Abstract
Since the first successful use of insulin in 1921 to treat diabetes at Toronto General Hospital, the major advances in development of the medication itself have taken place in parallel with equally significant developments in the means of delivery. Administration of insulin remains parenteral. This article reviews the main variants in prescription-available delivery technology: vial and syringe, pen injector, needle-free injection, and continuous subcutaneous insulin infusion pumps. For each of these, the background and major milestones are covered briefly and followed by a discussion of the latest product innovations, technologies, and implementations, which are all considered in the context of the interaction with users. The article concludes by reflecting upon how the progress in the technology of diabetes management can best serve the patient. The spectacular technological advances in medication, monitoring, and delivery since 1922 have transformed the lives of millions. However, the fact that we can add sophisticated technology to delivery devices and accessories does not mean it is always the best thing for the patient. Electronic sophistication may be welcomed by a young, eager type 1 diabetes patient, while a senior citizen who discovers he has type 2 diabetes may yearn for simplicity. Technology continues to provide great solutions, but the type of solution delivered must be matched to the user if the maximum benefit is to be achieved for all.
Keywords: continuous subcutaneous insulin infusion pump, insulin pen, needle-free injector, patch pump, syringe
Background
In 2012, insulin’s 90th anniversary year, the story of Leonard Thompson’s rescue from a painful and distressing death will be retold many times in the popular and professional media. The Internet is remarkable for, among other things, providing multiple, unverified versions of history, and this story, one of the most dramatic breakthroughs in medicine, is no exception. Therefore, the following abbreviated account has been taken from a 1983 copy of “The Discovery of Insulin,”1 by Michael Bliss, Professor of Canadian History at the University of Toronto.
On December 2, 1921, 14-year-old Leonard Thompson, diagnosed with diabetes in 1919, was admitted to Toronto General Hospital: frail, grossly underweight, listless, and losing his hair. A medical student at the time recalled, “We all knew he was doomed.” On January 23, 1922, Dr. Walter Campbell, founder of the hospital diabetes clinic, injected a refined extract from a beef pancreas into Leonard’s buttocks: 5ml at 11:00 AM, 20 ml at 5PM, and two further 10 ml injections the next day. Leonard’s glycosuria almost disappeared; his blood sugar levels dropped dramatically and he became brighter, looked better, and felt stronger. Insulin had arrived and diabetes was no longer an inescapable death sentence.
Delivery Devices and Systems
As with any medication that cannot be administered orally, the means of administration—the delivery device—is the sine qua non. Despite improvements in purity and insulin concentration, early injections were intramuscular, twice-daily, and with injected volumes between 5 and 18 ml to already emaciated patients. Today we may regard this as eye-watering; however, given the alternative of a slow and painful death, discomfort during administration was probably regarded as a minor concern. Banting, Campbell, and other insulin pioneers used traditional, reusable glass-bodied syringes. Within a year, however, the first purpose-designed, reusable glass and metal insulin syringes appeared; an example is shown as Figure 1 (Eli Lilly and Company, Indianapolis, IN). Just as the development of diabetes medication since 1922 has not stood still, development of delivery devices and systems has continued in parallel. Innovation and evolution of delivery devices—from “basic” syringes to the most sophisticated automated devices—have been and will continue to be affected by developments across a range of fields. These include the “pull” of biopharmaceuticals and regenerative medicine, human factors engineering, and health economics and the enabling (“push”) from materials and manufacturing, pumps and fluidics, sensors and displays, microelectronics and batteries, and information and communications technologies. Above all, the objective of insulin delivery technology development continues to be that of improving therapy by reducing demands upon the patient.
Figure 1.

Early 1920s insulin syringe kit. Photo courtesy of Eli Lilly and Company archives.
Syringes
The first disposable glass syringe, the Hypak™ (Becton, Dickinson and Co. Franklin Lakes, NJ), was introduced in 1954 for use in polio vaccination but also found use in insulin delivery. The all-plastic Monoject™ syringe (Roehr Products Inc., Waterbury, CT) appeared in 1955, and by the mid-1960s, disposable plastic syringes from several manufacturers were in widespread use. Fast-forward to today’s purpose-designed plastic syringes for U100 insulin, marked in IUs and in 30, 50, and 100 U sizes (0.3, 0.5, and 1.0 ml), and the lineage is easy to trace. While improvements in medication, blood glucose meters, and hypodermic needle technologies all enabled improvements in diabetes treatment, “traditional” syringe delivery technology per se has not changed significantly since the last quarter of the 20th century. Therefore, this article will not comment further on syringe use, except that “vial and syringe” remains the most common means of insulin administration in the United States (approximately 75% of all insulin use in the United States in 20102,3).
