Abstract
While suberin is an insoluble heteropolymer, a number of soluble lipids can be extracted by rapid chloroform dipping of roots. These extracts include esters of saturated long-chain primary alcohols and hydroxycinnamic acids. Such fatty alcohols and hydroxycinnamic acids are also present in suberin. We demonstrate that alkyl coumarates and caffeates, which are the major components of Arabidopsis (Arabidopsis thaliana) root waxes, are present primarily in taproots. Previously we identified ALIPHATIC SUBERIN FERULOYL TRANSFERASE (At5g41040), a HXXXD-type acyltransferase (BAHD family), responsible for incorporation of ferulate into aliphatic suberin of Arabidopsis. However, aliphatic suberin feruloyl transferase mutants were unaffected in alkyl hydroxycinnamate ester root wax composition. Here we identify a closely related gene, At5g63560, responsible for the synthesis of a subset of alkyl hydroxycinnamate esters, the alkyl caffeates. Transgenic plants harboring PAt5g63560::YFP fusions showed transcriptional activity in suberized tissues. Knockout mutants of At5g63560 were severely reduced in their alkyl caffeate but not alkyl coumarate content. Recombinant At5g63560p had greater acyltransferase activity when presented with caffeoyl-Coenzyme A (CoA) substrate, thus we have named this acyltransferase FATTY ALCOHOL:CAFFEOYL-CoA CAFFEOYL TRANSFERASE. Stress experiments revealed elevated alkyl coumarate content in root waxes of NaCl-treated wild-type and fatty alcohol:caffeoyl-CoA caffeoyl transferase plants. We further demonstrate that FATTY ACYL-CoA REDUCTASEs (FARs) FAR5 (At3g44550), FAR4 (At3g44540), and FAR1 (At5g22500) are required for the synthesis of C18, C20, and C22 alkyl hydroxycinnamates, respectively. Collectively, these results suggest that multiple acyltransferases are utilized for the synthesis of alkyl hydroxycinnamate esters of Arabidopsis root waxes and that FAR1/4/5 provide the fatty alcohols required for alkyl hydroxycinnamate synthesis.
Suberin is an extracellular lipid-rich heteropolymer that is deposited abutting the inner surface of the primary cell wall of certain tissues (Kolattukudy, 2001). More specifically, suberin is deposited in endodermal cells of the elongation zone of young developing roots and the periderm of roots and stems undergoing secondary growth. Solvent-soluble, lipophilic compounds associated with suberized tissues, typically peridermal tissues, have been described and have been loosely termed suberin-associated waxes or, in the case of roots, root waxes (Espelie et al., 1980; Li et al., 2007; Molina et al., 2009; Serra et al., 2010). Root wax and suberin-associated wax constituents include fatty acids (typically C16–C22), fatty alcohols (≥C18), and monoacylglycerols (typically with C20 and C22 acyl chains). Alkanes (typically C29 and C31) and their midchain oxidized (keto or hydroxy) derivatives have been reported in root- and suberin-associated waxes. Root- and suberin-associated waxes also contain alkyl hydroxycinnamate esters (Bernards and Lewis, 1992; Schreiber et al., 2005; Li et al., 2007). These alkyl hydroxycinnamate esters are comprised of phenylpropanoids, typically coumaric, ferulic, or caffeic acids, esterified to fatty alcohols. While there are many reports of alkyl hydroxycinnamate esters as natural products (García-Argáez et al., 1999; Freire et al., 2002; del Río et al., 2004; Yunoki et al., 2004; Santos et al., 2007), few studies have explicitly investigated their presence in suberized tissues. Nonetheless, alkyl hydroxycinnamate esters are reported to be present in the suberized periderm of both aboveground (bark) and underground (tuber) plant organs (Kawanishi et al., 1990; Bernards and Lewis, 1992; Schreiber et al., 2005; Sun et al., 2006; Freire et al., 2007). A 22-caffeoyloxy-docasanoyl glycerol has been reported in waxes extracted from suberized fibers of green cotton (Gossypium hirsutum; Schmutz et al., 1994).
To date, only three genes involved in alkyl hydroxycinnamate ester synthesis have been identified (Gou et al., 2009; Molina et al., 2009; Serra et al., 2010; Rautengarten et al., 2012). All three are members of the BAHD family of HXXXD-type acyltransferases (St Pierre and De Luca, 2000; D’Auria, 2006; Tuominen et al., 2011). These genes are primarily involved in the formation of feruloyloxy aliphatics (i.e. ferulate linked to the ω-terminus of ω-hydroxy fatty acids) in suberin and cutin polymers. In Arabidopsis (Arabidopsis thaliana), alkyl ferulates comprise only a small proportion of the alkyl hydroxycinnamate esters extracted from roots (Li et al., 2007; Molina et al., 2009). Instead, Arabidopsis root waxes are dominated by alkyl caffeate and alkyl coumarate esters. BAHD acyltransferases with activity toward caffeoyl-CoA (acyl donor) and polyamines and other types of acyl acceptors have been identified (Comino et al., 2009; Grienenberger et al., 2009; Sander and Petersen, 2011); however, of these, none have been shown to utilize fatty alcohols as acyl acceptors. Here we first describe the distribution of root waxes along the root axis. We then describe the identification and characterization of a BAHD acyltransferase, encoded by the At5g63560 locus, with caffeoyl transferase activity toward fatty alcohols. We have named this enzyme FATTY ALCOHOL:CAFFEOYL-CoA CAFFEOYL TRANSFERASE (FACT). It is a member of clade Va of the BAHD family of acyltransferases (St Pierre and De Luca, 2000; D’Auria, 2006; Tuominen et al., 2011) that also contains ALIPHATIC SUBERIN FERULOYL TRANSFERASE (ASFT) and DEFICIENT IN CUTIN FERULATE (DCF). We further demonstrate the involvement of the FATTY ACYL-CoA REDUCTASEs (FARs) FAR1, FAR4, and FAR5 in root wax alkyl hydroxycinnamate synthesis. Finally, we describe the effect of salt stress on alkyl hydroxycinnamate composition in roots.
RESULTS
Arabidopsis Root Waxes Are Enriched in the Mature Taproot
Previously we analyzed the waxes released by rapid chloroform extraction of the entire root mass from mature Arabidopsis plants. The dominant lipid class within these waxes was the alkyl hydroxycinnamates. Additional components included sterols, primary alcohols, free fatty acids, and monoacylglycerols. In two separate studies we reported that the relative amounts of alkyl hydroxycinnamates subclasses were either coumarates > caffeates > ferulates or caffeates > coumarates > ferulates (Supplemental Fig. S1; Li et al., 2007; Molina et al., 2009). These analyses quantified alkyl hydroxycinnamates using primary alcohols and other related compounds as internal standards. In this study we synthesized and purified both tridecyl (C13) ferulate and heptadecyl (C17) coumarate, to be used as internal standards (Supplemental Figs. S2 and S3) to facilitate a more accurate quantification of alkyl hydroxycinnamate esters.
Mature portions of Arabidopsis roots are covered by a suberized cell layer or periderm (Franke et al., 2005; Li et al., 2007). This periderm has been shown to extend to roughly 4 cm below the base of the rosette in 5-week-old plants (Höfer et al., 2008). To investigate the spatial distribution of waxes along the root axis of Arabidopsis, we compared the rapidly solvent-extractable lipids of primary taproots (3–5 cm below the rosette) to remaining younger roots (>5 cm below the rosette). This comparison indicated a nearly 5-fold enrichment of root wax aliphatics from taproot (primary root) surfaces compared with younger roots, including a nearly 9-fold enrichment in alkyl hydroxycinnamates. These results, and the nature of the extraction employed (dipping of entire roots in chloroform), suggest that root waxes might be derived from the peridermal region (Fig. 1) of the root. These comparisons are made using wax loads expressed on a lipid mass per unit fresh weight basis. The surface area per unit fresh weight for the younger roots will be many-fold greater than for the taproot, so that on a mass per tissue surface area basis this difference will be greatly magnified.
