During the course of this century, increasing human population and economic development will continue to put pressure on agricultural systems for increased crop yields (Rosegrant and Cline, 2003; Foley et al., 2005). Grains produced for livestock feed and biofuels are now competing for space with crops intended for human consumption (Foley et al., 2011), which means that in order to meet increasing global food demand and multiple objectives for arable land, new crop varieties with improved performance will have to be developed. At the same time, the use of fertilization, irrigation, and pesticides is very likely to increase (Oerke, 2006; Jaggard et al., 2010). The intensification of agricultural practices, however, creates environmental and health concerns, which are already a matter of significant scientific and social debate. Alternative methods to increase crop yields without agricultural expansion and under conditions of reduced chemical use are urgently required.
A particular concern for the growing intensity of agricultural practices is the management of pests and diseases, which can account for up to 25% of preharvest crop losses in agricultural areas managed with well-developed crop protection technologies (Pimentel, 1997; Oerke, 2006). In order to minimize these losses, expensive and potentially hazardous chemical control strategies are routinely applied, which are becoming increasingly regulated due to their negative impacts on human health and ecosystems (Birch et al., 2011). Furthermore, a recent analysis suggests that despite the massive (greater than 15-fold) increase in the use of pesticides during the past four decades, the overall proportion of crop losses to pests and diseases has not decreased (Oerke, 2006). It is interesting that while these levels of crop losses in agroecosystems are maintained at a high cost, in natural ecosystems plant biomass losses due to insect herbivory are typically fairly small (approximately 10%; Schoonhoven et al., 2005). It seems possible that lessons could be learned from natural defense mechanisms to reduce negative impacts of pests and pathogens and, consequently, the inputs of toxic chemicals used to protect crops and crop yield from biotic stressors. Indeed, plants have demonstrated a broad repertoire of extremely effective tactics that allow them to fight off natural enemies (Karban and Baldwin, 1997; Schoonhoven et al., 2005; Jones and Dangl, 2006; Wu and Baldwin, 2010; Agrawal and Heil, 2012).
The mechanisms responsible for plant defense are becoming increasingly well understood at the molecular level (Jones and Dangl, 2006; Howe and Jander, 2008; Panstruga et al., 2009; Wu and Baldwin, 2010). Major hormonal players in the regulation of immune responses have been identified (Erb et al., 2012; Pieterse et al., 2012), and the functions of defense-related molecules produced by plants are gradually becoming elucidated (D’Auria and Gershenzon, 2005; Mithöfer and Boland, 2012). Our understanding of the regulation of defense systems by environmental signals or conditions is also increasing rapidly, and the discipline of plant biology is creating the knowledge base and conceptual foundation for the purposeful utilization of natural defense mechanisms in agriculture.
Light has emerged as a key modulator of plant immunity. However, until very recently, the beneficial effects of light on plant resistance to pests and disease have been largely unappreciated. In this Update, we briefly highlight recent findings in this area, focusing on how changes in the canopy light environment, caused by the proximity of other plants, regulate plant immunity. We propose that a better understanding of the underlying mechanisms is essential for the development of healthier crop systems necessary for reducing the environmental costs of modern agriculture.
PROXIMITY EFFECTS
A major imperative for modern agriculture is to increase crop yield per unit area, due to the escalating demand of agricultural commodities and the simultaneous need for preservation of natural ecosystems. A common strategy in the management of many crops for enhancing yield has been the implementation of practices that increase canopy light interception, such as higher planting density, reduced row spacing, and fertilization (Harder et al., 2007; De Bruin and Pedersen, 2008). All of these practices, which result in increased leaf area index (LAI), can substantially reduce individual plant exposure to solar radiation, particularly early in the growing season (Flénet et al., 1996; Maddonni et al., 2001).
The effects of plant density on the severity of plant disease caused by microbial pathogens are well documented in natural and managed ecosystems (Burdon and Chilvers, 1975; Augspurger and Kelly, 1984; Bell et al., 2006; Jurke and Fernando, 2008), with a general pattern of increased disease at high plant densities (for review, see Burdon and Chilvers, 1982; Alexander and Holt, 1998; Gilbert, 2002). Similarly, high density and shading frequently increase plant damage by herbivorous insects and/or reduce the expression of antiherbivore defenses (Karban et al., 1989; Cipollini and Bergelson, 2001, 2002; Yamamura, 2002; Roberts and Paul, 2006; Agrawal et al., 2012). The mechanisms that mediate these effects of crowding on plant health are elusive. In the case of microbial pathogens, microenvironmental factors such as air humidity and leaf surface wetness are likely to play an important role. However, several studies have shown that infection by a range of pathogens, and the success of several insect herbivores, can be affected by the light environment of the host before contact with the consumer organism (Roberts and Paul, 2006). In the following sections, we will discuss how changes in the canopy light environment, caused by increased LAI and perceived by specific photoreceptors, affect the expression of plant defenses against biotic stressors.
UV-B AND UVR8
Overcrowding, air pollution, and poorly lit urban environments are widely accepted to have played a role in the increased incidence of rickets, a bone disorder of childhood, during the early days of the industrial revolution in the 19th century (Holick, 2004). A link between sunlight deprivation and rickets was originally postulated in the early 1800s and experimentally confirmed nearly a century later by studies showing that the disease could be cured by exposing children with rickets to the radiation produced by a mercury arc lamp. These observations led, perhaps, to the first appreciation of the beneficial effect of sunlight and UV radiation on human health, an effect that is now documented and mechanistically linked with the photosynthesis of vitamin D driven by UV-B radiation (280–315 nm; Holick, 2004, 2007; Juzeniene et al., 2011).
Positive effects of UV-B radiation on plant health have also been demonstrated, in spite of the fact that the vast majority of the studies of UV-B impacts were originally aimed to detect deleterious effects of UV-B on plant growth. Most examples of “beneficial” effects of UV-B on plants come from studies of plant-herbivore interactions. Field experiments in which losses to phytophagous insects were compared between plants grown under either ambient or attenuated levels of solar UV-B radiation have shown that plant exposure to solar UV-B radiation typically increases plant resistance to insect herbivory (for review, see Caldwell et al., 2003; Ballaré et al., 2011; Kuhlmann and Müller, 2011; Fig. 1). UV-B radiation has also been reported to increase plant resistance to microbial pathogens (Gunasekera et al., 1997; Wargent et al., 2006; Gunasekera and Paul, 2007; Kunz et al., 2008; Demkura and Ballaré, 2012) and can interact with the plant to modify the composition of microbial communities in the phyllosphere (Kadivar and Stapleton, 2003; Balint-Kurti et al., 2010). Treatment with unnaturally high doses of UV-B radiation, or with UV wavelengths not present in the daylight spectrum (UV-C, λ < 280 nm), has also been reported to activate defense-related pathways in several species (Bridge and Klarman, 1973; Brederode et al., 1991; Conconi et al., 1996; Mert-Turk et al., 2003; Glawischnig, 2007); however, the ecological significance of these responses is not clear.
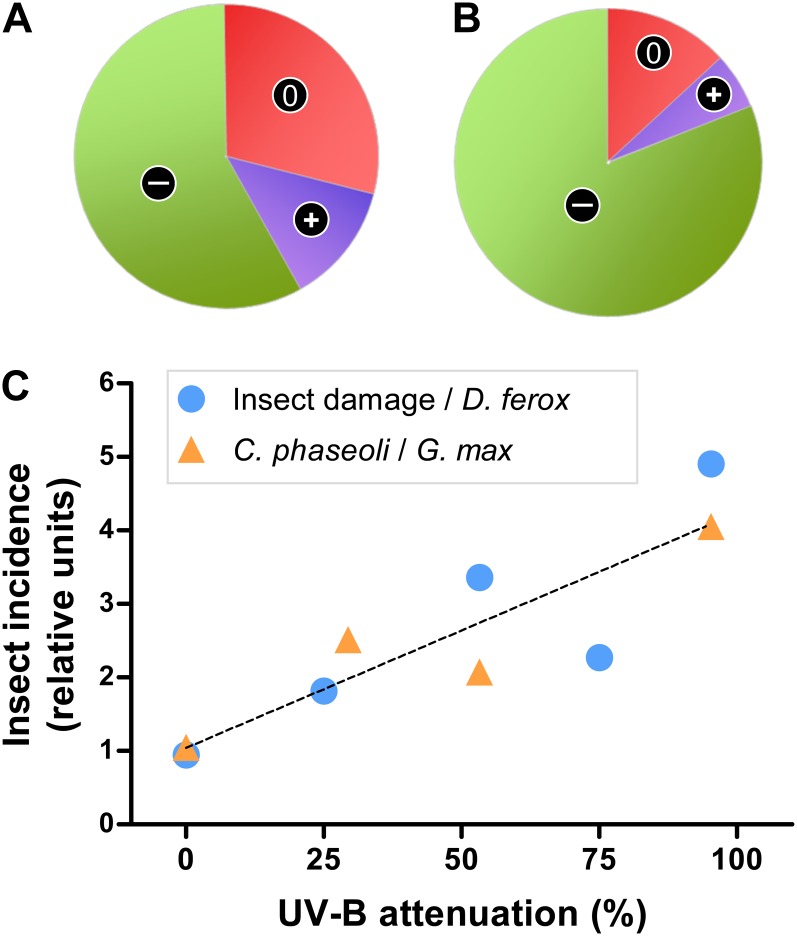
Figure 1.
