Abstract
The family of plant membrane transporters named HKT (for high-affinity K+ transporters) can be subdivided into subfamilies 1 and 2, which, respectively, comprise Na+-selective transporters and transporters able to function as Na+-K+ symporters, at least when expressed in yeast (Saccharomyces cerevisiae) or Xenopus oocytes. Surprisingly, a subfamily 2 member from rice (Oryza sativa), OsHKT2;4, has been proposed to form cation/K+ channels or transporters permeable to Ca2+ when expressed in Xenopus oocytes. Here, OsHKT2;4 functional properties were reassessed in Xenopus oocytes. A Ca2+ permeability through OsHKT2;4 was not detected, even at very low external K+ concentration, as shown by highly negative OsHKT2;4 zero-current potential in high Ca2+ conditions and lack of sensitivity of OsHKT2;4 zero-current potential and conductance to external Ca2+. The Ca2+ permeability previously attributed to OsHKT2;4 probably resulted from activation of an endogenous oocyte conductance. OsHKT2;4 displayed a high permeability to K+ compared with that to Na+ (permeability sequence: K+ > Rb+ ≈ Cs+ > Na+ ≈ Li+ ≈ NH4+). Examination of OsHKT2;4 current sensitivity to external pH suggested that H+ is not significantly permeant through OsHKT2;4 in most physiological ionic conditions. Further analyses in media containing both Na+ and K+ indicated that OsHKT2;4 functions as K+-selective transporter at low external Na+, but transports also Na+ at high (>10 mm) Na+ concentrations. These data identify OsHKT2;4 as a new functional type in the K+ and Na+-permeable HKT transporter subfamily. Furthermore, the high permeability to K+ in OsHKT2;4 supports the hypothesis that this system is dedicated to K+ transport in the plant.
High-affinity K+ transporters (HKTs) in plants and their K+ transporter (Trk and Ktr) counterparts in fungi and bacteria form a superfamily of membrane transport systems permeable to monovalent cations, K+ and/or Na+ essentially (Rodríguez-Navarro, 2000; Corratgé-Faillie et al., 2010). These systems, forming the HKT/Trk/Ktr superfamily, are involved in physiological functions such as nutritional ion uptake from the external medium, control of membrane polarization, or adaptation to osmotic and salt stresses (Corratgé-Faillie et al., 2010). In plants, the most documented roles are those played in adaptation to salinity constraint: By mediating Na+ retrieval (desalinization) from the ascending xylem sap (Ren et al., 2005; Sunarpi et al., 2005) and Na+ recirculation from leaves to roots via the phloem sap (Berthomieu et al., 2003), some members from the HKT family are involved in control of Na+ accumulation in leaves (Berthomieu et al., 2003; Ren et al., 2005; Huang et al., 2006; Byrt et al., 2007). Evidence is also available that plant HKT transporters can play a role in nutritional Na+ uptake in roots facing K+ shortage in the presence of low external Na+ concentrations (Horie et al., 2007).
The first identified HKT transporter, the wheat (Triticum aestivum) TaHKT2;1 transporter, was cloned by functional complementation of a yeast (Saccharomyces cerevisiae) mutant defective for K+ transport and was initially proposed to behave as H+-K+ symport (Schachtman and Schroeder, 1994). It was later shown to be able to mediate Na+-K+ symport, at least when expressed in yeast or Xenopus oocytes (Rubio et al., 1995; Gassmann et al., 1996). A Na+-selective HKT transporter was then identified in Arabidopsis (Arabidopsis thaliana; Uozumi et al., 2000) where it constitutes the sole HKT-type system. Cereals possess a much larger number of HKT genes, e.g. five to 11 genes in the different wheat genomes (Huang et al., 2008) and nine genes in rice (Oryza sativa; Garciadeblás et al., 2003), leading to functional diversity within this family (Jabnoune et al., 2009; Corratgé-Faillie et al., 2010). Based on functional studies in heterologous systems (Xenopus oocytes or yeast cells) and phylogenetic analyses, higher plant HKT transporters have been sorted out into two subfamilies (Platten et al., 2006). Subfamily 1 is present in both dicotyledonous and monocotyledonous species and comprises Na+-selective transporters. Subfamily 2 is specific to monocotyledonous species and comprises transporters permeable to both Na+ and K+ and, at least for some of them, able to display Na+-K+ symport activity when heterologously expressed in yeast or Xenopus oocytes (Hauser and Horie, 2010). Within the HKT/Trk/Ktr superfamily, transporters endowed with Na+-K+ symport activity (at least when expressed in heterologous systems) have been identified in bacteria, fungi, and plants, while Na+-selective transporters have been identified only in plants so far, and H+-K+ symporters only in bacteria and fungi (Corratgé-Faillie et al., 2010).
Sequence analyses, in silico modeling, and site-directed mutagenesis experiments have led to the conclusion that HKT/Trk/Ktr transporters share a typical structure, which probably derived from that of an ancestral K+ channel subunit (Durell et al., 1999; Durell and Guy, 1999). The shared structure comprises a channel-like hydrophobic core region composed of four successively arranged MPM domains (abbreviation for transMembrane segment, pore loop, transMembrane segment). Each MPM domain of HKT/Trk/Ktr transporters harbors sequence and structure similarities with K+ channel subunits that display a single MPM domain and associate into tetramers to form functional channels (Durell et al., 1999; Durell and Guy, 1999). The eight transmembrane segments of the core region of HKT/Trk/Ktr transporters are organized with a 4-fold symmetry around a central permeation pathway (pore) lined by the four pore loop domains (Durell and Guy, 1999; Kato et al., 2001). This model has recently been confirmed by the elucidation of the crystal structure of a bacterial member from the HKT/Trk/Ktr superfamily, VpTrkH from Vibrio parahaemolyticus (Cao et al., 2011). In the pore sequence of each MPM domain, a short stretch, which includes a Gly residue, can be identified as the counterpart of the GYGD motif that forms the selectivity filter in K+ channels (Doyle et al., 1998; Cao et al., 2011). Although they display such a channel-like structure, HKT/Trk/Ktr systems can be considered as transporters rather than channels since they are able, at least for some of them, to mediate active K+ uptake (Corratgé-Faillie et al., 2010).
