Abstract
Background
The clinical significance of blood glucose meter (BGM) error in the presence of increasing carbohydrate errors in diabetes patients who use both the BGM result and the carbohydrate estimation to dose insulin is unknown.
Methods
This Monte Carlo simulation modeled diabetes patients who calculate insulin dosages based on BGM results and carbohydrate estimations. It evaluated the likelihood of on-target insulin dosing and clinically significant insulin dose errors based on data from five BGMs with different levels of performance (expressed as bias and imprecision [coefficient of variation (%CV)]) and increasing levels of carbohydrate estimation errors. The study was performed across three separate preprandial glucose (PPG) ranges (90–150, 150–270, and 270–450 mg/dl).
Results
When carbohydrate estimation is accurate (%CV = 0%), the likelihood for on-target insulin doses ranged 50.1–98.5%. The likelihood depended on BGM performance and PPG range. In the presence of carbohydrate estimation errors (%CV = 5–20%), the likelihood of on-target insulin dosages markedly decreased (range, 27.2–80.1%) for all BGMs, the likelihood of insulin underdosing (range, 0–12.8%) and overdosing (range, 0–32.3%) increased, and the influence of BGM error on insulin dosing accuracy was blunted. Even in the presence of carbohydrate error, the BGM with the best performance (bias 1.35% and %CV = 4.84) had the highest probability for on-target insulin dosages.
Conclusions
Both BGM and carbohydrate estimation error contribute to insulin dosing inaccuracies. The BGM with the best performance was associated with the most on-target insulin dosages.
Keywords: blood glucose monitoring, carbohydrate counting, in silico modeling, postprandial glucose, preprandial glucose
Introduction
The American Diabetes Association recommends that self-monitoring of blood glucose (SMBG) be carried out three or more times daily for patients using multiple insulin injections or pump therapy to help optimize glycemic control.1 Many of these patients calculate their preprandial insulin doses based on SMBG measurements and estimates of meal carbohydrate content. If successful, these insulin adjustments will lead to a hemoglobin A1c level of ≤7%, minimized weight gain, and minimized hyperglycemia and hypoglycemia.2 Insulin dose calcula-tions must be tailored to individual needs because there is large interindividual variation.3 The two main causes of insulin dosage errors are inaccurate carbohydrate counting and glucose meter analytical error.4
Several studies have assessed the ability of people to estimate carbohydrate meal content accurately. In one study, only 32% of subjects could correctly calculate the total amount of carbohydrate in a multiserving soda label; most subjects failed to account for multiple servings per container or made calculation errors.5 Graff and colleagues6 found that patients overestimated carbohydrate content at breakfast, on average, by +8.5% (range, -93% to +100%) and underestimated carbohydrate content at lunch, on average, by -28% (range, -97% to +43%). Kildegaard and associates4 observed an average intra-individual variation of 30% for estimates of carbohydrate meal content.4 Methods for better estimating carbohydrate and improving insulin dosages are under investigation.7
Establishing analytical performance of blood glucose meters (BGMs) is the subject of regulatory and clinical debate.8 The current International Organization for Standardization (ISO) performance standard ISO 15197 for BGMs is 95% of test results be within ±15 mg/dl (when the blood glucose concentration is <75 mg/dl) or ±20% (when the blood glucose concentration is ≥75 mg/dl) of a reference glucose result.9 The next revision of the ISO 15197 criteria is expected to establish tighter meter performance criteria (proposal as of December 31, 2011, is ±15% instead of ±20%), but the clinical basis for any specific level of meter performance is not clear.
LifeScan is distributing a new BGM (OneTouch® Verio™IQ, LifeScan Europe, Division of Cilag GmbH International, Zug, Switzerland). A compilation of internal studies on the OneTouch Verio platform (this includes OneTouch VerioIQ, OneTouch VerioPro [available in certain European countries], and two experimental meters not yet approved for use) found an overall bias of -1.35%, a coefficient of variation (%CV) of 4.84%, and ≥95% of the results within ±10% of a reference glucose result. Monte Carlo modeling was used to compare this level of BGM performance (i.e., ±10% total error) to BGMs that have ±20% error and ±15% total error. The model (where absolute differences for glucose error <75 mg/dl were not applied) combined various levels of BGM performance, preprandial glucose (PPG), and carbohydrate estimation error in order to predict the likelihood of either on-target insulin dosing, insulin overdosing, or insulin underdosing.
