Abstract
Metabolites and derivatives of vitamin D are well-known inducers of monocytic differentiation, but the mechanistic basis for their action is not fully elucidated. Here we show that the product of protooncogene Cot1 represses the monocytic phenotype in human acute myeloid leukemia (AML) cells induced to differentiate by 1,25-dihydroxyvitamin D3 (1,25D), even though the expression of cellular Cot1 increases early in the process of 1,25D-induced differentiation. Interestingly, the expression of the two members of the Kinase Suppressor of Ras (KSR) family of molecular scaffolds, known to be positive regulators of Ras signaling and of 1,25D-induced differentiation, increases in parallel with Cot1 in 1,25D-treated cells. However, KSR1/2 are negatively regulated by Cot1, as determined by transfection of siCot1, and confirmed by a reverse effect of ectopic expression of Cot1. The effect of Cot1 in AML cells appears to be cell-type specific, as previous reports in other cell types found KSR-2 to be a negative regulator of Cot1, a reverse relationship. Also in contrast to findings in other cells, in AML cells Cot1 exerts negative control on the MAP kinase pathways, since siCot1 increases the levels of activated Raf1, p90RSK, JNK1, c-jun, and p38, though not of MEK/ERK. These findings have implications for therapy of AML, since in AML cells active MAPKs hasten cell differentiation, and specific pharmacological inhibitors of Cot1 kinase activity have recently became available, thus making Cot1 a “druggable” target.
Acute myeloid leukemia (AML) is a disease with a poor overall prognosis and limited options for successful treatment. Cytotoxic therapy alone results in only a few complete and durable remissions, and bone marrow transplantation, although often effective, is hazardous and can only be offered to a subset of patients (Koreth et al., 2009). An alternative approach to treatment of AML is exemplified by the success with the vitamin A derivative all-trans retinoic acid (ATRA) in acute promyelocytic leukemia, a subset of AML, whereby the immature myeloid hematopoietic cells, known as blasts, are induced to differentiate into cells with granulocytic phenotype, resulting in long lasting remissions (Tallman et al., 2002). However, APL accounts for only 10% of AML cases, so other agents for differentiation therapy of AML are urgently needed.
A candidate for another differentiation therapy agent for AML is 1,25-dihydroxyvitamin D3 (1,25D), which in supra-physiological concentrations effectively induces differentiation of rodent and human AML cell lines (Abe et al., 1981; Tanaka et al., 1982; Studzinski et al., 1985; Munker et al., 1986), and its analogs can achieve similar results at lower dosages, especially if supplemented by other agents, such as plant polyphenols or glucocorticoid derivatives (Miyoshi et al., 1997; Danilenko et al., 2001; Danilenko and Studzinski, 2004). Such regimens are also effective in animal experiments (Sharabani et al., 2006; Shabtay et al., 2008). However, although limited success has been reported (Beer et al., 2001), the above approaches have been insufficient to lower the risk of life-threatening hypercalcemia when administered to patients with a variety of malignant diseases (Koeffler et al., 1985).
A possible reason for the inability to improve the therapeutic regimens, and thus successfully bring 1,25D or its analogs to the clinic, may be the lack of information regarding the mechanistic basis of their ability to induce differentiation in AML blasts. When one considers that the block to cell differentiation is the result of highly heterogeneous genetic aberrations in these cells, it may seem strange that a single compound could overcome the effects of diverse lesions. In the case of ATRA, the simple explanation is that the disease-causing mutations interfere with the function of retinoic acid receptor (RAR), a critical component of granulocytic differentiation signaling by retinoic acid, but an excess of ATRA can force the signals to be recognized (Chen et al., 1991; Degos and Wang, 2001; Schlenk et al., 2004). Perhaps somewhat analogously, 1,25D can negate the mutations that interfere with signaling of monocytic differentiation, and overcome the differentiation block by upregulating the expression of the transcription factors (TFs) which are essential for monocyte/macrophage phenotype, such as the components of c-jun/AP-1 TFs or members of the C/EBP family, as previously suggested (Studzinski et al., 2005; Zhang et al., 2009). The question then remains how the upregulation of these TFs is achieved by an exposure to 1,25D.
Several laboratories, including ours, have focused on the MAPK pathways, particularly the Raf1/MEK1/ERK1/2 pathway, as transducers of signals for 1,25D-induced differentiation (Marcinkowska et al., 1997; Wang et al., 2000; Wang and Studzinski, 2001). These studies revealed ERK participation which is transient, and a more long lasting involvement of Raf1 and p90RSK (Wang and Studzinski, 2001, 2006). Also, there is a less well-defined role for JNK and p38MAPK pathways (Wang et al., 2000, 2003; Chen-Deutsch et al., 2009). Regarding the Raf1/MEK1/ERK1/2 pathway, we have also found that the so-called scaffold proteins, KSR1 and hKSR2, enhance the efficiency of Raf1-dependent 1,25D signaling of differentiation, but only at low 1,25D concentrations. KSR1 and hKSR2 are evolutionarily conserved enhancers of Ras signaling, most likely by providing a platform that permits efficient interactions between Raf1 and its substrates for phosphorylation, resulting in the activation of the Raf1 pathway targets (Wang and Studzinski, 2004; Wang et al., 2006, 2007). However, the details, especially as they pertain to 1,25D-induced differentiation of human AML cells are far from clear.
To explore these mechanisms further, we focus here on the recent finding of potential translational importance, that the Ser–Thr kinase (MAP3K8) with oncogenic properties, designated Cot1 (Cancer Osaka Thyroid-1) in humans, and Tpl2 (tumor progression locus-2) in rodents (Miyoshi et al., 1991; Patriotis et al., 1993), is regulated by 1,25D in human AML cells (Wang and Studzinski, 2010). Seemingly paradoxically, while its expression increased in cells exposed to 1,25D, the inhibition of its kinase activity by a pharmacological agent (Cot Inh) increased the effectiveness of 1,25D as a differentiation agent. We now report that this effect can be explained by the down-regulation by Cot1 of at least two other MAPK pathway regulators, KSR1 and hKSR2, known to be required for optimal 1,25D-induced differentiation of AML cells, as mentioned above (Wang et al., 2006, 2007). Although the initial focus of this study was on hKSR2 because of its reported direct and functional interaction with Cot1 (Channavajhala et al., 2003, 2005), it appears that KSR-1 is also under negative control by Cot1, and therefore both may contribute to the enhancement of monocytic differentiation when Cot1 enzyme activity is inhibited, perhaps by facilitating Raf1 functions, and influencing interactions with MAPKs, through the increased abundance of both KSR proteins.