Solar UV-B radiation increases plant resistance to herbivorous insects in the field. A, Fraction of published studies showing that UV-B radiation reduced (−), increased (+), or did not affect (0) herbivory levels or insect performance (adapted from Kuhlmann and Müller, 2011). B, Same as in A, but analysis restricted to studies that tested the effects of solar (ambient) UV-B radiation (i.e. excluding UV-B lamp supplementation studies). C, Quantitative relationship between solar UV-B attenuation and insect herbivory under field conditions. UV-B attenuation, which was accomplished with plastic films that reduced the UV-B component of solar radiation with minimal effects on other wavelengths, increased the density of insect herbivores (Caliothrips phaseoli) in a soybean canopy and damage caused by leaf beetles on Datura ferox plants. Zero percent attenuation corresponds to full sunlight. Primary data can be found in Ballaré et al. (1996) and Mazza et al. (1999).
The effect of natural levels of UV-B radiation boosting plant resistance to biotic stress has been linked with increased expression of jasmonate (JA) responses and with the induction of increased levels of phenolic compounds. Indirect evidence for a connection with JA responses originated from the observation that plant exposure to solar UV-B can increase the expression of JA-responsive markers, such as proteinase inhibitors, in tomato (Solanum lycopersicum) and Nicotiana spp. (Stratmann et al., 2000; Izaguirre et al., 2003; Demkura et al., 2010). However, synergistic effects of UV-B and JA elicitation have not been observed for several classic markers of the JA response in Arabidopsis (Arabidopsis thaliana; Demkura and Ballaré, 2012). Similarly, mixed results have been obtained regarding the effects of UV-B radiation on JA accumulation (A-H-Mackerness et al., 1999; Demkura et al., 2010). The strongest evidence for a role of JA in some defense responses triggered by natural doses of UV-B radiation comes from studies using mutants or transgenic lines impaired in JA biosynthesis or signaling. Disruption of the JA signaling pathway in Arabidopsis (Caputo et al., 2006) and Nicotiana attenuata (Demkura et al., 2010) was shown to eliminate the effect of solar UV-B inducing plant protection against herbivorous insects. In contrast, a recent case was reported in which a positive effect of UV-B radiation on plant resistance against pathogens was conserved in JA-response mutants (Demkura and Ballaré, 2012), suggesting that UV-B radiation likely influences plant defense via multiple pathways.
Soluble phenolic compounds typically accumulate in plants exposed to solar UV-B radiation and play a central role in photoprotection (Caldwell et al., 1983; Mazza et al., 2000; Kotilainen et al., 2009). Interestingly, it has been noted that some of the phenolic compounds that are induced by UV-B exposure are also induced by insect herbivory and other biotic stressors (Izaguirre et al., 2007; Demkura et al., 2010). The partial convergence in the induction of phenolic metabolites, and the fact that some of these compounds may have a role as defenses against pests and pathogens, have lent support to the idea that at least part of the effect of UV-B radiation on plant resistance to biotic stressors is mediated by the enhanced production of phenylpropanoid derivatives. For example, enhancement of JA- and insect-induced phenolic responses by solar UV-B radiation has been documented for conjugated polyamines in N. attenuata (Demkura et al., 2010). Recent studies in this species have identified MYB8 as a critical transcription factor controlling polyamine synthesis in response to herbivory (Onkokesung et al., 2012), and the MYB8 gene is known to be positively regulated at the transcriptional level by UV-B radiation (Pandey and Baldwin, 2008). It is worth noting that stress-specific alterations in phenolic profiles have also been reported (Demkura et al., 2010; Kuhlmann and Müller, 2011): for example, flavonoids and sinapates typically accumulate in response to UV-B irradiation but not in response to herbivory or JA treatments (Demkura et al., 2010; Demkura and Ballaré, 2012).
The mechanism of perception of UV-B radiation is becoming increasingly elucidated (Jenkins, 2009; Heijde and Ulm, 2012), and a UV-B photoreceptor, UV-RESISTANCE LOCUS8 (UVR8), has been recently characterized at the molecular level (Rizzini et al., 2011). UVR8 is a β-propeller protein that under UV-B-free light conditions exists as a homodimer. Absorption of UV-B quanta by UVR8 induces instant monomerization of the photoreceptor, its accumulation in the nucleus, and interaction with the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC1 (Rizzini et al., 2011), which is required for UV-B-stimulated gene activation in light-grown seedlings (Oravecz et al., 2006). This increased understanding of UV-B perception will greatly facilitate research on the mechanism by which UV-B radiation affects the expression of plant immune responses.
Whereas the role of UVR8 in UV-B-induced gene expression is now well established (Jenkins, 2009; Heijde and Ulm, 2012), the participation of this photoreceptor in the activation of plant defenses against biotic stress requires further study. Conceivably, the effects of UV-B on plant defense might also result from pleiotropic consequences of UV-induced cellular damage and activation of a “generalized” stress response (Brown and Jenkins, 2008; González Besteiro et al., 2011). Nevertheless, it is important to note that, under field conditions, strong impacts on plant defense and health are induced by relatively low UV-B irradiances (less than 1% of the total short-wave sunlight photon flux), which do not cause visible stress symptoms or plant damage (discussed in Demkura et al., 2010). Therefore, a specific, photoreceptor-activated signaling pathway is likely to be involved in the regulation of plant immunity by UV-B radiation.
Recently, activation of UVR8 was linked to the production of chemicals involved in plant defense against pathogens (Demkura and Ballaré, 2012; Fig. 2). Small, ecologically meaningful doses of UV-B radiation (approximately 1 µmol m−2 s−1: λ > 290 nm) were shown to increase the resistance of Arabidopsis plants to the necrotrophic fungus Botrytis cinerea in a UVR8-dependent manner. The same study showed that UVR8 was required for UV-B-induced accumulation of sinapates (sinapoyl malate and sinapoyl Glc) and that the UV-B effect on fungal resistance was absent in fah1, a mutant deficient in ferulic acid 5-hydroxylase, which is essential for sinapate biosynthesis. Sinapates are important precursors in the synthesis of syringyl-type (“defense”) lignin, which is used to strengthen the plant cell walls and prevent penetration by fungal hyphae (Lloyd et al., 2011). Collectively, these results indicate that UVR8 plays an important role in mediating the effects of solar UV-B radiation on pathogen resistance by controlling the expression of the sinapate biosynthetic pathway. The UV-B effect on plant resistance to B. cinerea was conserved in transparent testa4 (deficient in chalcone synthase), which suggests that this effect is likely independent of UV-B-induced flavonoid accumulation (Demkura and Ballaré, 2012). However, it is important to note that flavonoid biosynthesis is also controlled by UVR8 (Jenkins, 2009) and that flavonoids can play a role in plant defense against insect herbivores (Hoffmann-Campo et al., 2001). Studies under artificial laboratory conditions show that cellular responses triggered by microbe-associated molecular patterns (such as the flagellin-derived elicitor peptide flg22) can depress flavonoid responses triggered by UV-B radiation, presumably to save phenylpropanoid precursors for the synthesis of lignin and other antimicrobial compounds (Schenke et al., 2011; Serrano et al., 2012). If corroborated in the field, these studies suggest the potential for significant interactions between different biotic stressors (e.g. pathogens and herbivores) mediated by the regulation of plant responses to UV-B radiation.