Figure 1.
Comparison of waxes extracted from Arabidopsis taproots versus remaining roots. Data are presented as the mean of four biological replicates + sd. The inset graph compares total amounts of waxes extracted from taproots versus remaining roots. Alkn, Alkanes; FFAs, free fatty acids; MAGs, monoacylglycerols.
Identification of a Candidate Gene for Alkyl Hydroxycinnamate Ester Synthesis and Demonstration of Its Expression in Suberized Tissues
Previously, transcript coexpression analysis was used to identify a BAHD acyltransferase, ASFT (At5g41040), responsible for the incorporation of ferulic acid into root and seed coat suberin (Molina et al., 2009). Although recombinant ASFT protein was capable of catalyzing an acyl transfer between feruloyl-CoA and primary alcohols, asft mutant plants did not manifest any detectable reduction in alkyl hydroxycinnamate ester root waxes (Molina et al., 2009). The coexpression analysis used to discover ASFT also identified a closely related member of the BAHD acyltransferase superfamily encoded by the Arabidopsis locus At5g63560. Extension of the coexpression analysis to include recently identified ASFT and CYP86B1 (Molina et al., 2009) genes as bait showed further correlation between At5g63560 and characterized suberin biosynthetic gene transcript abundance (Supplemental Table S1).
Information on the transcriptome of mature roots is largely absent from publically accessible gene-expression databases (Winter et al., 2007; http://bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi). As such, we created a transcriptional fusion (Supplemental Table S2) to determine if At5g63560 expression was associated with mature roots. Transcriptional fusions were made by adding the 1,583-nucleotide putative promoter sequence of At5g63560 upstream of the enhanced yellow fluorescent protein (eYFP) coding sequence (PAt5g63560::eYFP). Confocal laser-scanning microscopy (CLSM) analysis of transgenic Arabidopsis plants harboring PAt5g63560::eYFP revealed transcriptional activity mainly in the outermost circumference of mature roots (suggestive of a peridermal localization; Fig. 2). Additionally, At5g63560 promoter activity was observed in the endodermis of young roots and in the seed coat of developing seeds (Fig. 2). It is important to note that in seeds the FACT promoter is active only in outer integument layer 1 of the seed coat, which is the same localization as seen for promoters of the suberin biosynthetic genes GPAT5 (Molina et al., 2008) and ASFT (Molina et al., 2009).
Figure 2.

Analysis of At5g63560 (FACT) promoter activity. Live tissues from plants transformed with PAt5g63560::eYFP were analyzed by CLSM. A, Extended focus image of taproot cross section (approximately 1 cm below rosette) from mature root of transgenic plants showing eYFP expression primarily in the outer circumference of the root (compiled from 33 optical sections, 2.43 μm each). B, Extended focus image of young roots (24 optical sections, 2 μm each). YFP expression is localized to the endodermis. C, Confocal sections of transgenic seeds entering desiccation stage showing YFP expression in the outer integument 1 (oi1) and the chalazal region of the seed coat. Propidium iodide was used to visualize cell walls (red fluorescence). Scale bars = 100 μm (A), 10 μm (B), and 50 μm (C). en, Endodermis; ep, epidermis; oi1, outer integument 1; oi2, outer integument 2.
Alkyl Caffeates Esters Are Absent in Solvent-Soluble Root Wax Extracts of fact Mutants
Given the observed promoter activity of At5g63560 in suberized tissues, it seemed likely that this locus was involved in a suberin-related process. Here we describe the chemical characterization of mutant alleles of At5g63560. We have named this gene FACT based on our findings.
Two mutant alleles of FACT were obtained by PCR screening of populations segregating for transposon/transferred DNA (T-DNA) insertions obtained from the Arabidopsis Biological Research Center (Supplemental Table S2). These were designated fact-1 (SM_3_35551) and fact-2 (WiscDsLoxHS125_07F). Reverse transcription (RT)-PCR of root mRNA extracts with FACT-specific primers revealed no transcript present in the fact-1 line. Roots of the fact-2 allele had a lower abundance of the entire FACT transcript but elevated amounts of a truncated (first exon) transcript (Supplemental Fig. S4).
Analysis of the lipids obtained from rapid dipping of the roots of these two mutant alleles in chloroform demonstrated a near-complete lack of all alkyl caffeate esters, a slight reduction in alkyl ferulate esters, and an increase in alkyl coumarate esters (Fig. 3). A concomitant increase in the proportion of C20 and C22 primary alcohols and eicosyl (C20) coumarate and docosyl (C22) coumarate was evident in root waxes of the mutant alleles. The fact mutants also presented a clear increase in total long-chain fatty alcohol content over the wild type.
Figure 3.
Comparison of waxes extracted from mature roots of fact mutant and wild-type (Col-0) Arabidopsis plants. A, Total wax components in each class per g of fresh weight. B, Chain length distribution of individual wax components as mole percent composition. Data are presented as the mean of four biological replicates + sd. Alkn, Alkane; FFAs, free fatty acids; MAGs, monoacylglycerols.
Although we previously reported that sodium-methoxide catalyzed transmethylation gave good recoveries of coumarate, ferulate, and sinapate monomers from suberin (Molina et al., 2006), we never tested caffeate. Recovery of the methyl ester of this particular hydroxycinnamic acid is very poor with base-catalyzed depolymerization of suberized tissues, so we reverted to acid-catalyzed methods (5% sulfuric acid in methanol and 1 n methanolic hydrogen chloride) where caffeate could be efficiently recovered (Supplemental Tables S3 and S4; Supplemental Materials and Methods S1). Analysis of the aliphatic components released by depolymerization of delipidated taproot residues revealed only minor changes in taproot (periderm) suberin content or composition in fact mutants (Fig. 4). However, as expression databases and confocal imaging of PAt5g63560::eYFP-containing transgenic plants revealed FACT expression in the seed coat of developing seeds, we also analyzed the suberin of delipidated seed residues for changes in caffeate content. Analysis of fact-1 seed suberin monomers revealed reduced amounts of caffeate (Fig. 5). In wild-type Arabidopsis seeds the level of caffeate is low, at about 61 ± 8 μg/g seed residue (n = 3), but this is reduced to 19 ± 2 μg/g seed residue (n = 3) in the fact-1 mutant (Fig. 5). For comparison, wild-type Arabidopsis seeds have 702 μg of ferulate per gram of seed residue (Molina et al., 2009).
Figure 4.
Suberin monomer composition of fact mutant versus wild-type (Col-0) taproots (periderm). Inset graph shows caffeate content. Data are presented as the mean of four biological replicates + sd. PPs, Phenylpropanoids; FAs, fatty acids; ω-OH FAs, ω-hydroxy fatty acids; DCAs, dicarboxylic acids.
Figure 5.
Caffeate content of seed suberin in fact-1 versus wild-type (Col-0). Data are presented as means of triplicate determinations + sd.
As other BAHD enzymes have been implicated in lignin synthesis (Hoffmann et al., 2003, 2005) we analyzed the lignin content of one fact mutant allele. Analysis of fact-1 acetyl-bromide soluble lignin and lignin monomer composition by thioacidolysis did not reveal significant alteration of root or seed lignin content or composition (Supplemental Fig. S5; Supplemental Materials and Methods S1). Seed surface waxes of fact-1 were not substantially different from those of the wild type (Supplemental Fig. S6). Collectively, these data demonstrated that a fully functional FACT was necessary for production of alkyl caffeate root waxes and the incorporation of caffeate into seed coat suberin.
