Abstract
Background
Impaired dexterity has been reported to be prevalent in diabetes patients independent from the existence of diabetic neuropathy. This study was performed to investigate the impact of dexterity impairment on patient preference for two insulin pen injection devices (InnoLet and FlexTouch).
Methods
Ninety patients [54 male/36 female; age (mean ± standard deviation), 62 ± 8 years; disease duration, 18 ± 11 years; hemoglobin A1c, 7.2 ± 1.0%] were included in this investigation and were stratified into four different groups based on the results of a dexterity test (Jebsen–Taylor Hand Function Test) and assessment of visual impairment: 15 type 1 (group A) and 30 type 2 (group B) patients with impaired dexterity, 30 type 1/type 2 patients with visual impairment (group C), and 15 type 1/type 2 patients without any impairment (group D). The patients performed a cognitive function test (number connection test), were introduced to the devices in random order, and were asked to perform some mock injections before completing a six-item standardized preference questionnaire.
Results
There was a strong preference for FlexTouch in all groups. All unimpaired patients (100%, group D) preferred FlexTouch, as did the vast majority in all other groups. Only 11% of the patients with impaired cognitive function preferred InnoLet, as did a few patients with more severely impaired dexterity or with visual impairment (group A, 13%; group B, 3%; group C, 14%).
Conclusions
Patient dexterity skills may have an influence on device preference, especially if the impairment is more pronounced.
Keywords: dexterity, insulin injection device, patient preference
Introduction
Many insulin-treated patients with type 1 or type 2 diabetes mellitus (T1DM or T2DM) have to perform complex diagnostic and therapeutic procedures, e.g., glucose measurements with blood glucose meters for patient self-testing or insulin injections with pen devices, several times per day. The preference for specific devices and the final choice for these diagnostic and therapeutic tools have an influence on the type of insulin or the blood glucose strip brand used for many years until a potential change may happen due to further technological device improvements. Therefore, the manufacturers of blood glucose meters and insulin pen injection devices try to develop products that might be preferred by patients in comparison with competitive devices. Many studies have been published wherein the participants showed a greater preference for the pen device of the study sponsor.1–5 There are some confounding/biasing factors that may have influenced the results of some studies, and it is important to observe the study setup closely. Factors leading patients to prefer an insulin pen in comparison with other pen devices include, but are not limited to, ease of use, reliability and accuracy of dosing, required manual force for a complete injection, ease of dose selection, feedback mechanisms after completion of the injection, maximal single dose, memory function for the last dose, and acoustic or tactile feedback during dose dialing. However, haptic impressions while using the pen as well as the design and appearance of the pen may also influence the choice for a given device.
Many other factors with potential influence on device preference are still unexplored, and it might be helpful for the development engineers of the medicinal device companies to gain an understanding of how patient skills and acute or chronic disease-related conditions may interfere with device preference. It can be expected that patient dexterity skills, visual impairment, or diabetic neuropathy may influence the device selection by the patients with a high probability. However, systematic investigations about these conditions are barely found in the current literature or international research databases.
Little is known about the prevalence of dexterity impair-ment in patients with diabetes. In an earlier study, we investigated the dexterity skills of T1DM and T2DM patients with different age ranges in comparison with a healthy control group and observed impaired dexterity skills in the diabetes patient populations6 by means of validated dexterity tests, such as the Jebsen–Taylor Hand Function Test (JHFT).7–9
In this study, we stratified 90 insulin-treated patients by T1DM or T2DM, and by patients with or without visual impairment with respect to their dexterity skills based on the results of the JHFT and investigated the self-awareness of the defined affected or unaffected groups for their dexterity impairment. In a first analysis, we found that only a third of the patients with significant dexterity problems were aware of their impairments, and existence of this condition was widely underestimated by the study participants.10 Here we report on additional data of the investigation, which was performed to investigate whether impaired dexterity or impaired cognitive function may influence patient preference for one of two pen devices from the same manufacturer (InnoLet® and FlexTouch®, both Novo Nordisk A/S, Søborg, Denmark) at all, and if so, to which degree.