Injector Pens
The launch of NovoPen® (Figure 2; NovoNordisk A/S, Bagsvaerd, Denmark) in 1985 established a new template in diabetes treatment. A compact, convenient, and discreet alternative to the vial and syringe, the NovoPen had a cartridge format insulin primary container, a short needle, and incremental “one click per unit” dosing. This was shortly followed by the NovoPen2®, incorporating dial-up setting of the full required dose. Similar fundamental features, together with finer, sharper, lubricated needles, are exhibited on the wide range of pen injectors now available in most developed markets. By 2008, pens were used by 88% of people with diabetes in Europe, 95% of those in Japan, yet only by 17% of users in the United States.2,3 Anecdotally, pen use may have increased to as much as 30% of the U.S. diabetes market; however, limited adoption of pens is related not just to clinical practice, but also to insurance plans not paying for pens. A “dial-up, dial-down, press-to-deliver” sequence of pen use has emerged as a de facto standard operating procedure; numerous interviews with patients across a range of ages, including those with type 1 and type 2 diabetes, suggest that alternative operating sequences have not been well received.
Figure 2.

Original NovoPen. Image reproduced with kind permission of NovoNordisk A/S.
A core objective of pen injectors is to reduce the physical, cognitive, and emotional burden of diabetes management. Manual needle insertion and injection is almost universal; however, products that automate parts of the operating sequence are becoming more common. Automatic dose delivery of the set dose at the touch of a button, once the needle has been manually inserted, is a feature of the Autopen® (Figure 3; Owen Mumford Ltd., Oxford, United Kingdom) and the prefilled FlexTouch® from NovoNordisk. The Softpen®, marketed in Germany as Diapen® (Figure 4; Haselmeier GmbH, Stuttgart, Germany), has fully automated needle insertion and dose delivery. Pens are essentially mechanical devices in terms of adjustment and delivery. Dose display is generally mechanical, although an electronic dose display is featured on some products, including the Humapen Memoir® (Eli Lilly and Company, Indianapolis, IN), which retains an electronic memory of past doses, and Novo Nordisk’s NovoPen Echo®, which shows time elapsed since last dose.
Figure 3.
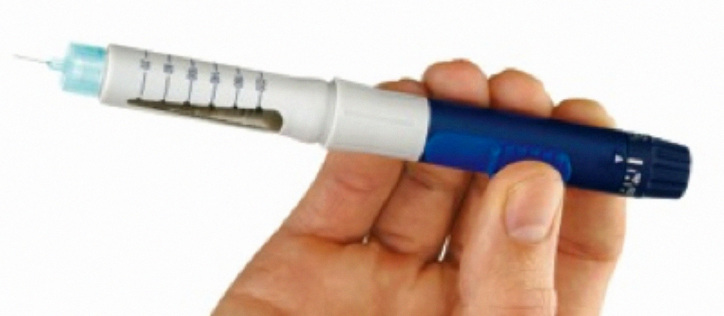
Owen Mumford Autopen. Image reproduced with kind permission of Owen Mumford Ltd.
Figure 4.

Haselmeier Diapen. Image reproduced with kind permission of Haselmeier GmbH.
Overall, pens are now either reusable items with replaceable insulin cartridges or are supplied as prefilled devices to be disposed of when empty. Adjustment, display, and overall usability are broadly similar in either case, and both use short, ultra-fine, siliconized needles for ease of insertion and minimal pain. Some reusable pens are adjustable in half-unit increments, principally for pediatric use. Sales of disposable pens are exhibiting stronger growth than the forecasted growth of reusable pen sales. Patients interviewed by drug delivery device specialists Team Consulting (Team Consulting Ltd., Cambridge, United Kingdom) describe keeping “one in the office, one in the kitchen, and one in my pocket,” in the same manner that many asthma sufferers treat inhalers, which may be a potential benefit to users of disposable format products.
Diabetes nurses interviewed commented that a wide variety of devices is a real advantage, enabling a more individualized matching of the device to the patient. One size does not fit all, and the care, expertise, and experience of the diabetes nurse is instrumental in understanding individual patient needs and enabling better selection of the most suitable pen. Selecting a pen that a patient engages with fully is seen as the crucial factor in supporting compliance and hence managing the condition, even if this means––in the case of the more popular prefilled pens––changing to an equivalent but different insulin on the basis of better patient-to-device engagement.
