Abstract
Tillering (branching) is a major yield component and, therefore, a target for improving the yield of crops. However, tillering is regulated by complex interactions of endogenous and environmental signals, and the knowledge required to achieve optimal tiller number through genetic and agronomic means is still lacking. Regulatory mechanisms may be revealed through physiological and molecular characterization of naturally occurring and induced tillering mutants in the major crops. Here we characterize a reduced tillering (tin, for tiller inhibition) mutant of wheat (Triticum aestivum). The reduced tillering in tin is due to early cessation of tiller bud outgrowth during the transition of the shoot apex from the vegetative to the reproductive stage. There was no observed difference in the development of the main stem shoot apex between tin and the wild type. However, tin initiated internode development earlier and, unlike the wild type, the basal internodes in tin were solid rather than hollow. We hypothesize that tin represents a novel type of reduced tillering mutant associated with precocious internode elongation that diverts sucrose (Suc) away from developing tillers. Consistent with this hypothesis, we have observed upregulation of a gene induced by Suc starvation, downregulation of a Suc-inducible gene, and a reduced Suc content in dormant tin buds. The increased expression of the wheat Dormancy-associated (DRM1-like) and Teosinte Branched1 (TB1-like) genes and the reduced expression of cell cycle genes also indicate bud dormancy in tin. These results highlight the significance of Suc in shoot branching and the possibility of optimizing tillering by manipulating the timing of internode elongation.
Tillering, or branching from the nodes of shoots in monocots, is closely associated with crop yield because of its involvement in determining the final number of productive (grain bearing) and unproductive shoots. Excessive levels of tillering in the major cereal crops can lead to yield reductions because most of the tillers compete for resources with the main shoot during vegetative growth, but senesce before reaching maturity and so may not contribute to yield. Therefore, the development of crop varieties with optimal tiller number for a specific environment could play an important role in efforts to increase yield. This has not yet been achieved, mainly due to incomplete knowledge of the physiology and genetics of tillering/branching.
Tiller or branch development is a two-step process: initiation of a meristem in the axil of a developing leaf to form a bud, followed by bud outgrowth. In some cases, the bud is dormant as a result of complex interactions between endogenous developmental signals and environmental factors. Inhibition of bud outgrowth by endogenous developmental signals, such as apical dominance in pea (Pisum sativum), has been the focus of research on bud dormancy in annuals for more than eight decades (Beveridge et al., 2009). Early decapitation experiments in beans (Vicia faba) revealed that the apex of an actively growing dominant shoot produces auxin that inhibits axillary bud outgrowth (Thimann and Skoog, 1933). Subsequent studies indicated that the inhibition of bud outgrowth by auxin from the shoot apex was indirect (Prasad et al., 1993) and caused in part by reduced synthesis of a bud outgrowth-promoting hormone, cytokinin, in the roots and nodes (Nordström et al., 2004; Tanaka et al., 2006). Analyses of the highly branched ramosus (rms) mutants of pea and the decreased apical dominance (dad) mutant of petunia (Petunia hybrida) led to the discovery of another hormone-like signal known as shoot multiplication signal (SMS) that inhibits bud outgrowth in response to auxin (Napoli, 1996; Beveridge et al., 1997; Beveridge et al., 2000; Beveridge, 2006). The identification of a series of branching mutants in Arabidopsis (Arabidopsis thaliana) known as max (for more axillary growth) and cloning of the MAX genes (Stirnberg et al., 2002; Sorefan et al., 2003; Booker et al., 2004; Booker et al., 2005), as well as related genes from pea (Sorefan et al., 2003; Johnson et al., 2006), rice (Oryza sativa; Ishikawa et al., 2005; Zou et al., 2006; Arite et al., 2007), and petunia (Snowden et al., 2005; Simons et al., 2007) led to the discovery that the SMS signal is a new hormone, strigolactone (Gomez-Roldan et al., 2008; Umehara et al., 2008). Therefore, apical dominance in annuals is regulated by auxin, cytokinin, and strigolactones and their interactions (Beveridge et al., 2009; Domagalska and Leyser, 2011). The involvement of other factors may also be important; for example, the early events of decapitation-induced bud outgrowth in pea are independent of auxin (Morris et al., 2005).
Bud dormancy in annual plants grown at high density is also influenced by environmental signals such as shade, but it is only recently that the downstream molecular and genetic mechanisms have been investigated (Kebrom and Brutnell, 2007). The red (R) to far-red (FR) light ratio (R:FR) in dense stands is lower because of absorption by leaves of R light and reflection of FR (Ballaré et al., 1990). A low R:FR serves as a signal for shade or competition for light, and plants respond by inhibiting axillary bud outgrowth, growing taller, and flowering earlier to avoid the detrimental effect of shade. This set of responses is known as shade avoidance syndrome (SAS), and it is mediated by the R and FR absorbing photoreceptor phytochrome B (phyB; Smith and Whitelam, 1997). The inhibition of bud outgrowth in plants growing at high density and enriched with FR is linked to the expression of orthologs of TB1-like genes in the buds of sorghum (Sorghum bicolor; SbTB1) and Arabidopsis BRANCHED1 (BRC1; Kebrom et al., 2006; Finlayson et al., 2010). The TB1 gene was discovered in studies on the evolution of apical dominance in maize (Zea mays ssp. mays) during its domestication from teosinte (Zea mays ssp. parviglumis; Doebley et al., 1997). In the absence of shade, a higher proportion of phyB is in the active form and induces bud outgrowth by downregulating SbTB1; shade signals with their low R:FR lower the proportion of the active form of phyB, which enhances the expression of SbTB1 leading to bud dormancy.
In monocot and eudicot species, the TB1-like genes inhibit bud outgrowth in response to several dormancy-inducing hormonal and environmental signals, suggesting a role as an integrator of signals controlling the dormancy versus outgrowth fates of an axillary bud (Kebrom et al., 2006; Aguilar-Martínez et al., 2007; Finlayson, 2007; Martín-Trillo et al., 2011). In sorghum, dormancy induced by shading was associated with changes in expression of SbTB1, although dormancy induced by defoliation was not. In contrast, the expression of the SbMAX2 and SbDRM1 (for Dormancy-associated1) genes was associated with both shade- and defoliation-induced dormancy (Kebrom et al., 2010). SbMAX2 is a putative ortholog of Arabidopsis MAX2 and encodes an F-box protein, which probably functions in the perception or signal transduction of strigolactones (Stirnberg et al., 2002; Stirnberg et al., 2007). SbDRM1 is a putative ortholog of the DRM1 gene of pea that encodes a protein of unknown function (Stafstrom et al., 1998). Because the roles of MAX2 and DRM1 appear to be more closely aligned to dormancy than TB1, signal integration may occur downstream of TB1. This is further supported by the recent discovery of another transcription factor in maize, Grassy Tillers1 (GT1), which controls apical dominance downstream of TB1 (Whipple et al., 2011).