Recombinant FACT Protein Functions as a Fatty Alcohol:Hydroxy Cinnamoyl CoA Acyltransferase with Apparent Preference for Caffeoyl-CoA
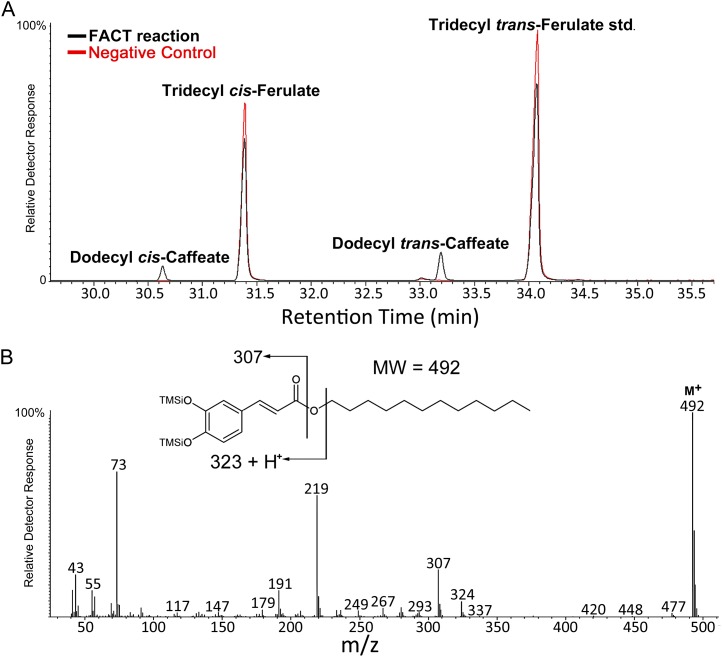
In part by analogy to its close homolog, ASFT, root wax data from the two mutant alleles suggested that FACT was most likely responsible for catalyzing an acyl transfer of the caffeoyl group from caffeoyl-CoA to a fatty alcohol acceptor. To test this hypothesis, we expressed the protein-coding sequence of At5g63560 in Escherichia coli. After recombinant protein induction, soluble protein extracts were tested for acyltransferase activity. We employed a coupled enzyme assay system using a preincubation step with recombinant tobacco (Nicotiana tabacum) 4-COUMARATE:COENZYME A LIGASE (4CL; Lee and Douglas, 1996; Beuerle and Pichersky, 2002) to generate CoA esters of specific phenylpropanoids (ferulate, coumarate, or caffeate) in vitro. Figure 6 shows the product recovered from assay with dodecan-1-ol (C12) and caffeic acid. The corresponding electron impact mass spectrum shows the expected molecular ion for bis-trimethylsilyl dodecyl caffeate at mass-to-charge ratio (m/z) = 492, plus diagnostic fragmentation. Elimination of an alkene produces the ion radical at m/z = 324, while elimination of an alkoxy radical produces the ion at m/z = 307. The origin of the m/z = 219 ion is uncertain, but it may occur from nominal loss of Me4Si from the m/z = 307 ion.
Figure 6.
In vitro formation of dodecyl caffeate by recombinant FACT. A, Partial GC-MS chromatogram of FACT reaction extract showing dodecyl caffeate (cis- and trans-isomers) formed by recombinant FACT and tridecyl ferulate internal standard used for quantification. Black line represents extract from a recombinant FACT-containing reaction. The red line represents an extract from a negative control reaction containing soluble crude protein extracted from nontransgenic (WT) BL21 E. coli cells. B, EI mass spectrum of trans-dodecyl caffeate from FACT reaction extract and dodecyl caffeate molecular structure showing diagnostic ions.
Despite a preference for coumarate and ferulate over caffeate by the 4CL employed in the coupled assays (Lee and Douglas, 1996), we consistently found higher amounts of alkyl hydroxycinnamate product formed with caffeate than with either coumarate or ferulate (Fig. 7). No activity was detected when cell-free protein extracts from FACT-expressing E. coli were excluded from the assay, nor when cell-free protein extracts from untransformed E. coli, that is without the FACT expression vector, were used instead. These results, in conjunction with the root wax data, strongly implicate a preference for caffeoyl-CoA by FACT and FACT’s function as a caffeoyl-CoA-dependent caffeoyl transferase toward fatty alcohol substrates. We also tested a range of alkyl chain lengths, both for recombinant FACT and for ASFT, in the coupled assay (Supplemental Fig. S7). In both cases activity fell off dramatically as the carbon chain length of the primary alcohol acyl acceptor increased from C8 or C12 to C18, such that no activity was detected, even in the much more active ASFT preparation, for octadecan-1-ol. The inclusion of CHAPS detergent (0.001%–1% [w/v]) in the assay failed to enhance activity toward a longer-chain fatty alcohol substrate, although no inhibition was observed either (Supplemental Fig. S7).
Figure 7.
Substrate specificity of the recombinant FACT enzyme. Coupled cell-free assays were performed using different acyl-CoA donors. Recombinant 4CL was used to generate corresponding CoAs of different phenylpropanoids (i.e. ferulate, coumarate, or caffeate) in vitro via a preincubation step. FACT activity was measured in a second step by the addition of total protein extracts from BL21 E. coli-expressing recombinant FACT or from wild-type (nontransgenic) BL21 cells (WT Crude) and dodecan-1-ol. N.D., Not detected. Data represent the means of quadruplicate assays + sd.
FAR1, FAR4, and FAR5 Provide Alkan-1-ols for Alkyl Hydroxycinnamate Synthesis
Transcript coexpression analysis revealed a strong correlation between FACT and two FARs, FAR4 and FAR5 (Supplemental Table S1). Recently, FAR5, FAR4, and FAR1 were demonstrated to produce the C18, C20, and C22 alkan-1-ols, respectively, found in Arabidopsis seed, root, and wound suberin (Domergue et al., 2010). The alkyl functions of alkyl hydroxycinnamates, derived from fatty alcohols, possess the same carbon chain lengths as those found in suberin. Hence we investigated far1, far4, and far5 for their effects on alkyl hydroxycinnamate synthesis. The reported chain length specificity of each respective FAR was closely reflected in the alkyl hydroxycinnamate waxes of the far1, far4, and far5 mutants (Fig. 8). far5 mutants lacked all C18 alkyl hydroxycinnamates, far4 mutants had reduced levels of C20 alkyl hydroxycinnamates, and far1 mutants had reduced levels of C22 alkyl hydroxycinnamates. These results suggest that FAR1, FAR4, and FAR5 donate the alcohols necessary for alkyl hydroxycinnamate synthesis to FACT for alkyl caffeate synthesis and the yet to be identified acyltransferase(s) required for alkyl coumarate and alkyl ferulate synthesis. These results also demonstrate overlap between suberin and root wax biosynthetic pathways.
Figure 8.
Root wax alkan-1-ol and alkyl hydroxycinnamate compositions from taproots of far1, far4, far5 mutants, and wild-type (Col-0) plants. Data are presented as the mean of four biological replicates + sd.
Alkyl Coumarate But Not Alkyl Caffeate Root Waxes Are Induced by Salt Stress
Little is known about the function of alkyl hydroxycinnamate ester root waxes. To determine if alkyl hydroxycinnamates in root waxes could play a role in abiotic stress responses, 6-week-old wild-type and fact mutant plants were treated twice with 200 mm NaCl over a 1-week period. A concentration of 200 mm was selected based on previous stress experiments related to root suberin and leaf cuticle lipids (Beisson et al., 2007; Kosma et al., 2009). NaCl treatment resulted in an increased proportion of root wax alkyl coumarates and a slight reduction in alkyl caffeate content in wild-type plants (Fig. 9) but did not affect the proportions of other root wax components (Supplemental Fig. S8). A higher proportion of alkyl coumarates in the root waxes of NaCl-treated plants was also evident in fact mutant alleles. What can be surmised from these experiments is that alkyl caffeates are not induced by salt stress and by proxy FACT and alkyl caffeates are not involved in NaCl stress responses.
Figure 9.
Alkan-1-ol and alkyl hydroxycinnamate composition from roots of 200 mm NaCl-treated wild-type (Col-0) and fact mutant plants. The inset graph shows total wax content, in μg per g fresh weight, for all genotypes and treatments. Data are presented as the mean of four biological replicates + sd.