We hypothesize that both BGM and carbohydrate estima-tion error can, when considered separately or together, influence the probability of insulin dosing accuracy.
Methods
This Monte Carlo analysis was performed to evaluate the expected likelihood of insulin dosing errors in the presence of BGM and carbohydrate estimation inaccuracies. Simulated patient data were used to compare the performance of five BGMs with different performances as outlined in Table 1. All calculations were performed using the TIBCO Spotfire® S+ 8.1 statistical software package for Windows (TIBCO Software Inc., Somerville, MA).
Table 1.
Listing of Blood Glucose Meter Performances Used in the Monte Carlo Model
| % Within | ||||||
|---|---|---|---|---|---|---|
| BGM | Bias, % | %CV | ±5% | ±10% | ±15% | ±20% |
| BGM 1a | -1.35 | 4.84 | 67.99 | 95.35 | 99.72 | 99.99 |
| BGM 2 | -2.0 | 6 | 56.98 | 88.60 | 98.26 | 99.85 |
| BGM 3 | +2.0 | 6 | 56.98 | 88.60 | 98.26 | 99.85 |
| BGM 4 | -4.0 | 7 | 45.75 | 78.16 | 93.86 | 98.86 |
| BGM 5 | +4.0 | 7 | 45.75 | 78.16 | 93.86 | 98.86 |
OneTouch VerioIQ, LifeScan Inc., Milpitas, CA
Blood glucose meter accuracy can be described by two components: bias (how close the average result is to the actual result) and imprecision (how scattered the individual results are around the average). The bias is represented as a percentage of the mean at each reference glucose level. Imprecision is represented as %CV (standard deviation [SD]/mean). The larger the %CV, the more scattered the results are around the average blood glucose result.
Blood glucose meter 1 refers to the new LifeScan meter as described in the previous section (i.e., ±10% total error). Blood glucose meters 2 and 3 represent meters that have ≥95% of results within ±15% of the reference value. Blood glucose meters 4 and 5 represent meters that have ≥95% of results within ±20% of the reference value—the current ISO 15197 standard for performance (Table 1). The performances of BGMs 2–5 are hypothetical and are based on the variation of bias and %CV of BGMs described in the study by Freckmann and coworkers.10 All BGMs met the current ISO 15197 standard. It was assumed that the bias and %CV are relatively stable across the blood glucose range 90–450 mg/dl. This is generally true for BGM 1; however, not all BGMs have a relatively consistent bias and %CV profile.
The simulation model represents the scenario in which a person, about to consume a meal, takes an insulin dose via insulin pen (to minimize the contribution of inaccurate insulin dosing) based on the PPG reading and the estimated carbohydrate content of a meal. The true blood glucose concentration will range from 90 to 450 mg/dl (uniformly distributed). The true carbohydrate content of the meal will range from 30 to 100 g (uniform distribution).
Both BGM readings and carbohydrate estimates incorporate some level of error. The BGM accuracy profile is provided in Table 1. With regards to carbohydrate estimation error, it was assumed that patients can compensate for consistent overestimates and underestimates (bias). Therefore, this model only includes the imprecision of carbohydrate estimation represented as %CV.
Few studies have measured carbohydrate error. As noted earlier, Kildegaard and associates4 did measure intra-individual variation in carbohydrate estimation, but they did not clearly state whether the variation observed represented %CV, SD, or some other measure of variation. Due to the poor ability of patients to accurately estimate carbohydrate content, a wide imprecision range was incorporated in the final simulation. The carbohydrate estimation %CVs modeled are 0% (no error), 5%, 10%, and 20%. A %CV of 20% means that 95% of carbohydrate estimates are within 40% of the average estimate. To simplify the model, only carbohydrate imprecision was included.
The person being simulated has type 1 diabetes with an average sensitivity to insulin. The person will calculate an insulin dose using a correction factor of 40 mg/dl per unit of insulin (IU), an insulin-to-carbohydrate ratio (I:C) of 1 IU insulin:10 g, and a target glucose of 100 mg/dl. The following formula will be used to calculate the insulin dose: insulin dose = (premeal blood glucose – target blood glucose)/correction factor + (carbohydrate/I:C).
The insulin dose is rounded to the nearest 0.5 IU, which is a typical level of precision on insulin pens. An optimal insulin dose is also calculated using the actual blood glucose and carbohydrate content values; the optimal value is not rounded.