Materials and Methods
Cell culture
HL60-G cells, subcloned from HL60 cells derived from a patient with promyeloblastic leukemia (Gallagher et al., 1979), and U937 cells, derived from human histiocytic lymphoma (Sundstrom and Nilsson, 1976), were cultured in suspension in RPMI 1640 medium supplemented with 10% bovine calf serum (Hyclone, Logan, UT) in a humidified atmosphere of 5% CO2 at 37°C. Cells were passaged 2–3 times a week and were used in the exponential growth phase. Routine microbiology testing for Mycoplasma was performed by selective culture techniques (Garner et al., 2000). For all experiments the cells were suspended for the indicated times in fresh medium containing 1,25D or the equivalent volume of ethanol as a vehicle control. Each experimental condition was repeated at least three times.
Reagents and antibodies
1,25D was a kind gift from Dr. Milan Uskokovic (Bioxell, Nutley, NJ). The following antibodies COT1, KSR1, KSR2, and Crk-L were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Raf1 (Ser338), phospho-MEK1/2 (Ser217/221), phospho-ERK (Thr202/Tyr204), phospho-p90RSK (Ser380), Phospho-SAPK/JNK (Thr183/Tyr185), Phospho-c-Jun (Ser63), Phospho-p38 (Thr180/Tyr182), anti-rabbit and anti-mouse antibodies linked to HRP were purchased from Cell Signaling Technologies (Danvers, MA). Nitrocellulose membranes were purchased from Amersham BioSciences (Piscataway, NJ). The pharmacological inhibitor of COT-1 kinase, 4-(3-chloro-4-fluorophenylamino)-6-(pyridin-3-yl-methylamino-3-cyano-[1-7]-naphthyridine (Cot1 Inh), was purchased from EMD Chemical (Gibbstown, NJ).
Small interfering (si) RNA and antisense oligonucleotides
siRNAs targeting KSR1, KSR2, and COT1 and generic scrambled siRNA as control were purchased from Dharmacon (Lafayette, CO). Phosphorothioate antisense oligonucleotides to KSR1: 5′-TGATGTGTGCCGGAATTC-3′, to hKSR-2, 5′-GTCCTCGTTGTCCCTCTCA-3′, and scrambled control oligonucleotides were synthesized by Molecular Resource Facility of the NJ Medical School. Each oligonucleotide was incubated with the cells at 10 μM for 48 h before adding 1,25D. siRNAs (5 μM) were transfected into cells using Amaxa nucleofector (solution T and program T-19) according to the manufacturer’s protocol. The cells were allowed to recover for 48 h and checked for the reduction of mRNA and protein expression. Transfected cells were seeded at a concentration of 2 × 10cells/ml and exposed to the specified agents for the indicated times. Transfection efficiency was monitored by using a co-transfected GFP plasmid; the mean efficiency was 51% in HL60 cells and 79% in U937 cells. Cell viabilities in all samples, determined by scatter plots on flow cytometry, were above 90%.
Transfection of COT1 and cell sorting
Full-length COT was generated by PCR and subcloned into the pcDNA3 expression vector (Invitrogen). All constructs generated were verified by DNA sequencing. COT1 stable transfectants were generated using a pEF-IRES2-EGFP expression vector containing COT1 fragment (pIRES-COT1), the vector was transfected into either HL-60 or U937 cells by using Amaxa Nucleofector according to Company’s protocol. Cells were rest for 48–72 h after transfection. The transfectants were then sorted on the basis of EGFP fluorescence by flow cytometry to derive a population exceeding 95% positive for EGFP. COT1 transfected cells were further selected with G418 (10 ng/ml) selection for 2–3 weeks. Enhanced COT1 expression was confirmed by Western analysis. The stably transfected cells were then treated with 1,25D to check the effect of ectopic expression of COT1 on differentiation. Samples were checked for cell intensity as well as cell viability with both FACS and hemacytometer.
Markers of differentiation
Aliquots of 10cells were harvested, washed twice with phosphate buffered saline (PBS), resuspended in PBS and incubated for 45 min at room temperature with 0.5 μl MY4-RD-1 and 0.5 μl MO1-FITC antibodies to analyze the expression of surface cell markers CD14 and CD11b, respectively. The cells were then washed three times with ice-cold PBS, resuspended in 1 ml PBS and analyzed using EPICS XL Flow Cytometer (Beckman Coulter). Isotypic mouse IgG1 was used to set threshold parameters. Monocyte-specific esterase cytochemical reaction was performed as described before (Wang et al., 1997).
Cell cycle analysis
The DNA content of HL60 cells was determined as follows: Cells (10) were harvested and washed with PBS and fixed in 75% ethanol at −20°C for 24 h. The cells were then re-harvested and resuspended in 1 ml of PBS with RNase (at 1 μg/ml, Sigma) and propidium iodide (PI at 10 μg/ml, Sigma) for 30 min at 37°C. PI stained cells were analyzed using an EPICS Flow Cytometer. The resultant histogram of DNA content was gated and analyzed using the multicycle program to determine the proportion of cells in each phase of the cell cycle.
Quantitative real-time PCR
Real-time PCR was carried out by using a lightcycler with Faststart DNA SYBR Green PCR kit (Roche Diagnostics, Indianapolis, IN) as described before (Wang et al., 2006). Fold changes of mRNA levels in COT1 target gene relative to the RNA polymerase II (RPII) control were calculated by relative quantification analysis. Primers used for real-time PCR for were: COT1, upstream 5′-CAAGGCCGCAGATGCAATCTT-3′, downstream 5′-AGTCAGACTCCTGGCTTTGCA-3′; KSR-1 upstream primer 5′-AGCAAGTCCCATGAGTCTCA-3′ and downstream primer 5-CAACCTGCAATGCTTGCACT-3; hKSR-2 upstream primer 5′-CCGACACAGAGGAGGATAAG-3′ and downstream primer 5′-TTCAAAGGCCCAGCAGAAG-3′; Raf1 upstream primer 5′-CGCTTCCGAGCCATCCTT-3′ and downstream primer 5′-GTCAGCGTGCAAGCATTGAT-3′; MEK1 upstream primer 5′-GCATGCTTTGCTGCTATAAAAA-3′ and downstream primer 5′-AAGGGCTCTGGCTAGATTTTG-3′; ERK upstream primer 5′-GCTGAATCACATCCTGGGTAT-3′ and downstream primer 5′-AGATCTGTATCCTGGCTGGAA-3′ (detects both isoforms of ERK), p90RSK upstream primer 5′-TCTCTGTCCAGCGGCGGGTGA-3′ and downstream primer 5′-GCATTCACAGCGCCCATGCG-3′, For RNA Pol II the primers were: upstream 5-GCACCACGTCCAATGACAT-3, downstream 5-GTGCGGCTGCTTCCATAA-3. The quality of PCR product was monitored using post-PCR melting curve analysis.
