Abstract
Background
The accuracy of continuous glucose monitoring (CGM) in non-critically ill hospitalized patients with heart failure or severe hyperglycemia (SH) is unknown.
Methods
Hospitalized patients with congestive heart failure (CHF) exacerbation (receiving IV or subcutaneous insulin) or SH requiring insulin infusion were compared to outpatients referred for retrospective CGM.
Results
Forty-three patients with CHF, 15 patients with SH, and 88 outpatients yielded 470, 164, and 2150 meter–sensor pairs, respectively. Admission glucose differed (188 versus 509 mg/dl in CHF and SH, p < .001) but not the first sensor glucose (p = .35). In continuous glucose error grid analysis, 67–78% of pairs during hypoglycemia were in zones A+B (p = .63), compared with 98–100% in euglycemia (p < .001) and 98%, 92%, and 99% (p = .001) during hyperglycemia for the CHF, SH, and outpatient groups, respectively. Mean absolute relative difference (MARD) was lower in the CHF versus the SH group in glucose strata above 100 mg/dl, but there was no difference between the CHF and outpatient groups. Linear regression models showed that CHF versus outpatient, SH versus CHF, and coefficient of variation were significant predictors of higher MARD. Among subjects with CHF, MARD was not associated with brain natriuretic peptide or change in plasma volume, but it was significantly higher in subjects randomized to IV insulin (p = .04).
Conclusions
The results suggest that SH and glycemic variability are more important determinants of CGM accuracy than known CHF status alone in hospitalized patients.
Keywords: continuous glucose monitoring, heart failure, hospital
Introduction
Current guidelines highlight the need to manage hyperglycemia effectively while avoiding hypoglycemia in hospitalized patients.1 Frequent glucose monitoring is essential for monitoring and adjusting therapy; however, this requires extensive nursing efforts, which may be limited, especially outside of the intensive care unit (ICU). Continuous subcutaneous glucose monitoring (CGM) may be an important tool for use in addition to point-of-care glucose measurements in hospitalized patients. According to a consensus panel on CGM, the mean absolute relative difference (MARD) for specific glucose ranges varies from 10% to 20%. Furthermore, only 60% to 80% of the glucose readings fall in the Clarke A zone, which is significantly lower than what can be achieved with capillary blood glucose (BG).2 Hence, CGM has not been used for stand-alone glucose measurement. These estimates are generally higher for professional CGM compared with real-time CGM, because calibration is performed retrospectively.
In the ICU, various factors, including hypotension, hydration, acid–base disturbances, oxygenation, and drug interferences, are known to affect the accuracy of point-of-care devices.3 These factors may similarly affect the accuracy of CGM. In the ICU, various studies have demonstrated limited, but “acceptable,” accuracy.3–9 However, there are no data to support the use of CGM outside of the ICU (non-ICU), where fewer nursing resources are available and where fewer interfering conditions may exist. In particular, heart failure and severe hyperglycemia (SH) are common outside of the ICU and may pose unique challenges to the sensor environment and signal stability. No study has specifically addressed these conditions separately. Interim data from an ongoing study to compare intravenous (IV) and SQ insulin in patients hospitalized with hyperglycemia and heart failure exacerbation (ClinicalTrials.gov, NCT00812253) suggested that CGM accuracy would be comparable to outpatient CGM. Therefore, a comparison was performed with existing outpatient data and with a small sample of hospitalized patients without heart failure.
The objective of this study was to evaluate the accuracy of interstitial glucose measurements in hospitalized non-ICU patients with SH or congestive heart failure (CHF) exacerbation and to compare with nonhospitalized outpatients with diabetes.
Methods
Study subjects consisted of three groups (CHF, SH, and outpatients). The CHF group consisted of insulin-requiring subjects with symptomatic CHF exacerbation who were randomly assigned to IV or SQ insulin as part of an ongoing study. The SH group consisted of patients admitted to the medical floor who were requiring an insulin infusion for uncontrolled hyperglycemia. Severe hyperglycemia was defined, therefore, as that which is sufficient to warrant an insulin infusion at admission and is determined by the admitting team. Exclusion criteria for both groups included critical illness (ventilator, hypotension requiring pressors), end-stage renal or liver disease, and pregnancy. The outpatient group consisted of patients with diabetes who were referred by the patient’s endocrinologist to the institution’s diabetes research center for placement of a retrospective (professional) CGM device. All studies were approved by the Institutional Review Board at Ohio State University, and all prospectively studied patients signed informed consent.