Dormancy of axillary meristems is classified as endo-, para-, and ecodormancy (Lang, 1987). Endodormancy is caused by factors within the bud, paradormancy by factors within the plant but outside the bud, and ecodormancy is caused by unfavorable environmental conditions. As an example, in paradormant buds of pea, the expression levels of cell cycle genes are repressed by auxin synthesized in the shoot apex. In this case, bud dormancy is due to suspension of cell division (Devitt and Stafstrom, 1995). However, studies in other species indicate that axillary meristem dormancy is not always associated with downregulation of cell cycle genes. The expression of cell cycle genes was downregulated in dormant buds of the FR-treated wild type but not in a phyB null mutant of sorghum (Kebrom et al., 2010). Similarly, endodormancy but not ecodormancy of the cambial meristem in hybrid aspen (Populus tremula × Populus tremuloides) was associated with downregulation of the cell cycle-related genes (Espinosa-Ruiz et al., 2004). Further study may explain why the expression level of cell cycle-related genes is downregulated in some but not all types of dormancy.
The architecture of the modern maize plant, with one strong main shoot and limited tillering, is the best example of how the selection for an allele of TB1 that inhibits bud outgrowth under both favorable and unfavorable environmental conditions can enhance the productivity of the main shoot in the grasses (Doebley et al., 2006). Detailed studies of reduced tillering mutants in other major crops may reveal additional regulatory mechanisms and genes that can be deployed to improve yield. In wheat (Triticum aestivum), tillers normally develop during the vegetative phase, prior to the elongation of stem internodes. During the reproductive phase, internodes elongate, tiller formation ceases, and young tillers senesce (McMaster, 1997). The senescence of young tillers is likely to be due, at least in part, to their inability to compete with the rapidly elongating internodes for photoassimilate.
A wheat mutant with reduced tillering, thicker stems, darker leaves, and larger spikes with more and larger grains was identified and characterized (Atsmon and Jacobs, 1977; Richards, 1988). However, its use in breeding has been restricted because of stunted growth (Supplemental Fig. S1) under long photoperiod and at low temperature (Atsmon et al., 1986; Duggan et al., 2002). The mutation was designated tin (for tiller inhibition) and was mapped as a single gene trait to the short arm of chromosome 1A after it was transferred into Australian wheat varieties (Fig. 1; Richards, 1988; Spielmeyer and Richards, 2004). The reduced tiller number of tin is due to early cessation of tillering (Duggan et al., 2002), but the underlying physiological and molecular aspects have not been investigated.
Figure 1.
A, Wild-type (WT; left) and tin mutant (right) wheat plants (var Banks). B, The spike of the main stem of WT (left) and tin mutant (right).
To better understand tiller inhibition in tin lines and to advance our knowledge of branching regulation in the grasses, we have conducted detailed physiological and molecular studies using near-isogenic lines. Our results reveal that tiller inhibition in tin is associated with the precocious elongation of basal internodes, which are solid rather than hollow, and we suggest that the arrest of bud growth is due to diversion of Suc away from the axillary bud to support internode elongation.
RESULTS
Characterization of Tiller Development in tin and the Wild Type
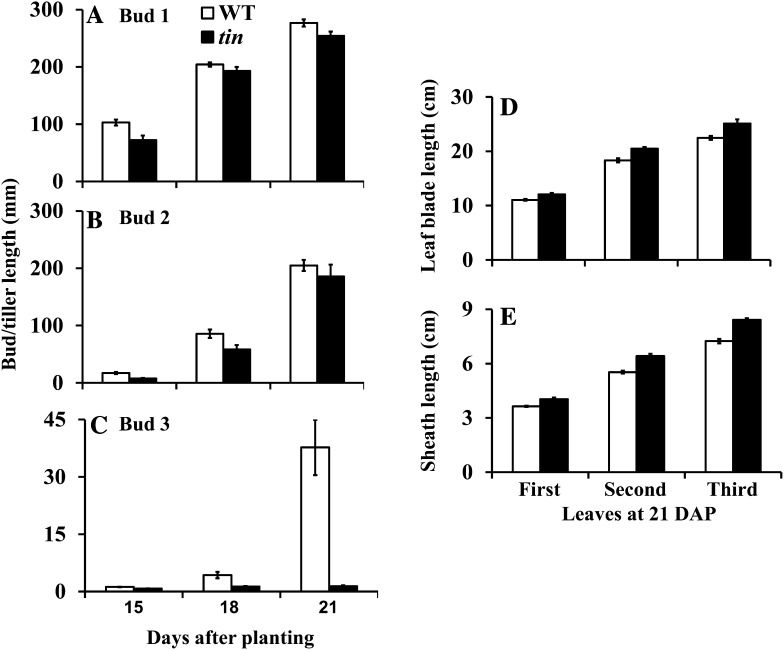
Tillers of wheat form sequentially, starting with the tiller in the axil of the first leaf, so it is easy to follow the developmental progression of tiller buds in the axils of successive leaves. In the wild type, about three to four primary tillers develop, and its bushy appearance (Fig. 1) is due to the growth of higher order tillers from the primary tillers that are absent in tin. In tin, the growth of buds in the first and second leaf axils was similar to the wild type (Fig. 2, A and B). However, the bud in the third leaf axil of tin lines stopped growing around 18 d after planting (DAP), soon after its formation (Fig. 2C), whereas the bud at the same position in the wild type continued growth and formed a tiller. For both tin and the wild type, growth of the first and second leaf blade and sheath was completed by 15 DAP or earlier, and growth of the third leaf blade and sheath by 18 DAP. The leaf blades and sheaths of tin were slightly longer than the wild type, which may have contributed to a small delay in the growth of the first and second tillers of tin (Fig. 2, A and B). The coleoptile tiller also frequently grows from a bud in the axil of the coleoptile in both tin and the wild type that may reach maturity and produce grains or senesce depending on growing conditions. Overall, because only the coleoptile tiller and one or two primary tillers grow, the tin line has a reduced tillering (oligoculm) phenotype.
Figure 2.
Development of tiller buds and tillers in the first (A), second (B), and third (C) leaf axil of tin and the wild type (WT) at 15, 18, and 21 DAP. Leaf blade (D) and sheath (E) length of the first, second, and third leaves at 21 DAP. The first and second leaves attained their final leaf blade and sheath length by 15 DAP or earlier and the third leaf by 18 DAP. Data are mean ± se; n = 10.
The Onset of Bud Dormancy in tin Coincides with the Transition of the Shoot Apex from Vegetative to Reproductive Stage
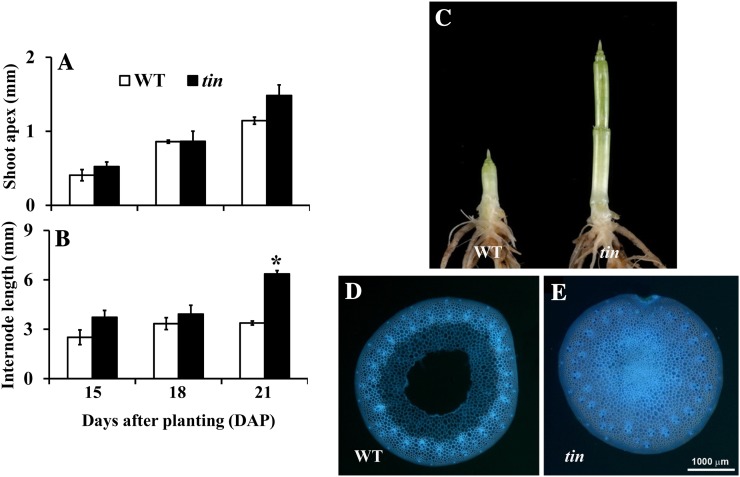
The buds in the first and second leaf axil of tin and the wild type continue to grow and form tillers of normal size. The buds in the third leaf axil of tin and the wild type are also well formed at about 2 weeks after planting, but in tin this bud stops growing when the shoot apex of both tin and the wild type starts to elongate. An increase in apex length is an accepted indicator of the transition from the vegetative to the flowering stage. When we determined the status of the shoot apex of tin and wild-type lines at 15, 18, and 21 d, there was no significant difference in the length of the shoot apex (Fig. 3A). However, elongation of stem internodes occurred earlier in tin than in the wild type (Figs. 3, B and C), and they were solid rather than hollow (Figs. 3, D and E). Further examination of the internodes of mature tin and wild-type plants grown in a glasshouse showed that out of five to six internodes of a mature shoot, only the three basal internodes of tin were solid (Supplemental Fig. S2).