Western blotting
Western blotting was performed using whole cell extracts. Proteins in 35 μg of cell extract were separated on 10% SDS–PAGE gel, then transferred to nitrocellulose membranes (Amersham). The membranes were blocked with 5% milk in TBS/0.1% Tween-20 for 1 h, incubated overnight with primary antibodies, and then blotted with a horseradish peroxidase-linked secondary antibody for 1 h. The protein bands were visualized using a chemiluminescence assay system (Pierce Biotechnology, Rockford, IL), and the optical density of each band was quantitated using an ImageQuant 5.0 (Molecular Dynamics, Sunnyvale, CA).
Statistical methods
Each experiment was performed at least three times and the results were expressed as percentages (mean ± SD) of the vehicle controls. Significance of the differences between mean values was assessed by a two-tailed Student’s t-test. All computations were performed with an IBM-compatible personal computer and used Microsoft EXCEL.
Results
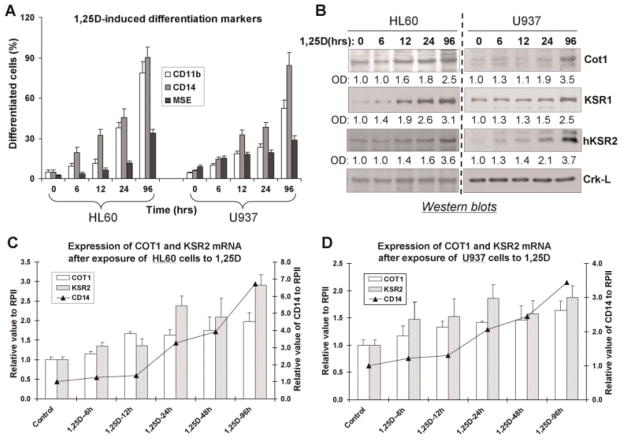
Expression of Cot1 increases in parallel with KSR1 and hKSR2 during 1,25D-induced differentiation of human AML cells
We have previously shown that the expression of KSR1 and hKSR2 increases coordinately with markers of monocytic differentiation in HL60 cells treated with low doses of 1,25D, and found that these proteins facilitate the appearance of the monocytic phenotype (Wang et al., 2006, 2007). Following the report that hKSR2 is a negative regulator of Cot1 in transiently transfected cells (Channavajhala et al., 2003) we initiated studies to determine if this applies to endogenous hKSR2 and Cot1 in HL60 and U937 cells, the frequently used in vitro models of human AML (Sundstrom and Nilsson, 1976; Gallagher et al., 1979). First, we demonstrated that in each cell line the expression of Cot1 and hKSR2 both parallel 1,25D-induced differentiation (Fig. 1A) at protein (Fig. 1B) and mRNA levels (Fig. 1C,D), particularly up to 24th hour of exposure to 1,25D, after which hKSR2 tends to become more upregulated than Cot1. Additionally, the kinetics of the upregulation of the mRNA for monocytic marker CD14 tended to approximate the upregulation of the mRNA for hKSR2, consistent with its positive role in 1,25D-induced differentiation (Wang et al., 2007). However, the increase in Cot1 expression in differentiating cells was puzzling in view of our previous finding that the exposure of AML cells to an inhibitor of Cot1 kinase activity, Cot1 Inh, increased the effectiveness of 1,25D as a differentiation agent, suggesting that Cot1 should prevent differentiation. Thus, further exploration by alternative approaches of the findings obtained with its pharmacological inhibitor seemed essential.
Fig. 1.
Protein and mRNA levels of COT1, KSR1, and hKSR2 increase in parallel with 1,25D-induced differentiation of AML cells. A: HL60 and U937 cells were treated with 1 nM 1,25D, and samples were taken at the indicated times to determine the surface differentiation markers of monocyte lineage CD11b and CD14, and the cytoplasmic monocyte-specific enzyme MSE. B: Protein was extracted from these samples for Western blot analysis of the indicated proteins. Crk-L served as a loading control, and optical densities of each signal relative to Crk-L are shown below the band. C: Expression of COT1 and KSR2 mRNA after exposure to 1 nM 1,25D in HL60 cells (bars) and CD14 mRNA (lines). RNA polymerase II (RPII) was used to obtain the relative values for the expression of genes of interest. D: Similar experiments using U937 cells. Mean values ± SD are shown, n = 3.
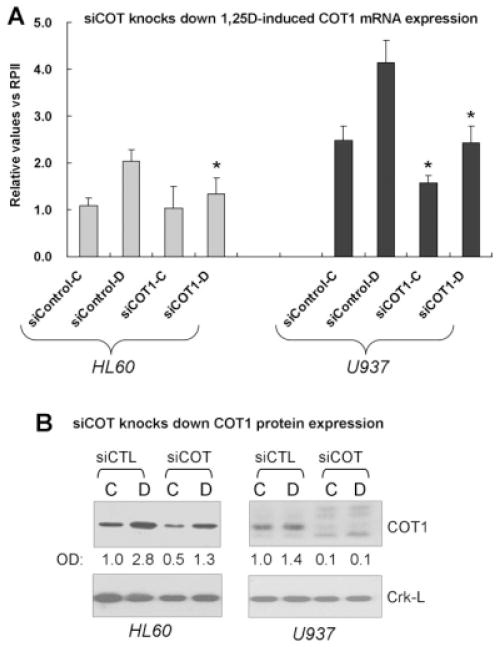
Down-regulation of Cot1 expression enhances 1,25D-induced monocytic differentiation
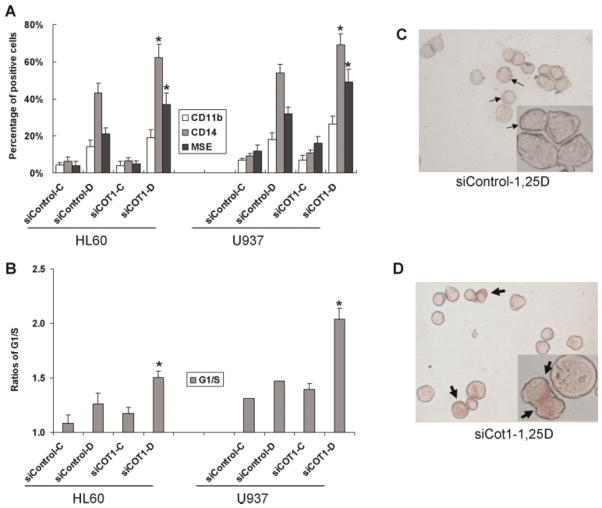
To further our understanding of the role of Cot1 in 1,25D-induced differentiation we reduced the endogenous expression of Cot1 using a pool of siRNA constructs. This was possible to a greater extent in U937 cells (more than 90% knock down at protein level) than in HL60 cells (ca. 50% knock down), since HL60 are well known to be difficult to transfect without inducing major cytotoxicity (Fig. 2, and data not shown). Consistent with the result obtained using Cot1 Inh (Wang and Studzinski, 2010), knock down of Cot1 significantly increased differentiation, as demonstrated by the expression of monocytic surface markers and the cytoplasmic staining for monocyte-specific esterase (MSE) and by the G1 cell cycle arrest, in both HL60 and U937 cells (Fig. 3). Thus, somewhat surprisingly, although Cot1 expression is induced by 1,25D, Cot1 inhibits the effect of 1,25D on differentiation. This could serve to provide a negative feedback that restricts the rate of differentiation to allow coordination of the emergence of different functionalities of the differentiated cells.
