Abstract
Introduction
In times of short health care budgets, reimbursement for self-monitoring of blood glucose (SMBG) in diabetes patients without insulin treatment is subject to debate. The Structured Testing Program (STeP) trial found a positive correlation of test frequency and improved hemoglobin A1c (HbA1c) levels in poorly controlled type 2 diabetes patients not treated with insulin.
Methods
A structured literature search for other clinical studies reporting on SMBG frequency was performed.
Results
There is scarce evidence: three trials, including STeP, noted a significant and relevant correlation between testing frequency and improved HbA1c levels (FA effect), whereas two studies did not. The comparability between the identified studies is problematic.
Conclusion
Future research should consider correlations between testing frequency and level of glycemic control. More emphasis should be placed on a structured approach to use SMBG and to address adherence to testing and therapy.
Keywords: glycosylated hemoglobin A, NCT00674986, self-monitoring of blood glucose, type 2 diabetes mellitus
Introduction
Due to budget restrictions, more and more countries have begun to question the use of self-monitoring of blood glucose (SMBG) in their health care systems, especially for patients with type 2 diabetes who are not treated with insulin [non-insulin-treated diabetes mellitus (NITDM)].1 Apparently, a multifaceted discussion on what is the best available medical evidence on SMBG2 meets the pressing financial burdens of third-party payers.
Still, optimal blood glucose management is of utmost importance in diabetes care. Data from the United Kingdom Prospective Diabetes Study revealed that diabetes patients with tighter blood glucose control [expressed as hemoglobin A1c (HbA1c) 7.0%] for 10 years have a 15–33% lower risk of myocardial infarction and a 13–27% lower risk of death than those receiving conventional care (HbA1c 7.9%).3,4 On the other hand, epidemiological studies demonstrate that a considerable percentage of diabetes patients do not reach their glycemic targets.5
Self-monitoring of blood glucose is widely accepted as a tool for effective diabetes management in patients with multiple daily insulin injections (American Diabetes Association guidelines: evidence level A6). Self-monitoring of blood glucose represents an efficient health technology in type 1 diabetes, as this source of information triggers the correct use of insulin and patient behavior in routine care. Through that, medical outcomes and treatment safety are supported positively.7
This information-based mechanism holds true in NITDM patients, but the effects must be smaller, as their bodies can still produce insulin and regulate blood glucose. Nevertheless, treatment errors of patients, e.g., taking oral antidiabetic drugs like sulfonylurea, playing sports, and not eating, may lead to serious acute complications.8
The Structured Testing Program (STeP) study, a 1-year, prospective, cluster-randomized, multicenter clinical trial that evaluated the impact of SMBG9 is a thought-provoking investigative piece of information in the ongoing debate to solve the jigsaw puzzle on the value of SMBG.
STeP Study
Polonsky and colleagues9 examined in this study (trial registration NCT00674986) the impact of SMBG upon glycemic control and general well- being in 483 poorly controlled NITDM patients. In this two-armed study, physician practices were assigned to integrated treatment protocols:
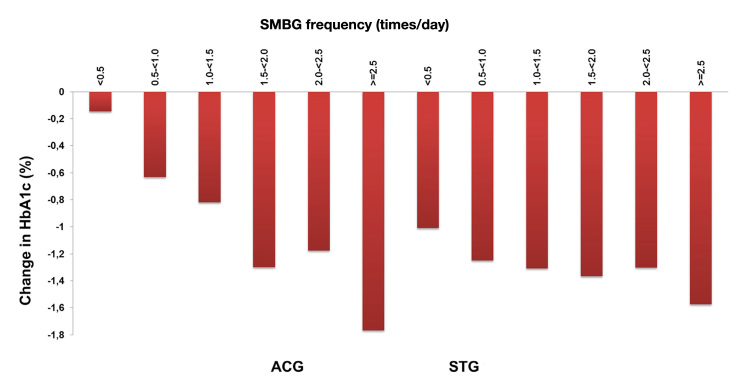
The so-called active controlled group (ACG) can be described as being a diabetes guideline approach, with quarterly visits of patients and training of patients. This protocol included SMBG, and it showed a drop of -0.9% in HbA1c levels over 52 weeks.