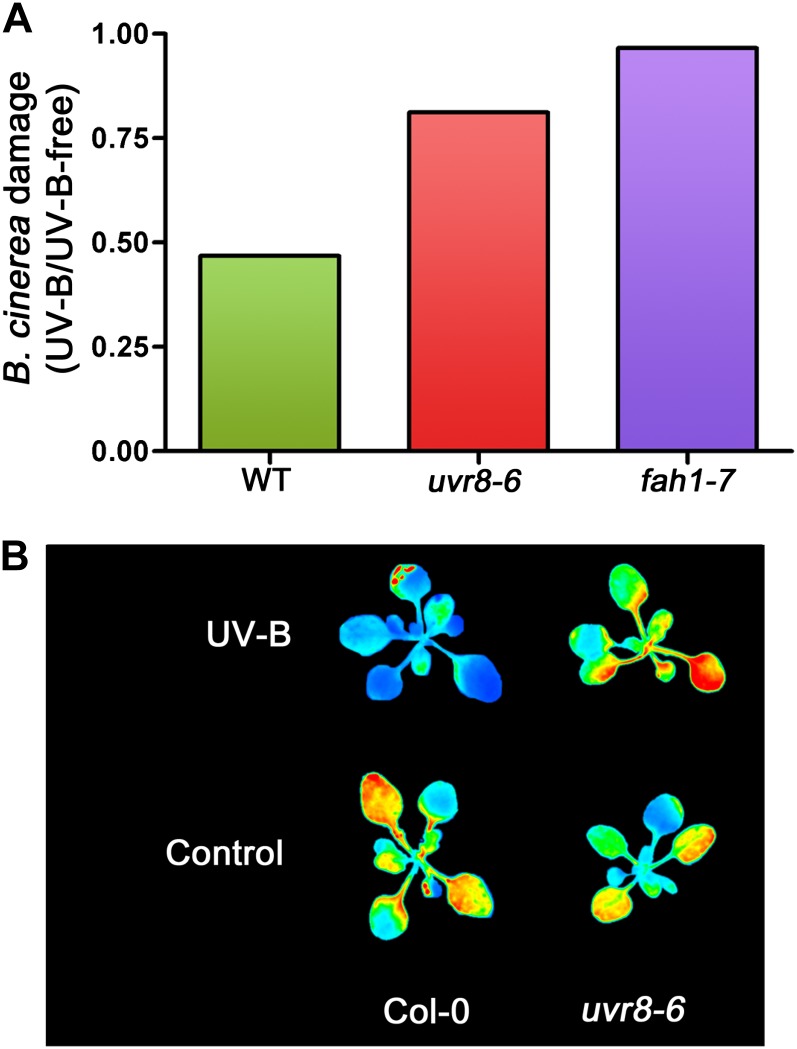
Figure 2.
UV-B radiation increases Arabidopsis resistance to the necrotrophic pathogen B. cinerea; this effect is mediated by UVR8 via increases in sinapate accumulation. Original data can be found in Demkura and Ballaré (2012). A, Damage by B. cinerea in plants grown with exposure to UV-B radiation relative to damage in control plants grown under UV-B-free conditions for wild type (WT) ecotype Columbia (Col-0) plants and mutants deficient in UV-B perception (uvr8-6; Favory et al., 2009) and sinapate biosynthesis (fah1-7; Meyer et al., 1996). B, UV-B induces the accumulation of phenolic sunscreens (mostly flavonoids and sinapates) in a UVR8-dependent manner. Sunscreen accumulation is revealed here by a decrease in the intensity of UV-induced chlorophyll fluorescence. Blue color indicates higher accumulation of phenolic compounds.
The UVR8 pathway has not been directly linked with the regulation of any of the principal hormonal pathways involved in plant defense (i.e. JA and salicylate [SA]) as of yet. Demkura and Ballaré (2012) showed that the UVR8 effect on Arabidopsis resistance to B. cinerea was independent of JA signaling, but because interactions between natural levels of UV-B radiation and JA-induced responses have been reported in other studies (see above), further work is needed to establish the influence of UVR8 activation on the regulation of defense signaling pathways.
As discussed previously, increased crop density has strong effects reducing the penetration of sunlight (including UV-B) into the canopy. The extent to which such a reduction in UV-B radiation affects plant defense needs to be quantified. All studies to date that manipulated UV-B levels have used filters placed above the canopy, which do not strictly mimic the effects of neighboring plants on the UV-B fluxes received by different plant organs. In spite of these limitations, recent UV-B exclusion experiments carried out with soybean (Glycine max) crops demonstrated that the well-documented negative effect of solar UV-B radiation on crop yield was reversed when soybean pests were not controlled by pesticide applications (Mazza et al., 2012). This observation suggests that natural levels of UV-B radiation may have a significant effect protecting crop plants from insect pests under field conditions, presumably through mechanisms involving UV-B-induced enhancement of plant defenses. The potential value of capitalizing on plants’ natural responses to increased defenses with exposure to solar UV-B radiation should not be underestimated and could serve as a starting point for strategic crop improvement for maximizing these natural defense mechanisms. Moreover, some of the compounds that are induced by ambient levels of UV-B radiation, such as phenolic compounds (Mazza et al., 2000; Wargent et al., 2006; Berli et al., 2008) and antioxidants (Giordano et al., 2004), may have nutraceutical and organoleptic value (Jansen et al., 2008; Schreiner et al., 2012). Therefore, in horticultural crops, manipulation of plant responses to solar UV-B radiation could have significant implications for food quality.
RED TO FAR-RED RATIO AND PHYTOCHROME B
The red (R) to far-red (FR) ratio (R:FR) of sunlight is a critical environmental signal for plants. Low values of R:FR indicate the proximity of other plants, because plant tissues strongly absorb R photons, while FR quanta are either reflected or transmitted (Ballaré et al., 1990). In crop canopies, the R:FR of horizontally propagated radiation is directly related to the LAI of the plant stand. Plants sense the changes in R:FR using the phytochromes, particularly phytochrome B (phyB). Low R:FR values inactivate phyB by transforming the active form of the photoreceptor, Pfr, into the inactive from, Pr (Smith, 1995). Upon inactivation of phyB, basic helix-loop-helix transcription factors known as phytochrome-interacting factors accumulate in a dephosphorylated form and activate the expression of growth-promoting genes (Lorrain et al., 2008; Hornitschek et al., 2012; Li et al., 2012). This transcriptional reprogramming leads to increased production and activity of growth hormones such as auxins and GAs, which accelerate cell expansion and promote the elongation of stems and petioles (Djakovic-Petrovic et al., 2007; Tao et al., 2008; Keuskamp et al., 2010; Hornitschek et al., 2012; Li et al., 2012). For plants, a fast rate of elongation is essential for rapid colonization of the upper canopy strata and a central element of a suite of phenotypic changes that are collectively known as the shade-avoidance syndrome (SAS; Franklin, 2008; Ballaré, 2009).
Whereas R:FR, perceived by phyB, plays a well-known role in neighbor detection and the elicitation of adaptive morphological responses (Ballaré, 1999; Dorn et al., 2000), its role as a modulator of defense expression is only beginning to be appreciated. A high R:FR (typical of open canopies) is a positive regulator of plant defense against herbivorous insects. Experiments with cucumber (Cucumis sativus), tomato, N. attenuata, and Arabidopsis demonstrate that inactivation of phyB, either by mutation or by exposing the plants to supplemental FR to lower the R:FR, decreases plant resistance to herbivory (McGuire and Agrawal, 2005; Izaguirre et al., 2006; Moreno et al., 2009; Fig. 3). Down-regulation of the expression of antiherbivory traits under low R:FR has been proposed to be a key element in the mechanism by which plants resolve the tradeoff between resource allocation to growth or defense (i.e. the “dilemma” of plants; Ballaré, 2009; Moreno et al., 2009).
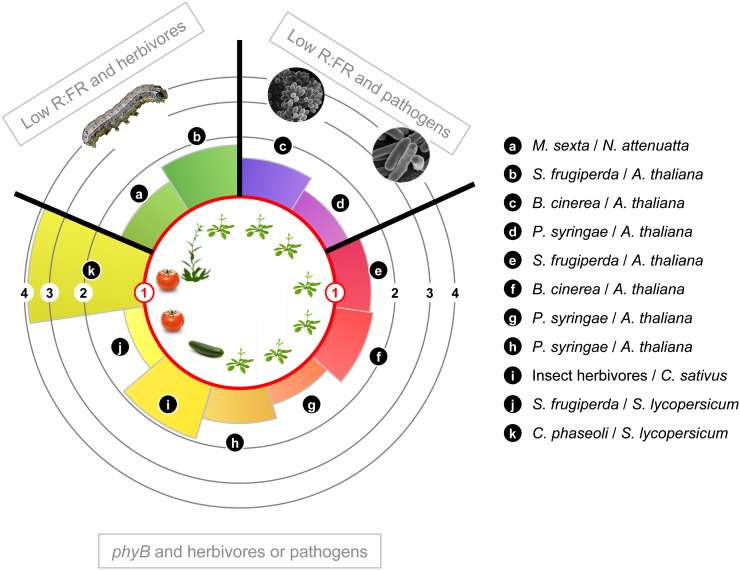
Figure 3.