Fig. 2.
Inhibition of COT1 mRNA and protein expression by silencing COT1 RNA (siCOT1). A: siRNAs were transfected into cells using Amaxa nucleofector and incubated for 48 h, then 1 nM 1,25D was added for 48 h. The cells were harvested to determine efficiency of knock down of COT1 mRNA. siControl is a non-specific siRNA used here as a control. “C” represents vehicle controls; “D,” cells treated with 1,25D. Asterisks () indicate significant (P < 0.05) difference from cells transfected with control RNA. B: Immunoblots for the target protein after transfection with the silencing RNAs showed that siCOT1 effectively (~50% HL60, ~90% U937 cells) knocked down COT1 protein. OD = optical density signals for COT1 relative to the internal control, Crk-L protein.
Fig. 3.
Inhibition of COT1 expression by siCOT1 enhances 1,25D-induced differentiation. A: Effect of siCOT1 on the expression of differentiation markers CD11b, CD14, and MSE. Cells were exposed to 1 nM 1,25D for 48 h. The asterisk () denotes P < 0.05 for siControl RNA-1,25D versus siCOT1 RNA-1,25D; n = 3. B: The effect of siCOT1 on the ratios of the proportion of cells in the G1 phase compartment to the S phase compartment. The asterisk () denotes P < 0.05 for siControl RNA-1,25D versus siCOT1 RNA-1,25D; n = 3. C: A microscopic field, with a high power inset, showing weak cytoplasmic staining for the differentiation marker, monocyte-specific esterase (thin arrows) in HL60 cells treated with 1 nM 1,25D. D: Cells treated as in (C), but exposed to siCOT1 RNA. Note the increased staining intensity in the cytoplasm (thick arrows). The quantitation of MSE data illustrated here is included in part A. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
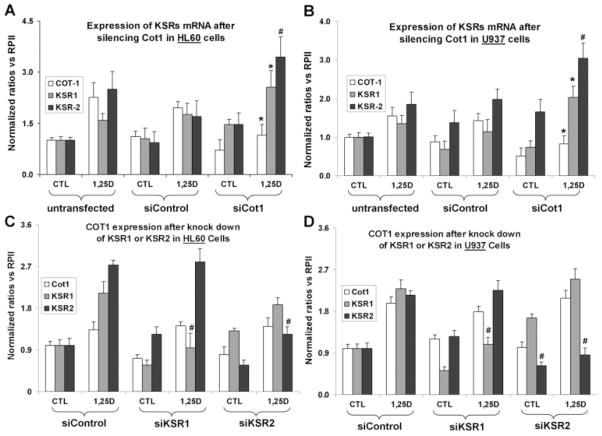
siCot1 increases the expression of KSR scaffold proteins
Since the KSR proteins enhance 1,25D-induced monocytic differentiation and hKSR2 has been reported to interact with Cot1, we tested if the inhibitory effect of Cot1 on differentiation could be due to the repression of KSR expression. Indeed, exposure of both HL60 and U937 cells to siCot1 resulted in a highly significant increase in KSR1 and hKSR2 mRNAs, consistent with the hypothesis that the effects of Cot1 on differentiation are mediated by the KSR proteins (Fig. 4A,B). Conversely, knock down of KSR1 or KSR2 had no effect of the expression of Cot1 (Fig. 4C,D), and this was also observed when antisense oligos to KSR1 and KSR2 were used (data not shown). This shows that unlike in the system studied by (Channavajhala et al., 2003), Cot1 is upstream of hKSR2, not the reverse.
Fig. 4.
Knock down of Cot1 by siCot1 enhances 1,25D-induced expression of KSR1 and hKSR2 mRNA, while the knock down of KSR1 or hKSR2 has no effect on the expression of Cot1. A: Knock down of Cot1 by siCot1 RNA in HL60 cells produced a significant increase in KSR1 and hKSR2 mRNA levels, as determined by RT-qPCR. B: A similar experiment to part A, but using U937 cells. C: Silencing RNAs to KSR1 and KSR2 reduce the 1,25D-induced expression of the cognate KSR mRNAs, but have no effect on the expression of COT-1 mRNA in HL60 or D, U937 cells. The cells were treated with 1 nM 1,25D or vehicle (CTL) for 48 h. The comparisons were made between cells treated with siControl (vector) and target gene (KSR1, KSR2, or Cot1) in cell treated with 1,25D. The asterisk () denotes P < 0.05, and # denotes P < 0. 01, n = 3.
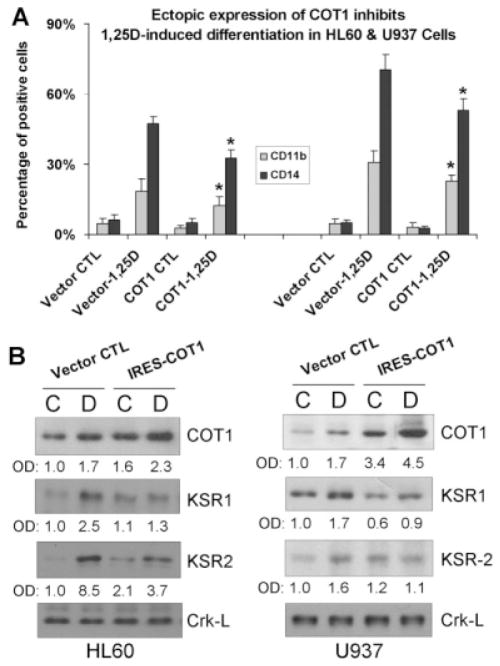
Ectopic expression of Cot1 represses the 1,25D-increased expression of KSR1 and hKSR2, and inhibits 1,25D-induced monocytic differentiation of AML cells
The unexpected results obtained in our studies of the effects of inhibiting the activity, or reducing the expression of Cot1 in AML cells, in which the efficiency of transfection is limited by the fragility of these cells, prompted a confirmation of the key results by the complementary approach of over-expression of Cot1. Transfection of IRES-Cot1 (Fig. 5A), but not of its mutated version (data not shown), resulted in a significantly reduced differentiation response to 1,25D, though the reduction was modest, probably because in cells that already express high levels of Cot1, its over-expression does not markedly influence the downstream signaling events. The extent of the increase in Cot1 protein levels, shown in Figure 5B, indicated that in both HL60 and U937 cells Cot1 levels were considerably increased by transfection of Cot1, though the further increase by exposure of the transfected cells to 1,25D produced a smaller increase, suggesting that there is a cellular “ceiling” on 1,25D-induced Cot1 levels. In contrast, the levels of KSR1 and hKSR2 proteins were decreased (Fig. 5B, lower parts), consistent with the results of experiments in which an increase in KSR1/2 mRNA and protein levels was achieved by the knock down of Cot1 (Figs. 7 and 8).