The other so-called structured testing group (STG) of patients received the same handling, but on top of it, patients were asked at months 1, 3, 6, 9, and 12 to perform a seven-point blood glucose profile on three consecutive days and to document this on a paper form before seeing their doctor. The adherent patients in this study arm improved even more to -1.2% in HbA1c levels (Δ = -0.3%, p = .04).
Test strip consumption decreased during the course of the study in both treatment arms. Overall, mean SMBG use over 12 months was 1.2 tests/day for ACG versus 0.9 tests/day for the STG cohort, which represents a 25% difference in annual test strip consumption.
Several results of this study were discussed as being remarkable,10 but one, which, so far, has only been published in a poster at the European Association for the Study of Diabetes congress 2010,11 is particularly inspiring:
Although the test frequency in the STG group was significantly lower than in the ACG group, a statistically significant “dose-dependent” response expressed as a number of applied glucose tests per year and the reduction of HbA1c level was noted for both groups (Figure 1).
Figure 1.
Correlation of HbA1c levels and SMBG testing frequency in the STeP study.
Dose–effect relations play an important role in clinical drug testing. In phase II trials, future drug compounds shall demonstrate a dose–effect relation in order to find out the best dosage for patients. In many cases, effect and adverse reactions have to be balanced.
Objective
The STeP study results suggest a working hypothesis: a positive correlation between frequency of SMBG and improving HbA1c levels (FA effect) can be found in managed diabetes care settings involving structured information exchange between patients and physicians.
Available Evidence
A structured literature search for frequency of SMBG and HbA1c based on the search string “diabetes mellitus, type 2 AND blood glucose self-monitoring AND hemoglobin A, glycosylated AND frequency” reveals only a few studies and publications in this field (96 hits in PubMed). Bonomo and associates12 also found a positive correlation between frequent SMBG tests and better HbA1c levels in a small monocentric Italian study on 273 type 2 diabetes patients performing a structured SMBG protocol.
Apart from this we see scarce and conflicting evidence. Karter and coworkers13 reported in their retrospective study the association between SMBG test frequency and glycemic control in two cohorts during a 4-year observation period. One cohort included 16,091 SMBG naïve patients starting SMBG use at baseline (“new users”). A second cohort with 15,347 “prevalent users” included diabetes patients already using glucose test devices at baseline. There was a prominent and test-frequency-dependent improvement in HbA1c after implementation of SMBG. This SMBG-frequency-dependent effect was particularly observed in drug-treated patients, which suggests that SMBG-related feedback may help patients to understand the impact of lifestyle and treatment on their glycemic control.
Schutt and associates14 found a positive correlation between testing frequency and HbA1c levels in a large sample of 24,500 diabetes patients from the German DPV-Wiss database in type 1 diabetes patients and insulin-treated type 2 diabetes patients but not in NITDM patients. An assessment of National Health and Nutrition Examination Survey III data did not find any relation between frequency of SMBG and HbA1c levels.15 A small study by Scherbaum and colleagues16 evaluated 202 type 2 diabetes patients over 12 months. They had a stable metabolic control and a mean HbA1c of 7.2% at the beginning of the trial. This experiment did not show a frequency effect of SMBG on HbA1c.
Discussion
The hypothesis of the FA effect is far from being established because of scarce available evidence. One cannot claim causation at this time. Yet the possible relation between testing frequency and HbA1c should not be neglected easily and should be studied in future trials. The Karter and coworkers13 study based on a large data sample of the Kaiser Permanente health maintenance organization is by far the study with the most type 2 diabetes patients analyzed for the relation of SMBG frequency and HbA1c levels. These findings and the significant as well as relevant results of the STeP study direct the researcher’s attention to the question, how is SMBG information utilized in the most efficient way?
Medicare NITDM beneficiaries may use 100 test strips and 100 lancets every 3 months.17 Instead of testing regularly once a day, the STeP study results suggest using a cluster approach: a seven-point blood glucose profile on three consecutive days had been performed, documented on a simple paper form, and presented to the treating physician. Polonsky and colleagues18 explain the positive effect of SMBG in their study with better patient adherence and the increased use of SMBG information for treatment decisions by physicians in the STeP study. This efficacy of a cluster approach is supported by other authors.19 The study by Bonomo and associates12 suggests that trained patients are competent in using the available blood glucose information.