DISCUSSION
The BAHD acyltransferase encoded by At5g63560, a very close homolog to the ASFT At5g41040 (Molina et al., 2009), also has a very strong correlation with suberin biosynthesis genes (Beisson et al., 2012). To track down the function of At5g63560, we examined the alkyl hydroxycinnamates, constituents of root waxes. We describe the chemistry and localization of these molecules, and then experiments that show At5g63560 (FACT) is indeed an aliphatic caffeoyl transferase. We also implicate FAR1, FAR4, and FAR5 as providing the fatty alcohols required for alkyl hydroxycinnamate synthesis. Finally, we briefly discuss aspects of alkyl hydroxycinnamate biosynthesis and function.
FACT Is Involved in Alkyl Caffeate Ester Synthesis in Roots
FACT, a member of the BAHD superfamily and of the same clade as suberin-associated ASFT, was identified as playing a role in the synthesis of alkyl caffeate esters found in root waxes based on chemical analysis of mutant plants and in vitro activity of recombinant proteins. Since alkyl caffeate esters were virtually absent from fact mutant plants (Fig. 3), and asft mutant plants were reported to be unaffected in their root wax composition (Molina et al., 2009), there appears to be little or no functional redundancy between FACT and ASFT.
Several lines of evidence designate the in planta function of FACT as a caffeoyl O-acyltransferase required for the synthesis of alkyl caffeate esters of root waxes. (1) fact mutant alleles demonstrated a near-complete lack of alkyl caffeate esters in their root waxes. (2) We found no apparent reduction in the 3′-hydroxylated phenylpropanoids of lignin monomers (syringyl or guaiacyl monomers) in one fact allele, making it unlikely that FACT is directly involved in the synthesis of caffeoyl-CoA. Furthermore, HYDROXYCINNAMOYL-CoA SHIKIMATE/QUINATE HYDROXYCINNAMOYL TRANSFERASE, a BAHD acyltransferase, has already been identified to participate in the synthesis of caffeoyl-CoA (catalyzing the reversible transfer of hydroxyl-cinnamoyl groups between CoA thioester and shikimic/quinic acid conjugates, allowing 3-hydroxylation of coumarate to produce caffeate). (3) FACT is phylogenetically distant from HYDROXYCINNAMOYL TRANSFERASE (Tuominen et al., 2011). (4) Our enzymology results demonstrate a preference for caffeoyl-CoA and catalysis of the transfer of caffeate from its CoA thioester to a fatty alcohol acyl acceptor by recombinant FACT.
To date, four BAHD acyltransferases have been implicated in suberin and cutin biosynthesis (Gou et al., 2009; Molina et al., 2009; Panikashvili et al., 2009; Serra et al., 2010; Rautengarten et al., 2012). However, three of these acyltransferases are primarily involved in the transfer of the feruloyl group from feruloyl-CoA to the ω-terminus of ω-hydroxy fatty acids to form feruloyloxy aliphatics. The other is implicated as catalyzing the transfer of aliphatics to diacylgylcerols (Rani et al., 2010). Of these BAHDs, potato (Solanum tuberosum) FATTY OMEGA-HYROXYACID/FATTY ALCOHOL HYDROXYCINNAMOYL TRANSFERASE (FHT) is the only acyltransferase that affects the accumulation of simple alkyl ferulate esters (i.e. ferulate linked to a fatty alcohol). This was demonstrated both in planta by analysis of mutant chemical phenotypes and in vitro using fatty alcohols as acyl acceptors (Serra et al., 2010). FHT acyl acceptors in planta are C18 to C31 primary alcohols and thus are extremely hydrophobic. For ASFT, in planta the suberin acyl acceptors were inferred to be largely ω-hydroxyfatty acids and possibly fatty alcohols from the stoichiometric reduction of these monomers that occurred together with the reduction in ferulate in seeds of asft lines (Molina et al., 2009). BAHD acyltransferases behave as soluble acyl transferases in vitro, and are generally known to function with more hydrophilic acyl acceptors (D’Auria, 2006). The fact mutant phenotype provides an additional example that BAHD enzymes can utilize very hydrophobic aliphatic substrates in vivo. However, in vitro assays of FHT, ASFT, DCF, and FACT are problematic with more hydrophobic substrates. ω-Hydroxypalmitate appears to be the best acyl acceptor in multiple studies (Lofty et al., 1994; Gou et al., 2009; Molina et al., 2009; Serra et al., 2010; Rautengarten et al., 2012). Recombinant ASFT is reported to have activity toward C12 and C14 fatty alcohols in one study (Molina et al., 2009), but to only have activity toward C7 and C8 in a C3 to C18 range tested in an independent study (Gou et al., 2009). For recombinant FHT, activity toward C12 and C14 fatty alcohols was reported from a range of C8 to C20 substrates tested (Serra et al., 2010). This is in close agreement with the specificity of the partially purified enzyme from wound-healing potato discs (Lofty et al., 1994). C1 to C18 alkan-1-ols were tested with DCF but activity was only detected with C1 to C6 substrates. Thus assays of FHT, ASFT, DCF, and FACT all suffer from lack of activity toward long-chain fatty alcohols, indicative of a substrate solubility issue; whereas we know that in vivo long-chain aliphatics are the natural substrates. Assay of ASFT with the detergent CHAPS to act as a fatty alcohol solubilizing agent did not alleviate the problem. Here we have demonstrated that the acyl-CoA reductases that provide FACT with fatty alcohol substrates are FAR1, FAR4, and FAR5. Thus we expect that there must either be some type of fatty alcohol channeling or carrier protein between FAR and FACT, or that in vivo both enzymes associate with a specific membrane domain to produce hydroxycinnamate esters.
Connections between Alkyl Hydroxycinnamate Ester Synthesis and Suberin Synthesis
Alkyl hydroxycinnamate esters represent the junction of two distinct metabolisms, namely phenylpropanoid biosynthesis and very-long-chain fatty acid and fatty alcohol biosynthesis. A number of genes and enzymes have been shown to participate in the synthesis of long-chain aliphatic suberin monomers. FAR genes (FAR5, FAR4, and FAR1) have been shown to synthesize the C18, C20, and C22 saturated fatty alcohols, respectively. These fatty alcohols are typically found in methanolysates of Arabidopsis suberin (Domergue et al., 2010). DAISY/KETOACYL CoA SYNTHASE2, is involved in the initial condensation reaction for elongation reactions leading to C22 and C24 suberin monomers (Franke et al., 2009). The fatty alcohols present in Arabidopsis root and seed suberin are largely straight-chain C18, C20, and C22 saturates (Franke et al., 2005; Molina et al., 2006; Beisson et al., 2007). This is the same suite of chain lengths found in the hydroxycinnamic acid esters of Arabidopsis roots (Li et al., 2007; Molina et al., 2009; this study). In addition, we have also noted that gene expression between FAR4/5 and FACT is highly correlated (Supplemental Table S1). This correlation is directly demonstrated by the fact that far1, far4, and far5 mutants have exactly the same effect on alkyl chain length for suberin monomers and hydroxycinnamate esters. Thus we infer that these FAR enzymes provide fatty alcohols for the biosynthesis of both suberin and solvent-soluble hydroxycinnamate esters.
Mature portions of Arabidopsis taproots are covered by an exposed and suberized periderm (Franke et al., 2005; Li et al., 2007; Höfer et al., 2008) and, as illustrated here, are also enriched in root waxes. Our eYFP reporter localization experiments demonstrated FACT transcriptional activity in suberized tissues including the outer perimeter of mature roots (suggestive of a periderm localization). We have shown that mutations in suberin-associated genes FAR1, FAR4, and FAR5 have a large impact on alkyl hydroxycinnamate accumulation in root waxes. Furthermore, gpat5 mutants, known to be severely affected in suberin monomer accumulation (Beisson et al., 2007), are also reduced in their root wax monoacylglycerol content (Li et al., 2007). Collectively, these results are suggestive of a biosynthetic overlap between root waxes and suberin. How specific this overlap is to the suberized periderm remains to be determined.