FR supplementation treatments, designed to mimic the proximity of neighboring plants, or mutational inactivation of the PHYB gene increase the levels of insect herbivory and disease severity in a variety of herbaceous species. Concentric circles indicate the level of enhancement (fold increase) of plant susceptibility to attack, expressed as a ratio between the treatment (low R:FR or phyB) and control (high R:FR, PHYB) conditions. Plant susceptibility was estimated on the basis of bioassay results that measured the growth of the consumer organism or the severity of plant damage. Sources of primary data are as follows: a, j, and k, Izaguirre et al. (2006); b and e, Moreno et al. (2009); c and f, Cerrudo et al. (2012); d and g, De Wit (2012); h, Faigón-Soverna et al. (2006); i, McGuire and Agrawal (2005).
The effect of variations of R:FR on resistance to microbial pathogens has received little attention until recently. Previous studies with a variety of horticultural species have shown that treatment of leaves with R stimulates plant resistance to a number of pathogens, compared with leaves incubated in the dark or visible light of other wavelengths (Islam et al., 1998, 2002). However, because the control for the R treatment was not established as a low-R:FR treatment, the results are difficult to interpret in terms of plant ecology and specific phytochrome action. In fact, some of the protective effects of R in those experiments have been attributed to the stimulation of photosynthesis (Rahman et al., 2002). Very recent studies have shown that plants exposed to low R:FR values (R:FR < 1), which were designed to mimic realistic competition scenarios, are more susceptible to subsequent infection by necrotrophic pathogens (such as B. cinerea) than plants exposed to white light (R:FR > 1; Cerrudo et al., 2012; De Wit, 2012; Fig. 3). Similarly, phyB mutants of Arabidopsis were found to be more susceptible to necrotrophs than the corresponding wild types (Kazan and Manners, 2011; Cerrudo et al., 2012). Recent experiments demonstrate that low-R:FR treatments also reduce Arabidopsis resistance to the hemibiotrophic pathogen Pseudomonas syringae pv tomato DC3000 (M. De Wit and R. Pierik, unpublished data), which is consistent with the susceptibility phenotype of phyB mutants in P. syringae bioassays (Faigón-Soverna et al., 2006; Fig. 3). Moreover, in these experiments (De Wit, 2012; De Wit and Pierik, unpublished data), the effect of low R:FR depressing plant resistance to the pathogen was observed even if the change in light conditions (from high to low R:FR) was applied concomitantly with the infection treatment.
The mechanisms by which variations in R:FR regulate plant resistance to pests and pathogens have been connected with modulation effects of phyB Pfr on the signaling networks activated by the major defense hormones JA and SA.
R:FR and JA
Shading by a forest leaf canopy, which reduces both total irradiance and R:FR, can reduce JA production and the expression of JA-dependent defenses in plants of the understory (Agrawal et al., 2012). Furthermore, even in the absence of shading (i.e. at constant levels of photosynthetically active radiation [PAR]), the reduction of R:FR caused by the proximity of neighboring plants in an even-height canopy can depress plant responses to JA (Moreno et al., 2009). This effect of low R:FR is mediated by the inactivation of phyB and has been demonstrated at the level of gene expression (Moreno et al., 2009; Suzuki et al., 2011; Cerrudo et al., 2012; De Wit, 2012) and the accumulation of secondary metabolites likely to be involved in direct defense against pathogens and pests, such as phenylpropanoids, anthocyanins, and glucosinolates (Izaguirre et al., 2006; Moreno et al., 2009; Cerrudo et al., 2012; Fig. 4).
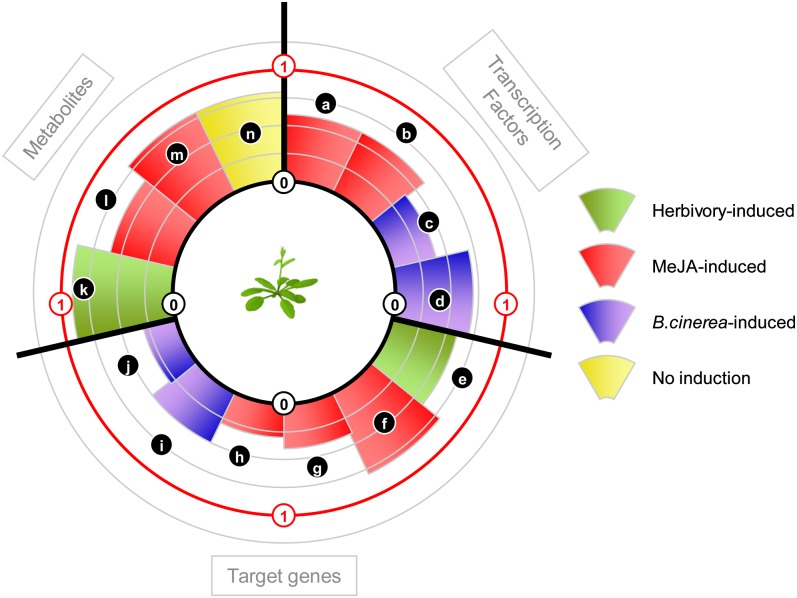
Figure 4.
Exposure of Arabidopsis plants to low-R:FR treatments that mimic the proximity of neighboring plants depresses the levels of defense markers associated with JA signaling: a, MYC2; b, ERF1; c, ERF1; d, ORA59; e, HEL; f, ASA1; g, PDF1.2; h, HEL; i, HEL; j, PDF1.2; k, leaf phenolics; l, leaf phenolics; m, anthocyanins; n, gluconasturtiin. Concentric circles indicate the level of depression of the defense response caused by low R:FR, expressed as a ratio between the treatment (low R:FR or phyB) and control (high R:FR, PHYB) conditions. The elicitor used to activate the JA response is indicated by different colors. Also shown is the effect of low R:FR on the concentration of gluconasturtiin, a glucosinolate that is particularly abundant in watercress (Nasturtium officinale). MeJA, Methyl jasmonate. Sources of primary data are as follows: a to d, f, g, i, j, l, and m, Cerrudo et al. (2012); b, e, h, g, and k, Moreno et al. (2009); n, Engelen-Eigles et al. (2006).
The mechanisms that link phyB Pfr with JA signaling are not completely clear and are likely to involve several layers of regulation (Ballaré, 2011). Perception of jasmonoyl-Ile, the bioactive amino acid conjugate of jasmonic acid, is achieved by a coreceptor formed by the ubiquitin ligase SCFCOI1 (for S-phase kinase-associated protein1-Cullin1-F-box protein CORONATINE INSENSITIVE1) complex and JASMONATE ZIM DOMAIN (JAZ) proteins (for review, see Browse, 2009). Jasmonoyl-Ile stimulates the specific binding of COI1 and JAZ proteins, which leads to the ubiquitination of JAZs by SCFCOI1 and their subsequent degradation by the 26S proteasome (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007, 2009; Melotto et al., 2008; Pauwels et al., 2010; Sheard et al., 2010). JAZ proteins are repressors of JA-responsive transcription factors; therefore, degradation of JAZ proteins initiates the activation of the JA response (Pauwels and Goossens, 2011; Shyu et al., 2012). Regulation of plant defense by phyB has been recently linked to JAZ activity, because the effect of low R:FR values reducing Arabidopsis resistance to B. cinerea was found to be significantly attenuated in a jaz10 null mutant and in RNA interference lines disrupted for the expression of the JAZ10 gene (Cerrudo et al., 2012). A possible mechanism behind this connection may be based on phyB-mediated changes in JAZ gene expression or JAZ protein stability. Increased expression of certain JAZ genes has been observed in response to low R:FR values (Moreno et al., 2009). A phytochrome effect on JAZ stability has been demonstrated for phytochrome A (phyA). Thus, COI1-mediated degradation of JAZ1 in response to JA treatment was found to require active phyA (Robson et al., 2010). However, it remains to be demonstrated whether in fully deetiolated plants, where responses to low R:FR are controlled predominantly by phyB (Ballaré, 1999), changes in the levels of phyB Pfr in response to R:FR affect the turnover of JAZ repressors.