Surprisingly, a subfamily 2 rice HKT transporter that remained to be characterized, OsHKT2;4, was recently reported to form a calcium-permeable weakly selective cation channel (Lan et al., 2010) or a Ca2+-permeable K+ transporter (Horie et al., 2011) when heterologously expressed in Xenopus oocytes. These results were exciting for the plant ion transport community, since in addition to the fact that they raised questions of structural evolution within the HKT family, at least for the former of these studies, they identified OsHKT2;4 as a potential actor of plant cell Ca2+ signaling, in which very few proteins playing a role as Ca2+ transporters or channels have been clearly identified at the molecular level so far (White et al., 2002; Ali et al., 2007; Cho et al., 2009; Laohavisit et al., 2010, 2012).
Here, we have reexamined the functional properties of OsHKT2;4 when heterologously expressed in Xenopus oocytes. Using selected batches of oocytes with low Ca2+ endogenous currents, we observed that OsHKT2;4 did not provide any detectable pathway for Ca2+ transport, even at very low K+ concentration, a result discarding the hypothesis that this system would play a role in Ca2+ signaling in planta. OsHKT2;4 is shown to be permeable to both K+ and Na+, as other members from HKT subfamily 2, but to constitute a new functional type within this subfamily. It is endowed with high permeability to K+: It transports Na+, besides K+, only at high external Na+, and mediates K+-selective transport at low external Na+.
RESULTS
Expression of OsHKT2;4 in Oocytes Gives Rise to an Instantaneously Activating Non-Voltage-Regulated Conductance
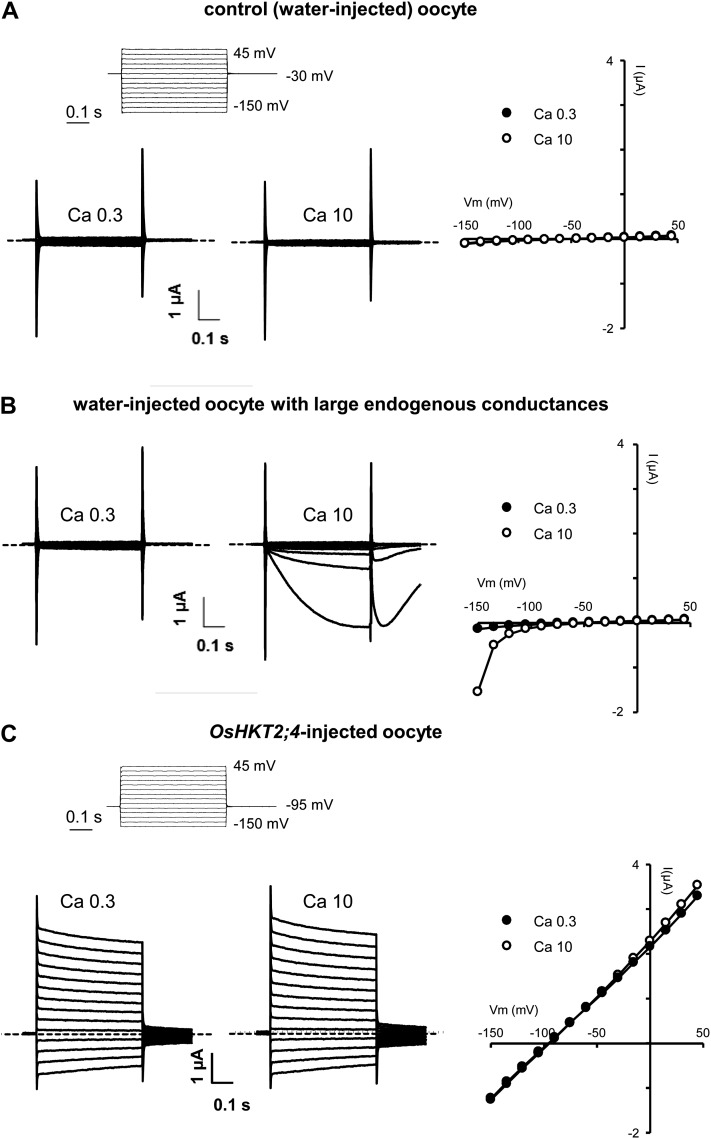
OsHKT2;4 expression in Xenopus oocytes being expected not to lead to large exogenous currents (Lan et al., 2010), we paid special attention to the quality of oocytes used. Figure 1A provides examples of currents recorded in a control (water-injected) oocyte from a batch selected for OsHKT2;4 expression. In the presence of 3 mm NaCl and either 0.3 or 10 mm calcium gluconate, the level of endogenous currents was very low and independent of the external Ca2+ concentration. Besides selected oocyte batches, other ones displayed significant endogenous currents in the presence of a high (10 mm) external Ca2+ concentration upon membrane hyperpolarization (Fig. 1B). These currents, displaying slow kinetics of both activation and deactivation upon return to weakly polarized conditions (Fig. 1B), were reminiscent of a reported oocyte endogenous anionic conductance activated by an increase in cytosolic calcium concentration (Kuruma and Hartzell, 1999; Hartzell et al., 2005). Being regulated by cytosolic Ca2+, this conductance is in particular strongly increased in bath solutions containing a high calcium concentration, if a Ca2+ conductance is also present at the membrane. The level of currents in the presence of high external calcium was therefore always checked in control oocytes. When control oocytes displayed in the presence of 10 mm Ca2+ at membrane potentials less negative than −165 mV slowly activating and deactivating currents reminiscent of typical Ca2+-activated endogenous anionic conductance (Fig. 1B; Kuruma and Hartzell, 1999), revealing a substantial permeability to Ca2+ in those oocytes, the corresponding batch of oocytes was systematically discarded for OsHKT2;4 expression.
Figure 1.