All patients receiving IV insulin (half of the CHF group and all of the SH group) were managed using our hospital’s standard nursing run protocol, which was adapted from a published protocol and has a target glucose of 110–150 mg/dl.10 Subjects treated with IV insulin also received dextrose 5% in half-normal saline at 10 ml/h, according to hospital guidelines. Among patients receiving SQ insulin, basal and prandial insulin were administered in approximately equal total daily doses with adjustments based on a published algorithm.11 The major exceptions were that prandial insulin was delivered according to carbohydrate intake based on total daily dose (as opposed to fixed meal doses) and that the target glucose range was 100–150 mg/dl (as opposed to 140 mg/dl).
All subjects wore the CGMS iPro® (Medtronic, Minneapolis, MN), which was inserted on the abdomen and downloaded using CGMS solutions software according to manufacturer guidelines. The CGM sensors (Sof-SensorTM, Medtronic, Minneapolis, MN) were worn for at least 24 h. Capillary glucoses were analyzed with the ACCU-CHEK Inform® system (Roche, Indianapolis, IN) in hospitalized patients and the FreeStyle Lite (Abbott, Abbott Park, IL) in outpatients and were used to calibrate the sensor according to manufacturer guidelines. Capillary glucoses were collected hourly during insulin infusions and every 4–6 h otherwise. However, only glucoses at four predetermined time points per day (premeal and at bedtime closest to 7 am, 11 am, 4 pm, and 9 pm) within the allowable glucose limits of the software (40–400 mg/dl) were used to calibrate the CGM device. Points of time with rapid change in glucose were not excluded. In the CHF group, brain natriuretic peptide (BNP) was analyzed at baseline as well as change in plasma volume, which was calculated with the hemoglobin and hematocrit from successive days, as published previously.12 No such patients were actively bleeding or received blood transfusions.
Glucose readings were compared by calculating the mean absolute difference (MAD) and MARD between interstitial glucose (Medtronic, Minneapolis, MN) and the reference capillary glucose method. Pearson’s correlation coefficients were calculated overall and for each group. The data were further stratified by glucose ranges and analyzed separately. There were insufficient glucose values in the hypoglycemic range for adequate comparisons. Therefore, glucose ranges were chosen so that direct comparison could be made with a previous ICU study.5 Clarke error grid analysis (EGA) and the continuous glucose error grid analysis (CG-EGA) were performed for standard accepted accuracy criteria for interstitial and capillary glucose monitors.13,14
Comparisons between groups were conducted using analysis of variance with adjustment for multiple comparisons using the Tukey–Kramer honestly significant difference method.15 A p value < 0.05 was considered statistically significant. Multiple linear regression analyses were conducted using least squares linear regression with backward stepwise method. The dependent variable was MARD and the independent variables were group, age, race, gender, duration of diabetes, coronary artery disease, body mass index, hemoglobin A1c (HbA1c), first sensor glucose, sensor mean glucose, sensor glucose coefficient of variation (CV) as a measure of glycemic variability,16 treatment type, and creatinine. The variables were chosen based on effect estimates from univariable analyses. Statistical analyses were performed using JMP 8.0 software. The EGAs were performed using static glucose EGA and CG-EGA software (The Epsilon Group).
Results
The study sample included 43, 15, and 88 patients in the CHF, SH, and outpatient groups, respectively. Baseline characteristics for all groups are shown in Table 1.
Table 1.