Separate Genes/Enzymes Are Required for Alkyl Coumarate and Alkyl Caffeate Synthesis
Alkyl coumarate esters were not eliminated or reduced by fact mutations. In fact, eicosan-1-ol, docasan-1-ol, and their corresponding alkyl coumarate esters were present in amounts higher than those observed in the wild type. This is suggestive of a redirection of eicosan-1-ol and docosan-1-ol that would normally be destined for alkyl caffeate synthesis, to alkyl coumarate synthesis. Furthermore, our enzymology data indicated that FACT was capable of using both feruloyl- and coumaroyl-CoAs yet had a preference for caffeoyl-CoA. Previously characterized suberin biosynthetic enzymes ASFT (Gou et al., 2009) and HYDROXYCINNAMOYL-CoA:ω-HYDROXYPALMITIC ACID O-HYDROXYCINNAMOYLTRANSFERASE (HHT) (Lofty et al., 1994) showed a clear preference for feruloyl- and coumaroyl-CoA and had no activity with caffeoyl-CoA. ASFT and HHT also preferred ω-hydroxy fatty acids over primary alcohols, while asft mutants had no effect on the levels of any of the alkyl hydroxycinnamate esters present in taproots (Molina et al., 2009). Thus ASFT and HHT can be excluded as candidates for caffeoyl-CoA caffeoyl transferases. Collectively, these results suggest a significant degree of hydroxycinnamate specificity by the acyltransferases involved in the synthesis of each respective class of root wax alkyl hydroxycinnamates, namely the caffeates, coumarates, and ferulates. This hydroxycinnamate preference can also be seen in vivo for suberin synthesis with asft, fht, and fact mutants. Clearly, additional alkyl hydroxycinnamate ester biosynthetic genes remain to be identified, specifically for the synthesis of alkyl coumarates, which are not reduced in asft or fact mutants. The identification of these genes will help clarify questions on the physiological function of alkyl hydroxycinnamate esters and whether alkyl hydroxycinnamates are truly associated with suberin, as their gene expression and chemistry suggests. We are currently exploring candidate genes.
The Physiological Functions of Alkyl Hydroxycinnamate Esters Remain an Enigma
Little is known about the function of alkyl hydroxycinnamate esters in roots or other organs with a suberized periderm (i.e. bark). Given that the periderm is the interface between the plant root and its environment, one generic possibility is that root waxes function to augment the barrier properties of suberin (Schreiber et al., 2005; Schreiber, 2010). This possibility could imply colocalization to the suberin lamellae. Function may be dependent on localization. The kinetics of solvent extraction (Li et al., 2007) and a recent imaging study using surface-sputtering mass spectrometry of silver ion adducts (Jun et al., 2010) suggest rather superficial localizations. However, the exact localization of the alkyl hydroxycinnamates, both in terms of extra- versus intracellular deposition, and lateral heterogeneity, remains unclear. Our current analyses show that Arabidopsis taproots (periderm) contain 13-fold greater suberin monomer load (9,411 ± 2,028 μg/g dry weight taproot, n = 4) than rapidly extractable wax (703 ± 170 μg/g dry weight taproot, n = 4). However, our analysis of root waxes from plants grown at different times and in different growth environments revealed a large variability in alkyl hydroxycinnamate composition (Supplemental Fig. S1; Li et al., 2007; Molina et al., 2009). Independent studies have reported difficulty in verifying the presence of alkyl hydroxycinnamates in Arabidopsis root waxes (Domergue et al., 2010; Schreiber, 2010). Our analyses show that the rapidly extracted alkyl hydroxycinnamate esters are detectable in taproots of mature Arabidopsis plants (approximately 7 weeks and older) germinated and grown in peat-based potting medium (i.e. not germinated and grown on Murashige and Skoog plates, or transferred to potting medium from Murashige and Skoog plates, etc.). At present, the conditions that lead to alkyl hydroxycinnamate production are, like their physiological function, not clearly understood.
The lack of knowledge on root physiology that might be attributed to alkyl hydroxycinnamates and the general lack of information on mature Arabidopsis roots (transcriptome, stress experiments, etc.) prompted us to explore a condition that might induce root alkyl hydroxycinnamate production, namely NaCl stress. Both wild-type and fact mutant plants exhibited proportional increase in root wax alkyl coumarate content under NaCl stress. fact mutant plants did not appear more sensitive to NaCl treatment than the wild type based on visual observations. Thus, alkyl caffeates do not appear to play a role in functions related to salt-stress tolerance and by implication neither does the FACT gene. Whether alkyl coumarates play a direct role in NaCl stress responses remains to be determined. Identification of the coumaroyl transferases will aid this endeavor.
Arabidopsis has clearly evolved distinct genes for coumaroyl, caffeoyl, and feruloyl transfer to aliphatic acceptors. The evolution of such genes argues that each class of alkyl hydroxycinnamate has a distinctly different functional role. The discovery of a caffeoyl transferase is of particular interest given the new roles being discovered for caffeate derivatives in lignin (Chen et al., 2012). While the physiological function of alkyl hydroxycinnamate esters in the root periderm remains unknown, it is possible that these specialized lipids play a role in preventing decay or breakdown of the root by soil-dwelling microorganisms, thereby protecting the reserves stored in an important sink organ, the taproot. This may prove important in the vegetative overwintering undergone by many ecotypes of Arabidopsis in nature and by cruciferous vegetables in general. Clearly further investigation into the physiological and ecological roles of root wax alkyl hydroxycinnamate esters is warranted.
CONCLUSION
Here we have identified four genes required for root wax alkyl hydroxycinnamate ester synthesis. First, we have characterized an acyltransferase, FACT, which catalyzes the ultimate step of alkyl caffeate synthesis, the transfer of a caffeoyl group from the caffeoyl-CoA thioester to a fatty alcohol acceptor. We have also implicated FACT as important for the incorporation of caffeate into seed coat suberin. Furthermore, we have shown that FAR1, FAR4, and FAR5 each provide a specific chain length of fatty alcohol required for alkyl hydroxycinnamate synthesis. We have also demonstrated that alkyl coumarate production, and not alkyl caffeate production, appears to be induced by NaCl stress, excluding FACT involvement in NaCl-stress responses. Collectively, these results suggest that separate acyltransferases are required for the synthesis of each respective class of alkyl hydroxycinnamate (coumarate versus caffeate versus ferulate) and that a biosynthetic overlap exists between suberin and root wax biosynthesis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type (Columbia-0) seeds were stratified at 4°C for 2 to 3 d and grown in 3.5-inch pots of a mixture of Promix PGX soil-less medium (Premier Horticulture), vermiculite, and perlite (1:1:1, v/v) or, for root wax and suberin analysis, in Promix PGX soil-less medium and calcined clay granules (1:1, v/v; Profile Greens Grade) at a density of four to five plants per pot. All plants were grown in a growth chamber at 21°C to 22°C, 40% to 60% humidity, a 16/8-h light/dark cycle, and a fluorescent light intensity of 80 to 100 μmol m−2 s−1. T1 and T2 seeds of transgenic plants harboring PAt5g63560::eYFP constructs were surface sterilized, selected on 0.7% agar (one-half-strength Murashige and Skoog salts, 1.5% Suc, supplemented with 100 μg mL−1 Timentin or Carbenicllin, and 15–20 μg mL−1 Hygromycin B or 100 μg mL−1 Gentamicin), transferred to soil, and grown as described above.