Fig. 5.
Ectopic expression of Cot1 inhibits 1,25D-induced monocytic differentiation and represses the 1,25D-increased expression of KSR1 and hKSR2 protein. A: HL60 or U937 cells were stably transfected with either vector control, or with the IRES2-Cot1 vector, then 1 nM 1,25D was added for 48 h, and monocytic differentiation markers CD11b and CD14 were determined by flow cytometry. B: The expression of Cot1, KSR1, and KSR2 proteins were determined by Western blots in HL60 and U937 cells. Crk-L protein was used as internal control for gel loading. Experiments were repeated at least three times, and differentiation data are presented as the mean ± SD (n = 3). The asterisk () denotes P < 0.05 for the difference in the expression of differentiation markers in cells transfected with the empty vector or with IRES-Cot1, and then treated with 1,25D.
Fig. 7.
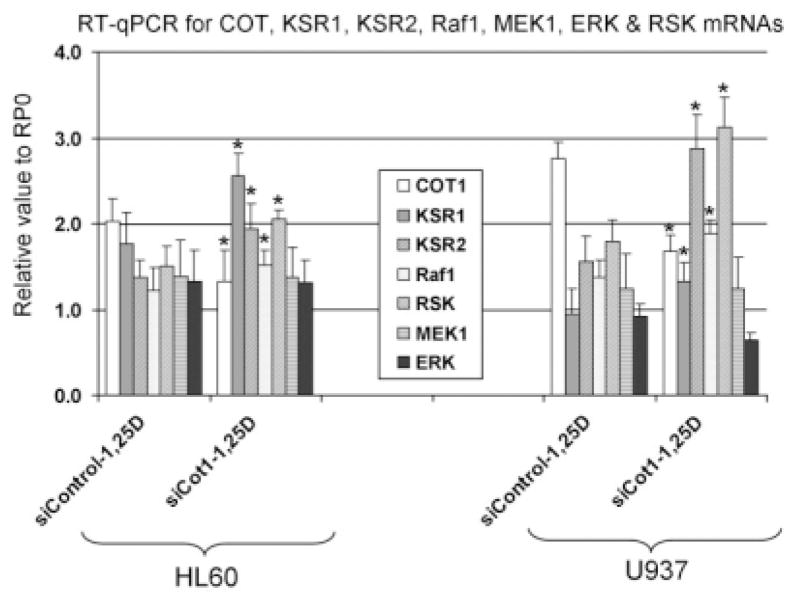
Knock down of Cot1 increases the level of Raf1 and p90RSK mRNAs, but not of MEK1 and ERK mRNAs. Cells were transiently transfected with either siControl, or with the siCOT1 RNA for 48 h, then 1 nM 1,25D was added for 48 h, and mRNA expression of KSRs and the core components of Raf MAP kinase pathway were determined by RT-qPCR.P < 0.05; siControl RNA-1,25D versus siCOT1 RNA-1,25D. n = 3.
Fig. 8.
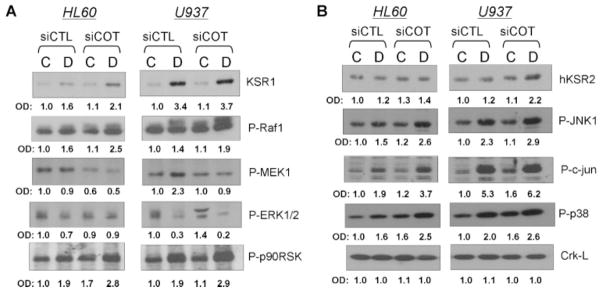
Knock down of COT1 in AML cells by silencing COT1 RNA (siCOT1) enhances Raf, JNK, and p38 MAP kinase pathways. siRNAs were transfected into HL60 and U937 cells using Amaxa nucleofector and incubated for 48 h, then 1 nM 1,25D was added for 48 h to the groups marked “D.” Cells exposed to ethanol, the vehicle control are indicated by “C.” siControl is the non-specific siRNA used here as a control. The levels of proteins activated by phosphorylation were determined by Western blots. A: KSR1 and components of the Raf1 pathway. B: hKSR2 and the “stress-activated” JNK kinase and p38MAPK pathways.
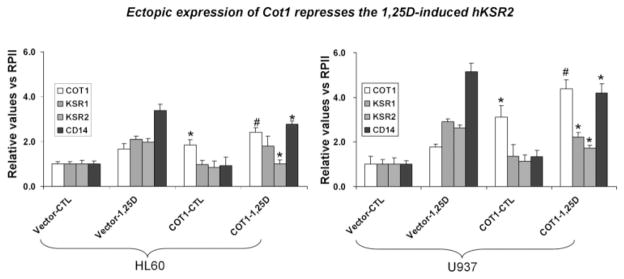
To ensure that the changes in KSR1 and hKSR2 protein levels in the Cot1 over-expression experiments were not just due to protein degradation, we determined the effect of Cot1 transfection on the levels of the cognate mRNAs by RT-qPCR. Figure 6 shows that, as expected, the exposure of either HL60 or U937 cells to 1 nM 1,25D for 48 h increased the mRNA levels for KSR1 and hKSR2, as well as for CD14, the monocytic marker. Importantly, transfection of Cot1 reduced this effect of 1,25D, consistent with the antagonistic effect of Cot1 on differentiation. Although the difference between the vector-only and Cot1-transfected HL60 cells in KSR1 mRNA levels was not significant in these experiments, the sharp decrease in KSR1 protein level in the experiment shown in Figure 5B suggests that here is indeed a decrease in the expression of KSR1 in HL60 cells, as is apparent in U937 cells (Fig. 6). Together, our results show that Cot1 antagonizes differentiation, at least in part, by reducing the expression of KSR1 and hKSR2.
Fig. 6.