Figure 3.
Shoot apex (A) and internode (B) length of the wild type (WT) and tin at 15, 18, and 21 DAP. Data for A and B are mean ± se; n = 3. Only the internode length at 21 DAP, shown by the asterisk, is statistically significantly different (P value = 0.001) between the wild type and tin. C, Internode development of WT (left) and tin (right) at 28 DAP. Hollow internode of WT (D) and solid internode of tin (E). The first three basal internodes of tin are solid, and the sections of WT and tin are from the third internodes at 5 weeks after planting.
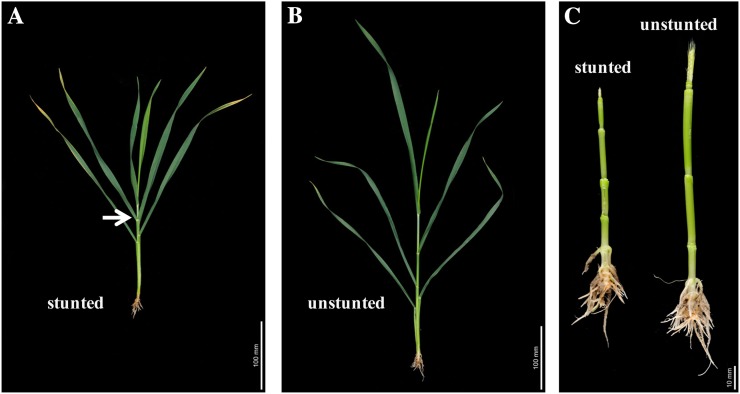
As in the wild type, a tin plant has ligules of successive leaves spaced apart from one another (Fig. 4B). However, tin plants may stunt and display a rosette-like growth habit of leaves under long photoperiod and lower temperatures (Supplemental Fig. S1). Stunted plants either die or recover and set grains. Stunted tin plants that recover can be identified by examining the distance between ligules of successively formed leaves. As shown in Fig. 4A, there is no separation between the ligules of two successive leaves, indicating that the plant experienced stunting during the development of those leaves, whereas the ligule of the next leaf was higher, indicating that the plant recovered from stunting. We examined the stems of the stunted and normal tin plants to see if stunting was associated with changes in internode development. Stems from plants that stunted but then recovered had a greater number of elongated internodes (Fig. 4C, left) than those from plants that did not stunt (Fig. 4C, right). They also showed much less advanced development of the reproductive apex compared with unstunted plants (Fig. 4C). Both the early growth of solid internodes associated with tiller inhibition and the elongation of additional internodes during stunting suggest direct effects of the tin mutant on internode development.
Figure 4.
Internode development in tin plants. A, The main stem of a tin plant, which was stunted during its development and then recovered; an arrow indicates two successive leaves with no separation between their ligules. B, A tin plant that developed normally with distance between ligules of successive leaves. C, Stems of the stunted and recovered shoot (left) and unstunted shoot (right). The shoots are from 5-week-old plants.
Suc Contents of tin and Wild-Type Buds
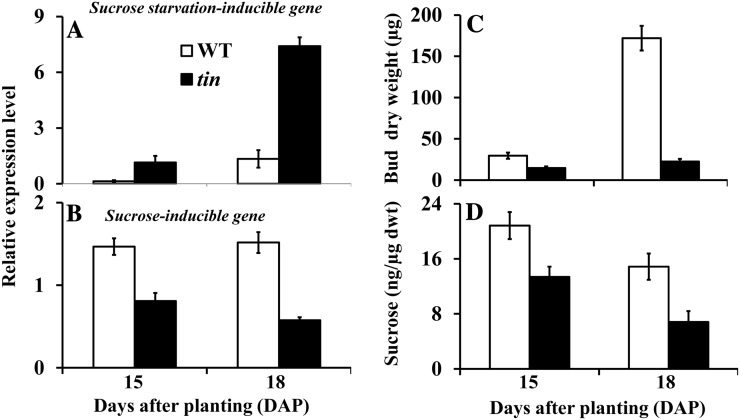
Since internode elongation occurs earlier in tin lines, we hypothesized that the inhibition of outgrowth of the bud in the third leaf axil of tin could be due to Suc starvation caused by competition from a strong sink such as the elongating solid stem. To investigate this hypothesis, we identified wheat complementary DNAs (cDNAs) AK334107 and AK332443, which are most similar to Arabidopsis genes responsive to Suc, and examined their expression level in axillary buds of tin and the wild type. The AK334107 cDNA (hereafter called Suc starvation-inducible gene) has been previously annotated as an Asn synthase gene (TaASN1), which is closely related to the Arabidopsis gene that encodes a Gln-dependent Asn synthase1 (ASN1) and is upregulated by salt stress, osmotic stress, and abscisic acid (ABA; Wang et al., 2005). The ASN1 gene, also known as dark inducible6 (DIN6), is induced by Suc starvation (Gonzali et al., 2006; Rolland et al., 2006). The AK332443 cDNA (hereafter called Suc-inducible gene) is similar to the Arabidopsis At1g20950 gene (a putative pyrophosphate-Fru-6-P 1-phosphotransferase) that is involved in Glc metabolism, and is induced by Suc (Gonzali et al., 2006). Expression of the wheat Suc starvation-inducible gene was upregulated in buds in the third leaf axil of tin at 18 DAP (Fig. 5A). Conversely, the Suc-inducible gene was expressed at a lower level in tin buds (Fig. 5B). These results suggest a lower level of Suc in the arrested buds of tin. To confirm this, we determined by gas chromatography-mass spectrometry (GC-MS) the Suc contents per unit dry weight of axillary buds of tin and the wild type at 15 and 18 DAP (Fig. 5C). Consistent with the gene expression results, the level of Suc in tin was significantly lower at 18 DAP (Fig. 5D), at a time when the buds in tin stopped growing. A comparable reduction in Suc content in tin buds was obtained in two further experiments.
Figure 5.
Gene expression and Suc contents in axillary buds of the wild type (WT) and tin. Relative expression levels of (A) Suc starvation-inducible and (B) Suc-inducible genes, and bud weight (C) and Suc contents (D) in buds in the third leaf axil of WT and tin at 15 and 18 DAP. Data for gene expression are mean ± sem; n = 3 biological replicates from two independent experiments. Data for bud weight and Suc are mean ± se; n = 9 or 10 buds for bud weight and from at least four buds for Suc contents.