Mutant Isolation
Seed stocks of transposon/T-DNA insertion lines (Alonso et al., 2003) for FACT (AT5G63560; John Innes Centre SM_3_35551/CS122262 and WiscDsLoxHs125_07F/CS911958) were obtained from the Arabidopsis Biological Resource Center seed collection (http://www.biosci.ohiostate.edu). Primers to amplify the wild-type genomic DNA were FACT_F1 and FACT_R1, and FACT_F2 and FACT_R2, respectively (Supplemental Table S2). To check for the T-DNA insertion in SM_3_35551/CS122262, FACT_F1, specific for the 3′ untranslated region gene sequence, and T-DNA left-border-specific primer Spm32 were used. To check for the T-DNA insertion in WiscDsLoxHs125_07F/CS911958, FACT_F2, specific for the first exon of At5g63560, and T-DNA left-border-specific primer L4 were used. For RT-PCR, RNA was isolated from 100 mg of 3-week-old Arabidopsis roots using Trizol, following manufacturer’s instructions. SuperScript II reverse transcriptase (Invitrogen) was used to synthesize the first-strand complementary DNA (cDNA) using a polyT(15) primer following manufacturer’s protocol using 300 ng of total RNA extract. Two-microliter aliquots of the RT reactions were used as template for PCR. Primers for FACT transcript/cDNA amplification were FACT_RT_F and FACT_RT_R1, specific for the first exon, and exon-spanning FACT_RT_F and FACT_RT_R2 (Supplemental Table S2).
Root Wax and Suberin Analysis
Seven-week-old Arabidopsis plants were used for root wax extractions and suberin preparations. All Arabidopsis plants used for root wax extraction were germinated and grown in soil-less potting medium as described above. Each replicate consisted of the roots from all plants in three to four pots (12–20 plants). Genotypes were blocked by replicate in a randomized complete block design. Arabidopsis root waxes were extracted by submerging roots in chloroform for 1.5 min. This time point was chosen to maximize alkyl hydroxycinnamate extraction based on release/dipping kinetics of Arabidopsis root waxes by Li et al. (2007). Internal standards included pentadecanoic acid (C15:0), tricosan-1-ol (C23), monoheptadecanoin (17:0 monoacylglycerol), and octacosane (C28). Tridecyl ferulate and heptadecyl coumarate were also used as internal standards for alkyl hydroxycinnamate esters. After extraction, internal standards were added to chloroform extracts, the volume of each extract reduced by evaporation under nitrogen, and extracts filtered through glass wool. Samples were evaporated to dryness and silylated with 100 μL, each, of pyridine and N,O-bis(trimethylsilyl)trifluoroacetamide at 110°C for 10 min. Derivatized samples were evaporated to dryness (without heating) and dissolved in heptane:toluene (1:1, v/v) for analysis by electron impact (EI) gas chromatography-mass spectrometry (GC-MS).
Root waxes were analyzed on an Agilent 6850 gas chromatograph equipped with an Agilent 5975 mass spectrometer. Splitless injection was used with a 30-m HP5-MS column. Temperature settings were as follows: inlet 350°C, detector 320°C, oven temperature program was set to 130°C for 2 min and increased to 325°C at a rate of 5°C min−1. The helium flow rate was set at 1.5 mL min−1.
Acid-catalyzed transmethylation was used to affect the depolymerization of Arabidopsis taproot and seed coat suberin. Taproot reactions consisted of approximately 30 mg dried, delipidated root tissue, 3 mL of 1 n methanolic-hydrogen chloride (Supelco), and 50 μL of 0.2% (w/v) butylated hydroxytoluene (dissolved in methanol). Reactions were heated overnight at 60°C, allowed to cool to room temperature, and extracted (2×) with dichloromethane. Organic phases were collected, dried over anhydrous sodium sulfate, and evaporated to dryness. Saline washes (0.9% NaCl, w/v) were found to remove caffeate from the organic phase (Supplemental Table S4) so were omitted from the protocol. Dried extracts were silylated and analyzed by GC-MS as described above. Arabidopsis seed suberin was analyzed via acid-catalyzed transmethylation (5% sulfuric acid in methanol, v/v) using toluene as a cosolvent in a 2:1 ratio (v/v). Reactions were heated overnight at 60°C, allowed to cool to room temperature, and extracted (2×) with dichloromethane. Organic phases were collected, dried over anhydrous sodium sulfate, and evaporated to dryness. Dried extracts were silylated and analyzed by GC-MS (EI) as described above. Methyl heptadecanote and pentadecalactone were used as internal standards.
Synthesis and Purification of Tridecyl Ferulate and Heptadecyl Coumarate Standards
Alkyl hydroxycinnamates were synthesized by acid-catalyzed esterification as per the method of Trombino et al. (2009) with slight modification. Either 1-tridecanol (22 mmol), ferulic acid (20 mmol), and p-toluene sulfonic acid (1.5 mmol), or 1-heptadecanol (11 mmol), coumaric acid (10 mmol), and p-toluene sulfonic acid (0.75 mmol) were refluxed together in 50 mL of toluene under nitrogen gas for 10 h with azeotropic distillation to remove water. The solvent-extracted crude reaction product was purified by preparative thin-layer chromatography on silica thin-layer chromatography plates (PK6F; Whatman) using multiple development with 1.5% ethanol in chloroform. Alkyl hydroxycinnamate bands were identified by fluorescence quenching under UV light. Alkyl hydroxycinnamates were eluted from the silica with chloroform:methanol:water (5:5:1, v/v/v), and recovered in the chloroform phase after phase partitioning by addition of chloroform and 0.88% KCl (w/v), then washing the chloroform phase twice with methanol:water (1:1, v/v). Extracts were evaporated to dryness under nitrogen gas, recovered as waxy solids, desiccated for 2 d, and dry weights collected. Purity as determined by GC-MS of the silylated product was >95% (Supplemental Figs. S3 and S4; Supplemental Materials and Methods S1).
eYFP Reporter Construct Design and Plant Transformation
The reporter gene eYFP was released from pAN59 by restriction endonuclease digestion and ligated into BamHI- and EcoRI-digested pPZP221. The 1.583-kb 5′ DNA sequence upstream of the FACT open reading frame was amplified by PCR using genomic DNA as a template and subcloned into the pGEM-Teasy vector (Promega Corporation). Cloning primers were FACTpro_SalI_F and FACTpro_BamHI_R (Supplemental Table S2). All amplifications used a proofreading DNA polymerase (Phusion, New England Biolabs). A PAt5g63560::eYFP cassette was generated by releasing the FACT promoter from the pGEM-Teasy (Promega) vector and ligating the FACT promoter upstream of the eYFP sequence into BamHI/SalI-digested, eYFP-containing pPZP221. The PAt5g63560::eYFP cassette was amplified from pPZP221 binary vector by PCR using FACTpro_HindIII_F and pAN59_eYFP_SACI_R primers, digested with restriction endonucleases, and ligated into HindIII/SacI-digested pGWB540 (Nakagawa et al., 2007) and sequenced. The binary plasmid was introduced into Agrobacterium tumefaciens strain C58CI by electroporation. Arabidopsis Columbia-0 wild-type transformation was performed by the vacuum-infiltration method (Bechtold et al., 1993). Both PAt5g63560::eYFP-containing pPZP221 and pGWB540 binary vectors were used, in separate transformations, to transform Col-0 plants. T1 and T2 plants that were hygromycin or gentamicin resistant were analyzed by CLSM to evaluate eYFP expression. Wild-type plants grown under similar conditions were used for comparison.
CLSM Analysis of Fluorescent Protein Expression
Imaging of whole or hand-sectioned, transgenic roots mounted in water was performed with Olympus FluoView FV1000 confocal laser-scanning microscope. For visualization of YFP fluorescence (digitally colored green) samples were excited with 515-nm argon ion laser line and emission was collected at 525 to 555 nm or 535 to 565 nm. For visualization of propidium iodide staining (digitally colored red), samples were excited with a diode laster at 559 nm with emission collected at 650 to 750 nm (roots) or 580 to 630 nm (seeds). Propidium iodide staining was performed by submerging tissues in 0.5 mg mL−1 aqueous propidium iodide for 10 min. Individual optical sections were used to create extended focus images using Olympus FluoView ASW software (ver. 3.1a; Olympus). Images were transformed to TIFF format files and processed with Adobe Photoshop Elements 10.