The risks of hypoglycemia or hyperglycemia were considered as possible outcomes of interest; however, many additional factors can influence postprandial blood glucose, such as accuracy of the insulin dose, changes in insulin sensitivity, and variation in absorption of carbohydrates and insulin. Therefore, the primary outcome of interest in this study is the proportion of insulin doses within 0.5 IU of the optimal dose. Secondary outcomes are the proportion of insulin overdoses exceeding the optimal insulin dose by ≥1 IU and the proportion of insulin underdoses of >2 IU compared with the optimal insulin dose.
The thresholds for overdose and underdose are based on the following rationale. Assuming that no other factors are involved, an insulin overdose of ≥1 IU would result in a postprandial blood glucose concentration of <70 mg/dl. That is >30 mg/dl below the target of 100 mg/dl, which may be regarded as hypoglycemia. Similarly an underdose of ≥2 IU would result in a postprandial blood glucose concentration of ≥180 mg/dl, which may be regarded as hyperglycemia.
The performance of the BGMs was compared across the glucose range (90–450 mg/dl) and within each of the following discrete ranges: 90–150, 150–270, and 270–450 mg/dl. The probability estimates (%) are based on 50,000 simulated patients in each blood glucose range. Based on this sample size, the standard error of an individual probability estimate is 0.22%. Differences greater than ±0.63% are considered statistically significant (95% confidence).
Results
Table 2 summarizes the likelihood of on-target insulin dosages (i.e., within ±0.5 IU of optimal insulin dose) using BGMs 1–5 when carbohydrate estimation was ideal (i.e., %CV = 0%). The likelihood for on-target insulin dosages ranged from 50.1–98.5%, depending on meter performance and the PPG range. Across the entire glycemic range (90–450 mg/dl), insulin doses from BGM 1 were on target 81.8% of the time versus 64.3–74.1% for BGMs 2–5.
Table 2.
Percentage Likelihood of On-Target Insulin Dosages Based on Blood Glucose Meter Error but no Carbohydrate Error
| Glucose, mg/dl | ||||
|---|---|---|---|---|
| BGM | 90–150 | 150–270 | 270–450 | 90–450 |
| BGM 1 | 98.5a | 89.8a | 71.0a | 81.8a |
| BGM 2 | 96.2 | 83.1 | 60.9 | 74.1 |
| BGM 3 | 96.3 | 82.9 | 61.0 | 74.1 |
| BGM 4 | 91.9 | 73.4 | 50.1 | 64.6 |
| BGM 5 | 92.2 | 73.6 | 50.2 | 64.3 |
Differences between BGM 1 and BGMs 2–5 are statistically significant (p < .05).
Figure 1A shows the probability of on-target insulin dosages (range, 27.2–98.5%) in the presence of various carbohydrate estimation errors (%CV = 0–20%). Over the entire glucose range (90–450 mg/dl), BGM 1 had the highest frequency of on-target insulin doses (range, 31.0–81.8%) compared with BGMs 2–5 (range, 29.2–74.1%). At 5% carbohydrate error, BGM 1 was on-target 68.4% of the time (versus 56.7–63.6% for BGMs 2–5). As carbohydrate error increased, the probability of on-target dosages decreased. At 10% carbohydrate error, the likelihood of being on target decreased to 51.0% for BGM 1 and 44.5–48.0% for BGMs 2–5. When carbohydrate estimation error was high (%CV = 20%), the likelihood of being on target decreased to 31.0% for BGM 1 compared with 29.2–30.4% for BGMs 2–5.
Figure 1.