Patients and Methods
The study was conducted in compliance with ethical standards as set forth by the Declaration of Helsinki and by the guidelines of good clinical practice. The project was approved by the responsible ethics committee, and written informed consent was obtained from all participants prior to any study procedure. We enrolled a total of 90 patients (36 females, 54 males) who were stratified into four different groups based on the results of a dexterity test (JHFT, discussed later) and visual assessment by an ophthalmologist: group A, patients with T1DM and dexterity impairment (JHFT), without visual impairment; group B, patients with T2DM and dexterity impairment (JHFT), without visual impairment; group C, patients with T1DM or T2DM and visual impairment (visus < 0.3); and group D, control subjects with diabetes but without dexterity impairment (JHFT) or visual impairment. Further inclusion criteria were hemoglobin A1c < 10% and insulin therapy for at least 1 year. Patients with neuropathy of other origin than diabetes, dementia, M. Parkinson’s or other neurological disorder with influence on dexterity, or patients with drug or alcohol abuse were excluded from study participation.
The primary objective of the study was to collect information regarding patient preference for two pen devices in patients with T1DM and T2DM, with or without visual impairment, as compared with nonimpaired diabetes subjects in relation to their dexterity skills. The secondary objectives were to evaluate the impact of dexterity and cognitive function on potential problems with needle attachment, dose setting, dose delivery, general handling of the devices, and type and number of errors occurring during testing both devices.
After enrollment and dexterity assessment, the patient performed a cognitive function test (number connection test, discussed later) and neuropathy was assessed by determining temperature, pain, and vibration perception thresholds with the Medoc TSA 2001 device (Medoc Advanced Medical Systems, Eilat, Israel). Thereafter, the patients were introduced to the two test devices by a trained interviewer (FlexTouch and InnoLet, both NovoNordisk, Denmark). Patients were randomly allocated to start with either FlexTouch or InnoLet. The patients operated the first device and performed mock injections at different dose settings (10, 30, and 50 U). Subsequently, the patient assessed the handling of the tested device by means of a standardized questionnaire. After completion of the first assessment, the patients performed the same series of procedures with the second device. After individual testing of both pen-devices, the subjects completed a comparative final preference questionnaire.
Standardized Questionnaire
The patient device preference questionnaire contained six questions, which were to be answered by giving a preference for any of the two devices for each individual question. The options provided were “prefer to use,” “easiest to use,” “easiest to learn how to use,” “easiest to teach how to use,” “confidence in correct dose delivery,” and “recommend others to use.”
Number Connection Test (Cognitive Function)
In this test, numbers from 1–25 are arranged in an arbitrary sequence (scattered about) on a paper sheet. The numbers have to be connected with one another as quickly as possible in their correct sequence by using a pencil to draw a line between them, starting with the smallest one. The test is structured in such a way that a healthy individual will always be able to perform this task in less than 30 s.11
Jebsen–Taylor Hand Function Test (Dexterity Assessment)
The JHFT is a widely used assessment of common everyday motor skills.7–9 The test was developed to provide a standardized and objective evaluation of several major aspects of hand function using simulated activities of daily living. It has a good validity and reliability, and normative data are available for different ages and both genders.8 The test consists of seven subtests: writing a sentence, turning over cards, picking up small objects and placing them in a can, picking up small objects with a teaspoon and placing them in a can (simulated feeding), stacking checkers, moving large light cans, and moving heavy cans. Patients were instructed to perform the tasks as rapidly and accurately as possible according to standardized instructions.7–9 Subtest JHFT times were recorded with a stopwatch for analysis. In order to be considered “not impaired” regarding the dexterity skills in this study, a subject had to successfully perform at least four out of the seven subtests within the timeframe defined as “normal” in previous investigations.8
Statistical Analysis
Mean values of normally distributed parameters were compared by means of appropriate parametric tests, e.g., Student’s t-test, analysis of variance. Not-normally distributed parameters were compared by appropriate nonparametrical test methods, e.g., Mann–Whitney U test. A p-value < .05 was considered to be statistically significant and was interpreted in an exploratory sense.
Results
After dexterity assessment by means of the JHFT, the patients were allocated to one of the four patient groups. Demographic data and neuropathy assessment results of the study participants are provided in Table 1. It can be seen that there were no clinically relevant differences between the groups with respect to demographic data, glycemic control, or the results of the neuropathy tests. All patients completed all study procedures and were included in the final analysis.
Table 1.