In addition to Pfr effects on some of the early JA signaling components, light quality perceived by phyB could affect JA responses by affecting the levels of other hormones known to regulate JA signaling, and the list of these regulators includes GAs, brassinosteroids (BRs), and SA, among others (Bari and Jones, 2009; Ballaré, 2011; Erb et al., 2012; Pieterse et al., 2012). SA has been dismissed as a potential mediator of the effect of low R:FR values on JA signaling by studies demonstrating that this effect is conserved in SA synthesis and signaling mutants (Cerrudo et al., 2012). GAs are important growth-promoting hormones during SAS, accumulating in response to neighbor proximity and low R:FR values and participating in the elongation response (Djakovic-Petrovic et al., 2007). GAs can antagonize certain JA responses (Navarro et al., 2008), and this action is mediated by their effect on the DELLA proteins (Hou et al., 2010). GA perception leads to DELLA degradation in the proteasome (Hirano et al., 2008), and DELLAs are positive regulators of the JA response, as they prevent the repressive action of JAZ proteins on JA signaling (Hou et al., 2010). Whether the increased DELLA turnover caused by low R:FR values in canopies (Djakovic-Petrovic et al., 2007) is responsible for the depression in JA-mediated chemical defenses at high density has not been explicitly tested. BRs were recently found to participate in the orchestration of the SAS phenotype induced by low R:FR values in Arabidopsis (Kozuka et al., 2010). Interestingly, BRs have also been shown to antagonize JA-mediated growth and antiherbivore responses (Campos et al., 2009; Ren et al., 2009) and to modulate the efficiency of plant immune responses elicited by microbe-associated molecular patterns (Albrecht et al., 2012; Belkhadir et al., 2012; Wang, 2012). BRI1-ASSOCIATED KINASE1 (BAK1) is a BR coreceptor and also physically interacts with the flg22 receptor FLS2, positively regulating FLS2-mediated innate immunity in Arabidopsis (Chinchilla et al., 2009). In N. attenuata, BAK1 is a positive regulator of herbivory-induced JA accumulation and also a negative modulator of certain defense responses elicited by JA (Yang et al., 2011). Clearly, further work is needed to assess the effects of changes in BR signaling elicited by low R:FR values on the modulation of plant defense responses in dense canopies.
R:FR and SA
SA plays a major role in disease resistance signaling (Durrant and Dong, 2004; Vlot et al., 2009). The SA response pathway is typically effective against microbial pathogens with a biotrophic lifestyle (Glazebrook, 2005). Light, when compared with dark conditions, increases plant resistance to a variety of biotrophic pathogens (Zeier et al., 2004), and SA accumulation in healthy Arabidopsis plants has been shown to increase in response to increased PAR (Karpinski et al., 2003). Recent experiments with cucumber suggested that light-enhanced resistance may be specific for R, since other light treatments were less effective in reducing damage by Sphaerotheca fuliginea (powdery mildew) as compared with R irradiation (Wang et al., 2010). Infected plants responded with increased free SA levels, and this response, together with the induction of a range of SA-responsive genes, was enhanced in R as compared with other wavelengths (Wang et al., 2010). The apparently specific effects of R suggest the involvement of phytochromes. Indeed, phy mutants have reduced SA-mediated resistance against (hemi)biotrophic pathogens. Thus, the Arabidopsis phyB mutant allows stronger proliferation of an avirulent P. syringae strain (Faigón-Soverna et al., 2006), and phyAphyB double mutants are impaired in SA-dependent systemic responses and more susceptible to pathogens with a biotrophic lifestyle (Genoud et al., 2002; Griebel and Zeier, 2008). Interestingly, defense responses at the direct sites of P. syringae inoculation appear to be mostly phytochrome independent (Griebel and Zeier, 2008). Triple phyAphyBphyC mutants of rice (Oryza sativa) were also shown to be more susceptible to blast fungus (Magnaporthe grisea) than the cv Nipponbare wild type (Xie et al., 2011). Consistent with enhanced susceptibility to this fungus, the phyAphyBphyC mutant showed reduced induction of PATHOGENESIS-RELATED1 gene expression in response to SA spray treatment (Xie et al., 2011). Recent studies in Arabidopsis indicate that low-R:FR treatments that simulate neighbor proximity also lead to enhanced susceptibility to P. syringae and reduced defense gene induction in response to SA treatment (De Wit, 2012; De Wit and Pierik, unpublished data). Collectively, these results indicate that phyB Pfr is a positive regulator of SA-mediated defense responses. The mechanism of SA perception has been clarified only very recently (Fu et al., 2012), and the signaling networks that connect phyB and SA signaling are at present largely unknown. The fact that phyB inactivation depresses both JA and SA responses suggests that the effect of low R:FR values on plant defense does not result from a simple shift of balance between different immune branches. Elucidation of the molecular links between light and defense signaling might reveal new connections between defense hormone pathways.
PAR, PHOTOSYNTHESIS, AND BLUE LIGHT RECEPTORS
Chloroplast-derived signals can also modulate defense responses, although a clear picture of the adaptive significance of this regulation under canopy light conditions is not yet available (Karpinski et al., 2003; Kangasjärvi et al., 2012). Similarly, the role of blue light (B) and B photoreceptors in the regulation of defense responses is not well established. Jeong et al. (2010) reported that cryptochrome 2 and phototropin 2 are specifically required for resistance protein-mediated Arabidopsis defense against Turnip crinkle virus. There is also evidence that cryptochrome1 (cry1) is a positive modulator of Arabidopsis defense against P. syringae pv tomato DC3000 when plants are exposed to continuous light after pathogen infection (Wu and Yang, 2010). However, other studies, carried out under day/night light cycles, failed to detect any effects of mutations in B photoreceptors on Arabidopsis resistance to this bacterial pathogen (Griebel and Zeier, 2008; Jeong et al., 2010). Moreover, cry1 mutants did not show any obvious susceptibility phenotype in bioassays with the necrotrophic fungus B. cinerea (Cerrudo et al., 2012). Therefore, while the participation of cryptochromes in the regulation of SAS responses at high planting density is well documented (Pierik et al., 2004, 2009; Sellaro et al., 2010; Keller et al., 2011; Keuskamp et al., 2011), the effects of variations of B levels caused by the proximity of neighbors on plant immune responses requires further study.
CONCLUSION
In order to achieve elevated yields per unit area, plants must be grown at high density, but increasing crop density and LAI may have negative effects on plant resistance to pests and diseases that are reminiscent of the effects of sunlight deprivation on human health. There is now ample evidence that light, and light signals associated with open space, are positive regulators of plant defense against a broad spectrum of enemies via mechanisms triggered by specific photoreceptors for UV-B and R:FR (Fig. 5). From an evolutionary perspective, this beneficial effect of light might reflect the activity of an optimization strategy that distributes limited resources between growth and defense as a function of the risk of competition that the plant senses using its photoreceptors (Ballaré, 2009). Whether the plant’s solution to this dilemma could be manipulated in species of economic interest to reduce pesticide loads without greatly forfeiting crop yields requires further investigation.
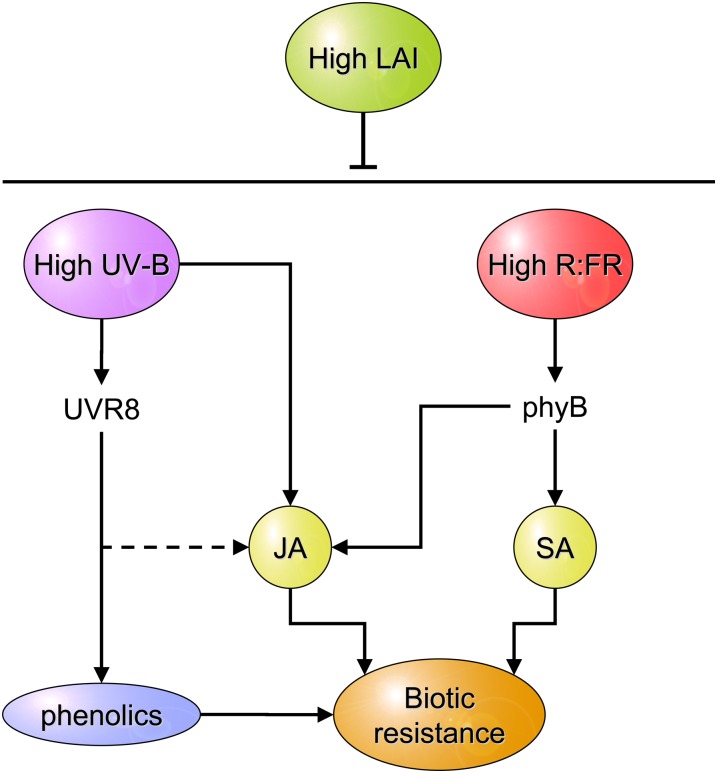
Figure 5.
