Abstract
Since 2000, there has been an ongoing debate regarding tightness of glycemic control in critically ill patients. An increased risk of hypoglycemia is observed in patients treated with an intensive insulin protocol targeting “normoglycemia,” probably accounting for a reduction of the overall benefit. Hypoglycemia is associated with neurological side effects and is found to be an independent predictor of mortality in most trials; however, long-term sequelae are rare if glucose is administered early. We describe a case of prolonged, extreme hypoglycemia in a critically ill patient treated according to an intensive insulin protocol who recovered without any neurological deficit at discharge.
Keywords: continuous glucose monitoring system, critical illness, hypoglycemia, iatrogenic disease, insulin
Introduction
A 35-year-old woman with a past medical history of morbid obesity (body mass index, 40 kg/m2), type 2 diabetes mellitus treated with insulin and metformin, hypothyroidism treated with levothyroxine, depression treated with venlafaxine, a cholecystectomy, and an appendectomy presented for elective bariatric surgery. She underwent a Roux-en-Y gastric bypass. On the fifth postoperative day, emergency laparotomy was performed because of signs of localized peritonitis; leakage in the enteroenterostomy was found as well as a partial dehiscence of the gastroenterostomy with diffuse peritonitis. A new gastrogastrostomy was created, the blind loop was connected to an external drain, and the peritoneal cavity was thoroughly washed out. From the peritoneal fluid cultures, Escherichia coli was grown. Further inspections and washouts were planned over the following days.
In the intensive care unit, the patient was sedated and mechanically ventilated, and received hemodynamic support with dopamine. Hyperglycemia was treated with intravenous insulin, following an intensive protocol aiming for glycemia between 80 and 110 mg/dl,1 using a GEM Premier 3000 blood gas analyzer (Instrumentation Laboratory, Bedford, MA). On the eighth postoperative day, a sudden rise in blood pressure and bigeminy with a pulse rate of 34 beats per minute developed. Dopamine was ceased, but nonetheless, blood pressure increased further to 247/94 mm Hg. Neurological examination revealed no focal signs but fixed and dilated pupils. An acute intra-cranial process was suspected, and sedation was deepened. Arterial oxygen pressure (PaO2) and arterial carbon dioxide pressure (PaCO2) were within normal limits on an arterial blood gas sample. However, concomitantly, a blood glucose level of 0.0 mg/dl was reported on the blood gas readout but was overlooked by the nurse, not reported to the physician, and thus not acted upon. After reviewing the correct administration of sedative and vasoactive drugs, the patient was taken for an urgent computed tomography (CT) scan of the brain, which was unremarkable. Infusions and medications that were administered around the time of the incident are listed in Table 1.
Table 1.
Infusions Administered at the Time of the Incident
| Drug/infusion | Rate at time of incident |
|---|---|
| Total parenteral nutrition (Clinomel N7-1000®, Baxter) | 80 ml/h |
| Insulin (Actrapid®, Novo Nordisk) | 3 U/h |
| Sodium bicarbonate 8.4% | 10 ml/h |
| Propofol 2% | 60 mg/h |
| Midazolam | 6 mg/h |
| Sufentanil | 30 μg/h |
| Furosemide | 10 mg/h |
| Dopamine | 2.5 μg/kg/min |
| Somatostatin | 240 μg/h |
Half an hour after performing the scan, profound hypo-glycemia (13 mg/dl) was noted for the first time on an arterial blood gas analysis and was promptly treated with administration of 12 g glucose intravenously. The size and shape of the pupils became normal, and responsiveness to light reappeared. Blood pressure and heart rhythm normalized. Because of agitation and spontaneous move- ments of the legs, sedation was increased. After reviewing the previous arterial blood gas samples, it became apparent that extreme hypoglycemia had been present for several hours. There had been a problem with the pump driver of the total parenteral nutrition, which had not been running continuously. Results and times of the samples taken for glucose measurement are listed in Table 2, and the glucose trend is depicted in Figure 1.
Table 2.
Time and Results of Glucose Measurement and Current Insulin Administration
| Time | 12 h 33 min | 16 h 43 min | 20 h 20 mina | 21 h 08 minb | 22 h 03 min | 22 h 50 min |
| Glucose (mg/dl)c | 97 | 77 | 2 | 0 | 13 | 97 |
| Insulin (U/h) | 6 | 6 → 3 | 3 | 3 | Ceased + 12 g intravenous glucose given | – |
At 20 h 40 min, onset of sudden hypertension and bradycardia; dopamine ceased.