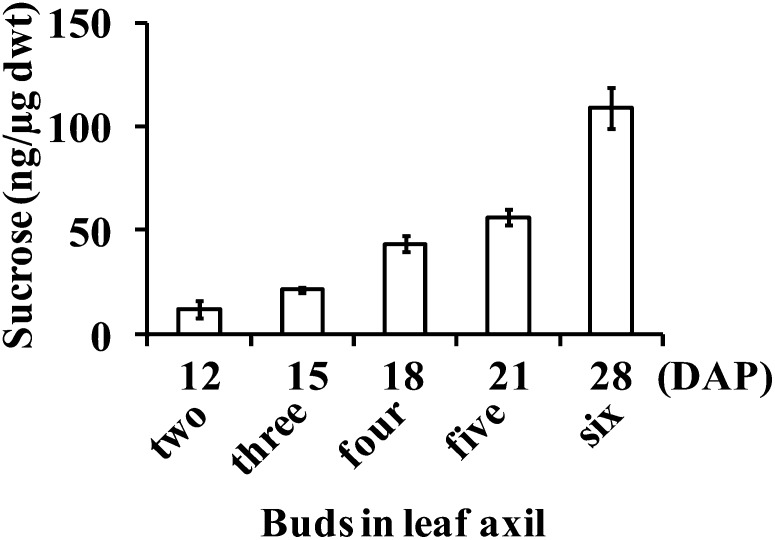
We investigated the effect of increasing the daytime growth temperature from 22°C to 24°C. At the higher temperature, growth rate was enhanced and development was more advanced during transition from vegetative to floral phase (data not shown). The buds in the third leaf axil elongated to about 1 to 2 cm, and their dry weight increased from 28 μg at 15 DAP to 45 μg at 18 DAP, values much greater than corresponding values at the cooler temperature. The Suc content per unit of bud dry weight also increased from 17 ng/μg at 15 DAP to 32 ng/μg at 18 DAP, instead of declining as it did at the cooler temperature. These results indicate that the bud in the third leaf axil is not inherently defective and is capable of continued growth, dependent on environmental conditions. Although the bud in the third leaf axil of tin elongated to 1 to 2 cm at higher temperatures, it failed to survive because it was compressed between the rapidly developing internode and the enclosing leaf sheath, causing it to fuse to the surface of the internode (Fig. 6B). In some cases, buds were crushed and only a mass of disorganized tissues remained in the axil of the leaf. The buds at higher nodes in tin were also crushed by the developing stem and sheath soon after they were initiated and could not be observed in mature tin stems (Fig. 6A, left). In contrast, the buds at higher nodes of wild-type stems were present even in mature plants (Fig. 6A, right). Because these do not form tillers, we asked if they stop growing because of Suc starvation. Contrary to expectations, the Suc contents of successively formed buds continued to increase (Fig. 7A), and, interestingly, they were floral (Fig. 6C). The continued increase in Suc content of the successively formed buds in the wild type could be associated with the increase in the photosynthetic leaf area of the whole plant. For tin at 18 DAP, the Suc synthesized by a smaller leaf area in the young plants may not have been enough to support the simultaneous growth of buds and solid internodes.
Figure 6.
Buds on nodes of elongated internodes of tin and the wild type (WT). A, Arrows on the right shoot indicate buds on nodes of elongated internodes of WT, whereas buds are absent on the nodes of tin (left). Sometimes the buds in tin are crushed when they are young, and there may not be any sign of a bud except a mass of disorganized tissues on the grooves of internodes. B, Arrow indicates a bud that was damaged by the stiff elongated internode and enclosing sheath of the parent shoot. The upper half of the bud is visible, whereas the lower was fused into the groove of the internode. C, The buds on nodes of elongated internodes in the WT are floral. The stems are from 5-week-old plants. The sheath was removed to show the buds.
Figure 7.
Suc contents in successively formed axillary buds of the wild type (WT) from the second to sixth leaf axils, sequentially from 12 to 28 DAP. The buds in leaf axils two, three, and four normally grow out and form tillers, whereas the buds in leaf axils five and six do not grow out. Data for Suc contents are means ± se from at least five buds.
Over a range of different growth conditions, we have observed higher order tiller buds in both tin and the wild type, but only the buds in the wild type formed visible tillers. Although two primary tillers are formed in tin, the second tiller usually senesces before reaching maturity and producing grains, indicating that it competes poorly for resources. Most of the secondary tillers and some primary tillers in the wild type senesce at latter stages of development, possibly due to competition for resources between parent and daughter tillers. It is plausible to speculate that secondary tillers do not develop in tin because competition for resources starts at a much earlier stage.
Expression of Cell Cycle-Related Genes in Dormant Buds of tin
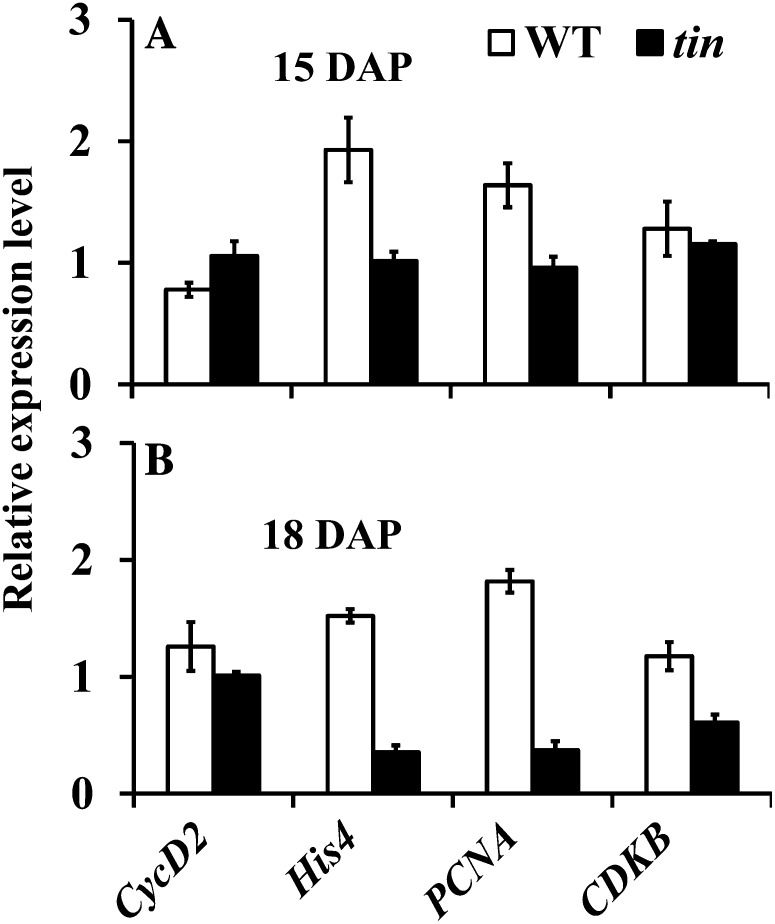
Studies on bud dormancy induced by apically derived auxin in pea and dormancy induced by shade signals and defoliation in sorghum have documented downregulation of cell division-related genes in dormant buds (Devitt and Stafstrom, 1995; Kebrom et al., 2010). However, results from other systems indicated that not all types of dormancy are associated with downregulation of the expression of cell cycle-related genes (Espinosa-Ruiz et al., 2004; Kebrom et al., 2010). Hence, the induction of different types or degrees of bud dormancy may involve distinct molecular mechanisms depending on the dormancy-inducing factors. We analyzed the expression of putative wheat orthologs of cell cycle genes in buds in the third leaf axil of tin and the wild type. Gene markers for DNA replication (S phase) histone H4 (His-4; expressed sequence tag [EST] accession no. CJ966253) and proliferating cell nuclear antigen (PCNA; EST accession no. CF182087) and the G2-M phase of the cell cycle, cyclin-dependent kinase B (CDKB; EST accession no. CD897467), were downregulated, but not Cyclin D2 (CycD2; accession no. AF512432), which is involved in the G1 phase of the cell cycle (Fig. 8). To confirm that the expression of CycD2 in wheat is specific to actively dividing tissues, we examined dividing and nondividing (mature) sections of a single leaf (Li et al., 2010). Consistent with its role in cell division, the expression of CycD2 was more than 20-fold higher in growing sections of the leaf compared with the mature section (data not shown). Therefore, although the bud in the third leaf axil of tin is dormant, the CycD2 (G1 phase) gene continued to be expressed at the same level as wild-type buds, whereas His-4, PCNA, and CDKB were downregulated.