Schematic representation of the positive effects of light signals of open space (high R:FR and UV-B) on plant health. UV-B affects plant resistance to insects and necrotrophic pathogens via mechanisms that involve interactions with JA signaling and JA-independent increases in the levels of certain phenolic compounds, the latter being mediated by UVR8. High R:FR values act through phyB increasing plant responses to JA and SA. Dashed lines indicate interactions that have not been explicitly demonstrated. For further explanation, see text.
The molecular mechanisms that mediate the effects of photoreceptor signals modulating the expression of plant defenses are beginning to be elucidated. Understanding these mechanisms may allow us to manipulate planting density and canopy structure to optimize light penetration for improved crop health. In addition, this understanding will provide key functional information for the design of crop varieties that maintain elevated levels of defense even at high planting density. In this regard, the rapid growth that we have witnessed in the last few years in the field of regulation of plant immunity suggests that, in the not very distant future, we will be able to identify targets for biotechnological manipulation to improve crop health at high LAI. These strategies may help us design agroecosystems that safely deliver healthy products to meet the nutritional demands of humankind in the following decades.
Acknowledgments
We thank Patricia Demkura, Mieke De Wit, and Mercedes Keller for helpful discussions and Patricia Demkura for providing the fluorescence image for Figure 2B.
Glossary
- LAI
leaf area index
- JA
jasmonate
- SA
salicylate
- R
red light
- FR
far-red light
- R:FR
red to far-red ratio
- SAS
shade-avoidance syndrome
- PAR
photosynthetically active radiation
- BR
brassinosteroid
- B
blue light
References
- A-H-Mackerness S, Surplus SL, Blake P, John CF, Buchanan-Wollaston V, Jordan BR, Thomas B. (1999) Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ 22: 1413–1423 [Google Scholar]
- Agrawal A, Kearney E, Hastings A, Ramsey T. (2012) Attenuation of the jasmonate burst, plant defensive traits, and resistance to specialist monarch caterpillars on shaded common milkweed (Asclepias syriaca). J Chem Ecol 38: 893–901 [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Heil M. (2012) Synthesizing specificity: multiple approaches to understanding the attack and defense of plants. Trends Plant Sci 17: 239–242 [DOI] [PubMed] [Google Scholar]
- Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez-Ibanez S, Chinchilla D, Rathjen JP, de Vries SC, Zipfel C. (2012) Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proc Natl Acad Sci USA 109: 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander HM, Holt RD. (1998) The interaction between plant competition and disease. Perspect Plant Ecol Evol Syst 1: 206–220 [Google Scholar]
- Augspurger CK, Kelly CK. (1984) Pathogen mortality of tropical tree seedlings: experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 61: 211–217 [DOI] [PubMed] [Google Scholar]
- Balint-Kurti P, Simmons SJ, Blum JE, Ballaré CL, Stapleton AE. (2010) Maize leaf epiphytic bacteria diversity patterns are genetically correlated with resistance to fungal pathogen infection. Mol Plant Microbe Interact 23: 473–484 [DOI] [PubMed] [Google Scholar]
- Ballaré CL. (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4: 97–102 [DOI] [PubMed] [Google Scholar]
- Ballaré CL. (2009) Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ 32: 713–725 [DOI] [PubMed] [Google Scholar]
- Ballaré CL. (2011) Jasmonate-induced defenses: a tale of intelligence, collaborators and rascals. Trends Plant Sci 16: 249–257 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Caldwell MM, Flint SD, Robinson SA, Bornman JF. (2011) Effects of solar ultraviolet radiation on terrestrial ecosystems: patterns, mechanisms, and interactions with climate change. Photochem Photobiol Sci 10: 226–241 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247: 329–332 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Stapleton AE, Yanovsky MJ. (1996) Solar ultraviolet-B radiation affects seedling emergence, DNA integrity, plant morphology, growth rate, and attractiveness to herbivore insects in Datura ferox. Plant Physiol 112: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Jones JD. (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69: 473–488 [DOI] [PubMed] [Google Scholar]
- Belkhadir Y, Jaillais Y, Epple P, Balsemão-Pires E, Dangl JL, Chory J. (2012) Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci USA 109: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T, Freckleton RP, Lewis OT. (2006) Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol Lett 9: 569–574 [DOI] [PubMed] [Google Scholar]
- Berli F, D’Angelo J, Cavagnaro B, Bottini R, Wuilloud R, Silva MF. (2008) Phenolic composition in grape (Vitis vinifera L. cv. Malbec) ripened with different solar UV-B radiation levels by capillary zone electrophoresis. J Agric Food Chem 56: 2892–2898 [DOI] [PubMed] [Google Scholar]
- Birch AN, Begg GS, Squire GR. (2011) How agro-ecological research helps to address food security issues under new IPM and pesticide reduction policies for global crop production systems. J Exp Bot 62: 3251–3261 [DOI] [PubMed] [Google Scholar]
- Brederode FT, Linthorst HJM, Bol JF. (1991) Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol Biol 17: 1117–1125 [DOI] [PubMed] [Google Scholar]
- Bridge MA, Klarman WL. (1973) Soybean phytoalexin, hydroxyphaseollin, induced by ultraviolet irradiation. Phytopathology 63: 606–609 [Google Scholar]
- Brown BA, Jenkins GI. (2008) UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol 146: 576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Burdon JJ, Chilvers GA. (1975) Epidemiology of damping-off disease (Pythium irregulare) in relation to density of Lepidium sativum seedlings. Ann Appl Biol 81: 135–143 [Google Scholar]
- Burdon JJ, Chilvers GA. (1982) Host density as a factor in plant disease ecology. Annu Rev Phytopathol 20: 143–166 [Google Scholar]
- Caldwell MM, Ballaré CL, Bornman JF, Flint SD, Björn LO, Teramura AH, Kulandaivelu G, Tevini M. (2003) Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem Photobiol Sci 2: 29–38 [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. (1983) Internal filters: prospects for UV-acclimation in higher plants. Physiol Plant 58: 445–450 [Google Scholar]
- Campos ML, de Almeida M, Rossi ML, Martinelli AP, Litholdo CG, Jr, Figueira A, Rampelotti-Ferreira FT, Vendramim JD, Benedito VA, Peres LE. (2009) Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J Exp Bot 60: 4347–4361 [DOI] [PubMed] [Google Scholar]
- Caputo C, Rutitzky M, Ballaré CL. (2006) Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia 149: 81–90 [DOI] [PubMed] [Google Scholar]
- Cerrudo I, Keller MM, Cargnel MD, Demkura PV, de Wit M, Patitucci MS, Pierik R, Pieterse CMJ, Ballaré CL. (2012) Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol 158: 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. (2009) One for all: the receptor-associated kinase BAK1. Trends Plant Sci 14: 535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Cipollini DF, Bergelson J. (2001) Plant density and nutrient availability constrain constitutive and wound-induced expression of trypsin inhibitors in Brassica napus. J Chem Ecol 27: 593–610 [DOI] [PubMed] [Google Scholar]
- Cipollini DF, Bergelson J. (2002) Interspecific competition affects growth and herbivore damage of Brassica napus L. in the field. Plant Ecol 162: 227–231 [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA. (1996) The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature 383: 826–829 [DOI] [PubMed] [Google Scholar]
- D’Auria JC, Gershenzon J. (2005) The secondary metabolism of Arabidopsis thaliana: growing like a weed. Curr Opin Plant Biol 8: 308–316 [DOI] [PubMed] [Google Scholar]
- De Bruin JL, Pedersen P. (2008) Effect of row spacing and seeding rate on soybean yield. Agron J 100: 704–710 [Google Scholar]
- Demkura PV, Abdala G, Baldwin IT, Ballaré CL. (2010) Jasmonate-dependent and -independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiol 152: 1084–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demkura PV, Ballaré CL. (2012) UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant 5: 642–652 [DOI] [PubMed] [Google Scholar]
- De Wit M. (2012) Neighbour detection and pathogen defence during competition. PhD thesis. Utrecht University, Utrecht, The Netherlands
- Djakovic-Petrovic T, de Wit M, Voesenek LA, Pierik R. (2007) DELLA protein function in growth responses to canopy signals. Plant J 51: 117–126 [DOI] [PubMed] [Google Scholar]