Recombinant Protein Expression
The FACT cDNA, designed de novo to optimize codon usage for Escherichia coli, was synthesized by GenScript. The synthetic FACT cDNA was cloned into pGS-21a expression vector (GenScript) using NcoI and XhoI restriction sites. A His tag/GST epitope was fused in frame at the N terminus. E. coli BL21 (DE3) LysS (Novagen) cells were transformed by heat shock with 0.2 μg of plasmid and grown in Luria-Bertani plates containing 100 mg L−1 carbenicillin and 34 mg L−1 chloramphenicol. A single bacterial colony was used to inoculate a liquid culture, which was in turn used to inoculate a subculture of Luria-Bertani medium containing 100 mg L−1 carbenicillin, which was grown at 20°C with shaking. When OD = 0.6, isopropyl β-d-thiogalactopyranoside (0.8 mm) was added for induction of protein expression, and the culture was grown for 24 h. Procedures for protein extraction are described elsewhere (Beuerle and Pichersky, 2002).
Enzyme Assay Conditions
Hydroxycinnamoyl-CoA thioester substrates were generated by incubating a reaction mixture (0.4 mL) containing 50 mm Tris-HCl pH 7.5, 2.5 mm MgCl2, 2.5 mm ATP, 0.2 mm caffeic (or coumaric or ferulic) acid, 0.2 mm CoASH (lithium salt), and 65 μg of whole soluble protein extract from E. coli expressing 4CL (Beuerle and Pichersky, 2002; Molina et al., 2009) for 40 min at room temperature. To each 4CL assay was then added, in this sequence and to a final concentration in a final volume of 0.5 mL, 1 mm dithiothreitol, 50 mm Tris-HCl (pH 7.5), and 140 μg of crude protein from FACT-expressing E. coli or from E. coli not harboring the FACT expression construct (wild-type crude). FACT acyltransferase reactions were initiated by the addition of 250 nmols alkan-1-ol to each reaction. Alkan-1-ols were added dissolved in acetone. When ethanol was used as a solvent, the corresponding ethyl esters of phenylpropanoids could be detected as FACT reaction products. Separate assays were used to test each specific phenylpropanoid CoA thioester. After a 90-min incubation at room temperature the reaction was stopped by addition of 1 mL of hot (85°C) iso-propanol and heating reactions at 85°C for 10 min. After cooling to room temperature tridecyl ferulate internal standard was added to each reaction. Reactions were phase partitioned by adding 1.5 mL of aqueous 6.7% (w/v) sodium sulfate and 1.5 mL hexane. The organic phase was removed to a new tube and the remaining aqueous phase was back extracted with 1 mL of hexane:isopropanol (7:2, v/v). Pooled organic phases were dried under a stream of N2. Samples were silylated by heating at 110°C for 10 min in N,O-bis(trimethylsilyl)trifluoroacetamide:pyridine (1:1, v/v) and analyzed by GC-MS on an HP-5MS capillary column temperature programmed from 130°C to 330°C at 5°C min−1.
Salt-Stress Treatments
Six-week-old plants of Columbia-0, fact1-1, and fact 1-2 were watered with 200 mm NaCl twice over a 1-week period to induce salt stress. Plants were grown as described above (“Plant Materials and Growth Conditions”). Each replicate consisted of the roots from all plants from three to four pots (approximately 12–20 plants). Genotypes and treatments were blocked by replicate in a randomized complete block design.
Sequence data from this article can be obtained from the Arabidopsis Genome Initiative database under the following accession numbers: ASFT (At5g41040), FACT (At5g63560), FAR5 (At3g44550), FAR4 (At3g44540), and FAR1 (At5g22500).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison of Arabidopsis (Col-0) root wax alkyl hydroxycinnamate composition from Li et al. (2007), Molina et al. (2009), and this study (from Figs. 1, 3, and 8).
Supplemental Figure S2. Chromatogram (A) and EI mass spectrum (B) from GC-MS analysis of purified tridecyl ferulate standard.
Supplemental Figure S3. Chromatogram (A) and EI mass spectrum (B) from GC-MS analysis of purified heptadecyl coumarate standard.
Supplemental Figure S4. RT-PCR analysis of At5g63560 transcript from 3-week-old roots of Columbia-0 and fact mutant plants.
Supplemental Figure S5. Lignin content and composition of fact-1 versus wild-type roots (A) and seeds (B).
Supplemental Figure S6. fact-1 seeds are unaffected in their surface wax composition.
Supplemental Figure S7. Effect of alkan-1-ol carbon chain length on FACT (A) and ASFT (B) activity, and of CHAPS detergent on ASFT activity (C).
Supplemental Figure S8. Root wax composition of 200 mm NaCl-treated Col-0 and fact mutant plants.
Supplemental Table S1. Coexpression analysis (http://cressexpress.org/) of suberin genes.
Supplemental Table S2. Primers used for mutant isolation, RT-PCR, and PAt5g63560::eYFP plasmid.
Supplemental Table S3. Comparison of phenylpropanoid methyl ester percent recoveries in base versus acid-catalyzed transmethylation reactions.
Supplemental Table S4. Effect of aqueous NaCl washing steps on phenylpropanoid methyl ester recovery from dichloromethane organic phases of different acid-catalyzed transmethylation procedures.
Supplementary Material
Acknowledgments
We thank Melinda Frame (Michigan State University Advanced Microscopy Center) for assistance/training with CLSM and Dr. Eran Pichersky (University of Michigan) for providing us with an E. coli strain expressing a tobacco 4CL. We also acknowledge and thank Cliff Foster of the Great Lakes Bioenergy Research Center Cell Wall Analysis Facility for assistance with lignin analysis, Linda Danhof of the Great Lakes Bioenergy Research Center for assistance with plant transformation, and Merissa Strawsine and Adam Rice (Michigan State University) for assistance with plant care. We thank Owen Rowland (Carleton University) and Frédéric Domergue (Université Victor Ségalan Bordeaux 2, Centre National de la Recherche Scientifique) for providing seeds of the far1, far4, and far5 mutants.