Figure 8.
Expression level of cell cycle-related genes in buds in the third leaf axil of the wild type (WT) and tin at (A) 15 and (B) 18 DAP. Data are means ± sem; n = three biological replicates from two independent experiments.
Expression of TB1- and DRM1-Like Genes in Axillary Buds of tin and the Wild Type
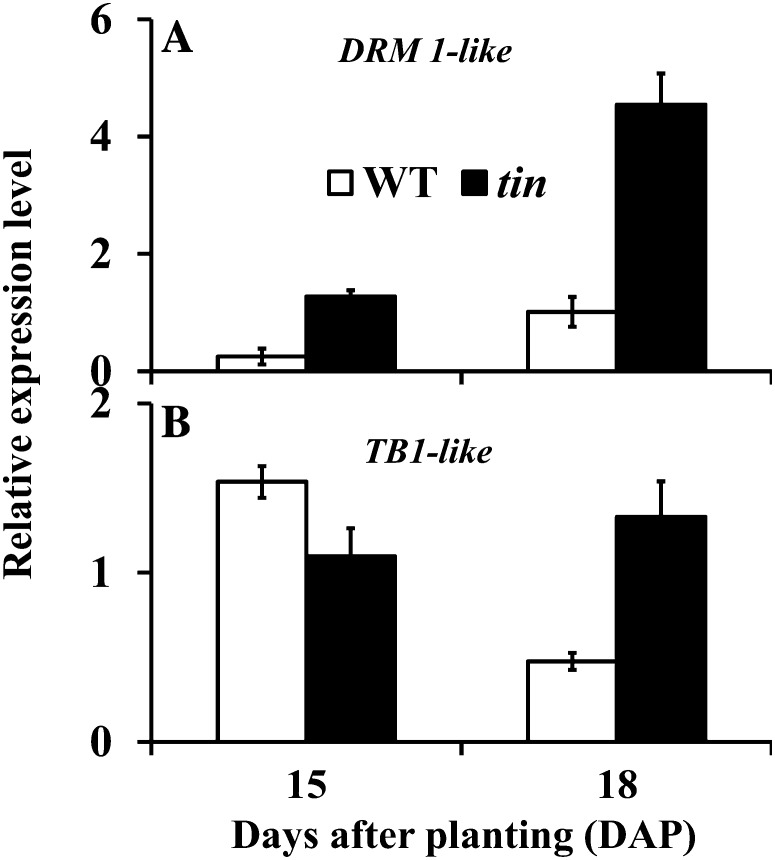
To investigate the possible role of TB1 and DRM1 genes in the inhibition of bud outgrowth in tin, we identified a wheat DRM1-like EST (accession no. DN828863) and a TB1-like EST (accession no. BJ314006). The wheat DRM1-like EST contains the entire deduced amino acid sequence of the DRM1 gene in other species (Stafstrom et al., 1998; Kebrom et al., 2006). The DRM1-like gene was upregulated at 18 DAP in the third leaf axil in tin compared with wild-type buds, consistent with the conserved function of this gene and the dormancy status of the tin buds (Fig. 9A). The wheat TB1-like EST contains the conserved helix-loop-helix TCP (for TB1, Cycloidea, and Proliferating cell factor) domain (Cubas et al., 1999). Based on similarity of the TCP domain, genes that belong to the TCP family are grouped into Class I or Class II (Martín-Trillo and Cubas, 2010). The TCP domain of the wheat TB1-like EST belongs to the Class II type, which includes TB1 in maize and BRC1 in Arabidopsis. In support of a role for TB1-like genes in bud dormancy of wheat, the expression pattern of this gene in different organs of the wild type at 18 DAP was similar to that observed in other species: high levels of expression were detected in axillary buds, and very low levels in the sheath, with no expression detected in the root and leaf blade. The TB1-like gene was expressed at high levels during early development of the buds in the third leaf axil at 15 DAP, and was downregulated in the growing buds of the wild type at 18 DAP, whereas it remained higher in dormant buds of tin (Fig. 9B). Therefore, changes in the expression levels of both DRM1-like and TB1-like genes were associated with the inhibition of bud outgrowth in tin.
Figure 9.
Expression level of (A) DRM1-like and (B) TB1-like genes in buds in the third leaf axil of the wild type (WT) and tin at 15 and 18 DAP. Data are means ± sem; n = three biological replicates from two independent experiments.
Detillering Wild-Type Plants Causes the Wheat Ear to Enlarge as in tin Plants
In addition to reduced tillering, the tin mutant displays enhanced growth of reproductive structures, including a longer spike (Fig. 1B) and a higher number of grains per spikelet. Whether this is due to pleiotropic effects of the tin gene on reproductive organs or to reduced intraplant competition associated with inhibition of tillering is an important biological question. Therefore, we excised tillers of wild-type plants as soon as they were initiated through a window cut at the base of the leaf sheath, and by doing so successfully converted the wild type into a monoculm plant (Fig. 10A). The head of the detillered plant (Fig. 10B) phenocopied that of the tin mutant (Fig. 1B) indicating the enhanced reproductive development of tin could result from reduced tillering. Therefore, the effects of the tin mutant on reproductive structures need not reflect a direct action of the mutant gene, but may be an indirect consequence of fewer tillers.
Figure 10.
A, Control (left) and detillered (right) wild type (WT) plants, and (B) main culm ear of control (left) and detillered (right) plants.
DISCUSSION
The onset of the inhibition of bud outgrowth in tin coincides in time with the initiation of development of solid stem internodes. Growth arrest of the buds was associated with a lowering of their Suc content, enhanced expression of a marker gene for Suc starvation, reduced expression of a Suc-inducible gene, and lower expression of genes involved in the S and G2-M phases of the cell cycle. Together, these results are consistent with the hypothesis that bud dormancy in tin lines is due to diversion of Suc away from the bud and into the elongating stem, leading to cell division arrest in the bud. The dormancy status of the tin bud was associated with increased expression of the TB1-like and DRM1-like genes, suggesting a role for these genes in mediating dormancy caused by Suc starvation. The main research focus of studies on paradormancy of axillary buds in annuals, also known as apical dominance, has been on hormonal signaling, especially for auxin, cytokinin, and strigolactone. Our study indicates that Suc supply or competition for Suc could also be a factor that causes paradormancy of axillary buds.