Whole-cell currents recorded in control or OsHKT2;4-expressing oocytes in the presence of varying external calcium concentrations. A, Typical examples of currents recorded in water-injected oocytes displaying low levels of endogenous conductances and used as controls. The voltage-clamp protocol (top section) consisted in 13 steps, the applied voltages ranging from −150 mV to +45 mV with an increment of +15 mV. The holding potential was −30 mV. The oocytes were perfused with bath solutions containing 3 mm NaCl, no Mg2+, and either 0.3 mm or 10 mm calcium gluconate. Left and middle sections: current traces recorded in the same oocyte in the presence of 0.3 or 10 mm calcium gluconate, respectively. Right section: current (I)-voltage (V) relationships derived from the recordings shown in the left and middle sections. B, Typical examples of currents recorded in water-injected oocytes displaying high levels of endogenous conductances. Same voltage-clamp protocol and same experimental conditions as in A. Left and middle sections: current traces recorded in the same oocyte in the presence of 0.3 or 10 mm calcium gluconate, respectively. Right section: I-V relationships derived from the recordings shown in the left and right sections. Oocyte batches in which water-injected oocytes were found to display such large endogenous conductances were not retained for functional characterization of OsHKT2;4. C, Typical examples of currents recorded in OsHKT2;4-injected oocytes. The voltage-clamp protocol (top section) was the same as that used for control oocytes (applied voltages from −150 mV to +45 mV) except concerning the holding potential, which was fixed to −95 mV (instead of −30 mV) to stay close to the current reversal potential. Bath solutions were as in A and B. Left and middle sections: current traces recorded in the same oocyte in the presence of 0.3 or 10 mm calcium gluconate, respectively. Right section: I-V relationships derived from the recordings shown in the left and middle sections.
Injection of 50 ng of OsHKT2;4 complementary RNA (cRNA) in oocytes from selected batches resulted in appearance of exogenous currents. Figure 1C shows representative examples of currents recorded 2 d after OsHKT2;4 cRNA injection, in the presence of 3 mm NaCl and either 0.3 or 10 mm calcium gluconate (same ionic conditions as for the recordings in water-injected oocytes shown in Fig. 1, A and B). Currents in this OsHKT2;4-expressing oocyte displayed instantaneous activation, no evolution with time at any imposed membrane potentials in the explored range of −150 to +45 mV, and no obvious rectification. Currents displaying such ohmic aspect were systematically observed in every OsHKT2;4-expressing oocytes, in all our experimental solutions (see current/voltage relationships in all figures).
Expression of OsHKT2;4 always induced a strong modification of oocyte membrane resting polarization in Na+-containing and/or low K+-containing media, indicating that it resulted in an important change in the nature of oocyte membrane ionic permeability. In the ionic conditions used in Figure 1 for instance (3 mm NaCl and either 0.3 or 10 mm calcium gluconate), the resting potential of the oocyte membrane was shifted negatively by more than 50 mV in oocytes expressing OsHKT2;4 when compared with control oocytes (Fig. 1, A and C): mean resting potential of −101 ± 4 or −48 ± 1 in OsHKT2;4-expressing or control oocytes, respectively. Analyzing currents in control and in OsHKT2;4-expressing oocytes also indicated that expression of OsHKT2;4 resulted in an increase in the oocyte conductance by about 5 to 40 times, depending on the external ionic conditions and the oocyte batch (20–fold increase in the inward direction and 34-fold increase in the outward direction in the examples shown in Fig. 1, A and C). In the subsequent functional characterization of OsHKT2;4 transport activity, for a precise analysis, OsHKT2;4 currents were extracted from total oocyte currents by subtracting the mean currents recorded in control oocytes from the same batch (Figs. 2, A and E; 3, A, C–E, and G; 4, A and D; and 5, A, D, and G).
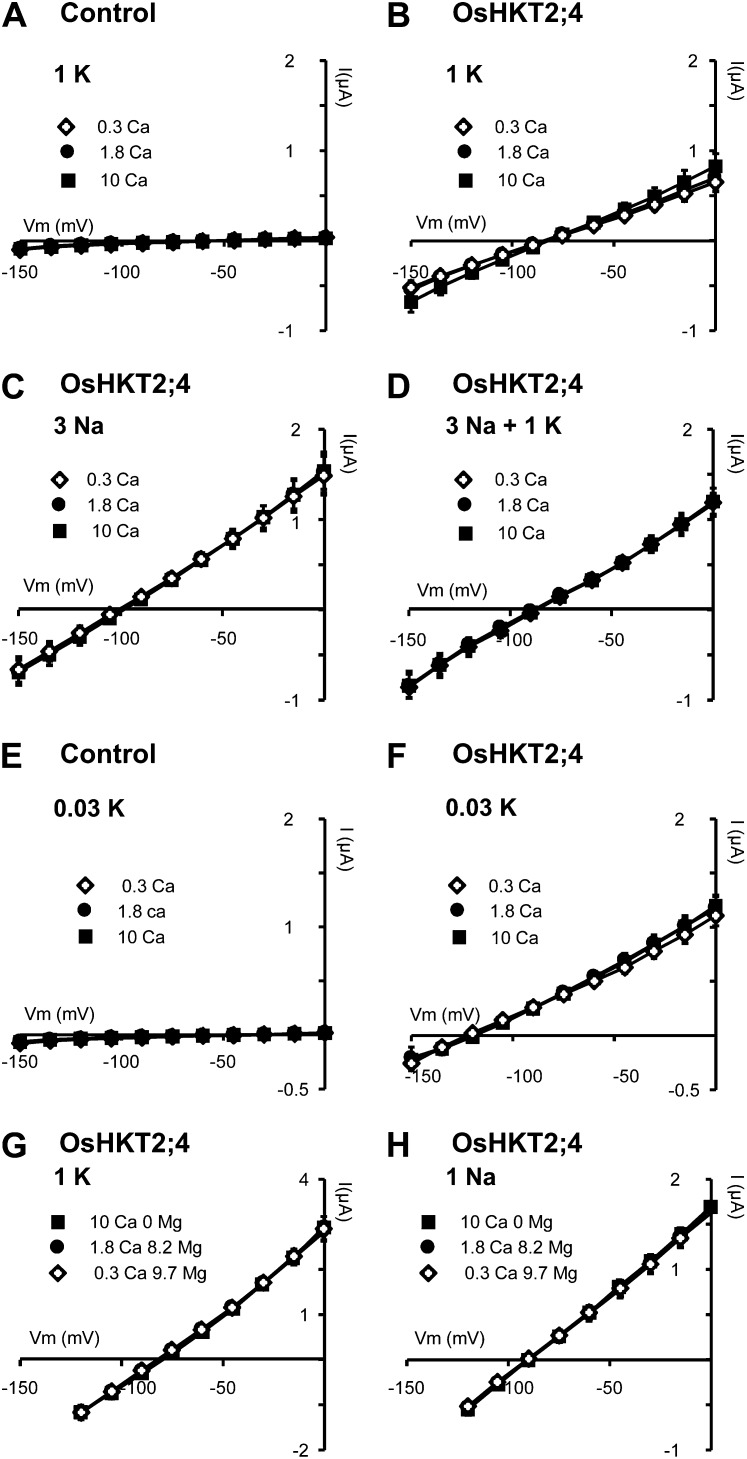
Figure 2.