Ectopic expression of Cot1 represses the 1,25D-increased expression of KSR1, hKSR2, and CD14 mRNA. HL60 and U937 cells were stably transfected with empty vector or IRES-Cot1 constructs, then 1 nM 1,25D was added for 48 h, and mRNA expression of COT1, KSR1, KSR2, and CD14 (a differentiation maker) was determined by RT-qPCR. The values relative to the expression of RPII are presented as means ± SD (n = 3). P < 0.01, and denotes the statistical significance of the increases in Cot1 mRNA levels between transfected cells (Vector + 1,25D vs. Cot1 + 1,25D); P < 0.05 denotes the statistical significance of the decreases in Cot1 mRNA levels in the same comparison. n = 3.
Cot1 negatively regulates MAPK pathways, principally downstream of Raf1 and JNK1, but excluding the MEK/ERK module
If the effects of Cot1 on differentiation are principally due to the inhibition of KSR function, the knock down of Cot1 should produce effects on MAPK pathways that are opposite to those of KSR knock down, previously shown to include down-regulation of Raf1 and p90RSK (Wang and Studzinski, 2004). We tested this by transfecting siCot1 and measuring the expression of Raf1 and p90RSK. Figure 7 shows that in addition to increasing the levels of KSR1 and KSR2 mRNAs, siCot1 also increased the level of Raf1 and p90RSK mRNAs in both HL60 and U937 cells, consistent with the premise that KSR family members mediate the effects of Cot1 in AML cells treated with 1,25D. However, the expression of MEK1 and ERK 1/2 was not significantly altered.
Activation of MAPK pathways was studied by the phosphorylation status of their selected components. As expected, siCot1 increased the protein levels of KSRs, and enhanced the 1,25D-induced activation of Raf1 and its downstream target p90RSK (Fig. 8A). However, activation of MEK1 and ERK1/2 by siCot1 was not observed. Since these determinations were made 48 h after addition of 1,25D to the cells, this is consistent with the previous demonstration that MEK1 and ERK1/2 do not participate in the later stages of 1,25D-induced monocytic differentiation of AML cells (Wang and Studzinski, 2006; Jamshidi et al., 2008). This confirms that in the second phase of 1,25D-induced differentiation Raf1 and p90RSK are the principal components of this branch of signal transmission network, perhaps by a direct interaction with each other (Wang and Studzinski, 2006), thus bypassing the usually required components of Raf1 signaling, that is, MEK1 and ERK1/2 (Dent et al., 1995; Marais and Marshall, 1996).
In addition, in AML cells exposed to siCot1 there were changes in the activation of representative members of the other main MAPK pathways, JNK1 and p38 (Fig. 8B). When AML cells were exposed to siCot1 each pathway showed an enhancement of the known 1,25D-induced increases in their activity (Wang et al., 2000, 2003; Wang and Studzinski, 2001; Zhang et al., 2010). Although the enhancement was apparent in each cell line, the extent of the increase was different. Thus, the knock down of Cot1 produced relatively greater increase in activation of various MAPKs in the myeloblastic HL60 than in the monoblastic U937 cells, even though transfection of siCot1 was more efficient in U937 cells. Overall, our data are consistent with a negative role of Cot1 on KSR enhancement of MAPK signaling of differentiation, especially in the more primitive HL60 cells.
Discussion
Our results highlight the importance of cell context on MAPK signaling and on its control. Although in studies which employed predominantly rodent cells, Cot1 has been found to be a positive regulator of several members of MAPK cascades including MEK1, SEK1, and p38MAPK (Salmeron et al., 1996; Hagemann et al., 1999; Chiariello et al., 2000), as well as ERK, JNK, and NFkB (Das et al., 2005), our results indicate that in human AML cells undergoing 1,25D-induced monocytic differentiation Cot1 inhibits activation of Raf1, p90RSK, JNK1, c-jun, and p38, though not of MEK1 or ERK1/2.
The differences between the signaling functions of Cot1 in hematopoietic cells and the fibroblasts used in previous studies have already been commented upon (Das et al., 2005). Also, there may be species differences in this regard, as macrophages and B cells from Tpl2−/− mice, missing the rodent homolog of Cot1 (Das et al., 2005), do not appear to have the same dependence on Cot1/Tpl2 signaling as the non-hematopoietic cells. These differences may also be due the fact that the previous studies examined the role of Cot1/Tpl2 in MAPK regulation during cell proliferation and/or neoplastic transformation, while our studies focused on MAPK activity in monocyte/macrophage differentiation, which entails a cessation of cell proliferation, the exact opposite. In this connection, it is interesting to note that our, and the previously reported findings of others (Das et al., 2005), coincide with regard to the MEK/ERK module, which is apparently activated by Cot1/Tpl2 pathway in both scenarios, as siCot1 results in a decrease in phosphorylation of MEK/ERK (Fig. 8).
Based on these data and on our earlier work (Wang and Studzinski, 2004, 2006), we propose that Raf1 can have a dual role in propagating 1,25D-derived signals. First, Raf1 can activate MEK–ERK for transmitting proliferation signals required for lineage amplification during the early stages of monocytic precursor differentiation, as previously reviewed (Gocek and Studzinski, 2009). However, in monocytic differentiation this is observed only in the first 24–36 h after the addition of 1,25D to AML cells, when activated ERK primarily stimulates signals for the maintenance of the cell cycle (Wang and Studzinski, 2001). Second, when abundance of KSR proteins increases due to the stimulation of their expression by 1,25D (Wang and Studzinski, 2004; Wang et al., 2007), and exceeds the levels of Cot1 (Fig. 1B), the KSR scaffolds are more likely to bind to Raf1 and MEK separately, thus diminishing the chance of activating ERK (Kolch, 2005). At this point Raf1 can bind p90RSK more effectively, and the activated p90RSK can promote the expression of proteins required for differentiation, including C/EBPβ (Ji and Studzinski, 2004; Wang and Studzinski, 2006). Thus, when Raf1 signals to p90RSK directly in these cells, differentiation proceeds while cell proliferation stops.
There are other reports of the involvement of Cot1 in differentiation. In PC12 cells Cot1 was found to activate MEK1 and this was linked to the induction of differentiation of these cells (Hagemann et al., 1999), and more recently also to the induction of differentiation of murine monocytes to osteoclasts (Hirata et al., 2010). It is tempting to speculate that this is the obverse of the situation observed here-Cot1 inhibits monocytic differentiation, yet according to Hirata and colleagues promotes further progression of the monocytic phenotype to the more complex phenotype of the osteoclast.