Tiller or branch development involves the initiation of an axillary meristem and the outgrowth of the axillary bud, and a number of genes and hormonal signals involved in regulating these two processes have been discovered and characterized (Schmitz and Theres, 2005; Wang and Li, 2008; Beveridge et al., 2009; Müller and Leyser, 2011). However, the tin phenotype does not appear to involve a general defect in either initiation of an axillary meristem or bud outgrowth, because tillers develop normally from the first and second leaf axils. Reduced tillering has also been related to earlier flowering (Gomez-Macpherson et al., 1998); however, we observed no difference in the timing of flowering and shoot apex development between tin and the wild type in this study. Previously, Duggan et al., (2002) also reported that the tin gene has no effect on the time from sowing to double ridge, a stage in shoot apex development that marks the beginning of development of the inflorescence. On the other hand, coincidence between the inhibition of bud outgrowth in tin (Fig. 2) and the early elongation of solid internodes (Fig. 3), as well as the switch to internode elongation in stunted plants (Fig. 4), suggest that tin is defective in regulating the timing of internode development, and this may indirectly affect bud outgrowth. In fact, tillering in wheat occurs during the vegetative phase from unelongated internodes. Tillering ceases and tiller senescence begins during the flowering phase at a time when internode elongation begins (McMaster, 1997), although tiller bud formation still occurs in the flowering phase (Fig. 6).
The early elongation of stem internodes in tin may inhibit bud outgrowth by diverting Suc away from axillary buds, which is consistent with the reduced Suc content, reduced expression of a Suc-inducible gene, and upregulation of a gene marker for Suc starvation in the repressed bud in the third leaf axil. However, buds in the wild type that develop after transition to flowering do not grow out, although their Suc content is high. This suggests that Suc is a necessary but not sufficient condition for bud outgrowth, which depends on additional factors including hormonal, environmental, and developmental signals. The internodes are solid and develop earlier in tin than in the wild type, making them a potentially stronger sink for Suc. Such sink effects are known for Fuchsia (Fuchsia hybrida), where enhanced stem elongation reduces assimilate supplied to the nearby shoot apex from the leaves (King and Ben-Tal, 2001), providing an example of paradormancy like we are suggesting for the tin buds. Interestingly, out of the five to six internodes formed at maturity, only the first three basal internodes of tin are solid. In rice, the EUI gene, which encodes a putative cytochrome P450 monooxygenase, controls the length of the uppermost internode (Rutger and Carnahan, 1981; Luo et al., 2006). This shows the presence of independent regulatory mechanisms for the growth of the different internodes in the grasses. Together these results support the hypothesis that the tin gene regulates the development of the basal internodes, and the early development of the solid basal internodes in tin reduces tillering by competing for Suc with the developing buds.
The growth arrest of axillary buds is due to suspension of cell division, and in pea it is associated with the downregulation of cell cycle-related genes (Devitt and Stafstrom, 1995). In sunflower (Helianthus annuus), cell division in dormant buds could be stimulated by Suc supply (Ballard and Wildman, 1964). Suc stimulates cell division by upregulating the expression of the CycD genes (Riou-Khamlichi et al., 2000). The CycD genes are important regulators of the G1 to S transition in response to Suc and hormonal factors (Dewitte and Murray, 2003; Inzé, 2005). However, the Suc level is also important for the mitosis (M) phase of the cell cycle (Skylar et al., 2011). In fact, the metabolic requirement is higher for the S and M phases than for the G1 and G2 phases (Van’t Hof, 1966). The inhibition of bud outgrowth in tin is associated with a reduction in the level of Suc and the expression of marker genes for the S (HIS4 and PCNA) and M (CDKB) stages of the cell cycle. The results suggest the inhibition of cell division in the buds of tin by reduced Suc contents. The expression level of CycD2 was not reduced in dormant tin buds, possibly due to levels of Suc that are adequate for entry into the cell cycle but inadequate to promote the progression of the cell cycle beyond the G1 phase. Results from studies in other species indicated that the expression level of the cell cycle genes is downregulated in some but not all dormant axillary meristems. In sorghum, inhibition of bud outgrowth by defoliation is associated with dramatic downregulation of the cell cycle genes, whereas the level of the cell cycle genes is slightly reduced in the dormant buds of phytochrome B (phyB-1) mutants (Kebrom et al., 2010). In hybrid aspen, the cell cycle genes are downregulated in endo- but not ecodormant cambial meristems (Espinosa-Ruiz et al., 2004). The differences in the expression levels of the cell cycle genes in dormant buds or axillary meristems could be related to the level of reduction of Suc caused by the dormancy inducing factors. In dormant axillary buds of sunflower, cell division can be induced by provision of Suc (Ballard and Wildman, 1964). It will be interesting to study if hormonal regulation of branching in Arabidopsis and pea is associated with changes in Suc level in the buds.
The dormancy versus outgrowth fates of a bud are determined by combined environmental and endogenous signals, and the integration of these signals within the bud is the subject of current research (Aguilar-Martínez et al., 2007; Kebrom et al., 2010). TB1 and TB1-like genes are important tillering genes that function within the bud, which could be a point of integration for some of the branching-regulating signals. To date, the regulation of branching by environmental shade signals through TB1-like genes has been reported in sorghum and Arabidopsis (Kebrom et al., 2006; Finlayson et al., 2010). In addition, the MAX-pathway (strigolactones) inhibits bud outgrowth in Arabidopsis by enhancing the expression of the TB1 ortholog, BRC1 (Aguilar-Martínez et al., 2007). This study suggests a role for TB1-like genes in mediating the response to changing Suc contents. Bud dormancy induced by different factors in diverse species has been associated with the expression level of DRM1, and a DRM1-like gene was upregulated in dormant axillary buds of tin. Functional studies of TB1 and DRM1 may reveal molecular mechanisms that integrate branching regulation signals within the bud.
The tin plants produce larger aboveground organs, including reproductive structures, and they have a higher root-to-shoot ratio and a deeper root system than the wild type (Atsmon and Jacobs, 1977; Duggan et al., 2005; Richards et al., 2007). In the rice OsSPL14 mutants, the reduced tillering and increased panicle branching is due to the role of the gene during both the vegetative and reproductive phases (Jiao et al., 2010; Miura et al., 2010). The enhanced development of the reproductive structures in tin could be phenocopied by detillering during the vegetative stage, indicating that reduced intraplant competition may be sufficient to enhance the development of the remaining shoot(s). Whether the enhanced growth of shoot and root is due to reduced tillering or due to pleiotropic effects of the tin gene during the reproductive stage needs to be investigated.