Needle-Free Injectors
Needle-free (NF) or jet injection technology has existed in various embodiments for over 60 years, starting with the Hypospray, developed by Robert A. Hingson in 1948.4 Multishot, all-stainless-steel, semiportable devices were used by clinicians in vaccination programs from the 1950s but were discontinued by the 1990s because of cross-contamination issues. Insulin delivery without needles seemed an obvious application to patients and device companies alike, and a number of spring-operated, self-administration devices began to emerge in the late 1970s. Needle-free injection has some unique features—no sharps, no needle phobia, and able to deliver high-viscosity products, some of which are of interest for insulin delivery (it would be nice to include “pain free,” but perhaps it is more accurate to say that there may be some discomfort with NF, though perceived differently from needle pain). However, in the years since the Medi-Ject (Antares Inc., Minneapolis, MN) appeared as the first jet injector system for insulin delivery, the NF market share has remained very limited and many manufacturers have withdrawn from the market. Needle-free offers attractive benefits for some applications; however, many of the boxes ticked by NF systems (such as high-viscosity capability) are of limited value for insulin delivery. Reported characteristics of NF insulin injection include faster absorption and localized bruising.
Needle-free devices for insulin delivery have to be filled from a vial, using an adaptor to make the transfer. The device also must be primed or “wound up” (most devices are spring operated) so the preparation for injection is already more complex and demanding than filling a traditional syringe. When compared with the compact size, easy dial-up and dial-down adjustment, and on-board insulin cartridge of an insulin pen, preparation for use of a NF device is significantly more complex. The very high pressures (typically hundreds of bar) required for NF delivery are one reason why a compact, multishot cartridge-based device—effectively a “NF pen”—has never been a commercial reality. Furthermore, pen devices are more or less fountain-pen sized, whereas most reusable NF devices are notably larger. The Injex™ (Figure 5; INJEX Pharma GmbH, Berlin, Germany) is the exception and is similar in size to some pens; however, it is reliant on a separate spring compressor for repriming, which brings the overall system’s bulk to a similar level as other NF devices.
Figure 5.

Injex 30 NF injector. Image reproduced with kind permission of INJEX Pharma GmbH.
Relatively high device cost has been cited as a barrier to adoption.5,6 Limited insurance reimbursement in the United States has deterred many from trying NF, while, in Europe, NF devices have not been widely promoted within public health systems. The U.K. National Health Service now offers the Injex system as the only NF option for people with diabetes, having deleted all other NF systems from the Drug Tariff in June 2010.
Among insulin users who have tried NF devices, there are undoubtedly some who are delighted and have no desire to revert to another system. However, they represent a small minority of the overall insulin user population. Across the parenteral delivery spectrum as a whole, NF offers real benefits in some applications, but diabetes management is perhaps not the area that displays these benefits to best effect.
Continuous Subcutaneous Insulin Infusion Pumps
The first attempted “portable” insulin pump was developed by Arnold Kadisch in 1963. It delivered both insulin and glucagon but was the size of a small suitcase and was never a commercial product. Attempts to create practical devices really began with the AutoSyringe AS6C (DEKA R&D Corporation, Manchester, NH) and the Mill Hill Infuser (National Institute for Medical Research, London, UK) in the late 1970s, setting the template for continuous subcutaneous insulin infusion (CSII). By the mid-1980s, a range of compact, wearable, electronically controlled CSII pumps were being offered by a number of manufacturers in the United States, Europe, and Japan. Issues of reliability, dosing control, and cost led to a drop in pump use and a thinning out of products and manufacturers toward the early 1990s. The Diabetes Control and Complications Trial,7 carried out between 1983 and 1993, highlighted the importance of tight control of blood glucose levels to limit eye, kidney, and nerve damage; in turn, this stimulated the development of the CSII pump as a means of delivering needs-based basal insulin. Significant progress in a number of areas has been made since the start of the 21st century.