Baseline Characteristics by Groupa
| Characteristic | Outpatient (n = 88) | CHF (n = 43) | Acute hyperglycemia (n = 15) | p value overall | p value CHF versus outpatient |
|---|---|---|---|---|---|
| Age (years) | 46.0 ± 14.1 | 60.2 ± 12.1 | 41.7 ± 11.4 | <0.0001 | <0.0001 |
| Male | 28 (31.8%) | 30 (69.8%) | 6 (40.0%) | 0.0002 | <0.0001 |
| Caucasian | 78 (88.6%) | 30 (69.8%) | 9 (60.0%) | <0.0001 | 0.013 |
| Body mass index (kg/m2) | 27.8 ± 6.1 | 37.3 ± 10.3 | 29.88 ± 9.59 | <0.0001 | <0.0001 |
| Diabetes duration (years) | 23.3 ± 12.9 | 13.8 ± 7.8 | 13.3 ± 9.88 | <0.0001 | <0.0001 |
| Type 2 diabetes | 17 (19.3%) | 43 (100.0%) | 12 (80%) | <0.0001 | <0.0001 |
| HbA1c (%) | 8.2 ± 1.3 | 7.77 ± 1.79 | 12.3 ± 2.1 | <0.0001 | 0.36 |
| Hypertension | 41 (46.5%) | 39 (90.7%) | 10 (66.7%) | <0.0001 | <0.0001 |
| Coronary artery disease | 22 (25.0%) | 26 (60.5%) | 4 (35.3%) | <0.0001 | <0.0001 |
| Retinopathy | 33 (37.5%) | 4 (9.3%) | 2 (17.6%) | 0.0016 | 0.0008 |
| Nephropathy | 30 (34.1%) | 7 (16.3%) | 5 (41.1%) | <0.0001 | 0.039 |
| Neuropathy | 31 (35.2%) | 17 (39.5%) | 13 (94.2%) | 0.95 | 0.85 |
| Creatinine (mg/dl) | 1.02 ± 0.69 | 1.5 ± 0.6 | 0.84 (±0.24) | <0.0001 | 0.0003 |
| Diabetic ketoacidosis | -- | -- | 6 (40%) | -- | -- |
| Glucose data | |||||
| Admission glucose (mg/dl) | -- | 188.4 ± 61.1 | 508.8 ± 193.7 | <0.0001 | -- |
| First sensor glucose (mg/dl) | 182.8 ± 77.5 | 163.0 ± 66.7 | 177.6 ± 50.6 | 0.35 | 0.32 |
| Mean sensor glucose (mg/dl) | 180.1 ± 41.5 | 150.6 ± 36.1 | 180.3 ± 52.5 | 0.0008 | 0.0007 |
| CV (%) | 40.9 ± 7.6 | 21.1 ± 9.1 | 23.4 ± 9.4 | <0.0001 | <0.0001 |
| % time in hypoglycemia | 5.4 ± 5.8 | 0.38 ± 1.3 | 3.02 ± 8.96 | <0.0001 | <0.0001 |
| MAD (mg/dl) | 16.3 ± 6.8 | 13.5 ± 5.3 | 30.0 ± 11.4 | <0.0001 | 0.098 |
| MARD (%) | 11.1 ± 5.0 | 9.6 ± 4.2 | 16.2 ± 4.4 | <0.0001 | 0.19 |
| Meter–sensor correlation (r) | 0.96 | 0.88 | 0.85 | ||
Data are reported as mean ± standard deviation or number (%). Glucose values are in mg/dl.
All together, the CHF, SH, and outpatient groups yielded 470, 164, and 2150 meter–sensor pairs, respectively. In light of the difference in indication for insulin infusion (research versus clinical indication), the admission glucose for the two hospitalized groups differed as expected (188 versus 509 mg/dl, p < .001). However, the first sensor glucose (163, 178, and 183 mg/dl in the CHF, SH, and outpatient groups) did not differ significantly (p = .35 overall, p = .32 for comparison between the inpatient groups). There were differences in mean glucose (151, 180, and 183 mg/dl in the CHF, SH, and outpatient groups) and CV (21.1%, 23.4%, and 40.9%) overall (p < .0001 for both) and between the CHF and outpatient groups (p = .007 for mean glucose and p < .0001 for CV). There were 3, 4, and 199 pairs with the reference value in the hypoglycemic range (<70 mg/dl in the CHF, SH, and outpatient groups, respectively, and there was more time spent in hypoglycemia in the outpatient group compared with the CHF group (p < .0001).