Demographic Data and Results of the Dexterity and Neuropathy Assessments
| Group A | Group B | Group C | Group D | |
|---|---|---|---|---|
| N | 15 | 30 | 30 | 15 |
| T1DM/T2DM | 15/0 | 0/30 | 7/23 | 4/11 |
| Impairment | Dexterity | Dexterity | Visual | None |
| Gender (male/female) | 10/5 | 14/16 | 19/11 | 11/4 |
| Age (years) | 59.6 ± 8.9 | 61.1 ± 9.5 | 63.7 ± 5.8 | 64.2 ± 5.4 |
| Hemoglobin A1c (%) | 6.9 ± 0.7 | 7.5 ± 1.2 | 7.3 ± 1.0 | 7.0 ± 1.0 |
| Body mass index (kg/m²) | 26.8 ± 4.1 | 36.3 ± 7.4 | 32.5 ± 6.9 | 30.5 ± 5.5 |
| Dexterity sum score | 6.8 ± 3.2 | 6.8 ± 3.2 | 6.5 ± 3.8 | 5.8 ± 3.8 |
| Impaired dexterity (%) | 100 | 100 | 33 | 0 |
| Neuropathy (AU) | ||||
| Sensitivity cold left hand | 29.7 ± 1.4 | 29.1 ± 1.9 | 29.8 ± 1.0 | 29.4 ± 1.5 |
| Sensitivity heat right hand | 34.5 ± 1.0 | 35.2 ± 2.1 | 34.7 ± 1.5 | 34.7 ± 1.0 |
| Pain cold left hand | 5.0 ± 4.7 | 6.8 ± 4.4 | 7.1 ± 5.5 | 5.6 ± 5.5 |
| Pain heat right hand | 46.2 ± 3.1 | 46.2 ± 3.6 | 45.4 ± 3.7 | 47.2 ± 2.7 |
| Vibration right palm of hand | 3.9 ± 4.5 | 5.2 ± 4.9 | 3.5 ± 3.1 | 3.9 ± 1.9 |
Summary results of the JHFT are also shown in Table 1. As to be expected from the inclusion procedures, there were significant differences in several of the JHFT subtests and in the sum score between patient control group D showing a normal sum score and the patients with dexterity or visual impairment. All patients in group A (100%), all patients in group B (100%), 33% in group C, and 0% in group D fulfilled the JHFT criteria defining “impaired dexterity.”
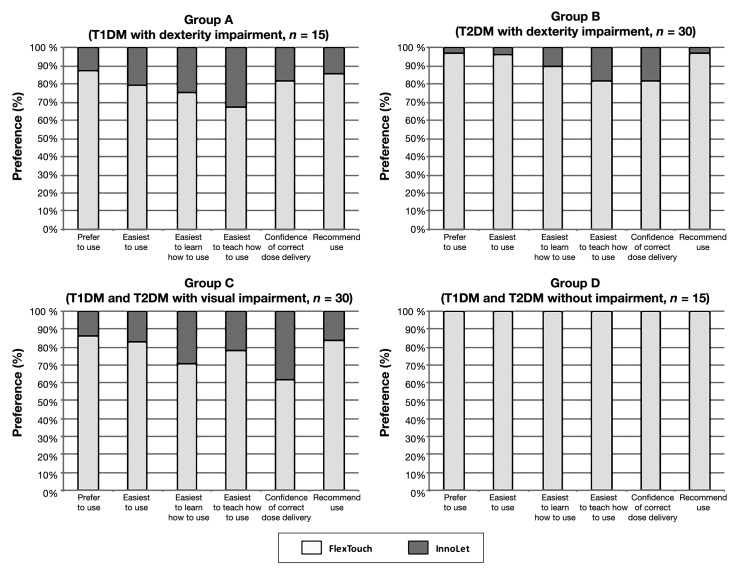
When asked for their preference for either FlexTouch or InnoLet, the patients preferred FlexTouch in all categories of the preference questionnaire. Results of the standardized questionnaire for all four patient groups are provided in Figure 1. It can be seen that InnoLet received only minor acceptance degrees of preference regarding “ease of learning” and “ease of teaching others” in patients with dexterity impairment and visual impairment. Confidence for a correct injection, however, was almost comparable between the two devices in patients with visual impairment (group C).
Figure 1.
Patient preference for FlexTouch or InnoLet with respect to certain device features as given in the patient device preference questionnaire in correlation with the degree of dexterity impairment (all presented differences are statistically significant in favor of FlexTouch).