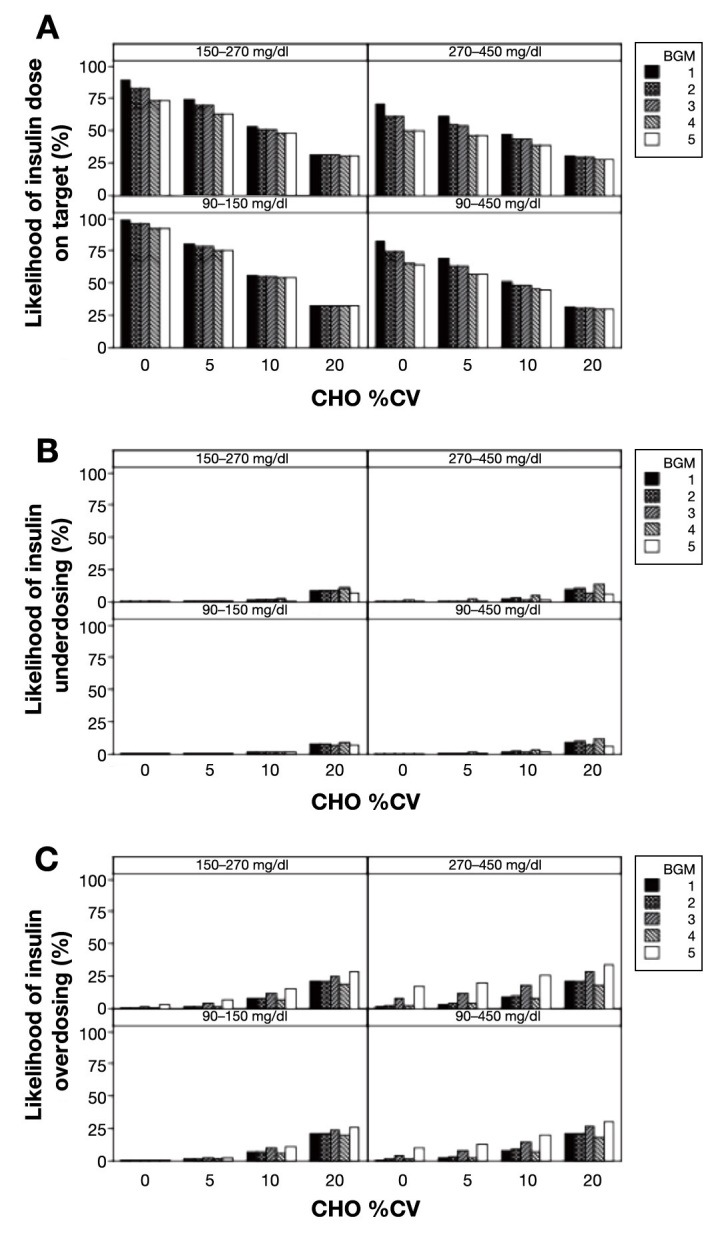
(A) Percentage likelihood of on-target insulin dosages with increasing BGM error and carbohydrate imprecision. (B) Percentage likelihood of insulin underdosing with increasing BGM error and carbohydrate imprecision. (C) Percentage likelihood of insulin overdosing with increasing BGM error and carbohydrate imprecision. CHO, carbohydrate.
Figure 1B shows that the probability of insulin under-dose was relatively low (range, 0–12.8%) but that the likelihood increased slightly when carbohydrate estimation error increased (i.e., %CV = 5–20%). Over the entire glucose range (90–450 mg/dl), at 5% carbohydrate error, BGM 1 (0%) was similar to all other meters (0–0.8%) for likelihood of insulin underdose. BGM 4 had the highest rate of underdosing in the presence of carbohydrate error. The frequency of underdosing increased as carbohydrate error increased as well as the PPG increased. At 20% carbohydrate error across the entire glucose range (90–450 mg/dl), the likelihood of insulin underdose increased to 8.0% for BGM 1, which was similar to BGM 2 (9.0%), higher than BGM 3 (6.5%) and BGM 5 (5.8%), and lower compared with BGM 4 (10.7%).
Figure 1C shows that the probability of insulin overdose ranged from 0–32.3% and that the likelihood generally increased for most meters when carbohydrate estimation error increased (%CV = 5–20%). At 5% carbohydrate error for the overall glucose range (90–450 mg/dl), BGM 1 (2.0%) was similar to BGM 2 (2.5%) and BGM 4 (2.2%) but different from BGM 3 (6.8%) and BGM 5 (12.0%). At 20% carbohydrate error, the likelihood of insulin overdose for BGM 1 increased to 19.9%, which was similar to BGM 2 (19.6%), slightly greater than BGM 4 (17.4%), and less than BGM 3 (25.3%) and BGM 5 (28.9%).
Figure 1 also illustrates that insulin dosing accuracy depends on the PPG level, such that, as PPG increases, the likelihood of on-target insulin dosages decreases for all meters. When carbohydrate %CV was 0%, BGM 1 was associated with higher rates of on-target insulin (Figure 1A) compared with all other meters at 90– 150 mg/dl (98.5% versus range, 91.9–96.2%), 150–270 mg/dl (89.8% versus range, 73.4–83.1%), and 270–450 mg/dl (71.0% versus range, 50.1–61.0%). When the carbohydrate %CV was 20%, the frequency of on-target insulin results decreased for all meters and, for PPG 90–450 mg/dl, BGM 1 had a slightly higher probability for on-target values (31.0%) compared with all other meters (range, 29.2–30.4%). The frequency of underdosing and over-dosing was also influenced by PPG.