The correlation coefficients were 0.88, 0.85, and 0.96 for the CHF, SH, and outpatient groups, respectively (p < .0001 for all). The MARD was 9.6%, 16.2%, and 11.1% in the CHF, SH, and outpatient groups, respectively, which was statistically significantly different overall (p < .0001) but not between the CHF and outpatient groups (p = .19). Similarly, the MAD was significantly different overall (p < .0001) but not between the CHF and outpatient groups (p = .098).
We further analyzed MARD by glucose strata (<100, 100–150, 151–200, >200 mg/dl, Table 2). The results showed a significant association between glucose stratum and MARD (p < .0001 in CHF and outpatient groups, p = .0498 in the SH group). Except for the stratum <100 mg/dl, MARD was higher in the SH group compared with the CHF group. There were no stratum-specific differences between the CHF and outpatient groups.
Table 2.
Mean Absolute Relative Difference by Glucose Category
| Outpatient (n = 88) | CHF (n = 45) | SH (n = 15) | p value | |||||
|---|---|---|---|---|---|---|---|---|
| mg/dl | Number of pairs n (%) | MARD (%) | Number of pairs n (%) | MARD (%) | Number of pairs n (%) | MARD (%) | Outpatient versus CHF | SH versus CHF |
| <100 | 506 (24) | 16.6 | 45 (10) | 19.6 | 14 (9) | 23.7 | 0.59 | 0.67 |
| 100–149 | 538 (25) | 12.3 | 197 (42) | 10.1 | 51 (31) | 21.6 | 0.13 | <0.0001 |
| 150–199 | 417 (19) | 10.2 | 147 (31) | 8.7 | 52 (32) | 14.3 | 0.26 | 0.002 |
| ≥200 | 689 (32) | 7.32 | 81 (17) | 9.1 | 47 (29) | 14.5 | 0.12 | 0.0003 |
An EGA was performed separately for each group (Figure 1). In the CHF group, 85.7% of pairs fell in zone A (indicating that the meter was within 20% of the reference or both meter and reference were <70 mg/dl, resulting in a correct and safe treatment decision) compared to 85.9% in outpatients and 69.5% in the SH group (p = .91 in CHF versus outpatient, p < .0001 overall). The percentage of pairs in zone A+B was 99.1%, 96.9%, and 97.7% in the CHF, SH, and outpatient groups, respectively (p = .09 overall). Zone A and zone B are considered clinically acceptable. Zone C accounted for 0%, 0.6%, and 0.1% of pairs in the CHF, SH, and outpatient groups, while zone D accounted for 0.9%, 2.4%, and 2.2% of pairs in the groups, respectively. There were no pairs in zone E, erroneous treatment.
Figure 1.
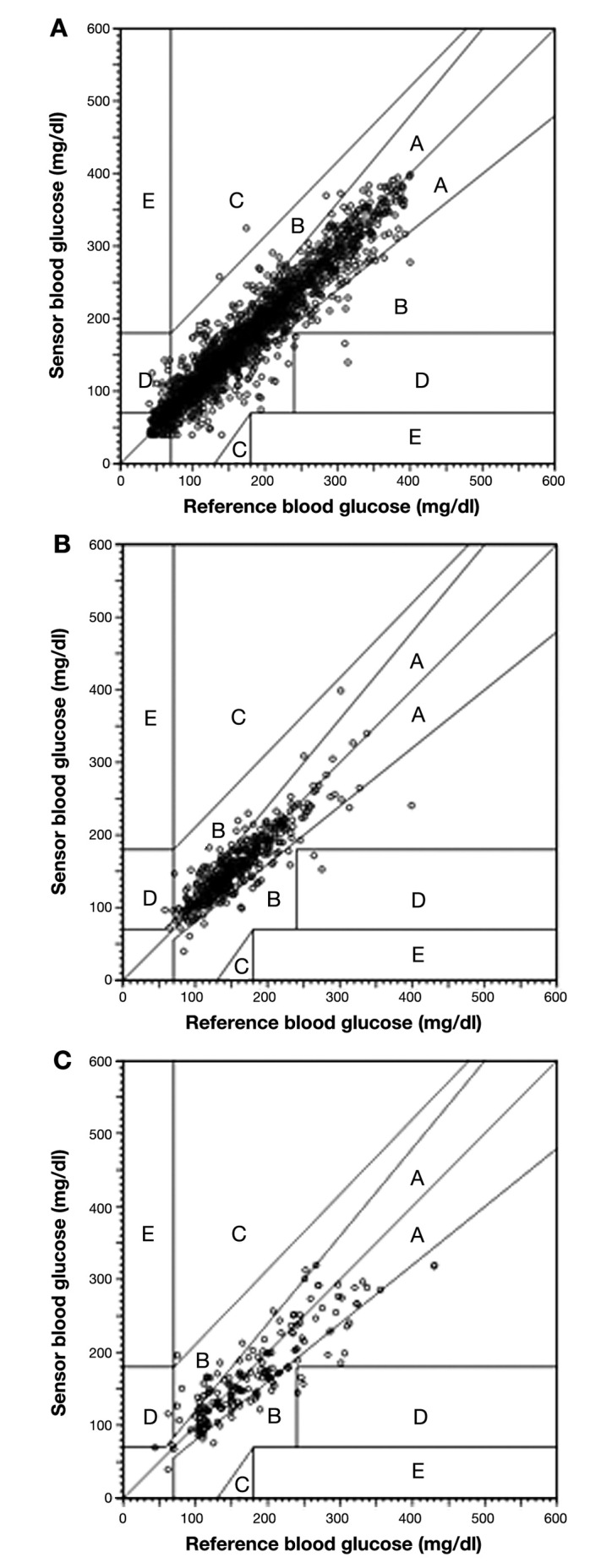
Static glucose EGA for (A) outpatient, (B) CHF, and (C) SH.