At 21 h 34 min, CT head performed.
For conversion of glucose units from mg/dl to mmol/liter, divide by 18.
Figure 1.
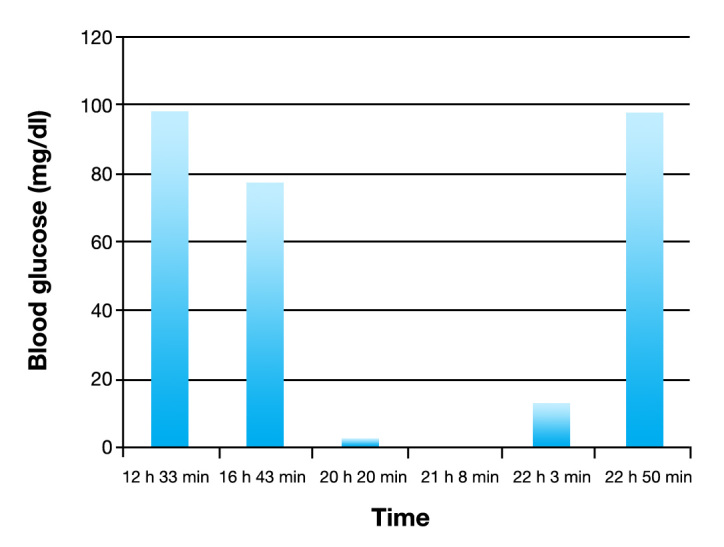
Blood glucose trend for the patient.
The next day, troponin levels increased (18.7 ng/ml). Contractility on echocardiography was slightly decreased in the inferoposterior segment. On day 10, continuous venovenous hemofiltration was commenced for acute renal failure. On day 13, the hemodynamic and renal function improved, and the patient was slowly weaned from sedation. The next day, she obeyed commands. She was extubated on day 16 and returned to the ward on day 20. Fourteen days later, she was discharged home without apparent neurological dysfunction. At a 6 month follow-up visit, she did not report any neurological or cognitive symptoms, and clinical neurological examination did not reveal abnormalities. When she presented for an unrelated problem 1 year later, she was assessed by a neurologist who performed a clinical examination, electroencephalogram, and CT scan of the brain, all of which were reported as normal.
Discussion
The brain relies almost exclusively on glucose for its metabolism, and there are several physiological mechanisms to prevent hypoglycemia. Therefore, significant hypoglycemia in a hospital setting is usually iatrogenic due to exogenous insulin administration. Very low glucose levels, be it a result of failure of neurohormonal defense mechanisms or of glucose-lowering therapy, can cause functional brain failure that can progress from cognitive impairment to aberrant behaviors, seizure, and coma. There seems to be a continuous spectrum with increasing risk of neuronal death at progressively lower glucose concentrations. Brain death is at the most extreme end of the spectrum, occurring solely when hypoglycemia is profound and prolonged.1
In this case, extreme hypoglycemia had been present and reported on the blood gas readout for at least 2 and probably 3 to 4 h before it was noticed and treated with intravenous glucose (see Table 2). A technical problem with the analyzer was unlikely, because there were no abnormal values in the analyses of other patients' samples, and glucose levels returned to normal after treatment. The hypertension and bradycardia can be retrospectively attributed to adrenergic and sympathetic activation and reflexive response, as is seen with the Cushing response. Both sinus tachycardia/bradycardia and ventricular/atrial ectopy have been described during acute insulin-induced hypoglycemia.2
In insulin-deficient diabetes, hypoglycemia is the result of the interplay of therapeutic insulin excess and compromised physiological (defective glucose counter regulation) and behavioral (hypoglycemia unawareness) defenses against falling plasma glucose concentrations due to a complex and not fully understood hypoglycemia-associated autonomic failure, the key feature of which is a downward shift of the glycemic thresholds for sympathoadrenal activation to lower plasma glucose concentrations.3 Furthermore, patients who underwent bariatric surgery are at two- to seven-fold increased risk of hypoglycemia, the mechanism of which is unclear but includes increased insulin secretion and decreased insulin resistance.4,5 Whether this is true in the acute postoperative setting is not known.
