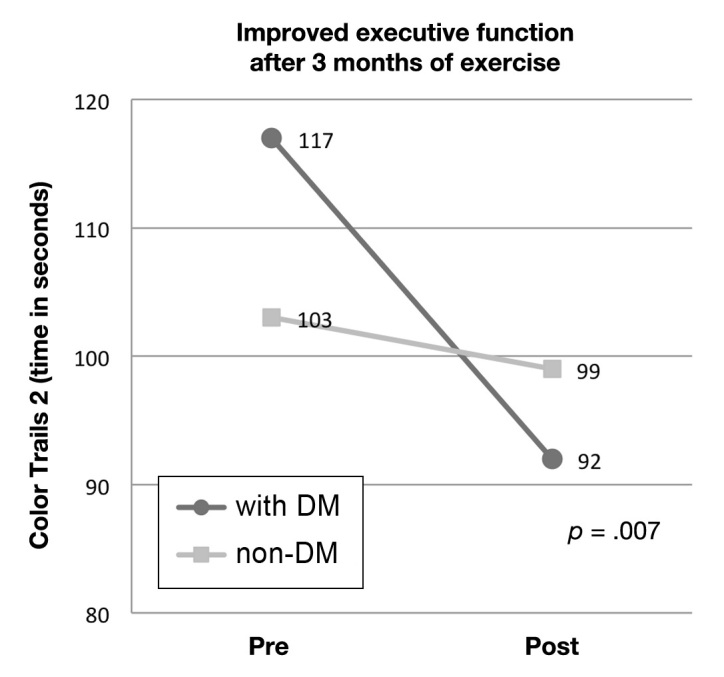
Means of Color Trails 2 by group and time. Lower scores indicate better performance (less time to complete the task).
Discussion
The U.S. population is aging, and dementia is on the rise;1 simultaneously, there is an increase in the incidence of diabetes, which has also been linked to dementia.3 Given the potential interconnection of the two conditions, aging and diabetes,3 there is an urgent need to develop interventions to address the cognitive decline associated with both. Exercise is an empirically supported inter-vention for curbing cognitive decline in later life,11 and cross-sectional research suggests exercise may also delay the cognitive decline associated with diabetes.29–31 One promising study has reported cognitive benefits of exercise in glucose intolerant older adults,31 but no prospective controlled study has been reported for T2DM.
Furthermore, while exercise may be well respected as a strategy for promoting a host of health benefits, and even widely prescribed by physicians, it is sorely underutilized, as only 7–14% of older adults engage in exercise at recommended frequency and exertion levels.18 Motivating older adults with DM to participate in exercise is daunting, but the novel technologies and compelling game challenges used in exergaming may improve motivation and behavior. The reinforcement potential of the videogame components and the playful and enjoyable aspects of the virtual reality environments could perhaps entice the most reluctant sedentary DM patient. To our knowledge, this is the first prospective study of the cognitive benefits of exercise for older adults with DM and the first to provide suggestive evidence that older adults both with and without diabetes can successfully use exergaming as a form of exercise.
In this exploratory study, 10 T2DM older adults were compared with 10 age-matched non-DM participants, all of whom exercised as part of a larger 3-month RCT that randomized participants to traditional stationary cycling or cybercycling. The 10 non-DM adults in this smaller study exhibited significant improvement on one of three measures of executive function (Color Trials 2). No significant group by time interactions were found for Digit Span Backwards or Stroop C. The impact of exercise on motor function has been observed elsewhere,37 but motor function did not change significantly for DM patients or non-DM participants in this study, so there may be other reasons for the differential effect on tasks of executive function between persons with and without diabetes. These preliminary and exploratory findings suggest that, for persons with DM, exercise may lead to significant improvement in some aspects of executive function. For older adults facing increased risk of cognitive decline due to age and diabetes, exercise may play an important role in staving off debilitating decline in cognitive function.