We then performed CG-EGA (Table 3), which calculates both point and rate accuracy in error matrices for determining the clinical accuracy of treatment decisions. The accuracy of CGM readings was evaluated separately in the following strata: hypoglycemia (BG ≤ 70 mg/dl), euglycemia (70 < BG ≤ 180 mg/dl), and hyperglycemia (BG > 180 mg/dl; Table 3). In the hypoglycemic range, 78%, 67%, and 75% of pairs were in zone A (p = .001 overall) compared with 82%, 91%, and 71% of pairs in the euglycemic range (p < .001) and 90%, 94%, and 70% in the hyperglycemic range (p < .001) in the outpatient, CHF, and SH groups, respectively. However, accurate readings (zone A+B) occurred in 78%, 67%, and 75% of pairs in the hypoglycemic range; 100%, 100%, and 98% of pairs in the euglycemic range; and 99%, 98%, and 92% of pairs in the hyperglycemic range.
Table 3.
Static and Continuous Glucose Error Grid Analyses
| Outpatient (%) | CHF (%) | SH (%) | p value overall | p value CHF versus outpatient | |
|---|---|---|---|---|---|
| Static EGA | |||||
| Zone A | 85.9 | 85.7 | 69.5 | <0.0001 | 0.91 |
| Zone A+B | 97.7 | 99.1 | 96.9 | 0.09 | 0.053 |
| CG-EGA | |||||
| <70 mg/dl | |||||
| Zone A | 78.4 | 66.7 | 75.0 | 0.88 | 0.63 |
| Zone A+B | 78.4 | 66.7 | 75.0 | 0.88 | 0.63 |
| Erroneous | 21.6 | 33.3 | 25.0 | ||
| 71–180 mg/dl | |||||
| Zone A | 82.2 | 90.9 | 70.7 | 0.001 | 0.004 |
| Zone A+B | 100 | 100 | 97.7 | <0.001 | 0.99 |
| Erroneous | 0.0 | 0.0 | 2.4 | ||
| >180 mg/dl | |||||
| Zone A | 89.8 | 94.4 | 70.3 | <0.001 | 0.13 |
| Zone A+B | 99.3 | 98.1 | 91.9 | 0.001 | 0.41 |
| Erroneous | 0.7 | 1.9 | 8.1 | ||
The initial linear regression model demonstrated a significant association between SH versus CHF (p = .008) and borderline trend for association with the CV (p = .07) and route of insulin (IV versus SQ, p = .07), but neither mean glucose nor first sensor glucose were significant predictors (Table 4). In the final model, CHF was associated with decreased MARD versus the SH group (an estimated 0.04% difference, p < .0001) and increased MARD versus the outpatient group (an estimated 0.07% difference, p = .002) after adjusting for CV. There was no interaction between mean glucose and CV (p = .20). Conversely, CV was statistically significantly associated with MARD (p = .014) after adjusting for patient groups, with an estimated 0.001% increase in MARD for every 1% increase in CV.