It is no coincidence that progress in insulin pumps has coincided with technology breakthroughs across the spectrum of computing, information technology, micro-electronics, and communications. The objective of emulating pancreatic functions within an extracorporeal device is creeping closer, but it is absolutely reliant upon the processing power of the control system. Sensor-augmented pump therapy is a major step forward, indeed the technology currently exists to operate “closed-loop” insulin pump control; within the European Union, “semi-closed-loop” systems are already out there, albeit these rely upon a confirmatory action by the patient (such systems are not yet approved within the United States). The same electronic manufacturing organizations producing smart phones in the millions are also producing many of the CSII devices and are utilizing similar technologies and techniques in system design, manufacture, and componentry. Low-power wireless systems such as Bluetooth, already a feature on mobile phones, enables communication between pumps and separate blood glucose meters; ultra-low-power versions of Bluetooth and similar technologies such as ANT™ (Dynastream Innovations Inc., Cochrane, Alberta, Canada8) are expected to minimize demand on batteries and extend overall endurance of pumps.
Wireless data exchange has enabled another major evolution—the patch pump. The Omnipod® (Insulet Corporation, Bedford, MA) is supplied empty, filled with insulin by the user, and attached directly to the skin to deliver up to a 3-day supply, at the end of which the entire patch pump is disposed of. The patch pump maintains near-field wireless connection with a separate “personal diabetes manager” that incorporates a blood glucose monitor. Wireless data exchange with one of the continuous glucose monitoring systems now available (typically wearable for up to 7 days) is also an option.
The JewelPUMP™ patch pump9 (Debiotech, Lausanne, Switzerland) incorporates a microelectromechanical system technology pump smaller than a match head that is disposed of together with the insulin reservoir when empty, while the casework containing the batteries, control, monitoring, alarm, and display are retained. The Cellnovo system (Figure 6) (Cellnovo, London, United Kingdom) also takes a “semi-disposable” approach. Their mobile diabetes management system comprises a pulsatile patch pump with a disposable insulin cartridge controlled by a mobile (Global System for Mobile Communications) handset that moves data to a secure Web site in real time. The Cellnovo system is unique in that it communicates both in near field and over mobile networks worldwide.
Figure 6.

Cellnovo pump and handset. Image reproduced with kind permission of Cellnovo Ltd.
Information capture, recall, and display features of CSII systems are increasingly sophisticated, with examples including
Alerts to users or, in many cases, to parents or caregivers;
Remote access to displayed data such as battery status, insulin reservoir content, and records of use (both for patch pumps and several “conventional” CSII products);
Food “libraries” to assist users in management of their insulin dosage; and
Capture of detailed records of device use, which can be reviewed by patients, caregivers, and clinicians to enable actual compliance to be monitored and treatment to be adjusted for best clinical outcome.
The development of patch pumps and wearable bolus delivery devices for conditions other than diabetes has also resulted in a range of simpler products, some of which have been developed for diabetes management. The V-Go™ patch pump (Figure 7; Valeritas Inc., Bridgewater, NJ) has been developed as a fully disposable unit for patients with type 2 diabetes and is designed to deliver steady–constant basal insulin over 24 h, with a press button for bolus delivery at mealtimes. The product has no electronics and no display and is entirely self-contained.
Figure 7.

Valeritas V-Go. Image reproduced with kind permission of Valeritas Inc.
Pumps still tend to be viewed as a solution aimed at the well-off, the well educated, and the young. It is hoped that this perception gap will narrow as technological opportunity converges with a more enlightened view of what a user has a right to expect.
Peering into the Crystal Ball
By 2030, 552 million people will have diabetes, a 51% increase on 2011 figures, according to the International Diabetes Federation Diabetes Atlas.10 How this is managed will depend on a spectrum of factors: technical, medical, economic, regulatory, and environmental. Today’s predictions will inevitably diverge from reality in 5, 10, or 20 years, but some observations on technologies in diabetes are given below as possible trajectories (not rigid predictions):
Pens will continue to dominate insulin delivery for the “middle classes” in developing economies. Conversely, take-up of pens will expand in the United States, but “vial and syringe” will retain followers well into the future.
Issues of sustainability, either imposed or driven by market concern, may lead to a resurgence of reusable pens in some markets.
Accurate, reliable, noninvasive continuous blood glucose monitoring should become a practical reality.
Closed-loop controlled insulin delivery (with safe-guards) is expected to become accepted practice by regulators.
Patch pumps, for both type 1 and type 2 diabetes, will dominate a growing CSII market as battery, pumping, and information technology advances continue.
Widespread use of mobile phone applications in managing a range of conditions, including diabetes, will be part of a universal culture of health consciousness.