- Dorn LA, Pyle EH, Schmitt J. (2000) Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution 54: 1982–1994 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X. (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Engelen-Eigles G, Holden G, Cohen JD, Gardner G. (2006) The effect of temperature, photoperiod, and light quality on gluconasturtiin concentration in watercress (Nasturtium officinale R. Br.). J Agric Food Chem 54: 328–334 [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigón-Soverna A, Harmon FG, Storani L, Karayekov E, Staneloni RJ, Gassmann W, Más P, Casal JJ, Kay SA, Yanovsky MJ. (2006) A constitutive shade-avoidance mutant implicates TIR-NBS-LRR proteins in Arabidopsis photomorphogenic development. Plant Cell 18: 2919–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al. (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flénet F, Kiniry JR, Board JE, Westgate ME, Reicosky DC. (1996) Row spacing effects on light extinction coefficients of corn, sorghum, soybean, and sunflower. Agron J 88: 185–190 [Google Scholar]
- Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, et al. (2005) Global consequences of land use. Science 309: 570–574 [DOI] [PubMed] [Google Scholar]
- Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O’Connell C, Ray DK, West PC, et al. (2011) Solutions for a cultivated planet. Nature 478: 337–342 [DOI] [PubMed] [Google Scholar]
- Franklin KA. (2008) Shade avoidance. New Phytol 179: 930–944 [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, et al. (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486: 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud T, Buchala AJ, Chua N-H, Métraux J-P. (2002) Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J 31: 87–95 [DOI] [PubMed] [Google Scholar]
- Gilbert GS. (2002) Evolutionary ecology of plant diseases in natural ecosystems. Annu Rev Phytopathol 40: 13–43 [DOI] [PubMed] [Google Scholar]
- Giordano CV, Galatro A, Puntarulo S, Ballaré CL. (2004) The inhibitory effects of UV-B radiation (280-315 nm) on Gunnera magellanica growth correlate with increased DNA damage but not with oxidative damage to lipids. Plant Cell Environ 27: 1415–1423 [Google Scholar]
- Glawischnig E. (2007) Camalexin. Phytochemistry 68: 401–406 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- González Besteiro MA, Bartels S, Albert A, Ulm R. (2011) Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J 68: 727–737 [DOI] [PubMed] [Google Scholar]
- Griebel T, Zeier J. (2008) Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147: 790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera TS, Paul ND. (2007) Ecological impact of solar ultraviolet-B (UV-B: 320-290 nm) radiation on Corynebacterium aquaticum and Xanthomonas sp. colonization on tea phyllosphere in relation to blister blight disease incidence in the field. Lett Appl Microbiol 44: 513–519 [DOI] [PubMed] [Google Scholar]
- Gunasekera TS, Paul ND, Ayres PG. (1997) The effects of ultraviolet-U (UV-B: 290-320 nm) radiation on blister blight disease of tea (Camellia sinensis). Plant Pathol 46: 179–185 [Google Scholar]
- Harder DB, Sprague CL, Renner KA. (2007) Effect of soybean row width and population on weeds, crop yield, and economic return. Weed Technol 21: 744–752 [Google Scholar]
- Heijde M, Ulm R. (2012) UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci 17: 230–237 [DOI] [PubMed] [Google Scholar]
- Hirano K, Ueguchi-Tanaka M, Matsuoka M. (2008) GID1-mediated gibberellin signaling in plants. Trends Plant Sci 13: 192–199 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Campo CB, Harborne JB, McCaffery AR. (2001) Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomol Exp Appl 98: 181–194 [Google Scholar]
- Holick MF. (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr (Suppl) 80: 1678S–1688S [DOI] [PubMed] [Google Scholar]
- Holick MF. (2007) Vitamin D deficiency. N Engl J Med 357: 266–281 [DOI] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, et al. (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J (in press) [DOI] [PubMed] [Google Scholar]
- Hou X, Lee LYC, Xia K, Yan Y, Yu H. (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 19: 884–894 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Islam SZ, Babadoost M, Honda Y. (2002) Effect of red light treatment of seedlings of pepper, pumpkin, and tomato on the occurrence of Phytophthora damping-off. HortScience 37: 678–681 [Google Scholar]
- Islam SZ, Honda Y, Arase S. (1998) Light-induced resistance of broad bean against Botrytis cinerea. J Phytopathol 146: 479–485 [Google Scholar]
- Izaguirre MM, Mazza CA, Biondini M, Baldwin IT, Ballaré CL. (2006) Remote sensing of future competitors: impacts on plant defenses. Proc Natl Acad Sci USA 103: 7170–7174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre MM, Mazza CA, Svatos A, Baldwin IT, Ballaré CL. (2007) Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Ann Bot (Lond) 99: 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL. (2003) Convergent responses to stress: solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol 132: 1755–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggard KW, Qi A, Ober ES. (2010) Possible changes to arable crop yields by 2050. Philos Trans R Soc Lond B Biol Sci 365: 2835–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK, Hectors K, O’Brien NM, Guisez Y, Potters G. (2008) Plant stress and human health: do human consumers benefit from UV-B acclimated crops? Plant Sci 175: 449–458 [Google Scholar]
- Jenkins GI. (2009) Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 60: 407–431 [DOI] [PubMed] [Google Scholar]
- Jeong RD, Chandra-Shekara AC, Barman SR, Navarre D, Klessig DF, Kachroo A, Kachroo P. (2010) Cryptochrome 2 and phototropin 2 regulate resistance protein-mediated viral defense by negatively regulating an E3 ubiquitin ligase. Proc Natl Acad Sci USA 107: 13538–13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Jurke CJ, Fernando WGD. (2008) Effects of seeding rate and plant density on sclerotinia stem rot incidence in canola. Arch Phytopathol Plant Prot 41: 142–155 [Google Scholar]
- Juzeniene A, Brekke P, Dahlback A, Andersson-Engels S, Reichrath J, Moan K, Holick MF, Grant WB, Moan J. (2011) Solar radiation and human health. Rep Prog Phys 74: 066701 [Google Scholar]
- Kadivar H, Stapleton AE. (2003) Ultraviolet radiation alters maize phyllosphere bacterial diversity. Microb Ecol 45: 353–361 [DOI] [PubMed] [Google Scholar]
- Kangasjärvi S, Neukermans J, Li S, Aro E-M, Noctor G. (2012) Photosynthesis, photorespiration, and light signalling in defence responses. J Exp Bot 63: 1619–1636 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin I. (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Karban R, Brody AK, Schnathorst WC. (1989) Crowding and a plant’s ability to defend itself against herbivores and diseases. Am Nat 134: 749–760 [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. (2003) Light perception in plant disease defence signalling. Curr Opin Plant Biol 6: 390–396 [DOI] [PubMed] [Google Scholar]
- Kazan K, Manners JM. (2011) The interplay between light and jasmonate signalling during defence and development. J Exp Bot 62: 4087–4100 [DOI] [PubMed] [Google Scholar]
- Keller MM, Jaillais Y, Pedmale UV, Moreno JE, Chory J, Ballaré CL. (2011) Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J 67: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Sasidharan R, Vos I, Peeters AJM, Voesenek LACJ, Pierik R. (2011) Blue-light-mediated shade avoidance requires combined auxin and brassinosteroid action in Arabidopsis seedlings. Plant J 67: 208–217 [DOI] [PubMed] [Google Scholar]
- Kotilainen T, Venäläinen T, Tegelberg R, Lindfors A, Julkunen-Tiitto R, Sutinen S, O’Hara RB, Aphalo PJ. (2009) Assessment of UV biological spectral weighting functions for phenolic metabolites and growth responses in silver birch seedlings. Photochem Photobiol 85: 1346–1355 [DOI] [PubMed] [Google Scholar]
- Kozuka T, Kobayashi J, Horiguchi G, Demura T, Sakakibara H, Tsukaya H, Nagatani A. (2010) Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol 153: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann F, Müller C. (2011) Impacts of ultraviolet radiation on interactions between plants and herbivorous insects: a chemo-ecological perspective. Prog Bot 72: 305–347 [Google Scholar]