Table 4.
Multiple Linear Regression for Mean Absolute Relative Difference
| Term | Estimate | Standard error | p value |
|---|---|---|---|
| Initial model | |||
| Outpatient (versus CHF) | -0.022 | 0.016 | 0.18 |
| SH (versus CHF) | 0.053 | 0.020 | 0.008 |
| Age | 0.0002 | 0.0004 | 0.55 |
| Nonwhite | -0.009 | 0.006 | 0.11 |
| Female | 0.005 | 0.005 | 0.29 |
| Type 1 diabetes | 0.001 | 0.007 | 0.87 |
| Diabetes duration | -0.0002 | 0.0004 | 0.63 |
| Coronary artery disease | -0.005 | 0.005 | 0.29 |
| Body mass index | -0.001 | 0.001 | 0.21 |
| IV insulin (versus SQ) | 0.016 | 0.008 | 0.07 |
| HbA1c | -0.001 | 0.003 | 0.73 |
| First sensor glucose | -0.0001 | 0.0001 | 0.40 |
| Sensor mean glucose | -0.0001 | 0.0001 | 0.33 |
| Sensor glucose CV | 0.001 | 0.001 | 0.07 |
| Creatinine | 0.004 | 0.007 | 0.58 |
| Final model | |||
| Intercept | 0.096 | 0.012 | <0.0001 |
| Outpatient (versus CHF) | -0.038 | 0.012 | 0.0020 |
| SH (versus CHF) | 0.066 | 0.014 | <0.0001 |
| Sensor glucose CV | 0.001 | 0.001 | 0.014 |
In the CHF group, MARD was not associated with baseline BNP (r = 0.15, p = .38). However, MARD was significantly higher in CHF subjects receiving IV insulin compared with SQ insulin (8.3% versus 11%, p = .04), and this remained significant after adjusting for CV. Change in plasma volume was not associated with MARD in patients with CHF (r = 0.11, p = .52) or in patients with SH (r = 0.31, p = .30).
Discussion
Congestive heart failure and SH requiring IV insulin are commonly encountered conditions in hospitalized non-ICU patients and may pose unique potential challenges to sensor accuracy. Overall, accuracy in subjects with CHF was acceptable, with most readings falling in EGA and CG-EGA zones A+B. The CHF group actually had more sensor–meter pairs in zone A of the CG-EGA euglycemic range than the other groups, but MARD was slightly higher in the CHF group compared with outpatients after accounting for differences in glycemic variability. In contrast, accuracy appeared to be suboptimal in subjects admitted with SH. The major exception was the hypoglycemic range, in which all groups appeared to have suboptimal accuracy, although solid conclusions can only be drawn for the outpatient group, where hypoglycemia was more frequent. Rapid glucose change affects the accuracy of CGM because of a time lag between sampling and signal detection and is a well-known phenomenon.17 This was reflected in the association between glucose CV and MARD overall in this study and after adjustment for multiple other variables. In comparison, the largest study in hospitalized patients to date reported 174 medical ICU patients requiring intensive insulin therapy, with 2045 sensor–meter pairs using similar real-time or retrospective CGM systems. The authors reported an overall MARD of 7.3%, 99.1% of subjects in insulin titration EGA zones A+B, and a correlation coefficient of 0.92.