Discussion
This in silico model demonstrates the impact of BGM performance in terms of bias (inaccuracy) and %CV (imprecision) on insulin dosing errors in the presence and absence of carbohydrate estimation errors. Boyd and Bruns11 found that meter bias and %CV of ≤1% is required for >95% on-target insulin dosages. This level of performance is unrealistic compared with the current ISO 15197 performance criteria or with the proposed revised criteria. In fact, this level of performance exceeds that of most laboratory diagnostic methods.12 In contrast, this Monte Carlo model compared an array of available BGMs—including a newly available model—and incorporates carbohydrate estimation error.
According to the modeling results, BGM performance, PPG, and carbohydrate estimation errors contribute to the likelihood of insulin dosing errors and, therefore, may also contribute to the risk for hypoglycemia and hyperglycemia. Table 2 shows that BGM 1 (meter bias -1.35%, %CV 4.84%; approximately ±10% total error) is superior to the other meters because it had a significantly higher likelihood of on-target insulin dosages.
When carbohydrate estimation is accurate (%CV = 0%), the likelihood for on-target insulin dosages (range, 50.1–98.5%) was highly dependent on performance and the PPG range. However, even accurate and precise BGM performance cannot overcome large carbohydrate estimation errors. In the presence of %CV of 5–20% carbohydrate estimation errors, the likelihood of on-target insulin dosages markedly decreased (range, 27.2–80.1%). Although the model supports the notion that BGM performance contributes to erroneous insulin dose calculations, it also demonstrates the clinical importance of accurate carbohydrate estimation when calculating insulin dosages. This was expected, as an insulin dose error of 1 IU required the BGM reading to be inaccurate by 40 mg/dl (based on the correction factor of 40 mg/dl/IU), whereas the carbohydrate estimate had to be inaccurate by 10 g (based on the I:C of 1 IU:10 g carbohydrate).
The likelihood of insulin underdosing of at least 2 IU was relatively small and mostly independent of meter performance across the range of carbohydrate estimation errors. It is only when carbohydrate estimations equal 20% that meter performance is an important factor. The positively biased (bias +4%) BGM 5 was somewhat protective (likelihood range, 5.6–6.0%) because it showed the lowest likelihood for insulin underdosing compared with all other meters.
The probabilities for insulin overdosing were larger than for insulin underdosing across the range of carbohydrate estimation errors compared with the insulin underdosing results. A negative bias was protective against insulin overdosing. For example, BGM 4, which was negatively biased (bias -4%), had the least likelihood of an insulin overdose even when the carbohydrate estimation error was high (%CV = 20%). However, when carbohydrate estimation errors were at 20%, likelihood for insulin overdosing did not exceed 32.3% for any BGM. The impact of BGM performance, although muted, was still important as the range in the frequency of insulin overdosing was quite large (16.9–32.3% in the PPG range 270–450 mg/dl).
This study analyzed the importance of meter performance at different PPG ranges. Although BGM and carbohydrate estimation accuracy is expressed in relative terms as a percentage, the likelihood of an insulin dose error depends on the absolute BGM error. For example, a +20% BGM error at 100 mg/dl (BGM would read 120 mg/dl) would lead to a 0.5 IU overdose. However, at 300 mg/dl, the BGM error (BGM would read 360 mg/dl) would lead to a 1.5 IU overdose.
Although some of the differences in likelihood rates in this study appear small (e.g., <5% difference), a person on multiple daily insulin injections or an insulin pump typically calculates >1000 insulin doses per year. For every 1000 calculations per year, each 1% increase in error rate might result in 10 additional hypoglycemic or hyper-glycemic events. Thus, as both blood glucose performance and carbohydrate estimation improve, associated clinical benefits would be expected for susceptible subpopulations.
Limitations of this study are that the results are based on a simulation, not on actual patient data, and that the bias and imprecision for BGMs 2–5 were estimated from a single evaluation of BGMs. Additionally, the simulation only included BGM and carbohydrate estimation errors. For this reason, the simulation focused on insulin dosing accuracy rather than outcomes such as hypoglycemia or hyperglycemia, which can be affected by changes in insulin sensitivity (related to exercise, stress, or menstruation). Other sources of error (pre- and post-analytical) and inaccuracies in insulin dose delivery (unrelated to the dosage calculation), which may also lead to significant glycemic variability, were not considered. This simulation also assumed that BGM errors were normally distributed across their dynamic glucose ranges, which may not be true for some BGMs, and modeled using a single PPG measurement. However, the assumptions seem reasonable and valid for the majority of diabetes management scenarios.