By study design, the three prospective clinical trials cannot be compared with the two retrospective registry studies that come from very different health care settings (United States and Germany).
Self-Monitoring of Blood Glucose, a Physician’s Dilemma
We regularly see that achievable treatment objectives are not met.5 Diabetes patients are dependent on themselves for 99.9% of their life with this chronic disease. It is a goal for patients to feel safe and to know what their “performance” is in preventing acute and late complications of diabetes. Adjusting medication or changing lifestyle requires knowledge about blood glucose as a prerequisite. Do patients have a “right to know their number”? Apparently, the alternatives to SMBG are not convincing, and this is why both patients and physicians oppose the threat of losing SMBG as treatment support.20,21
Since the very beginning of modern diabetes therapy, the discrepancy between blood glucose and urine glucose was detected and discussed, e.g., in the 1919 work of Williams and Humphreys.22 This effect is caused by multiple influences such as drinking, eating, physical activity, and the simple fact that the glomerular filtration rate varies.23 Change in the renal glucose thresholds caused by the progression of diabetic glomerulosclerosis and increasing age lead to relevant inter- and intra-individual variation of renal glucose excretion.24 An HbA1c value, ascertained two to four times a year,6,25 is an important screening and quality-assurance parameter. However, the HbA1c level is an aggregated indicator cumulating the blood glucose level over the past 3 months, and it is of lesser value when adjusting diabetes medications.
With the increasing demand of the health care systems for efficiency, i.e., a balance between medical outcomes and economic burdens, it is not enough just to prescribe glucose meters and test strips. Diabetes doctors need more clinical trials evaluating the best practice in SMBG, or they risk losing this health technology. The Canadian Optimal Medication Prescribing and Utilization Service report and the Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesenis (Institute for Quality and Efficiency in Healthcare) statement on SMBG versus urine glucose measurement pave the way to cancelling SMBG for NITDM.1,26
More research is needed evaluating structured SMBG in NITDM patients to consolidate or reject the hypothesis of the FA effect.
Conclusion
Data from the STeP study along with results from other clinical trials and registries suggest that more emphasis should be placed on a structured approach to use SMBG and to address adherence to testing and therapy. Future research on SMBG should consider a potential correlation between testing frequency and level of glycemic control.
Glossary
Abbreviations
- (ACG)
active controlled group
- (HbA1c)
hemoglobin A1c
- (NITDM)
non-insulin-treated diabetes mellitus
- (SMBG)
self-monitoring of blood glucose
- (STeP)
Structured Testing Program
- (STG)
structured testing group
Funding
This publication was supported by a grant of Roche Diagnostics GmbH, Germany.
Disclosure
Wendelin Schramm has received consulting fees from Roche Diagnostics GmbH, Germany.
References
- 1.Cameron C, Coyle D, Ur E, Klarenbach S. Cost-effectiveness of self-monitoring of blood glucose in patients with type 2 diabetes mellitus managed without insulin. CMAJ. 2010;182(1):28–34. doi: 10.1503/cmaj.090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb H, Kempf K, Martin S, Stumvoll M, Landgraf R. On what evidence-base do we recommend self-monitoring of blood glucose? Diabetes Res Clin Pract. 2010;87(2):150–156. doi: 10.1016/j.diabres.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 5.Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60(1):1–23. doi: 10.1016/j.metabol.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD, DARTS/MEMO Collaboration Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26(4):1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 9.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch IB. Home blood glucose monitoring in type 2 diabetes: broken health care system undermines study’s impact. Diabetes Care. 2011;34(2):527–528. doi: 10.2337/dc10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mast PW, Fisher L, Parkin C, Jelsovsky Z, Schweitzer M, Wagner R. Structured blood glucose monitoring reduces HbA1c levels and annual test strip consumption in poorly controlled, non-insulin treated type 2 diabetes: results from the STeP Study. Diabetologia. 2010;53(Suppl 1):S423–S424. [Google Scholar]