Glossary
- CLSM
confocal laser-scanning microscopy
- RT
reverse transcription
- m/z
mass-to-charge ratio
- T-DNA
transferred DNA
- cDNA
complementary DNA
- GC-MS
gas chromatography-mass spectrometry
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. (1993) In planta agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris Life Sci 316: 1194–1199 [Google Scholar]
- Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB. (2007) The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19: 351–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson F, Li-Beisson Y, Pollard M. (2012) Solving the puzzles of cutin and suberin polymer biosynthesis. Curr Opin Plant Biol 15: 329–337 [DOI] [PubMed] [Google Scholar]
- Bernards MA, Lewis NG. (1992) Alkyl ferulates in wound healing potato tubers. Phytochemistry 31: 3409–3412 [DOI] [PubMed] [Google Scholar]
- Beuerle T, Pichersky E. (2002) Enzymatic synthesis and purification of aromatic coenzyme a esters. Anal Biochem 302: 305–312 [DOI] [PubMed] [Google Scholar]
- Chen F, Tobimatsu Y, Havkin-Frenkel D, Dixon RA, Ralph J. (2012) A polymer of caffeyl alcohol in plant seeds. Proc Natl Acad Sci USA 109: 1772–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comino C, Hehn A, Moglia A, Menin B, Bourgaud F, Lanteri S, Portis E. (2009) The isolation and mapping of a novel hydroxycinnamoyltransferase in the globe artichoke chlorogenic acid pathway. BMC Plant Biol 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria JC. (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9: 331–340 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rodríguez IM, Gutiérrez A. (2004) Identification of intact long-chain p-hydroxycinnamate esters in leaf fibers of abaca (Musa textilis) using gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 18: 2691–2696 [DOI] [PubMed] [Google Scholar]
- Domergue F, Vishwanath SJ, Joubès J, Ono J, Lee JA, Bourdon M, Alhattab R, Lowe C, Pascal S, Lessire R, et al. (2010) Three Arabidopsis fatty acyl-coenzyme A reductases, FAR1, FAR4, and FAR5, generate primary fatty alcohols associated with suberin deposition. Plant Physiol 153: 1539–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelie KE, Sadek NZ, Kolattukudy PE. (1980) Composition of suberin-associated waxes from the subterranean storage organs of seven plants, parsnip, carrot, rutabaga, turnip, red beet, sweet potato and potato. Planta 148: 468–476 [DOI] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L. (2005) Apoplastic polyesters in Arabidopsis surface tissues—a typical suberin and a particular cutin. Phytochemistry 66: 2643–2658 [DOI] [PubMed] [Google Scholar]
- Franke R, Höfer R, Briesen I, Emsermann M, Efremova N, Yephremov A, Schreiber L. (2009) The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. Plant J 57: 80–95 [DOI] [PubMed] [Google Scholar]
- Freire CSR, Silvestre AJD, Neto CP. (2002) Identification of new hydroxy fatty acids and ferulic acid esters in the wood of Eucalyptus globulus. Holzforschung 56: 143–149 [Google Scholar]
- Freire CSR, Silvestre AJD, Neto CP. (2007) Demonstration of long-chain n-alkyl caffeates and delta7-steryl glucosides in the bark of Acacia species by gas chromatography-mass spectrometry. Phytochem Anal 18: 151–156 [DOI] [PubMed] [Google Scholar]
- García-Argáez AN, Pérez-Amador MC, Aguirre-Hernández E, Martínez-Vázquez M. (1999) Two new caffeate esters from roots of Merremia tuberosa and M. Dissecta. Planta Med 65: 678–679 [DOI] [PubMed] [Google Scholar]
- Gou JY, Yu XH, Liu CJ. (2009) A hydroxycinnamoyltransferase responsible for synthesizing suberin aromatics in Arabidopsis. Proc Natl Acad Sci USA 106: 18855–18860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienenberger E, Besseau S, Geoffroy P, Debayle D, Heintz D, Lapierre C, Pollet B, Heitz T, Legrand M. (2009) A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J 58: 246–259 [DOI] [PubMed] [Google Scholar]
- Höfer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R. (2008) The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid omega-hydroxylase involved in suberin monomer biosynthesis. J Exp Bot 59: 2347–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B, Legrand M. (2005) Acyltransferase-catalysed p-coumarate ester formation is a committed step of lignin biosynthesis. Plant Biosyst 139: 50–53 [Google Scholar]
- Hoffmann L, Maury S, Martz F, Geoffroy P, Legrand M. (2003) Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J Biol Chem 278: 95–103 [DOI] [PubMed] [Google Scholar]
- Jun JH, Song Z, Liu Z, Nikolau BJ, Yeung ES, Lee YJ. (2010) High-spatial and high-mass resolution imaging of surface metabolites of Arabidopsis thaliana by laser desorption-ionization mass spectrometry using colloidal silver. Anal Chem 82: 3255–3265 [DOI] [PubMed] [Google Scholar]
- Kawanishi K, Yasufuku J, Ishikawa A, Hashimoto Y. (1990) Long-chain alkyl ferulates in three varieties of Ipomoea batatas (l.) lam. J Agric Food Chem 38: 105–108 [Google Scholar]
- Kolattukudy PE. (2001) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71: 1–49 [DOI] [PubMed] [Google Scholar]
- Kosma DK, Bourdenx B, Bernard A, Parsons EP, Lü S, Joubès J, Jenks MA. (2009) The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol 151: 1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Douglas CJ. (1996) Two divergent members of a tobacco 4-coumarate:coenzyme A ligase (4CL) gene family: cDNA structure, gene inheritance and expression, and properties of recombinant proteins. Plant Physiol 112: 193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Beisson F, Ohlrogge J, Pollard M. (2007) Monoacylglycerols are components of root waxes and can be produced in the aerial cuticle by ectopic expression of a suberin-associated acyltransferase. Plant Physiol 144: 1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofty S, Negrel J, Javelle F. (1994) Formation of omega-feruloyloxypalmitic acid by an enzyme from wound-healing potato-tuber disks. Phytochemistry 35: 1419–1424 [Google Scholar]
- Molina I, Bonaventure G, Ohlrogge J, Pollard M. (2006) The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 67: 2597–2610 [DOI] [PubMed] [Google Scholar]
- Molina I, Li-Beisson Y, Beisson F, Ohlrogge JB, Pollard M. (2009) Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol 151: 1317–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina I, Ohlrogge JB, Pollard M. (2008) Deposition and localization of lipid polyester in developing seeds of Brassica napus and Arabidopsis thaliana. Plant J 53: 437–449 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, et al. (2007) Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 71: 2095–2100 [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shi JX, Schreiber L, Aharoni A. (2009) The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol 151: 1773–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani SH, Krishna TH, Saha S, Negi AS, Rajasekharan R. (2010) Defective in cuticular ridges (DCR) of Arabidopsis thaliana, a gene associated with surface cutin formation, encodes a soluble diacylglycerol acyltransferase. J Biol Chem 285: 38337–38347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Ebert B, Ouellet M, Nafisi M, Baidoo EE, Benke P, Stranne M, Mukhopadhyay A, Keasling JD, Sakuragi Y, et al. (2012) Arabidopsis deficient in cutin ferulate encodes a transferase required for feruloylation of ω-hydroxy fatty acids in cutin polyester. Plant Physiol 158: 654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Petersen M. (2011) Distinct substrate specificities and unusual substrate flexibilities of two hydroxycinnamoyltransferases, rosmarinic acid synthase and hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl-transferase, from Coleus blumei Benth. Planta 233: 1157–1171 [DOI] [PubMed] [Google Scholar]
- Santos S, Schreiber L, Graça J. (2007) Cuticular waxes from ivy leaves (Hedera helix L.): analysis of high-molecular-weight esters. Phytochem Anal 18: 60–69 [DOI] [PubMed] [Google Scholar]
- Schmutz A, Jenny T, Ryser U. (1994) A caffeoyl fatty-acid glycerol ester from wax associated with green cotton fiber suberin. Phytochemistry 36: 1343–1346 [Google Scholar]
- Schreiber L. (2010) Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci 15: 546–553 [DOI] [PubMed] [Google Scholar]
- Schreiber L, Franke R, Hartmann K. (2005) Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 220: 520–530 [DOI] [PubMed] [Google Scholar]
- Serra O, Hohn C, Franke R, Prat S, Molinas M, Figueras M. (2010) A feruloyl transferase involved in the biosynthesis of suberin and suberin-associated wax is required for maturation and sealing properties of potato periderm. Plant J 62: 277–290 [DOI] [PubMed] [Google Scholar]
- St Pierre B, De Luca V. (2000) Evolution of acyltransferase genes: origin and diversification of the bahd superfamily of acyltransferases involved in secondary metabolism. In, JJ Romeo, R Ibrahim, L Varin, V DeLucca, eds, Recent Advances in Phytochemistry, Vol 34. Elsevier Science, Oxford, UK, pp 285–315
- Sun W-X, Zhang Q, Jiang J-Q. (2006) Chemical constituents of daphne Giraldii nitsche. J Integr Plant Biol 48: 1498–1501 [Google Scholar]
- Trombino S, Cassano R, Muzzalupo R, Pingitore A, Cione E, Picci N. (2009) Stearyl ferulate-based solid lipid nanoparticles for the encapsulation and stabilization of beta-carotene and alpha-tocopherol. Colloids Surf B Biointerfaces 72: 181–187 [DOI] [PubMed] [Google Scholar]
- Tuominen LK, Johnson VE, Tsai C-J. (2011) Differential phylogenetic expansions in BAHD acyltransferases across five angiosperm taxa and evidence of divergent expression among Populus paralogues. BMC Genomics 12: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. (2007) An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunoki K, Musa R, Kinoshita M, Tazaki H, Oda Y, Ohnishi M. (2004) Presence of higher alcohols as ferulates in potato pulp and its radical-scavenging activity. Biosci Biotechnol Biochem 68: 2619–2622 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.