CONCLUSION
The yield increase that has come from enhancing apical dominance in crops like maize demonstrates the potential for improving the yield of crops such as wheat by manipulating tillering. Since the tillers in the first and second leaf axil of tin grow normally, we suggest that the tin gene has no direct effect on initiation of axillary meristems or bud outgrowth, but instead regulates tillering indirectly by controlling the timing of elongation of basal internodes of the main stem. These results reveal the possibility for manipulating tillering in the major crops by targeting the timing of internode elongation. In the future, we will focus on studying the early events that control internode development in wheat using tin mutant and wild-type plants. Hormonal regulation of apical dominance has been investigated for many decades using pea and Arabidopsis as models, and the role of auxin, cytokinin, and strigolactones in regulating the dormancy and outgrowth of an axillary bud has been demonstrated (Beveridge et al., 2009; Domagalska and Leyser, 2011; Müller and Leyser, 2011). The models developed from these studies do not entirely account for all aspects of apical dominance, and none have considered Suc supply as a factor involved in the dormancy versus outgrowth fates of an axillary bud. Determination of the Suc status of axillary buds may explain the unknown components of branching regulation, including early auxin-independent signals that promote bud outgrowth in response to decapitation (Morris et al., 2005; Beveridge et al., 2009). Therefore, our analysis of tin can provide impetus to research in understanding the physiological and molecular mechanisms regulating axillary bud outgrowth in model species and in designing strategies to improve crop yield by manipulating tillering in the major crops.
MATERIALS AND METHODS
Plant Materials and Growing Conditions
The tin mutation was first reported in line ‘492’ by Atsmon and Jacobs (1977). The gene was transferred through four backcrosses into an Australian spring bread wheat (Triticum aestivum ‘Banks’) and mapped to chromosome 1AS (Richards, 1988). A low tillering, near-isogenic line (BC4) carrying tin and the free tillering recurrent background Banks, which we refer to+ld type, were grown in a growth cabinet at 22/17°C day/night temperatures, 16-h photoperiod, and about 300 µmol m−2 s−1 photosynthetically active radiation. Seeds of tin and the wild type, two per tube, were sown at 2-cm depth in 8-cm diameter 15-cm long tubes filled with composted soil. The seedlings were thinned to one per pot after emergence.
Tiller Development in tin and the Wild Type
The developmental progression of tiller buds was studied in detail by recording the formation and outgrowth of buds in the axil of each leaf of tin and the wild type using a dissecting microscope. We also documented growth and development of the leaf blade, sheath, shoot apex, and stem internodes. For microscopic visualization of internode sections, hand sections were cut from the third internode and autofluorescence was visualized with UV excitation and a long pass emission filter on a Zeiss Axio Imager M1 compound microscope.
Suc Content in Buds Determined by GC-MS
The content of Suc in axillary buds was quantified by GC-MS using selected ion monitoring as described in King and Ben-Tal (2001). Buds from leaf axils were harvested and dried in an oven at 65°C for at least 48 h. The buds were weighed and stored in a desiccator. Suc from a single bud was extracted using 100 µL 80% HPLC-grade methanol and incubated at room temperature overnight. An aliquot of 1µg 13C12 standard Suc (Omicron Biochemicals, Inc.) dissolved in 10 µL of 80% methanol was added to each sample during extraction. The extract was transferred to a new vial, and the bud was washed using 50 µL of 80% methanol, which was added to the new vial. The solution was dried in a spin vac and derivatized using 10 µL of each of BSTFA (N,O-bistrimethylsilyltrifluoroacetamide) + 1% TMCS (trimethylchlorosilane) and pyridine (Alltech), with incubation at 90°C for 90 min. The samples were either dried in a spin vac and suspended in about 450 µL of hexane or dissolved directly in 450 µL of hexane and 1 µL was analyzed by GC-MS.
Quantitative PCR of Wheat Genes
We used tBLASTn searches of the National Center for Biotechnology Information database, to identify genes or ESTs in wheat that were closely related to genes that were previously isolated and characterized in other species. The closest matching EST was used as the putative ortholog in this study and the expression was monitored in axillary buds and in other tissues of tin and the wild type (Supplemental Table S1). Because primers were not tested for genome specificity, expression differences reported here may come from one or more of the three wheat homeologous genomes. RNA was extracted using TRIzol, treated with DNase I and transcribed using SuperScript III according to the manufacturer’s recommendations (Invitrogen). Quantitative PCR was performed in an 7900HT fast real-time PCR machine (Applied Biosystems), using18S ribosomal RNA (rRNA) to normalize the threshold values of each gene. The expression level was calculated using a slightly modified 2–(ΔΔCt) method, where ΔΔCt was the threshold cycle (Ct) of the target gene minus the Ct of rRNA of a sample, minus the average of the Ct of the target gene, minus the Ct of rRNA of all samples in each biological replicate. Instead of using one of the samples as a reference, the average of the Ct of the target gene minus the Ct of rRNA of all samples in each biological replicate, which is the mean normalized value, was used as a reference (as described in Kebrom et al., 2010). Quantitative PCR results are from three biological replicates, each with two technical replicates. The primer sequences used are shown in Supplemental Table S1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Normal and stunted tin plants of wheat.
Supplemental Figure S2. Mature stem internode length of the wild type and tin.
Supplemental Table S1. Summary of wheat ESTs used for expression analysis.
Supplementary Material
Acknowledgments
The authors thank Mark Talbot for helping with the microscopy and Carl Davies for taking excellent photographs. We also acknowledge the assistance provided by Rosangela Devilla to run GC-MS samples and thank Christine Beveridge for the critical reading of the manuscript.
Glossary
- SMS
shoot multiplication signal
- SAS
shade avoidance syndrome
- R
red
- FR
far-red
- phyB
photoreceptor phytochrome B
- DAP
d after planting
- ABA
abscisic acid
- GC-MS
gas chromatography-mass spectrometry
- cDNA
complementary DNA
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J. (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Atsmon D, Bush MG, Evans LT. (1986) Stunting in gigas wheat as influenced by temperature and daylength. Aust J Plant Physiol 13: 381–389 [Google Scholar]
- Atsmon D, Jacobs E. (1977) Newly bred gigas form of bread wheat (Triticum aestivum L): morphological features and thermo-photoperiodic responses. Crop Sci 17: 31–35 [Google Scholar]
- Ballard LAT, Wildman SG. (1964) Induction of mitosis by sucrose in excised and attached dormant buds of sunflower (Helianthus annuus L). Aust J Biol Sci 17: 36–43 [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247: 329–332 [DOI] [PubMed] [Google Scholar]
- Beveridge CA. (2006) Axillary bud outgrowth: sending a message. Curr Opin Plant Biol 9: 35–40 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Dun EA, Rameau C. (2009) Pea has its tendrils in branching discoveries spanning a century from auxin to strigolactones. Plant Physiol 151: 985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C. (1997) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol 115: 1251–1258 [Google Scholar]
- Beveridge CA, Symons GM, Turnbull CGN. (2000) Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol 123: 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8: 443–449 [DOI] [PubMed] [Google Scholar]
- Cubas P, Lauter N, Doebley J, Coen E. (1999) The TCP domain: a motif found in proteins regulating plant growth and development. Plant J 18: 215–222 [DOI] [PubMed] [Google Scholar]
- Devitt ML, Stafstrom JP. (1995) Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol 29: 255–265 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JAH. (2003) The plant cell cycle. Annu Rev Plant Biol 54: 235–264 [DOI] [PubMed] [Google Scholar]