- 12.Bonomo K, De Salve A, Fiora E, Mularoni E, Massucco P, Poy P, Pomero A, Cavalot F, Anfossi G, Trovati M. Evaluation of a simple policy for pre- and post-prandial blood glucose self-monitoring in people with type 2 diabetes not on insulin. Diabetes Res Clin Pract. 2010;87(2):246–251. doi: 10.1016/j.diabres.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Karter AJ, Parker MM, Moffet HH, Spence MM, Chan J, Ettner SL, Selby JV. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Diabetes Care. 2006;29(8):1757–1763. doi: 10.2337/dc06-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, Mayer I, Rosenbauer J, Wagner C, Zimmermann A, Kerner W, Holl RW, DPV Initiative Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384–388. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 15.Harris MI, National Health and Nutrition Examination Survey (NHANES III) Frequency of blood glucose monitoring in relation to glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;24(6):979–982. doi: 10.2337/diacare.24.6.979. [DOI] [PubMed] [Google Scholar]
- 16.Scherbaum WA, Ohmann C, Abholz HH, Dragano N, Lankisch M. Effect of the frequency of self-monitoring blood glucose in patients with type 2 diabetes treated with oral antidiabetic drugs-a multi-centre, randomized controlled trial. PloS One. 2008;3(8):e3087. doi: 10.1371/journal.pone.0003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services Review of Medicare claims for home blood-glucose test strips and lancets: durable medical equipment Medicare administrative contractor for Jurisdiction A. U.S. Department of Health and Human Services; 2010. http://oig.hhs.gov/oas/reports/region9/90800043.pdf.
- 18.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Axel-Schweitzer M, Petersen B, Wagner RS. A structured self-monitoring of blood glucose approach in type 2 diabetes encourages more frequent, intensive, and effective physician interventions: results from the STeP study. Diabetes Technol Ther. 2011;13(8):797–802. doi: 10.1089/dia.2011.0073. [DOI] [PubMed] [Google Scholar]
- 19.Pimazoni-Netto A, Rodbard D, Zanella MT, Diabetes Education and Control Group Rapid improvement of glycemic control in type 2 diabetes using weekly intensive multifactorial interventions: structured glucose monitoring, patient education, and adjustment of therapy-a randomized controlled trial. Diabetes Technol Ther. 2011;13(10):997–1004. doi: 10.1089/dia.2011.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diabetes.de Stellungnahme zur Verordnungsfähigkeit von Urin- und Blutzuckerteststreifen für nicht insulinpflichtige Typ-2-Diabetiker ab dem 1.10.2011. http://www.deutsche-diabetes-gesellschaft.de/fileadmin/Redakteur/Stellungnahmen/Informationen_zur_Verordnungsfaehigkeit_von_Urin_korrektur.pdf. Accessed on June 22, 2012.
- 21.Miller D, Berard L, Cheng A, Hanna A, Hagerty D, Knip A, McDougall P, Woo V. Self-monitoring of blood glucose in people with type 2 diabetes: Canadian Diabetes Association briefing document for healthcare providers. Canadian J Diabetes. 2011:317–319. http://www.diabetes.ca/documents/for-professionals/CJD--Sept_2011--SMBG.pdf. [Google Scholar]
- 22.Williams JR, Humphreys EM. Some observations on the clinical significance of blood sugar in nephritis and other diseases. Arch Int Med. 1919;23(5):537–545. [Google Scholar]
- 23.Lawrence RD. Cases of diabetes mellitus with a low renal threshold. Br Med J. 1929;1(3552):196–197. doi: 10.1136/bmj.1.3552.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walford S, Page MM, Allison SP. The influence of renal threshold on the interpretation of urine tests for glucose in diabetic patients. Diabetes Care. 1980;3(6):672–674. doi: 10.2337/diacare.3.6.672. [DOI] [PubMed] [Google Scholar]
- 25.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda O, Garber AJ, Hirsch IB, Horton ES, Ismail-Beigi F, Jellinger PS, Jones KL, Jovanovič L, Lebovitz H, Levy P, Moghissi ES, Orzeck EA, Vinik AI, Wyne KL, AACE Task Force for Developing Diabetes Comprehensive Care Plan American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 26.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG) Urin- und Blutzuckerselbstmessung bei Diabetes mellitus Typ 2. https://www.iqwig.de/download/A05-08_Abschlussbericht_Zuckerselbstmessung_bei_Diabetes_mellitus_Typ_2.pdf.