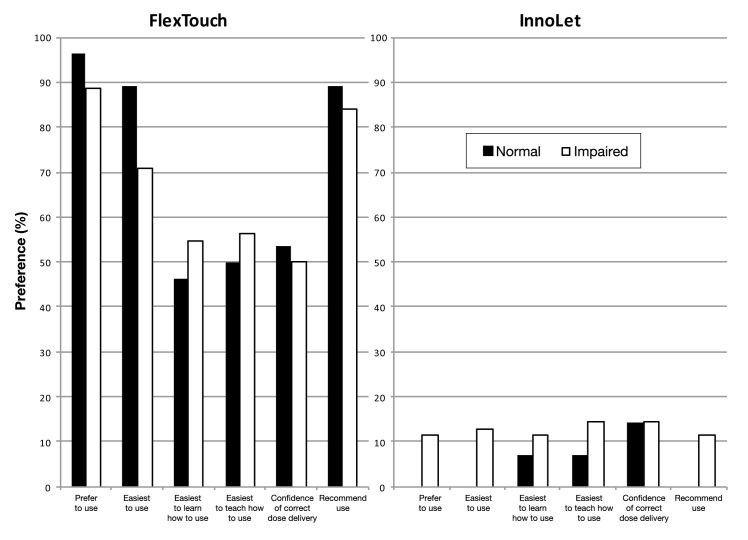
In the cognitive function assessment, all four groups stayed above the normal reference time of 30 s, including the unimpaired patient control group (mean time requirement: group A, 41 ± 20 s; group B, 40 ± 17 s; group C, 38 ± 16 s; group D, 39 ± 18 s; not significant for all group comparisons). In total, 62/90 (68.9%) of all tested patients had impaired cognitive function. When asked for their preference for either device, there was again a general and pronounced preference for FlexTouch in all groups. Although much less preferred than FlexTouch, InnoLet found a certain degree of acceptance, but only in patients with cognitive function impairment as shown in Figure 2. All (100%) of the unimpaired patients and 89% of the patients with impaired cognitive function preferred FlexTouch, while preference for InnoLet was observed only to a minor degree (11%) in patients with impaired cognitive function.
Figure 2.
Patient preference for FlexTouch or InnoLet in relation to the results of the cognitive function test (number connection test). All questions were answered with a statistically significant preference for FlexTouch independent of the cognitive function status. Percentages missing to 100% when adding the percentage responses of both devices represent “no preference for any of the two devices.”
Errors during the mock injections were observed in 32 patients with FlexTouch (35.6%) and 39 patients with InnoLet (43.3%). There were more patients producing errors with InnoLet in groups A (7 versus 13), B (18 versus 20), and C (18 versus 23), while one more patient produced an error in group D with FlexTouch (5 versus 4). Error types observed for injection preparation were “problems while disinfecting (FlexTouch 4 versus InnoLet 6), “problems while attaching a needle” (10 versus 8), and “problems while adapting the insulin dose” (7 versus 4). The number of problems increased when the injection process was started: “problems while injecting the insulin” (4 versus 15), “problems while generally handling the device” (8 versus 14), and “other problems” (15 versus 13). Thus the majority of errors with InnoLet occurred during the injection procedure itself (25.0%) and during general handling of the device (23.3%). Otherwise, no relevant differences with respect to potential errors could be observed between the two devices.