From the unadjusted analysis, glucose stratum-specific MARD differed substantially across the two inpatient groups, but the CHF and outpatient groups did not differ. However, adjusting for glycemic variability (which was higher in the outpatient group in which the majority of patients had type 1 diabetes) uncovered only a small difference between outpatients and subjects with CHF. Although no direct measures of edema, tissue oxygenation, or perfusion were available, neither BNP nor the indirect measure of change in plasma volume was associated with the MARD. However, in subjects with CHF who were randomly assigned to IV or SQ insulin, IV insulin was also independently associated with MARD, even after adjusting for CV. This raises the question of whether other factors, such as unmeasured fluid shifts, may be important, at least in subjects with CHF, even though the total amount of fluid obtained from IV insulin is small. One small study conducted in pediatric patients showed no significant association between sensor performance and a radiologic index of edema.6 Other studies have not identified associations between sensor accuracy and vasopressor use.7,8 More research is needed to further investigate these questions; however, this is the first such study of CGM accuracy in subjects with symptomatic CHF requiring hospitalization.
Perhaps more striking than the differences between the CHF and outpatient groups is that, even after adjustment for CV, there was still a significant difference between the SH and CHF groups, suggesting that known CHF alone may be less important than other factors that are specific to patients with SH, even among subjects who do not require ICU care. The relative importance of factors such as dehydration or mild acidosis are unclear. We did not identify an association between change in plasma volume and sensor accuracy in patients with SH, but the sample size was limited for making this conclusion. In addition, some effect of glucose legacy may play a role, as suggested by the dramatic difference in admission glucose and HbA1c (in comparison, the initial sensor glucose did not differ) among groups. This could be mediated by local tissue changes during prolonged hyperglycemia (such as glycosylation or inflammation). Of note, the most dramatic change in glucose in the SH group was probably not captured by CGM since subjects had been brought under more reasonable control by the time the sensor initialized. These data are novel in that previous studies in hospitalized patients have not specifically targeted enrollment to subjects with SH.3–9
The study is limited by the comparison of patients who underwent CGM for different indications, resulting in differing overall glycemic control and glucose variability. We did adjust for many factors in our models, but residual confounding is possible. It is possible that patients in the SH or outpatient groups could have had cardiac dysfunction since formal assessments were not performed, but none had active heart failure exacerbation on clinical grounds. In addition, the calibration of CGM was performed using a different glucometer in the inpatients and outpatients in accordance with the usual practice at our institution, and this could affect the results. Capillary instead of venous or arterial glucose readings were reported in many,4,6–9 but not all,5 ICU studies. Although we specifically excluded patients who were hypotensive, small studies suggest that edema may also affect the accuracy of capillary glucose values.18,19 Therefore, it may be possible that some of the inaccuracy attributed to CGM in the hospitalized patients may actually be due to the capillary glucose used for calibration. While non-critically-ill patients routinely undergo venipuncture once daily, it is unclear whether the additional accuracy provided by four blood draws per day would be cost-effective or practical for routine calibration of CGM on the wards. Regardless, the use of capillary glucose for calibration does not appear to explain all of the difference in MARD, which was still lower in patients with CHF compared with those with SH. Our conclusions are limited by the relatively small number of meter–sensor pairs in the hypoglycemic range.
It is worth emphasizing that conclusions using professional CGM may not be valid with real-time use because of differences in calibration that favor professional CGM. The iPro software, in particular, has the benefit of utilizing all calibration points in its recording period for calibration. However, the study offers novel insights that warrant additional study. Future comparisons between glucose-matched hospitalized patients with or without CHF or SH are needed.
Conclusions
This study demonstrates that glycemic variability, CHF, and SH requiring IV insulin were small but significant independent predictors of lower CGM accuracy and that sensor accuracy was suboptimal (at least in the outpatient group) during hypoglycemia. In subjects with CHF, MARD was associated with IV insulin but not BNP or change in plasma volume. The findings should be considered in the future application of CGM in hospitalized non-ICU patients.