OsHKT2;4 expressed in Xenopus oocytes displays no permeability to Ca2+ or Mg2+. A to F, Current-voltage (I-V) relationships obtained in oocytes bathed with solutions containing varying concentrations of calcium gluconate (0.3, 1.8, or 10 mm: 0.3Ca, 1.8Ca, and 10Ca solutions, respectively), no Mg2+, and either 1 mm KCl (1 K; A and B), 3 mm NaCl (3 Na; C), both 3 mm NaCl and 1 mm KCl (3 Na + 1 K; D), or 0.03 mm KCl (0.03 K; E and F). A and E, Control oocytes (injected with water). B to D and F, OsHKT2;4-expressing oocytes. G and H, Current-voltage (I-V) relationships in OsHKT2;4-expressing oocytes bathed with solutions containing varying concentrations of CaCl2 and MgCl2, the total divalent ion concentration being kept constant to 10 mm in all solutions (10 mm Ca2+, 1.8 mm Ca2+ + 8.2 mm Mg2+, or 0.3 mm Ca2+ + 9.7 mm Mg2+: 10 Ca 0 Mg, 1.8 Ca 8.2 Mg, and 0.3 Ca 9.7 Mg, respectively), and either 1 mm K-Glu (1 K; G) or 1 mm Na-Glu (1 Na; H). Currents flowing through OsHKT2;4 were extracted from whole oocyte currents by subtracting the mean currents recorded in the control oocytes. Data are means ± se (n = 4 in A, n = 6 in B, C, and G, n = 5 in D, n = 5 in E, n = 10 in F, and n = 3 in H) and are representative of at least three experiments performed on different oocyte batches.
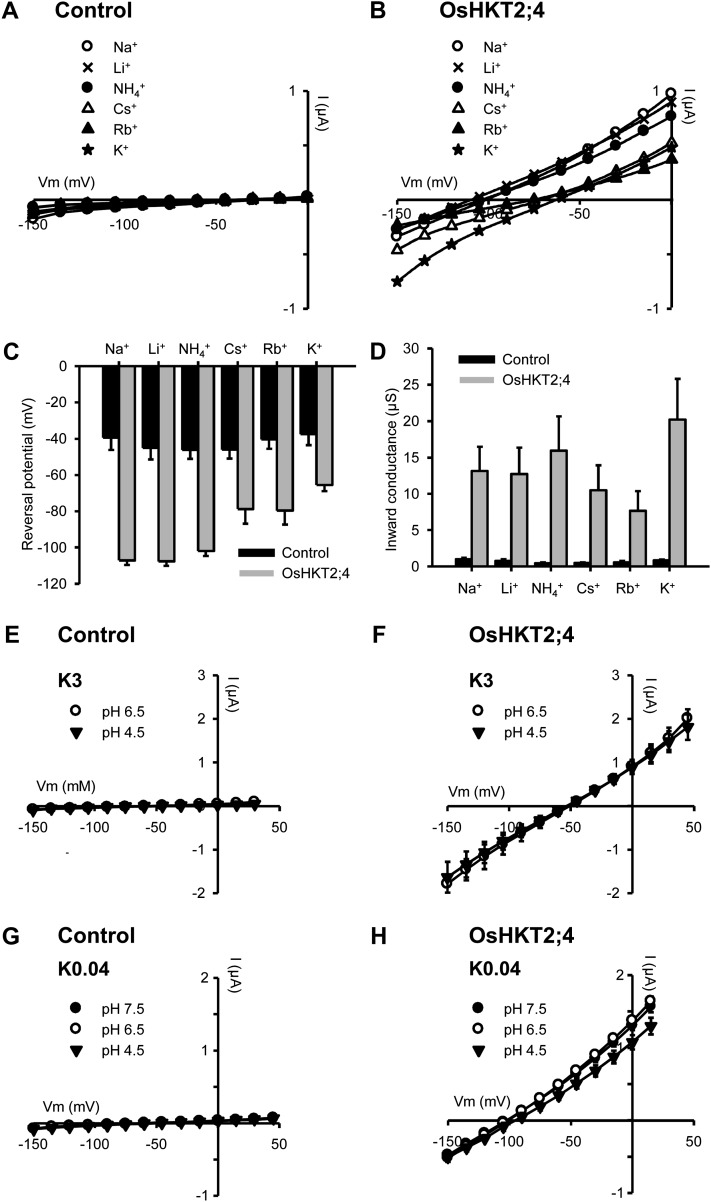
Figure 3.
Monovalent cation selectivity in OsHKT2;4. A to D, Xenopus oocytes were successively bathed with solutions containing 3 mm of either Na+, Li+, NH4+, Cs+, Rb+, or K+ (as chloride salts). A and B, I-V relationships. A, Control oocyte (injected with water). B, Oocyte expressing OsHKT2;4. Currents flowing through OsHKT2;4 were extracted from whole oocyte currents by subtracting the mean currents recorded in the control oocytes. C, Reversal potentials of currents flowing through OsHKT2;4 in the presence of the tested monovalent cations. D, Macroscopic inward conductance of OsHKT2;4 in the presence of the tested monovalent cations. Inward conductances were defined as slopes (of linear fits) of I-V relationships between the three imposed potentials closest to the reversal potential. Data are means ± se (n = 4) and are representative of three experiments performed on different oocyte batches. E to H, Effect of external pH on OsHKT2;4 I-V relationships. The bath solutions contained 3 mm K-Glu and 30 μm Na-Glu (K3) in E and F, and 40 μm K-Glu and 30 μm Na-Glu (K0.04) in G and H, at pH 7.5, 6.5, or 4.5. Currents were recorded in control oocytes (injected with water) in E and G, and in oocytes expressing OsHKT2;4 in F and H. Currents flowing through OsHKT2;4 were extracted from whole oocyte currents by subtracting the mean currents recorded in the control oocytes in the same conditions. Data are means ± se (n = 3 in E, n = 4 in F and G, and n = 6 in H).