Compared with prior research, these findings are consistent with the literature on exercise for older adults in general, which has found improvements in cognitive function with exercise.11 While the non-DM comparison group in this study did not show a significant improve-ment in executive function, as observed in the DM patient group, it is possible to interpret this short-term “maintenance” of function as a benefit in contrast with the decline sometimes reported in this age group.9 The current results are consistent with a controlled trial that found improvement in executive function following exercise for glucose intolerant older adults.31
Limitations of this quasi-experimental exploratory study include a small sample of T2DM participants and healthy age-matched non-DM participants; ideally, this study should be replicated with a larger sample of both groups. The lack of a nonexercising DM patient control sample is also a limitation, although the consistent research on cognitive benefit led us to contrast exercise types rather than exclude a group from exercise for ethical reasons. Combining the two types of exercise (cybercycling and traditional stationary cycling) is another limitation, although no significant differences were found in exercise behaviors or cognitive outcomes between these two types of exercise, so combining them was justified; however, with a larger sample, and thus greater statistical power, differences between types of exercise could be identified. While the data from this study are consistent with the literature on poorer cognitive function among older DM patients than their peers,5 it is additionally a limitation that DM patients and age-matched controls did not start from equivalent points on executive function or motor function (e.g., T2DM participants demonstrated slower performance on Color Trails 2 and Grooved Pegboard at pretest than non-DM participants, but the differences were nonsignificant; p = .45 and .83, respectively).
This study does exhibit several strengths. All participants engaged in 3 months of monitored exercise. This is fitting with calls for additional well-controlled research on effects of exercise in diabetes.15,16 Additionally, comprehensive evaluations pre- and post-exercise were performed, including neuropsychological evaluations consisting of alternate test forms across run-in, pre- and post-assessments, and behavioral data that were captured directly rather than only by self-report (e.g., heart rate, minutes). Comparison with age-matched non-DM controls within the same study protocol made the comparisons more stringent than comparison only with normative controls or nonexercising controls. While there are numerous reports of the benefits of exercise for older adults, specifically in executive function,13,14 this exploratory study is the first to our knowledge to find cognitive improvement for T2DM exercisers over and above that of normative exercising older adults. This exercise intervention was consistent with the 2007 ACSM and AHA recommended exercise frequency and intensity for older adults.18 While the findings need replication, it appears that only 3 months of exercise can produce benefit to executive functioning for older adults with diabetes.
Conclusions
This exploratory study of cognitive effects of exercise for older adults with and without diabetes lends additional support to the growing body of literature that suggests exercise can improve cognition later in life, and it shows that this effect may be even stronger for people with diabetes. This study also demonstrated the feasibility of utilizing game-based virtual-reality-enhanced exercise—cybercycling—in older adults with and without diabetes because they were able to use the equipment over an extended period of time and successfully complete the study. Additional research is needed to investigate why certain aspects of executive function might be differentially affected by exercise. The findings of this study could help justify physician prescription of exercise for diabetes management; and for DM patients, awareness of potential cognitive benefits, along with the use of novel and engaging exergame technologies, may motivate them to engage in physical activity as an ongoing lifestyle habit.
Acknowledgments
We acknowledge important technical assistance from Brian Button of Interactive Fitness Holdings regarding our use of the Expresso platform, Bruce Winkler and Ivjot Kholi from RA Sports LLC regarding our use of their NetAthalon cycling software and sensor kits, and Mark Martens regarding our pilot of the FitClub riding software from Pantometrics. We greatly appreciate the participation of the residents and essential facilitation of the site administrators from Beltrone Living Center, Glen Eddy, Hightpointe Apartments, Kingsway Village, Prestwick Chase, Schaffer Heights, Wesley Health Care (Embury Apartments and Woodlawn Commons), and Westview Apartments. This research could not have been possible without the dedication of many research assistants; in particular, we would like to acknowledge Lyndsay De Matteo, Ariele Gartenberg, Veronica Hopkins, Eric Hultquist, Dinesh Kommareddy, Darlene Landry, Shi Feng Lin, Mariale Renna, Michelle Russo, Ali Seiler, Nick Steward, Amanda Snyder, and Vadim Yerokhin. Earlier versions of this data were presented at the Annual Meetings of the ACSM, the American Psychological Association, and the Society of Behavioral Medicine.