Glossary
- (BG)
blood glucose
- (BNP)
brain natriuretic peptide
- (CG-EGA)
continuous glucose error grid analysis
- (CGM)
continuous glucose monitoring
- (CHF)
congestive heart failure
- (CV)
coefficient of variation
- (EGA)
error grid analysis
- (HbA1c)
hemoglobin A1c
- (ICU)
intensive care unit
- (IV)
intravenous
- (MAD)
mean absolute difference
- (MARD)
mean absolute relative difference
- (non-ICU)
areas outside of ICU
- (SH)
severe hyperglycemia
- (SQ)
subcutaneous
Funding
This work was funded by National Institutes of Health Grants 1K23DK080891 and R21DK081877 and the Ohio State University Clinical and Translational Research Center (supported by award UL1RR025755 from the National Center for Research Resources).
Disclosures
Kathleen M. Dungan reports research support from Novo Nordisk and consulting with Eli Lilly and Pfizer.
References
- 1.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 2.Blevins TC, Bode BW, Garg SK, Grunberger G, Hirsch IB, Jovanovič L, Nardacci E, Orzeck EA, Roberts VL, Tamborlane WV. AACE Continuous Glucose Monitoring Task Force, Rothermel C, Statement by the American Association of Clinical Endocrinologists Consensus Panel on continuous glucose monitoring. Endocr Pract. 2010;16(5):730–745. doi: 10.4158/EP.16.5.730. [DOI] [PubMed] [Google Scholar]
- 3.Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measure-ment: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 4.Corstjens AM, Ligtenberg JJ, van der Horst IC, Spanjersberg R, Lind JS, Tulleken JE, Meertens JH, Zijlstra JG. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit Care. 2006;10(5):R135. doi: 10.1186/cc5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA, Dziura JD, Inzucchi SE. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6(3):339–347. doi: 10.1089/152091504774198034. [DOI] [PubMed] [Google Scholar]
- 6.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, Jaksic T, Agus MS. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118(3):1176–1184. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 7.Brunner R, Kitzberger R, Miehsler W, Herkner H, Madl C, Holzinger U. Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med. 2011;39(4):659–664. doi: 10.1097/CCM.0b013e318206bf2e. [DOI] [PubMed] [Google Scholar]
- 8.Holzinger U, Warszawska J, Kitzberger R, Herkner H, Metnitz PG, Madl C. Impact of shock requiring norepinephrine on the accuracy and reliability of subcutaneous continuous glucose monitoring. Intensive Care Med. 2009;35(8):1383–1389. doi: 10.1007/s00134-009-1471-y. [DOI] [PubMed] [Google Scholar]
- 9.Logtenberg SJ, Kleefstra N, Snellen FT, Groenier KH, Slingerland RJ, Nierich AP, Bilo HJ. Pre- and postoperative accuracy and safety of a real-time continuous glucose monitoring system in cardiac surgical patients: a randomized pilot study. Diabetes Technol Ther. 2009;11(1):31–37. doi: 10.1089/dia.2008.0028. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12(Suppl 3):79–85. doi: 10.4158/EP.12.S3.79. [DOI] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34(2):256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol. 2002;39(12):1901–1908. doi: 10.1016/s0735-1097(02)01903-4. [DOI] [PubMed] [Google Scholar]
- 13.Clarke WL. The original Clarke Error Grid Analysis (EGA) Diabetes Technol Ther. 2005;7(5):776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 14.Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27(8):1922–1928. doi: 10.2337/diacare.27.8.1922. [DOI] [PubMed] [Google Scholar]
- 15.Pagano M, Gauvreau K. Principle of biostatistics. 2nd ed. Pacific Grove: Duxbury Press; 2000. [Google Scholar]
- 16.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S55–S67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 17.Kondepati VR, Heise HM. Recent progress in analytical instrumentation for glycemic control in diabetic and critically ill patients. Anal Bioanal Chem. 2007;388(3):545–563. doi: 10.1007/s00216-007-1229-8. [DOI] [PubMed] [Google Scholar]
- 18.Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33(12):2079–2084. doi: 10.1007/s00134-007-0835-4. [DOI] [PubMed] [Google Scholar]
- 19.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]