Discussion
A few previous studies in patients with T1DM and T2DM have already indicated that dexterity impairment is a frequent and underestimated phenomenon associated with significantly decreased hand function, limited joint mobility, impaired vibration thresholds, and other indicators of nerve malfunctions.6,12–14 It is of importance that patients with neuropathy and visual impairment have tactual deficits, which may lead to impaired dexterity,15 and there has been increased attention to disabilities affecting the use of insulin pens. First, diabetes technology device assessments with human subjects have included patients with visual impairment and/or dexterity problems.10,13–15 Commentaries are specifically requesting that disabled patients with both dexterity and visual impairment be included in device evaluation and patient preference studies, which would allow the linking of laboratory findings to human factors and provide a better understanding of the factors leading to patient preference for a given device.16
In this study, we tried to determine whether the degree of dexterity impairment and visual impairment would have an influence on injection pen device preference of patients with insulin-treated diabetes mellitus. It can be expected that a device that is easier to operate and that induces a feeling of confidence about an accurate and reliable injection may gain a higher acceptance rate. A confirmation for this hypothesis can be seen in those patient preference trials that compared pen injection devices in previous years with the classical vial-and-syringe application method. In all these studies, the pen devices were preferred to the vial-and-syringe injection systems, especially for reasons of accuracy and convenience.17–20
Clinically, insulin pens show an advantage through improved adherence and reduced hypoglycemic events. Furthermore, overall health care costs were either unchanged or improved in insulin pen users as compared with those using insulin vials and syringes, although little economic advantage was observed when switching from insulin vials to insulin pens.19,20 Patients tend to prefer insulin pen use based on patient satisfaction and ease of use. Through an understanding of the advantages and disadvantages of insulin pens and vials and syringes, physicians, educators, and pharmacists can help to advocate for the most appropriate insulin-delivery method to maximize clinical outcomes and to reduce overall health care spending.18 Improved patient adherence and better economics when using insulin pens versus vial and syringe were also confirmed in a meta-analysis by Asche and colleagues.21 Their analysis indicated that there was an improved adherence with insulin pen devices as opposed to insulin vials (syringes) and that the adherence to therapy and costs were found to decrease with the use of pen devices, compared with vials.20
In our trial, the new disposable FlexTouch device was clearly preferred to InnoLet by all the investigated subgroups of patients with different degrees of visual and dexterity impairment. More than 80% of each subgroup preferred FlexTouch, and even the patient groups with severe handicaps, such as visual impairment, showed a preference for this device in comparison to InnoLet. This is insofar remarkable, as the dose dialing procedure of the InnoLet device has been specifically designed to support patients with impaired visual sense and dexterity problems. In particular, the visually impaired patients were expected to express a higher preference for InnoLet prior to the investigation as compared with the later study result. It is possible that the degree of severity of dexterity and visual impairment in our patient population was not high enough to drive a general preference for InnoLet. This hypothesis could be supported by the finding that a preference for InnoLet was only reported by patients with serious dexterity impairment or eye problems. More important, patients with visual impairment showed a low degree of dexterity impairment and performed well in the majority of our tests, including the number connection test.
Our investigation has limitations with regard to the number of investigated patients in the investigated subgroups (only 15 patients in groups A and D), the selected methods (the JHFT and the number connection test still need to be further validated in diabetes patients), and the definition of the patient cohorts (some based on JHFT and diabetes type, some based on visual acuity) and the “control” group. However, all results confirm that impaired dexterity may be a highly prevalent problem and an entirely underestimated confounding factor influencing the capability of patients to operate medicinal devices and thus influencing their choice for the preferred device.
In conclusion, our study confirms that insulin-treated patients with both types of diabetes mellitus and with different dexterity skills showed a very high preference for the FlexTouch device in all groups in comparison with InnoLet. If at all, InnoLet was preferred by a few patients with serious visual and/or dexterity impairment. The device features of FlexTouch seem to overcome or mitigate previous difficulties expressed by impaired patients who used insulin injection devices.
Glossary
- (JHFT)
Jebsen–Taylor Hand Function Test