- Doebley J, Stec A, Hubbard L. (1997) The evolution of apical dominance in maize. Nature 386: 485–488 [DOI] [PubMed] [Google Scholar]
- Doebley JF, Gaut BS, Smith BD. (2006) The molecular genetics of crop domestication. Cell 127: 1309–1321 [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Duggan BL, Richards RA, Tsuyuzaki H. (2002) Environmental effects on stunting and the expression of a tiller inhibition (tin) gene in wheat. Funct Plant Biol 29: 45–53 [DOI] [PubMed] [Google Scholar]
- Duggan BL, Richards RA, van Herwaarden AF. (2005) Agronomic evaluation of a tiller inhibition gene (tin) in wheat. II. Growth and partitioning of assimilate. Aust J Agric Res 56: 179–186 [Google Scholar]
- Espinosa-Ruiz A, Saxena S, Schmidt J, Mellerowicz E, Miskolczi P, Bakó L, Bhalerao RP. (2004) Differential stage-specific regulation of cyclin-dependent kinases during cambial dormancy in hybrid aspen. Plant J 38: 603–615 [DOI] [PubMed] [Google Scholar]
- Finlayson SA. (2007) Arabidopsis Teosinte Branched1-like1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant Cell Physiol 48: 667–677 [DOI] [PubMed] [Google Scholar]
- Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ. (2010) Phytochrome regulation of branching in Arabidopsis. Plant Physiol 152: 1914–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Macpherson H, Richards RA, Masle J. (1998) Growth of near-isogenic wheat lines differing in development: spaced plants. Ann Bot (Lond) 82: 315–322 [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. (2006) Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res 119: 115–123 [DOI] [PubMed] [Google Scholar]
- Inzé D. (2005) Green light for the cell cycle. EMBO J 24: 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J. (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46: 79–86 [DOI] [PubMed] [Google Scholar]
- Jiao YQ, Wang YH, Xue DW, Wang J, Yan MX, Liu GF, Dong GJ, Zeng DL, Lu ZF, Zhu XD, et al. (2010) Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet 42: 541–544 [DOI] [PubMed] [Google Scholar]
- Johnson X, Brcich T, Dun EA, Goussot M, Haurogné K, Beveridge CA, Rameau C. (2006) Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol 142: 1014–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP. (2007) The molecular analysis of the shade avoidance syndrome in the grasses has begun. J Exp Bot 58: 3079–3089 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA. (2010) Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ 33: 48–58 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA. (2006) Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol 140: 1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Ben-Tal Y. (2001) A florigenic effect of sucrose in Fuchsia hybrida is blocked by gibberellin-induced assimilate competition. Plant Physiol 125: 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GA. (1987) Dormancy: a new universal terminology. HortScience 22: 817–820 [Google Scholar]
- Li PH, Ponnala L, Gandotra N, Wang L, Si YQ, Tausta SL, Kebrom TH, Provart N, Patel R, Myers CR, et al. (2010) The developmental dynamics of the maize leaf transcriptome. Nat Genet 42: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Luo AD, Qian Q, Yin HF, Liu XQ, Yin CX, Lan Y, Tang JY, Tang ZS, Cao SY, Wang XJ, et al. (2006) EUI1, encoding a putative cytochrome P450 monooxygenase, regulates internode elongation by modulating gibberellin responses in rice. Plant Cell Physiol 47: 181–191 [DOI] [PubMed] [Google Scholar]
- Martín-Trillo M, Cubas P. (2010) TCP genes: a family snapshot ten years later. Trends Plant Sci 15: 31–39 [DOI] [PubMed] [Google Scholar]
- Martín-Trillo M, Grandío EG, Serra F, Marcel F, Rodríguez-Buey ML, Schmitz G, Theres K, Bendahmane A, Dopazo H, Cubas P. (2011) Role of tomato BRANCHED1-like genes in the control of shoot branching. Plant J 67: 701–714 [DOI] [PubMed] [Google Scholar]
- McMaster GS. (1997) Phenology, development, and growth of the wheat (Triticum aestivum L) shoot apex: a review. Adv Agron 59: 63–118 [Google Scholar]
- Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M. (2010) OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet 42: 545–549 [DOI] [PubMed] [Google Scholar]
- Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA. (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138: 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Leyser O. (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot (Lond) 107: 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C. (1996) Highly branched phenotype of the petunia dad1-1 mutant is reversed by grafting. Plant Physiol 111: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G. (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101: 8039–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Li X, Abdelrahman AM, Hosokawa Z, Cloud NP, Lamotte CE, Cline MG. (1993) Does auxin play a role in the release of apical dominance by shoot inversion in Ipomoea-Nil. Ann Bot (Lond) 71: 223–229 [Google Scholar]
- Richards RA. (1988) A tiller inhibitor gene in wheat and its effect on plant-growth. Aust J Agric Res 39: 749–757 [Google Scholar]
- Richards RA, Watt M, Rebetzke GJ. (2007) Physiological traits and cereal germplasm for sustainable agricultural systems. Euphytica 154: 409–425 [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH. (2000) Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol 20: 4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Rutger JN, Carnahan HL. (1981) A 4th genetic element to facilitate hybrid cereal production: a recessive tall in rice. Crop Sci 21: 373–376 [Google Scholar]
- Schmitz G, Theres K. (2005) Shoot and inflorescence branching. Curr Opin Plant Biol 8: 506–511 [DOI] [PubMed] [Google Scholar]
- Simons JL, Napoli CA, Janssen BJ, Plummer KM, Snowden KC. (2007) Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol 143: 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skylar A, Sung F, Hong F, Chory J, Wu X. (2011) Metabolic sugar signal promotes Arabidopsis meristematic proliferation via G2. Dev Biol 351: 82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Whitelam GC. (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20: 840–844 [Google Scholar]
- Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ. (2005) The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17: 746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al. (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17: 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmeyer W, Richards RA. (2004) Comparative mapping of wheat chromosome 1AS which contains the tiller inhibition gene (tin) with rice chromosome 5S. Theor Appl Genet 109: 1303–1310 [DOI] [PubMed] [Google Scholar]
- Stafstrom JP, Ripley BD, Devitt ML, Drake B. (1998) Dormancy-associated gene expression in pea axillary buds. Cloning and expression of PsDRM1 and PsDRM2. Planta 205: 547–552 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Ottoline Leyser HM. (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50: 80–94 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, van De Sande K, Leyser HMO. (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45: 1028–1036 [DOI] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. (1933) Studies on the growth hormone of plants. III. The inhibiting action of the growth substance on bud development. Proc Natl Acad Sci USA 19: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Van’t Hof J. (1966) Experimental control of DNA synthesizing and dividing cells in excised root tips of pisum. Am J Bot 53: 970–976 [Google Scholar]
- Wang HB, Liu DC, Sun JZ, Zhang AM. (2005) Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA. J Plant Physiol 162: 81–89 [DOI] [PubMed] [Google Scholar]
- Wang YH, Li JY. (2008) Molecular basis of plant architecture. Annu Rev Plant Biol 59: 253–279 [DOI] [PubMed] [Google Scholar]
- Whipple CJ, Kebrom TH, Weber AL, Yang F, Hall D, Meeley R, Schmidt R, Doebley J, Brutnell TP, Jackson DP. (2011) grassy tillers1 promotes apical dominance in maize and responds to shade signals in the grasses. Proc Natl Acad Sci USA 108: E506–E512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JH, Zhang SY, Zhang WP, Li G, Chen ZX, Zhai WX, Zhao XF, Pan XB, Xie Q, Zhu LH. (2006) The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J 48: 687–698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.