In the current study, we have focused our attention on several upstream components of MAPK pathways in human myeloid leukemia cells, but further studies are needed to unravel how these signals impinge on the transcriptional and epigenetic factors which execute the programs for hematopoietic cell differentiation (e.g., Marcinkowska et al., 2006; Bakshi et al., 2010). In addition, since Cot1 activity promotes tumor cell proliferation, and pharmacological inhibitors of Cot1 kinase activity with exquisite specificity are already available (Gavrin et al., 2005; Hirata et al., 2010), Cot1 warrants timely consideration as a target for anti-cancer treatment.
Acknowledgments
We thank Dr. Milan Uskokovic, BioXell, Nutley, NJ, for the gift of 1,25-dihydroxyvitamin D3. We also thank Dr. Jonathan Harrison and Dr. Elzbieta Gocek for comments on the manuscript. This work was supported by grants RO1-CA-44722-20 and RO1-CA-117942-03 from the NIH/National Cancer Institute.
Abbreviations
- AML
acute myeloid leukemia
- Cot1 Inh
4-(3-chloro-4-fluorophenylamino)-6-(pyridin-3-yl-methylamino-3-cyano-[1-7]-naphthyridine (Cot Inh)
- 1,25D
1,25-dihydroxyvitamin D3
- KSR
kinase suppressor of Ras
- MSE
monocyte-specific esterase
- RAR
retinoic acid receptor
- siCot1
silencing Cot construct
- TF
transcription factor
Literature Cited
- Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R, Hassan MQ, Pratap J, Lian JB, Montecino MA, van Wijnen AJ, Stein JL, Imbalzano AN, Stein GS. The human SWI/SNF complex associates with RUNX1 to control transcription of hematopoietic target genes. J Cell Physiol. 2010;225:569–576. doi: 10.1002/jcp.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer TM, Munar M, Henner WD. A Phase I trial of pulse calcitriol in patients with refractory malignancies: Pulse dosing permits substantial dose escalation. Cancer. 2001;91:2431–2439. [PubMed] [Google Scholar]
- Channavajhala PL, Wu L, Cuozzo JW, Hall JP, Liu W, Lin LL, Zhang Y. Identification of a novel human kinase supporter of Ras (hKSR-2) that functions as a negative regulator of Cot (Tpl2) signaling. J Biol Chem. 2003;278:47089–47097. doi: 10.1074/jbc.M306002200. [DOI] [PubMed] [Google Scholar]
- Channavajhala PL, Rao VR, Spaulding V, Lin LL, Zhang YG. hKSR-2 inhibits MEKK3-activated MAP kinase and NF-kappaB pathways in inflammation. Biochem Biophys Res Commun. 2005;334:1214–1218. doi: 10.1016/j.bbrc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Zhu YJ, Tong JH, Dong S, Huang W, Chen Y, Xiang WM, Zhang L, Li XS, Qian GQ. Rearrangements in the second intron of the RARA gene are present in a large majority of patients with acute promyelocytic leukemia and are used as molecular marker for retinoic acid-induced leukemic cell differentiation. Blood. 1991;78:2696–2701. [PubMed] [Google Scholar]
- Chen-Deutsch X, Garay E, Zhang J, Harrison JS, Studzinski GP. c-Jun N-terminal kinase 2 (JNK2) antagonizes the signaling of differentiation by JNK1 in human myeloid leukemia cells resistant to vitamin D. Leuk Res. 2009;33:1372–1378. doi: 10.1016/j.leukres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiariello M, Marinissen MJ, Gutkind JS. Multiple mitogen-activated protein kinase signaling pathways connect the cot oncoprotein to the c-jun promoter and to cellular transformation. Mol Cell Biol. 2000;20:1747–1758. doi: 10.1128/mcb.20.5.1747-1758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko M, Studzinski GP. Enhancement by other compounds of the anti-cancer activity of vitamin D3 and its analogs. Exp Cell Res. 2004;298:339–358. doi: 10.1016/j.yexcr.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001;93:1224–1233. doi: 10.1093/jnci/93.16.1224. [DOI] [PubMed] [Google Scholar]
- Das S, Cho J, Lambertz I, Kelliher MA, Eliopoulos AG, Du K, Tsichlis PN. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280:23748–23757. doi: 10.1074/jbc.M412837200. [DOI] [PubMed] [Google Scholar]
- Degos L, Wang ZY. All trans retinoic acid in acute promyelocytic leukemia. Oncogene. 2001;20:7140–7145. doi: 10.1038/sj.onc.1204763. [DOI] [PubMed] [Google Scholar]
- Dent P, Reardon DB, Morrison DK, Sturgill TW. Regulation of Raf-1 and Raf-1 mutants by Ras-dependent and Ras-independent mechanisms in vitro. Mol Cell Biol. 1995;15:4125–4135. doi: 10.1128/mcb.15.8.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- Garner CM, Hubbold LM, Chakraborti PR. Mycoplasma detection in cell cultures: A comparison of four methods. Br J Biomed Sci. 2000;57:295–301. [PubMed] [Google Scholar]
- Gavrin LK, Green N, Hu Y, Janz K, Kaila N, Li HQ, Tam SY, Thomason JR, Gopalsamy A, Ciszewski G, Cuozzo JW, Hall JP, Hsu S, Telliez JB, Lin LL. Inhibition of Tpl2 kinase and TNF-alpha production with 1,7-naphthyridine-3-carbonitriles: Synthesis and structure-activity relationships. Bioorg Med Chem Lett. 2005;15:5288–5292. doi: 10.1016/j.bmcl.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci. 2009;46:190–209. doi: 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Troppmair J, Rapp UR. Cot protooncoprotein activates the dual specificity kinases MEK-1 and SEK-1 and induces differentiation of PC12 cells. Oncogene. 1999;18:1391–1400. doi: 10.1038/sj.onc.1202431. [DOI] [PubMed] [Google Scholar]
- Hirata K, Taki H, Shinoda K, Hounoki H, Miyahara T, Tobe K, Ogawa H, Mori H, Sugiyama E. Inhibition of tumor progression locus 2 protein kinase suppresses receptor activator of nuclear factor-kappaB ligand-induced osteoclastogenesis through down-regulation of the c-Fos and nuclear factor of activated T cells c1 genes. Biol Pharm Bull. 2010;33:133–137. doi: 10.1248/bpb.33.133. [DOI] [PubMed] [Google Scholar]
- Jamshidi F, Zhang J, Harrison JS, Wang X, Studzinski GP. Induction of differentiation of human leukemia cells by combinations of COX inhibitors and 1,25-dihydroxyvitamin D3 involves Raf1 but not Erk 1/2 signaling. Cell Cycle. 2008;7:917–924. doi: 10.4161/cc.7.7.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Studzinski GP. Retinoblastoma protein and CCAAT/enhancer-binding protein beta are required for 1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004;64:370–377. doi: 10.1158/0008-5472.can-03-3029. [DOI] [PubMed] [Google Scholar]
- Koeffler HP, Hirji K, Itri L. 1,25-Dihydroxyvitamin D3: In vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat Rep. 1985;69:1399–1407. [PubMed] [Google Scholar]
- Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H, Appelbaum FR, Dohner H, Antin JH, Soiffer RJ, Cutler C. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R, Marshall CJ. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- Marcinkowska E, Wiedlocha A, Radzikowski C. 1,25-Dihydroxyvitamin D3 induced activation and subsequent nuclear translocation of MAPK is upstream regulated by PKC in HL-60 cells. Biochem Biophys Res Commun. 1997;241:419–426. doi: 10.1006/bbrc.1997.7832. [DOI] [PubMed] [Google Scholar]
- Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBPbeta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006;312:2054–2065. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi J, Higashi T, Mukai H, Ohuchi T, Kakunaga T. Structure and transforming potential of the human cot oncogene encoding a putative protein kinase. Mol Cell Biol. 1991;11:4088–4096. doi: 10.1128/mcb.11.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Ohki M, Nakagawa T, Honma Y. Glucocorticoids induce apoptosis in acute myeloid leukemia cell lines with a t(8;21) chromosome translocation. Leuk Res. 1997;21:45–50. doi: 10.1016/s0145-2126(96)00089-6. [DOI] [PubMed] [Google Scholar]
- Munker R, Norman A, Koeffler HP. Vitamin D compounds. Effect on clonal proliferation and differentiation of human myeloid cells. J Clin Invest. 1986;78:424–430. doi: 10.1172/JCI112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriotis C, Makris A, Bear SE, Tsichlis PN. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc Natl Acad Sci USA. 1993;90:2251–2255. doi: 10.1073/pnas.90.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron A, Ahmad TB, Carlile GW, Pappin D, Narsimhan RP, Ley SC. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 1996;15:817–826. [PMC free article] [PubMed] [Google Scholar]
- Schlenk RF, Frohling S, Hartmann F, Fischer JT, Glasmacher A, del Valle F, Grimminger W, Gotze K, Waterhouse C, Schoch R, Pralle H, Mergenthaler HG, Hensel M, Koller E, Kirchen H, Preiss J, Salwender H, Biedermann HG, Kremers S, Griesinger F, Benner A, Addamo B, Dohner K, Haas R, Dohner H. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia. 2004;18:1798–1803. doi: 10.1038/sj.leu.2403528. [DOI] [PubMed] [Google Scholar]
- Shabtay A, Sharabani H, Barvish Z, Kafka M, Amichay D, Levy J, Sharoni Y, Uskokovic MR, Studzinski GP, Danilenko M. Synergistic antileukemic activity of carnosic acid-rich rosemary extract and the 19-nor Gemini vitamin D analogue in a mouse model of systemic acute myeloid leukemia. Oncology. 2008;75:203–214. doi: 10.1159/000163849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabani H, Izumchenko E, Wang Q, Kreinin R, Steiner M, Barvish Z, Kafka M, Sharoni Y, Levy J, Uskokovic M, Studzinski GP, Danilenko M. Cooperative antitumor effects of vitamin D3 derivatives and rosemary preparations in a mouse model of myeloid leukemia. Int J Cancer. 2006;118:3012–3021. doi: 10.1002/ijc.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski GP, Bhandal AK, Brelvi ZS. A system for monocytic differentiation of leukemic cells HL 60 by a short exposure to 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1985;179:288–295. doi: 10.3181/00379727-179-42098. [DOI] [PubMed] [Google Scholar]
- Studzinski GP, Wang X, Ji Y, Wang Q, Zhang Y, Kutner A, Harrison JS. The rationale for deltanoids in therapy for myeloid leukemia: Role of KSR-MAPK-C/EBP pathway. J Steroid Biochem Mol Biol. 2005;97:47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Nabhan C, Feusner JH, Rowe JM. Acute promyelocytic leukemia: Evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishii Y, Suda T. 1 alpha,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60) Biochem J. 1982;204:713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001;80:471–482. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Kinase suppressor of RAS (KSR) amplifies the differentiation signal provided by low concentrations 1,25-dihydroxyvitamin D3. J Cell Physiol. 2004;198:333–342. doi: 10.1002/jcp.10443. [DOI] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Raf-1 signaling is required for the later stages of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells but is not mediated by the MEK/ERK module. J Cell Physiol. 2006;209:253–260. doi: 10.1002/jcp.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Expression of MAP3 kinase COT1 is up-regulated by 1,25-dihydroxyvitamin D3 in parallel with activated c-jun during differentiation of human myeloid leukemia cells. J Steroid Biochem Mol Biol. 2010;121:395–398. doi: 10.1016/j.jsbmb.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gardner JP, Kheir A, Uskokovic MR, Studzinski GP. Synergistic induction of HL60 cell differentiation by ketoconazole and 1-desoxy analogues of vitamin D3. J Natl Cancer Inst. 1997;89:1199–1206. doi: 10.1093/jnci/89.16.1199. [DOI] [PubMed] [Google Scholar]
- Wang X, Rao J, Studzinski GP. Inhibition of p38 MAP kinase activity up-regulates multiple MAP kinase pathways and potentiates 1,25-dihydroxyvitamin D3-induced differentiation of human leukemia HL60 cells. Exp Cell Res. 2000;258:425–437. doi: 10.1006/excr.2000.4939. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang X, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D3. J Cell Biochem. 2003;89:1087–1101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang TT, White JH, Studzinski GP. Induction of kinase suppressor of RAS-1(KSR-1) gene by 1, alpha25-dihydroxyvitamin D3 in human leukemia HL60 cells through a vitamin D response element in the 5′-flanking region. Oncogene. 2006;25:7078–7085. doi: 10.1038/sj.onc.1209697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang TT, White JH, Studzinski GP. Expression of human kinase suppressor of Ras 2 (hKSR-2) gene in HL60 leukemia cells is directly upregulated by 1,25-dihydroxyvitamin D3 and is required for optimal cell differentiation. Exp Cell Res. 2007;313:3034–3045. doi: 10.1016/j.yexcr.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Harrison JS, Uskokovic M, Danilenko M, Studzinski GP. Silibinin can induce differentiation as well as enhance vitamin D3-induced differentiation of human AML cells ex vivo and regulates the levels of differentiation-related transcription factors. Hematol Oncol. 2010a;28:124–132. doi: 10.1002/hon.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Harrison JS, Studzinski GP. Isoforms of p38MAPK gamma and delta contribute to differentiation of human AML cells induced by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2010b doi: 10.1016/j.yexcr.2010.08.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]