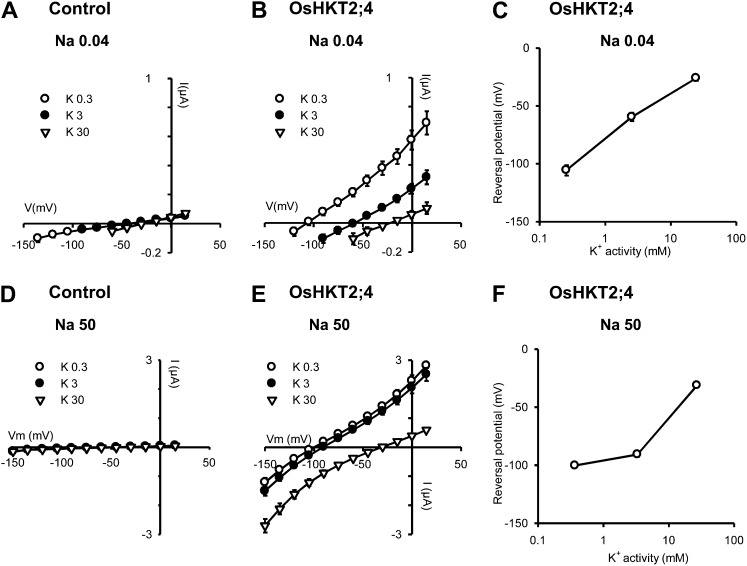
Figure 4.
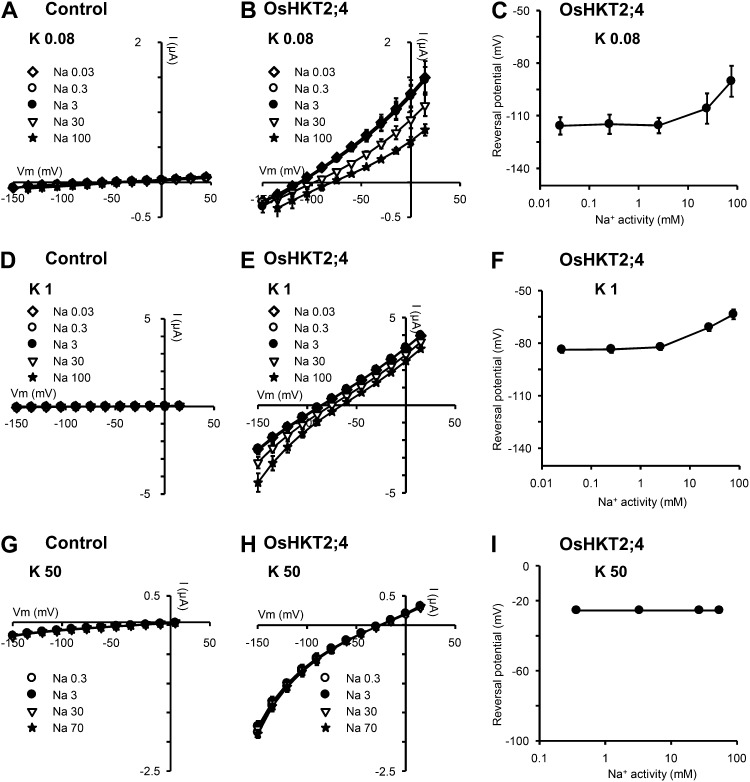
K+ transport through OsHKT2;4. A to C, K+ transport at low external Na+ concentration. D to F, K+ transport at high external Na+ concentration. A, B, D, and E, Effect of external K+ concentration on I-V relationships obtained in control (A and D) and in OsHKT2;4-expressing oocytes (B and E). Currents flowing through OsHKT2;4 were extracted from whole oocyte currents by subtracting the mean currents recorded in the control oocytes. Bath solutions contained Na-Glu at 40 μm (A–C) or at 50 mm (D–F) and varying concentrations of K-Glu (0.3, 3, or 30 mm: K0.3, K3, and K30, respectively). The Ca2+ and Mg2+ concentrations were the same in all solutions (1.8 and 6 mm, respectively). C and F, Effect of external K+ on current reversal potentials through OsHKT2;4. Reversal potentials in C and F were obtained from I-V data shown in B and E, respectively. Data are means ± se (n = 5 in A, n = 7 in B and C, n = 6 in D, and n = 11 in E and F) and are representative of at least two experiments performed on different oocyte batches.
Figure 5.
Na+ transport through OsHKT2;4. A to C, D to F, and G to I, Na+ transport in the presence of 80 μm K+, 1 mm K+, and 50 mm K+, respectively. A, B, D, E, G, and H, Effect of external K+ concentration on I-V relationships obtained in control (A, D, and G) and in OsHKT2;4-expressing oocytes (B, E, and H). Currents flowing through OsHKT2;4 were extracted from whole oocyte currents by subtracting the mean currents recorded in the control oocytes. Bath solutions contained K-Glu at 80 μm (A–C), 1 mm (D–F), or 50 mm (G–I) and varying concentrations of Na-Glu (0.03, 0.3, 3, 30, 70, or 100 mm: Na0.03, Na0.3, Na3, Na30, Na70, and Na100, respectively). The Ca2+ and Mg2+ concentrations were the same in all solutions (1.8 and 6 mm, respectively). C, F, and I, Effect of external Na+ on current reversal potentials through OsHKT2;4. Reversal potentials in C, F, and I were obtained from I-V data shown in B, E, and H, respectively. Data are means ± se (n = 5 in A, D, and G, n = 7 in B and C, n = 12 in E and F, and n = 14 in H and I) and are representative of at least two experiments performed on different oocyte batches.