- Kunz BA, Dando PK, Grice DM, Mohr PG, Schenk PM, Cahill DM. (2008) UV-induced DNA damage promotes resistance to the biotrophic pathogen Hyaloperonospora parasitica in Arabidopsis. Plant Physiol 148: 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ljung K, Breton G, Schmitz RJ, Pruneda-Paz J, Cowing-Zitron C, Cole BJ, Ivans LJ, Pedmale UV, Jung H-S, et al. (2012) Linking photoreceptor excitation to changes in plant architecture. Genes Dev 26: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AJ, Allwood JW, Winder CL, Dunn WB, Heald JK, Cristescu SM, Sivakumaran A, Harren FJM, Mulema J, Denby K, et al. (2011) Metabolomic approaches reveal that cell wall modifications play a major role in ethylene-mediated resistance against Botrytis cinerea. Plant J 67: 852–868 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Maddonni GA, Otegui ME, Cirilo AG. (2001) Plant population density, row spacing and hybrid effects on maize canopy architecture and light attenuation. Field Crops Res 71: 183–193 [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL. (2000) Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol 122: 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza CA, Gimenez PI, Kantolic AG, Ballaré CL. (2012) Beneficial effects of solar UV-B radiation on soybean yield mediated by reduced insect herbivory under field conditions. Physiol Plant (in press) [DOI] [PubMed] [Google Scholar]
- Mazza CA, Zavala J, Scopel AL, Ballaré CL. (1999) Perception of solar UVB radiation by phytophagous insects: behavioral responses and ecosystem implications. Proc Natl Acad Sci USA 96: 980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire R, Agrawal AA. (2005) Trade-offs between the shade-avoidance response and plant resistance to herbivores? Tests with mutant Cucumis sativus. Funct Ecol 19: 1025–1031 [Google Scholar]
- Melotto M, Mecey C, Niu Y, Chung HS, Katsir L, Yao J, Zeng W, Thines B, Staswick P, Browse J, et al. (2008) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J 55: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert-Turk F, Bennett MH, Mansfield JW, Holub EB. (2003) Quantification of camalexin in several accessions of Arabidopsis thaliana following inductions with Peronospora parasitica and UV-B irradiation. Phytoparasitica 31: 81–89 [Google Scholar]
- Meyer K, Cusumano JC, Somerville C, Chapple CCS. (1996) Ferulate-5-hydroxylase from Arabidopsis thaliana defines a new family of cytochrome P450-dependent monooxygenases. Proc Natl Acad Sci USA 93: 6869–6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Boland W. (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63: 431–450 [DOI] [PubMed] [Google Scholar]
- Moreno JE, Tao Y, Chory J, Ballaré CL. (2009) Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA 106: 4935–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, Jones JDG. (2008) DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol 18: 650–655 [DOI] [PubMed] [Google Scholar]
- Oerke EC. (2006) Crop losses to pests. J Agric Sci 144: 31–43 [Google Scholar]
- Onkokesung N, Gaquerel E, Kotkar H, Kaur H, Baldwin IT, Galis I. (2012) MYB8 controls inducible phenolamide levels by activating three novel hydroxycinnamoyl-coenzyme A:polyamine transferases in Nicotiana attenuata. Plant Physiol 158: 389–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oravecz A, Baumann A, Máté Z, Brzezinska A, Molinier J, Oakeley EJ, Adám E, Schäfer E, Nagy F, Ulm R. (2006) CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SP, Baldwin IT. (2008) Silencing RNA-directed RNA polymerase 2 increases the susceptibility of Nicotiana attenuata to UV in the field and in the glasshouse. Plant J 54: 845–862 [DOI] [PubMed] [Google Scholar]
- Panstruga R, Parker JE, Schulze-Lefert P. (2009) SnapShot: plant immune response pathways. Cell 136: 978.e971–978.e973 [DOI] [PubMed] [Google Scholar]
- Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, et al. (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Goossens A. (2011) The JAZ proteins: a crucial interface in the jasmonate signaling cascade. Plant Cell 23: 3089–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Cuppens MLC, Voesenek LACJ, Visser EJW. (2004) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136: 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Djakovic-Petrovic T, Keuskamp DH, de Wit M, Voesenek LACJ. (2009) Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and DELLA proteins in Arabidopsis. Plant Physiol 149: 1701–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, van der Does D, Zamioudis C, Leon-Reyes A, van Wees SCM. (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol (in press) [DOI] [PubMed] [Google Scholar]
- Pimentel D. (1997) Techniques for Reducing Pesticides: Environmental and Economic Benefits. Wiley, Chichester, UK
- Rahman MZ, Honda Y, Islam SZ, Arase S. (2002) Effect of metabolic inhibitors on red light-induced resistance of broad bean (Vicia faba L.) against Botrytis cinerea. J Phytopathol 150: 463–468 [Google Scholar]
- Ren C, Han C, Peng W, Huang Y, Peng Z, Xiong X, Zhu Q, Gao B, Xie D. (2009) A leaky mutation in DWARF4 reveals an antagonistic role of brassinosteroid in the inhibition of root growth by jasmonate in Arabidopsis. Plant Physiol 151: 1412–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Roberts MR, Paul ND. (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol 170: 677–699 [DOI] [PubMed] [Google Scholar]
- Robson F, Okamoto H, Patrick E, Harris SR, Wasternack C, Brearley C, Turner JG. (2010) Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 22: 1143–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosegrant MW, Cline SA. (2003) Global food security: challenges and policies. Science 302: 1917–1919 [DOI] [PubMed] [Google Scholar]
- Schenke D, Böttcher C, Scheel D. (2011) Crosstalk between abiotic ultraviolet-B stress and biotic (flg22) stress signalling in Arabidopsis prevents flavonol accumulation in favor of pathogen defence compound production. Plant Cell Environ 34: 1849–1864 [DOI] [PubMed] [Google Scholar]
- Schoonhoven LM, van Loon JJA, Dicke M. (2005) Insect-Plant Biology, Ed 2. Oxford University Press, New York
- Schreiner M, Mewis I, Huyskens-Keil S, Jansen MAK, Zrenner R, Winkler JB, O’Brien N, Krumbein A. (2012) UV-B-induced secondary plant metabolites: potential benefits for plant and human health. Crit Rev Plant Sci 31: 229–240 [Google Scholar]
- Sellaro R, Crepy M, Trupkin SA, Karayekov E, Buchovsky AS, Rossi C, Casal JJ. (2010) Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol 154: 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Kanehara K, Torres M, Yamada K, Tintor N, Kombrink E, Schulze-Lefert P, Saijo Y. (2012) Repression of sucrose/ultraviolet B light-induced flavonoid accumulation in microbe-associated molecular pattern-triggered immunity in Arabidopsis. Plant Physiol 158: 408–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu C, Figueroa P, Depew CL, Cooke TF, Sheard LB, Moreno JE, Katsir L, Zheng N, Browse J, Howe GA. (2012) JAZ8 lacks a canonical degron and has an EAR motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. Plant Cell 24: 536–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (1995) Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol 46: 289–315 [Google Scholar]
- Stratmann JW, Stelmach BA, Weiler EW, Ryan CA. (2000) UVB/UVA radiation activates a 48 kDa myelin basic protein kinase and potentiates wound signaling in tomato leaves. Photochem Photobiol 71: 116–123 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Suriyagoda L, Shigeyama T, Tominaga A, Sasaki M, Hiratsuka Y, Yoshinaga A, Arima S, Agarie S, Sakai T, et al. (2011) Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc Natl Acad Sci USA 108: 16837–16842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Wang H, Jiang YP, Yu HJ, Xia XJ, Shi K, Zhou YH, Yu JQ. (2010) Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur J Plant Pathol 127: 125–135 [Google Scholar]
- Wang Z-Y. (2012) Brassinosteroids modulate plant immunity at multiple levels. Proc Natl Acad Sci USA 109: 7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargent JJ, Taylor A, Paul ND. (2006) UV supplementation for growth regulation and disease control. Acta Hortic 711: 333–338 [Google Scholar]
- Wu J, Baldwin IT. (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44: 1–24 [DOI] [PubMed] [Google Scholar]
- Wu L, Yang H-Q. (2010) CRYPTOCHROME 1 is implicated in promoting R protein-mediated plant resistance to Pseudomonas syringae in Arabidopsis. Mol Plant 3: 539–548 [DOI] [PubMed] [Google Scholar]
- Xie X-Z, Xue Y-J, Zhou J-J, Zhang B, Chang H, Takano M. (2011) Phytochromes regulate SA and JA signaling pathways in rice and are required for developmentally controlled resistance to Magnaporthe grisea. Mol Plant 4: 688–696 [DOI] [PubMed] [Google Scholar]
- Yamamura K. (2002) Plant density. In D Pimentel, ed, Encyclopedia of Pest Management. Marcel Dekker, New York, pp 622–624
- Yan JB, Zhang C, Gu M, Bai ZY, Zhang WG, Qi TC, Cheng ZW, Peng W, Luo HB, Nan FJ, et al. (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE. (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DH, Hettenhausen C, Baldwin IT, Wu J. (2011) BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata’s responses to herbivory. J Exp Bot 62: 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Pink B, Mueller MJ, Berger S. (2004) Light conditions influence specific defence responses in incompatible plant-pathogen interactions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta 219: 673–683 [DOI] [PubMed] [Google Scholar]