Conclusions
Just because greater sophistication and more advanced technology is possible (such as electronic memory and display or patch pump format), it does not mean it is always the best answer. Technological advances have brought incalculable benefit—from the development of recombinant insulin, through handheld blood glucose meters, to pens and pumps—without which the lives of millions more people with diabetes would have been impaired. However, technology should exist to serve and liberate patients; not confuse and frighten them. People with type 2 diabetes in the autumn of their lives seek a device that they can read easily, grip readily, and use without awkward maneuvers. The InnoLet® device (Figure 8) from NovoNordisk (now discontinued in many variants but still known affectionately as “the kitchen timer”) remains popular with senior citizens, especially those with visual or dexterity issues. By comparison, a technology-savvy teenager who would be embarrassed to be seen with an InnoLet is likely to have a ready affinity with a CSII patch pump; however, the same patch pump would probably terrify a type 2 senior citizen.
Figure 8.

Novo InnoLet device. Image reproduced with kind permission of NovoNordisk A/S.
Technology has delivered great benefits, and it can continue to do so. What we must not overlook is that, for diabetes (and other chronic conditions), the delivery device is the drug-to-patient interface. The ultimate objective is to help patients comply with their therapy and manage their condition. Electronic memories and reminders to take a basal dose once or twice a day should be helpful features within an externally simple and reassuring device, configured for easy reading and secure grip. Quite separately, patients who are comfortable with a smart phone are likely to derive confidence and pride of ownership as well as therapeutic benefit from a well-designed pen or pump with a thoughtfully structured menu interface.
A wearable device with a simple interface, which emulates “normal” pancreatic functions, would restore normal life. Although we should not regard this as unachievable, it is still some way off, and entry cost is likely to be seen as a barrier. Nevertheless, in considering the lifetime cost of diabetes management (estimated as $465 billion in 201110), assessment of health economics for such a device must receive as equally detailed consideration as technical, clinical, and user aspects.
Today, however, for many patients “in the middle,” what is needed is a simple-to-use, “dial-up, dial-down, press-to-deliver” pen, likely to be disposable, hence containing no electronics. But to fully satisfy patient need, it must have a robust, repeatable, and accurate mechanism, a clear display, a modest operating force, and good ergonomics, i.e., an all-around excellent product. The technology for the “ideal disposable pen” is crucial, but it is the combined technology of excellent human factors engineering and industrial design together with outstanding mechanism design and production engineering that will deliver the real user benefit.
Acknowledgments
Special thanks are given for the insight, wisdom, and experience provided by the many people who gave generously of their time. Particular thanks are due to Christine Cottrell, Clinical Lead for Diabetes, Education for Health, Warwick, United Kingdom; John and Sue Reynard and George Ashford of Creative Medical Research, Ipswich, United Kingdom; and my colleagues at Team Consulting Ltd.: Diane Aston-James, Neil Cooper, Julian Dixon, and Andrew Pocock. Finally, I wish to thank the people around the world who have willingly shared their experiences of living with diabetes so as to assist in preparation of this article.
Glossary
- (CSII)
continuous subcutaneous insulin infusion
- (NF)
needle-free
References
- 1.Bliss M. The discovery of insulin. Edinburgh: Paul Harris; 1983. [Google Scholar]
- 2. IMS Health. www.imshealth.com. April 2011.
- 3. RNCOS Industry Research Solutions. Insulin delivery systems market analysis (2008-2012). RNCOS; 2011.
- 4.Hingson RA. The development of the Hypospray for parenteral therapy by jet injection. Anesthesiology. 1949;10(1):66–75. doi: 10.1097/00000542-194901000-00008. [DOI] [PubMed] [Google Scholar]
- 5. ShareCare.com. How does a jet injector for insulin use work? www.sharecare.com/question/how-jet-injector-insulin-work.
- 6. DiabetesExplained.com. Insulin jet injectors. www.diabetesexplained.com/insulin-jet-injector.html.
- 7. U.S. Department of Health and Human Services; National Diabetes Information Clearinghouse (NDIC). DCCT and EDIC: the Diabetes Control and Complications Trial and follow-up study. http://diabetes.niddk.nih.gov/dm/pubs/control/#DCCT.
- 8. Ant. ANT Wireless: providing technologies that lead the globe. www.thisisant.com/company.
- 9. Debiotech.com. JewelPUMP: value for life. www.debiotech.com/products/msys/insulinpump.html.
- 10.International Diabetes Federation . IDF Diabetes Atlas. 5th ed. Brussels: International Diabetes Federation; 2011. [PubMed] [Google Scholar]