Expression of OsHKT2;4 in Oocytes Does Not Elicit Significant Ca2+-Permeable Currents
OsHKT2;4 has been proposed to constitute a Ca2+-permeable cation/K+ channel or transporter when expressed in Xenopus oocytes (Lan et al., 2010; Horie et al., 2011). Currents attributed to OsHKT2;4 in the report by Lan et al. (2010) displayed a large slowly activating inwardly rectifying component and were thus very different from the instantaneously activating ohmic ones obtained in our study (compare with traces above). We therefore reexamined OsHKT2;4 permeability to Ca2+ (Fig. 2).
In a first series of experiments, Ca2+ was present in bath solutions as sole divalent cation (Fig. 2, A–F; Supplemental Fig. S1, A–C). Different sets of solutions were used. In each set of solutions, the concentration of the monovalent cations (either K+ or Na+ or both Na+ and K+) was fixed and that of Ca2+ was variable (0.3, 1.8, or 10 mm). It should be noted that two sets of solutions, those containing either 1 mm K+ or 3 mm Na+, displayed the same (or almost the same) cation composition as sets of solutions used by Lan et al. (2010) to address OsHKT2;4 permeability to Ca2+. Increasing external Ca2+ from 0.3 to 10 mm in the presence of 1 mm K+ and/or 3 mm Na+ was shown to have no significant effect on OsHKT2;4 current amplitude (Fig. 2, B–D; Supplemental Fig. S1, A and B). Furthermore, increasing external Ca2+ from 0.3 to 10 mm did not affect the reversal potential of current through OsHKT2;4 in any set of bath solutions (Fig. 2, B–D; Supplemental Fig. S1, A and B). The reversal potential of current through OsHKT2;4 was close to −100 mV in the set of solutions containing 3 mm Na+ (without K+), and close to −85 mV in the two other sets of solutions containing 1 mm K+ (Fig. 2, B–D; Supplemental Fig. S1, A and B), which was in all cases very far from the Ca2+ equilibrium potential (higher than +90 mV, assuming oocyte cytosolic free Ca2+ ≤ 400 nm and cytosolic ionic strength approximately 0.1 m; Dascal, 1987). It was very far also from the Cl− equilibrium potential (positive in all our conditions using a 35 mm cytosolic Cl− estimate; Barish, 1983), which confirmed that the activity of Ca2+-activated endogenous anionic conductances was very low in our experiments. The total absence of change in the reversal potential of current through OsHKT2;4 upon external Ca2+ increase by more than one decade of concentration strongly argues for an absence of significant Ca2+ permeability in OsHKT2;4. Horie et al. (2011) have proposed that the permeability to Ca2+ in OsHKT2;4 depended on the concentration of competing K+ ions. We therefore also examined Ca2+ permeability through OsHKT2;4 in the presence of very low K+ concentration (30 μm; Fig. 2F; Supplemental Fig. S1C). In these experiments, the mean reversal potential of currents through OsHKT2;4, more negative than −120 mV, was fully independent of the external calcium concentration in the range 0.3 to 10 mm. Furthermore, OsHKT2;4 current intensity also was independent of external calcium concentration (Fig. 2F; Supplemental Fig. S1C). This indicated that even in conditions of very low concentrations of competing cation for permeation through OsHKT2;4, Ca2+ was not significantly permeant.
We then examined a possible permeability to Mg2+ in OsHKT2;4 since this system had been reported to be permeable to Mg2+ as well (Lan et al., 2010; Horie et al., 2011). The bath solutions displayed varying Mg2+ concentrations (0, 8.2, or 9.7 mm), the total concentration of divalent cations (Ca2+ and Mg2+) being kept constant, 10 mm, and either 1 mm K+ (Fig. 2G; Supplemental Fig. S1D) or 1 mm Na+ (Fig. 2H). Within each set of solutions, changing the external Mg2+ concentration was without any effect on the macroscopic conductance of OsHKT2;4 and on the reversal potential of its currents (Fig. 2, G and H; Supplemental Fig. S1D). These results indicated that OsHKT2;4 was not significantly permeable to Mg2+ either.
OsHKT2;4 Is Selective for K+ among Monovalent Cations
To examine OsHKT2;4 permeability to monovalent cations, Na+, K+, Rb+, Cs+, Li+, and NH4+ currents were compared in bath solutions containing 3 mm of one of these cations and a fixed (standard) concentration of Ca2+ and Mg2+ (Fig. 3, B–D; Supplemental Fig. S2A). OsHKT2;4 current reversal potential and inward conductance depended on the monovalent cation present in the bath, indicating that the transporter discriminates between these cations. Three groups of monovalent cations could be distinguished from these experiments. K+ was the cation with which OsHKT2;4 conductance was the highest and the reversal potential of currents the less negative (approximately −65 mV; Fig. 3, B–D), indicating that this cation is the most permeant one through OsHKT2;4. When Na+, Li+, or NH4+ replaced K+, the reversal potential of currents was negatively shifted by approximately −45 mV (Fig. 3, B and C; Supplemental Fig. S2A) and the inward conductance was reduced by about 30% (Fig. 3, B and D). In the presence of Cs+ and Rb+ on the other hand, the reversal potential of OsHKT2;4 currents was only slightly different from that in the presence of K+ (shift by approximately −15 mV; Fig. 3, B and C), but the inward conductance was strongly reduced compared with that in the presence of K+ (by 50% and more than 60%, respectively; Fig. 3, B and D). Comparing OsHKT2;4 with other HKT transporters so far characterized and in particular with those described as K+ permeable (HKT2;1 from different cereals and OsHKT2;2), it could be noticed that OsHKT2;4 displayed the highest conductance to K+ relative to that to Na+: Indeed, the conductance to K+ was (slightly) higher than the conductance to Na+ in OsHKT2;4 (in bath solutions comprising only K+ or Na+ as monovalent cation), whereas it was strongly lower (by more than 4 times) than the conductance to Na+ in all other HKT transporters (Rubio et al., 1995; Gassmann et al., 1996; Uozumi et al., 2000; Horie et al., 2001; Jabnoune et al., 2009; Mian et al., 2011; Fig. 3D; Supplemental Fig. S2). Furthermore, the comparison of the reversal potential of currents in the presence of K+ or Na+ also suggested a selectivity for K+ higher in OsHKT2;4 than in other characterized HKT transporters (Gassmann et al., 1996; Jabnoune et al., 2009; Mian et al., 2011; Supplemental Fig. S2). Accurate permeability ratios can probably not be derived for all HKT transporters from the Goldman-Hodgkin-Katz equation classically used to describe ion channel permeability (Hille, 1992) since this equation assumes absence of interaction between permeant ions upon transport, which cannot be the case in HKT transporters from subfamily 2 displaying Na+-K+ symport activity (when expressed in oocytes, like the rice and barley [Hordeum vulgare] HKT2;1 transporters for instance). However, performing such crude calculations for broad comparison resulted in estimates of the K+ to Na+ permeability ratio higher in OsHKT2;4 than in the rice or barley HKT2;1 transporters, and much higher in these subfamily 2 transporters than in a member from subfamily 1, OsHKT1;3, as expected (Supplemental Fig. S2F).