This study was limited to exploring the impact of BGM performance on accuracy of insulin dosing. Patients also use BGM for reasons other than calculating insulin doses, such as hypoglycemia detection. In these circumstances, it is also important that a patient tests with a more accurate BGM. Breton and Kovatchev13 determined through another simulation study that, as accuracy diminished, the BGM was more likely to miss hypoglycemia.
In conclusion, this in silico study predicts the likelihood of insulin dosage errors associated with BGM and carbohydrate estimation errors. Blood glucose meter performance is important and its effect is most pronounced when carbohydrate estimation is accurate.
Acknowledgments
The authors thank S. Nandagopalan, Ph.D., for his statistical analysis and for manuscript review. The authors also received editorial and writing support from Excerpta Medica.
Glossary
- (%CV)
coefficient of variation
- (BGM)
blood glucose meter
- (I:C)
insulin-to-carbohydrate ratio
- (ISO)
International Organization for Standardization
- (IU)
unit of insulin
- (PPG)
preprandial glucose
- (SD)
standard deviation
- (SMBG)
self-monitoring of blood glucose
Funding
This work was funded by LifeScan Inc.
Disclosures
All authors are employees of LifeScan Inc. (a Johnson & Johnson company) and stockholders of Johnson & Johnson.
References
- 1.American Diabetes Association Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289(17):2254–2264. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- 3.Mooradian AD, Bernbaum M, Albert SG. Narrative review: a rational approach to starting insulin therapy. Ann Intern Med. 2006;145(2):125–134. doi: 10.7326/0003-4819-145-2-200607180-00010. [DOI] [PubMed] [Google Scholar]
- 4.Kildegaard J, Randløv J, Poulsen JU, Hejlesen OK. The impact of non-model-related variability on blood glucose prediction. Diabetes Technol Ther. 2007;9(4):363–371. doi: 10.1089/dia.2006.0039. [DOI] [PubMed] [Google Scholar]
- 5.Rothman RL, Housam R, Weiss H, Davis D, Gregory R, Gebretsadik T, Shintani A, Elasy TA. Patient understanding of food labels: the role of literacy and numeracy. Am J Prev Med. 2006;31(5):391–398. doi: 10.1016/j.amepre.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Graff MR, Gross TM, Juth SE, Charlson J. How well are individuals on intensive insulin therapy counting carbohydrates? Diabetes Res Clin Pract. 2000;50(Suppl 1):S238–S239. [Google Scholar]
- 7.Bao J, Gilbertson HR, Gray R, Munns D, Howard G, Petocz P, Colagiuri S, Brand-Miller JC. Improving the estimation of mealtime insulin dose in adults with type 1 diabetes: the Normal Insulin Demand for Dose Adjustment (NIDDA) study. Diabetes Care. 2011;34(10):2146–2151. doi: 10.2337/dc11-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klonoff DC. Regulatory controversies surround blood glucose monitoring devices. J Diabetes Sci Technol. 2010;4(2):231–235. doi: 10.1177/193229681000400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Organization for Standardization In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197:2003. http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=26309. Accessed December 22, 2011.
- 10.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, Heister F, Haug C. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;12(3):221–231. doi: 10.1089/dia.2009.0128. [DOI] [PubMed] [Google Scholar]
- 11.Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem. 2001;47(2):209–214. [PubMed] [Google Scholar]
- 12.Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Ehlers GW, Hassemer D, Lo SF, Seccombe D, Siekmann L, Thienpont LM, Toth A. State of the art in trueness and inter-laboratory harmonization for 10 analytes in general clinical chemistry. Arch Pathol Lab Med. 2008;132(5):838–846. doi: 10.5858/2008-132-838-SOTAIT. [DOI] [PubMed] [Google Scholar]
- 13.Breton MD, Kovatchev BP. Impact of blood glucose self-monitoring errors on glucose variability, risk for hypoglycemia, and average glucose control in type 1 diabetes: an in silico study. J Diabetes Sci Technol. 2010;4(3):562–570. doi: 10.1177/193229681000400309. [DOI] [PMC free article] [PubMed] [Google Scholar]