Glossary
- (ACSM)
American College of Sports Medicine
- (AHA)
American Heart Association
- (DM)
diabetes mellitus
- (non-DM)
patients without diabetes mellitus
- (RCT)
randomized clinical trial
- (T2DM)
type 2 diabetes mellitus
Funding
This study was funded by a grant from the Pioneer Portfolio of the Robert Wood Johnson Foundation, through the Health Games Research national program (#64449) and by faculty and student grants from Union and Skidmore Colleges.
References
- 1.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daffner KR. Promoting successful cognitive aging: a comprehensive review. J Alzheimers Dis. 2010;19(4):1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23(3):421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 5.Qiu WQ, Price LL, Hibberd P, Buell J, Collins L, Leins D, Mwamburi DM, Rosenberg I, Smaldone L, Scott TM, Siegel RD, Summergrad P, Sun X, Wagner C, Wang L, Yee J, Tucker KL, Folstein M. Executive dysfunction in homebound older people with diabetes mellitus. J Am Geriatr Soc. 2006;54(3):496–501. doi: 10.1111/j.1532-5415.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 6.Abbatecola AM, Paolisso G, Lamponi M, Bandinelli S, Lauretani F, Launer L, Ferrucci L. Insulin resistance and executive dysfunction in older persons. J Am Geriatr Soc. 2004;52(10):1713–1718. doi: 10.1111/j.1532-5415.2004.52466.x. [DOI] [PubMed] [Google Scholar]
- 7.Gregg EW, Yaffe K, Cauley JA, Rolka DB, Blackwell TL, Narayan KM, Cummings SR. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000;160(2):174–180. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 8.Royall D. Exercise and its mediating effects on cognition. Champaign: Human Kinetics; 2008. Diabetes, executive control, functional status, and physical activity; pp. 183–196. [Google Scholar]
- 9.Anderson-Hanley C, Arciero PJ, Brickman AM, Nimon JP, Okuma N, Westen SC, Merz ME, Pence BD, Woods JA, Kramer AF, Zimmerman EA. Exergaming and older adult cognition: a cluster randomized clinical trial. Am J Prev Med. 2012;42(2):109–119. doi: 10.1016/j.amepre.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(3) doi: 10.1002/14651858.CD005381.pub3. CD005381. [DOI] [PubMed] [Google Scholar]
- 11.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 12.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Eggermont LH, Milberg WP, Lipsitz LA, Scherder EJ, Leveille SG. Physical activity and executive function in aging: the MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57(10):1750–1756. doi: 10.1111/j.1532-5415.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman CH, Belopolsky AV, Snook EM, Kramer AF, McAuley E. Physical activity and executive control: implications for increased cognitive health during older adulthood. Res Q Exerc Sport. 2004;75(2):176–185. doi: 10.1080/02701367.2004.10609149. [DOI] [PubMed] [Google Scholar]
- 15.Orozco LJ, Buchleitner AM, Gimenez-Perez G, Roqué I, Figuls M, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;(3) doi: 10.1002/14651858.CD003054.pub3. CD003054. [DOI] [PubMed] [Google Scholar]
- 16.Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol. 2010;47(1):15–22. doi: 10.1007/s00592-009-0126-3. [DOI] [PubMed] [Google Scholar]
- 17.Kuo HK, Jones RN, Milberg WP, Tennstedt S, Talbot L, Morris JN, Lipsitz LA. Effect of blood pressure and diabetes mellitus on cognitive and physical functions in older adults: a longitudinal analysis of the advanced cognitive training for independent and vital elderly cohort. J Am Geriatr Soc. 2005;53(7):1154–1161. doi: 10.1111/j.1532-5415.2005.53368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman DA. Designing serious games for learning and health in informal and formal settings. In: Ritterfeld U, Cody M, Vorderer P, editors. Serious games: mechanisms and effects. New York: Routledge; 2009. pp. 117–130. [Google Scholar]