There are a number of plausible explanations for the occurrence of the incident we described. First, there were multiple breaches in protocol compliance: according to the local guideline, after the 16 h 43 min sample, a new analysis should have been performed 1 h later because of the downward trend in glycemia. Furthermore, the extremely low values on the 20 h 20 min and 21 h 08 min samples were thought to be factitious by the nurse and therefore not communicated but should at least have been checked with a plasma sample. Second, there was an obvious technical problem: the total parenteral nutrition had not been running continuously due to dysfunction of the pump driver, while the insulin infusion was not adjusted. In addition, somatostatin, a hormone that variably inhibits the release of several gastrointestinal and pancreatic hormones such as insulin and glucagon, has been established as a risk factor for developing hypoglycemia.6 Finally, dopamine, by suppressing growth hormone secretion from the pituitary, may have contributed to suppressed counter-regulatory responses to decreasing blood glucose levels.7
Treating hyperglycemia in a critically ill patient with intravenous insulin guided by an intensive protocol aiming for normal or near-to-normal glycemia increases the risk of hypoglycemia and its consequences.8 Even mild to moderate hypoglycemia has been independently associated with increased mortality in several post hoc analyses of large prospectively designed trials.9–11 However, it remains unproven whether this reflects a causative relationship or a marker of severity of illness, and individual outcome cannot be predicted.
Tight glucose control is a complex intervention not only requiring accurate infusion pumps and standardized blood glucose measurement systems, but also a guideline for the nursing staff who needs to be specifically trained for this intervention and allowed intuitive decision making.12 It is plausible to assume that current real-life technology and protocols limit the ability to reproduce study results found in the Leuven landmark study.
Our patient made an uneventful recovery. Was she just lucky, or could there be an explanation? Generally, complete recovery from hypoglycemia-induced brain failure is the rule if plasma glucose concentration is raised, while permanent neurological damage is rare.13 Intensive insulin therapy in critically ill children increased the incidence of hypoglycemia but did not increase levels of circulating S100B and neuron-specific enolase, markers of neurological damage.14 It also seems that there is a significant individual variability in tolerance of hypoglycemia. The time window for restoring glycemia without neurological sequelae is not clearly established in humans. In studies of insulin-induced hypoglycemia in monkeys, 5 to 6 h of blood glucose concentrations of less than 1.1 mmol/liter were required to provoke neurological damage.15 Other animal studies suggest that additional influences such as respiratory depression might potentiate hypoglycemia-induced brain injury. In a study comparing brain damage in cats exposed to severe hypoglycemia, respiratory depression, or both, all animals in the group exposed to both hypoglycemia and respiratory depression suffered from brain damage while the animals in the hypoglycemia group with preserved cardiorespiratory functions remained brain intact.16 The same study showed increased brain injury when hypoglycemia was corrected to hyperglycemia, compared with correction to euglycemia. Without extrapolating these data from animal studies to humans, the fact that our patient has always been adequately ventilated and that there was no overshooting in correcting her hypo-glycemia may have contributed to the favorable outcome.
Finally, a protective effect of the sedation may have played a role. Propofol has been shown to reduce cerebral glucose metabolism in both cortical and subcortical areas in healthy subjects.17 It remains difficult, however, to prove whether this attributed to our patient's recovery.