OsHKT2;4 permeability to H+ was also examined. Since some fungal HKT homologs have been described as H+-K+ symporters (Rodríguez-Navarro, 2000), and since K+ is the most permeant monovalent cation through OsHKT2;4 (Fig. 3, B–D), we tested whether K+ could be cotransported with H+. Two sets of bath solutions were used that displayed different pH, 7.5, 6.5, and 4.5, a fixed (standard) concentration of Ca2+ and Mg2+, and either 3 mm K+ (as in Fig. 3, B–D) or a very low concentration of K+ (0.04 mm; Fig. 3, F and H). In the presence of 3 mm K+, changing the external pH from 6.5 to 4.5 had no effect on OsHKT2;4 currents (Fig. 3F). In the presence of 0.04 mm K+, the decrease in external pH from 6.5 to 4.5 had a small effect on recorded currents: The reversal potential of OsHKT2;4 currents shifted positively by approximately 5 mV and OsHKT2;4 macroscopic conductance was slightly reduced (Fig. 3H). No effect of external pH was observed upon medium alkalinization from pH 6.5 to 7.5 (Fig. 3H). Thus, H+ is not permeant through OsHKT2;4 when the external concentration of K+ is higher than that of H+, but may be slightly permeant when both concentrations are similar. Taken as a whole, these results suggest that under our experimental and very likely most physiological ionic conditions, H+ is not significantly permeant through OsHKT2;4.
OsHKT2;4 Transports Na+ Along with K+ at High External Na+ Concentrations
Experiments using solutions containing either K+ or Na+ as sole monovalent cation, as shown in Figure 3, B to D, indicated that OsHKT2;4 is much more permeable to K+ than to Na+. OsHKT2;4 permeability to K+ and Na+ was then further analyzed by using sets of solutions containing both cations. The concentration of either Na+ or K+ was kept constant while that of the other ion was varied, the concentrations of Ca2+ and Mg2+ being kept constant, at their standard values.
In the presence of either 40 μm (Fig. 4, A–C) or 50 mm Na+ (Fig. 4, D–F), the concentration of K+ was successively set to 0.3, 3, and 30 mm. The increase in external K+ concentration positively shifted the reversal potential of OsHKT2;4 currents in every case. The mean shift was of about 40 mV upon 10-fold change in K+ activity, indicating that K+ was the main ionic species transported through OsHKT2;4 (theoretical shift of 58 mV for a purely K+-selective transporter), except in the presence of 50 mm Na+ when the concentration of K+ was varied between 0.3 and 3 mm. In these latter conditions, the shift in current reversal potential resulting from the 10-fold increase in K+ concentration, was reduced to about 10 mV, suggesting that Na+, and not K+, was then the main ionic species transported through OsHKT2;4.
In reciprocal experiments, OsHKT2;4 permeability to Na+ was analyzed by varying the external concentration of this cation from tens of μm to tens of mm, in the presence of a fixed concentration of K+, set to either 0.08 mm (Fig. 5, A–C), 1 mm (Fig. 5, D–F), or 50 mm (Fig. 5, G–I). Increasing the external concentration of Na+ shifted the current reversal potential when the concentration of Na+ was varied from 3 to 100 mm in the presence of 0.08 or 1 mm K+, i.e. when Na+ was brought in a range of concentrations much higher than the concentration of K+. The largest variation of current reversal potential was observed when the Na+ concentration was increased from 30 to 100 mm, in the presence of 0.08 mm K+. The shift in reversal potential, extrapolated over a 10-fold change in Na+ activity, was then close to +31 mV (Fig. 5C). This result, indicating an increased Na+ transport activity in OsHKT2;4 in low K+ and high Na+ conditions, is consistent with what was suggested by the reciprocal experiment described by Figure 4, E and F. It should be noted that HKT2;1-type transporters from different cereals have been proposed to be able to mediate Na+-K+ symport when expressed in yeast and/or Xenopus oocytes (Rubio et al., 1995; Haro et al., 2005; Jabnoune et al., 2009; Mian et al., 2011). Within the framework of the hypothesis that OsHKT2;4 could also mediate Na+-K+ symport activity with variable stoichiometry, as proposed for HvHKT2;1 transporter when expressed in oocytes (Mian et al., 2011), a shift in reversal potential of currents of 31 mV per 10-fold change in ionic activity is very close to the expected shift in a symport of monovalent cations with a 1:1 stoichiometry (i.e. 29 mV). Thus, OsHKT2;4 would display a Na+:K+ stoichiometry close to 1:1 in external solutions containing 30 to 100 mm Na+ and 80 μm K+, and possibly more generally when the external concentration of Na+ is about 102 to 103 times higher than that of K+. When the concentrations of K+ and Na+ in the external solutions were similar, or that of K+ was higher than that of Na+, the data shown in Figure 5 reveal that increasing the external concentration of Na+ was without any effect on the current reversal potential, leading to the conclusion that OsHKT2;4 was then behaving as a transporter essentially permeable to K+. Hence, taken as a whole, the data shown in Figures 4 and 5 indicate that OsHKT2;4 displays K+ selectivity but can significantly transport Na+ along with K+, and possibly mediate Na+-K+ symport, when the external concentration of K+ is at least one order of magnitude lower than that of Na+.