- 20.Debling D, Amelang M, Hasselbach P, Stürmer T. Diabetes and cognitive function in a population-based study of elderly women and men. J Diabetes Complications. 2006;20(4):238–245. doi: 10.1016/j.jdiacomp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen HT, Grzywacz JG, Arcury TA, Chapman C, Kirk JK, Ip EH, Bell RA, Quandt SA. Linking glycemic control and executive function in rural older adults with diabetes mellitus. J Am Geriatr Soc. 2010;58(6):1123–1127. doi: 10.1111/j.1532-5415.2010.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol. 1993;48(4):M117–M121. doi: 10.1093/geronj/48.4.m117. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman EA, Desemone J. Ideal management of diabetes mellitus and dementia: walking a tightrope between hyperglycemia and hypoglycemia. Arch Neurol. 2010;67(1):131–133. doi: 10.1001/archneurol.2009.302. [DOI] [PubMed] [Google Scholar]
- 24.Crooks VC, Buckwalter JG, Petitti DB. Diabetes mellitus and cognitive performance in older women. Ann Epidemiol. 2003;13(9):613–619. doi: 10.1016/S1047-2797(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 25.Gong B, Chen F, Pan Y, Arrieta-Cruz I, Yoshida Y, Haroutunian V, Pasinetti GM. SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer’s disease amyloidosis and improves synaptic function. Aging Cell. 2010;9(6):1018–1031. doi: 10.1111/j.1474-9726.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 28.Arciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol. 1999;86(6):1930–1935. doi: 10.1152/jappl.1999.86.6.1930. [DOI] [PubMed] [Google Scholar]
- 29.Colberg SR, Somma CT, Sechrist SR. Physical activity participation may offset some of the negative impact of diabetes on cognitive function. J Am Med Dir Assoc. 2008;9(6):434–438. doi: 10.1016/j.jamda.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Devore EE, Kang JH, Okereke O, Grodstein F. Physical activity levels and cognition in women with type 2 diabetes. Am J Epidemiol. 2009;170(8):1040–1047. doi: 10.1093/aje/kwp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirk A, Mutrie N, MacIntyre P, Fisher M. Increasing physical activity in people with type 2 diabetes. Diabetes Care. 2003;26(4):1186–1192. doi: 10.2337/diacare.26.4.1186. [DOI] [PubMed] [Google Scholar]
- 33.Wisse W, Rookhuizen MB, de Kruif MD, van Rossum J, Jordans I, Ten Cate H, van Loon LJ, Meesters EW. Prescription of physical activity is not sufficient to change sedentary behavior and improve glycemic control in type 2 diabetes patients. Diabetes Res Clin Pract. 2010;88(2):e10–e13. doi: 10.1016/j.diabres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Strauss E, Sherman EM, Spreen O. A compendium of neuro-psychological tests: administration, norms, and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 35.D’Elia LG, Satz P, Uchiyama CL, White T. Color trails test: professional manual. Odessa: Psychological Assessment Resources; 1996. [Google Scholar]
- 36.Houx PJ, Shepherd J, Blauw GJ, Murphy MB, Ford I, Bollen EL, Buckley B, Stott DJ, Jukema W, Hyland M, Gaw A, Norrie J, Kamper AM, Perry IJ, MacFarlane PW, Meinders AE, Sweeney BJ, Packard CJ, Twomey C, Cobbe SM, Westendorp RG. Testing cognitive function in elderly populations: the PROSPER study: PROspective Study of Pravastatin in the Elderly at Risk. J Neurol Neurosurg Psychiatry. 2002;73(4):385–389. doi: 10.1136/jnnp.73.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamijo T, Murakami M. Regular physical exercise improves physical motor functions and biochemical markers in middle-age and elderly women. J Phys Act Health. 2009;6(1):55–62. doi: 10.1123/jpah.6.1.55. [DOI] [PubMed] [Google Scholar]