This case report adds to the ongoing discussion regarding glycemic control in the intensive care unit. A continuous glucose monitoring system with a closed-loop feedback system could possibly have protected our patient from this iatrogenic episode of severe hypoglycemia. Development of such a device that provides frequent, timely, and precise measurements is a challenge, given the heterogeneity and other confounding factors presented by the critically ill population. Ideally, this could then be integrated in a closed-loop system with the insulin infusion pump to act as an acute beta-cell modulator or artificial pancreas, which could help to reduce human errors.18 One study in critically ill patients showed a strong correlation between glucose levels measured by a subcutaneous continuous glucose monitoring system and arterial blood gas analysis as a reference.19 A reliable continuous glucose monitoring system could also shed a whole new light on the tight glucose control debate because it would avoid the threat of severe hypoglycemia and imply better control of the prime variable, blood glucose, which has been inadequate in most studies. When one superimposes the recognized differences between the various sources of blood in which glucose is typically measured (capillary versus venous versus arterial), along with the imprecision of current analytical instruments, truly excellent glycemic manage-ment in the critically ill remains somewhat illusory.20
Glossary
Abbreviations
- (CT)
computed tomography
References
- 1.The Diabetes Control and Complications Trial Research Group Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial. Ann Int Med. 1996;124(4):379–388. doi: 10.7326/0003-4819-124-4-199602150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Schächinger H, Port J, Brody S, Linder L, Wilhelm FH, Huber PR, Cox D, Keller U. Increased high-frequency heart rate variability during insulin-induced hypoglycaemia in healthy humans. Clin Sci (Lond) 2004;106(6):583–588. doi: 10.1042/CS20030337. [DOI] [PubMed] [Google Scholar]
- 3.Cryer PE. Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog Brain Res. 2006;153:361–365. doi: 10.1016/S0079-6123(06)53021-3. [DOI] [PubMed] [Google Scholar]
- 4.Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia. 2010;53(11):2307–2311. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafian H, Athanasiou T, Li JV, Bueter M, Ahmed K, Nagpal K, Holmes E, Darzi A, Bloom SR. Diabetes resolution and hyper-insulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev. 2011;12(5):e257–e272. doi: 10.1111/j.1467-789X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 6.Vriesendorp TM, van Santen S, DeVries JH, de Jonge E, Rosendaal FR, Schultz MJ, Hoekstra JB. Predisposing factors for hypoglycemia in the intensive care unit. Crit Care Med. 2006;34(1):96–101. doi: 10.1097/01.ccm.0000194536.89694.06. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, de Zegher F, Lauwers P, Veldhuis JD. Growth hormone secretion in critical illness: effect of dopamine. J Clin Endocrinol Metab. 1994;79(4):1141–1146. doi: 10.1210/jcem.79.4.7962286. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 9.Krinsley JS, Schultz MJ, Spronk PE, Harmsen RE, van Braam Houckgeest F, van der Sluijs JP, Mélot C, Preiser JC. Mild hypo-glycemia is independently associated with increased mortality in the critically ill. Crit Care. 2011;15(4):R173. doi: 10.1186/cc10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85(3):217–224. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38(4):1021–1029. doi: 10.1097/CCM.0b013e3181cf710e. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, Mesotten D. Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94(9):3163–3170. doi: 10.1210/jc.2009-0663. [DOI] [PubMed] [Google Scholar]
- 13.Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117(4):868–870. doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhorebeek I, Gielen M, Boussemaere M, Wouters PJ, Grandas FG, Mesotten D, Van den Berghe G. Glucose dysregulation and neurological injury biomarkers in critically ill children. J Clin Endocrinol Metab. 2010;95(10):4669–4679. doi: 10.1210/jc.2010-0805. [DOI] [PubMed] [Google Scholar]
- 15.Kahn KJ, Myers RE. Brierly JB, Meldrum BS. Brain hypoxia. London: William Heinemann Medical Books; 1971. Insulin-induced hypoglycemia in the non-human primate. I. Clinical consequences. [Google Scholar]
- 16.De Courten-Myers GM, Xi G, Hwang JH, Dunn RS, Mills AS, Holland SK, Wagner KR, Myers RE. Hypoglycemic brain injury: potentiation from respiratory depression and injury aggravation from hyperglycemic treatment overshoots. J Cereb Blood Flow Metab. 2000;20(1):82–92. doi: 10.1097/00004647-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Zhang H, Gao C, Zhang G, Xu L, Lv M, Chai W. Imaging the effects of propofol on human cerebral glucose metabolism using positron emission tomography. J Int Med Res. 2008;36(6):1305–1310. doi: 10.1177/147323000803600618. [DOI] [PubMed] [Google Scholar]
- 18.Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37(5):1769–1776. doi: 10.1097/CCM.0b013e3181a19ceb. [DOI] [PubMed] [Google Scholar]
- 19.Brunner R, Kitzberger R, Miehsler W, Herkner H, Madl C, Holzinger U. Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med. 2011;39(4):659–664. doi: 10.1097/CCM.0b013e318206bf2e. [DOI] [PubMed] [Google Scholar]
- 20.Inzucchi SE, Kosiborod M. Continuous glucose monitoring during critical care. Anesthesiology. 2011;114(1):18–19. doi: 10.1097/ALN.0b013e3181ff419e. [DOI] [PubMed] [Google Scholar]