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
Funding
This work was funded by NovoNordisk.
Disclosures
Andreas Pfützner and Thomas Forst have received research grants, speaker fees, and travel support from Novo Nordisk. Marcus Niemeyer and Marianne Qvist are employees of Novo Nordisk.
References
- 1.Asakura T, Jensen KH. Comparison of intuitiveness, ease of use, and preference in two insulin pens. J Diabetes Sci Technol. 2009;3(2):312–319. doi: 10.1177/193229680900300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ignaut DA, Schwartz SL, Sarwat S, Murphy HL. Comparative device assessments: Humalog KwikPen compared with vial and syringe and FlexPen. Diabetes Educ. 2009;35(5):789–798. doi: 10.1177/0145721709340056. [DOI] [PubMed] [Google Scholar]
- 3.Clark PE, Valentine V, Bodie JN, Sarwat S. Ease of use and patient preference injection simulation study comparing two prefilled insulin pens. Curr Med Res Opin. 2010;26(7):1745–1753. doi: 10.1185/03007995.2010.489028. [DOI] [PubMed] [Google Scholar]
- 4.Pfützner A. FlexPen for the delivery of insulin: accuracy, injection force and patient preference. Expert Rev Med Devices. 2009;6(2):115–123. doi: 10.1586/17434440.6.2.115. [DOI] [PubMed] [Google Scholar]
- 5.Bailey T, Thurman J, Niemeyer M, Schmeisl G. Usability and preference evaluation of a prefilled insulin pen with a novel injection mechanism by people with diabetes and healthcare professionals. Curr Med Res Opin. 2011;27(10):2043–2052. doi: 10.1185/03007995.2011.616190. [DOI] [PubMed] [Google Scholar]
- 6.Pfützner J, Hellhammer J, Musholt PB, Pfützner AH, Böhnke J, Hero T, Amann-Zalan I, Ganz M, Forst T, Pfützner A. Evaluation of dexterity, in patients with insulin-treated type 1 and type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(1):158–165. doi: 10.1177/193229681100500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardised test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 8.Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Phys Ther. 1992;72(5):373–377. doi: 10.1093/ptj/72.5.373. [DOI] [PubMed] [Google Scholar]
- 9.Bovend’Eerdt TJ, Dawes H, Johansen-Berg H, Wade DT. Evaluation of the Modified Jebsen Test of Hand Function and the University of Maryland Arm Questionnaire for Stroke. Clin Rehabil. 2004;18(2):195–202. doi: 10.1191/0269215504cr722oa. [DOI] [PubMed] [Google Scholar]
- 10.Pfützner A, Musholt PB, Schipper C, Niemeyer M, Qvist M, Schorsch A, Forst T. Self-assessment and objective determination of dexterity in patients with type 1 or type 2 diabetes mellitus. Curr Med Res Opin. 2012;28(1):15–21. doi: 10.1185/03007995.2011.638911. [DOI] [PubMed] [Google Scholar]
- 11.Oswald WD, Roth E. Handanweisung. 2nd ed. Goettingen: Hogrefe; 1987. Der Zahlen-Verbindungs-Test (ZVT) (number connection test) [Google Scholar]
- 12.Casanova JE, Casanova JS, Young MJ. Hand function in patients with diabetes mellitus. South Med J. 1991;84(9):1111–1113. doi: 10.1097/00007611-199109000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Cederlund RI, Thomsen N, Thrainsdottir S, Eriksson KF, Sundkvist G, Dahlin LB. Hand disorders, hand function, and activities of daily living in elderly men with type 2 diabetes. J Diabetes Complications. 2009;23(1):32–39. doi: 10.1016/j.jdiacomp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Dahlin LB, Thrainsdottir S, Cederlund R, Thomsen NO, Eriksson KF, Rosén I, Speidel T, Sundqvist G. Vibrotactile sense in median and ulnar nerve innervated fingers of men with Type 2 diabetes, normal or impaired glucose tolerance. Diabet Med. 2008;25(5):543–549. doi: 10.1111/j.1464-5491.2008.02433.x. [DOI] [PubMed] [Google Scholar]
- 15.Travieso D, Lederman SJ. Assessing subclinical tactual deficits in the hand function of diabetic blind persons at risk for peripheral neuropathy. Arch Phys Med Rehabil. 2007;88(12):1662–1672. doi: 10.1016/j.apmr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Williams AS. Analysis: Linking laboratory data to human factors and inclusion of persons with disabilities in diabetes technology research. J Diabetes Sci Technol. 2011;5(5):1191–1194. doi: 10.1177/193229681100500524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buysman E, Conner C, Aagren M, Bouchard J, Liu F. Adherence and persistence to a regimen of basal insulin in a pre-filled pen compared to vial/syringe in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin. 2011;27(9):1709–1717. doi: 10.1185/03007995.2011.598500. [DOI] [PubMed] [Google Scholar]
- 18.Bastian MD, Wolters NE, Bright DR. Insulin pens vs. vials and syringes: differences in clinical and economic outcomes. Consult Pharm. 2011;26(6):426–429. doi: 10.4140/TCP.n.2011.426. [DOI] [PubMed] [Google Scholar]
- 19.Molife C, Lee LJ, Shi L, Sawhney M, Lenox SM. Assessment of patient-reported outcomes of insulin pen devices versus conventional vial and syringe. Diabetes Technol Ther. 2009;11(8):529–538. doi: 10.1089/dia.2009.0007. [DOI] [PubMed] [Google Scholar]
- 20.Ignaut DA, Schwartz SL, Sarwat S, Murphy HL. Comparative device assessments: Humalog KwikPen compared with vial and syringe and FlexPen. Diabetes Educ. 2009;35(5):789–798. doi: 10.1177/0145721709340056. [DOI] [PubMed] [Google Scholar]
- 21.Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: a review of the evidence. Diabetes Technol Ther. 2010;12(Suppl 1):S101–S108. doi: 10.1089/dia.2009.0180. [DOI] [PubMed] [Google Scholar]