DISCUSSION
OsHKT2;4 Does Not Display a Voltage-Gated Conducting Pathway Permeable to Ca2+
Heterologous expression of OsHKT2;4 in Xenopus oocytes was reported to give rise to Ca2+ and Mg2+ membrane transport activity (Lan et al., 2010; Horie et al., 2011). As discussed below, this transport activity was proposed to be mediated by OsHKT2;4 itself. The hypothesis that the Ca2+ and Mg2+ transport was mediated by endogenous channels whose activity was increased due to expression of OsHKT2;4 was discarded by Lan et al. (2010) and Horie et al. (2011), in part because Ca2+ and Mg2+ currents were not observed in oocytes expressing other members from the HKT family (e.g. AtHKT1;1 or OsHKT2;1), or observed at lower levels than in oocytes expressing OsHKT2;4 (e.g. in oocytes expressing OsHKT2;2 or TaHKT2;1). It is, however, known that heterologous expression in Xenopus oocytes can give rise to increased endogenous channel activity, depending on the exogenous protein (Shimbo et al., 1995; Tzounopoulos et al., 1995; Terhag et al., 2010).
In the reports by Lan et al. (2010) and Horie et al. (2011), the conductance to Ca2+ observed in oocytes expressing OsHKT2;4 was proposed to display unique features, when compared with the conductance to monovalent cations. Indeed, the currents carried by monovalent cations (K+ or Na+) in OsHKT2;4-expressing oocytes were found to display kinetics and pharmacological hallmarks (sensitivity to ion channel blockers) different from the currents carried by Ca2+: The former currents appeared as instantaneously activating, nonrectifying, and blocked by Ba2+, while the latter currents appeared as slowly activating, inwardly rectifying, and blocked by La3+ but not by Ba2+. This indicated that two different permeation pathways were open in OsHKT2;4-expressing oocytes, one permeable to monovalent cations, behaving as an instantaneously activating nonrectifying conductance (ohmic behavior) insensitive to the channel blocker La3+, and the other one permeable to Ca2+, behaving as slowly activating voltage-sensitive inwardly rectifying conductance blocked by La3+. Entry of Ca2+ through the latter pathway activated endogenous Ca2+-activated anion channels, which explained part of the large depolarizing effect of Ca2+ on OsHKT2;4-expressing oocyte membrane (Lan et al., 2010; Horie et al., 2011). The authors concluded that the two identified cation permeation pathways in OsHKT2;4-expressing oocytes coexisted in OsHKT2;4 transporter. Such a hypothesis, that OsHKT2;4 would harbor two distinct permeation pathways, is however not supported by the recent elucidation of the crystal structure of a bacterial member from the HKT/Ktr/Trk family, VpTrkH from V. parahaemolyticus (Cao et al., 2011). HKT/Ktr/Trk transporters have been proposed to originate from duplications of an ancestor channel gene, resulting in a channel-like structure with one central pore for ion permeation (Durell and Guy, 1999). A multimeric structure of these transporters may be hypothesized to allow additional permeation pathways at the multimer interfaces (Durell and Guy, 1999). Biochemical and structural analyses have evidenced dimeric structures in bacterial KtrB and TrkH transporters (Albright et al., 2007; Cao et al., 2011). However, the analysis of VpTrkH crystal structure, which confirms the presence of a single central pore in each monomeric transporter, does not support the hypothesis of an additional permeation pathway at the interface of two monomers, since large hydrophobic residues occlude the cavity formed at this interface (Cao et al., 2011).
In contrast to Lan et al. (2010) and Horie et al. (2011), we did not observe apparition of slowly activating inwardly rectifying conductance, or of a Ca2+- or Mg2+-permeable conductance, in OsHKT2;4-expressing oocytes (Figs. 1 and 2; Supplemental Fig. S1). Only instantaneously activating non-time-dependent and non- (or weakly) rectifying exogenous conductance was observed at low or high divalent cation concentrations. This instantaneously activating conductance was not permeable to Ca2+. This was ascertained by the fact that the reversal potential of currents through OsHKT2;4, as well as OsHKT2;4 conductance, appeared insensitive to large changes in external Ca2+ concentration (in the range 0.3–10 mm; Figs. 1 and 2; Supplemental Fig. S1). Horie et al. (2011) proposed that Ca2+ and Mg2+ competed with K+ for permeation through OsHKT2;4, and were more permeant at low external K+. However, in our experiments, an increase in external Ca2+ from 0.3 to 10 mm was shown to be without any effect on OsHKT2;4 conductance and on the current reversal potential even at very low external K+ concentration (30 μm; Fig. 2F; Supplemental Fig. S1C). The absence of permeability to Ca2+ was also evidenced by the fact that the reversal potential of OsHKT2;4 currents was highly negative (Figs. 1 and 2; Supplemental Fig. S1), i.e. very far from the positive value predicted for a Ca2+-selective conductance. Finally, absence of any significant permeability to Ca2+ in our OsHKT2;4-expressing oocytes was also indicated by absence of Ca2+-activated endogenous anion channel activity (in the explored experimental conditions).
It has been shown that expression in Xenopus oocytes of a number of membrane proteins, among which ion transport proteins, can stimulate endogenous hyperpolarization-activated currents mediated by two different types of conductances, a calcium-activated chloride conductance and a nonselective cation conductance permeable to Ca2+ (Tzounopoulos et al., 1995). It can therefore be hypothesized that both the Ca2+-permeable hyperpolarization-activated and the anionic hyperpolarization-activated components of the current observed by Lan et al. (2010) upon OsHKT2;4 expression were mediated by endogenous oocyte conductances. We hypothesize that selecting batches of oocytes with low levels of endogenous Ca2+ conductances, in this study, enabled to limit/avoid activation of the endogenous nonselective Ca2+-permeable cationic conductance upon OsHKT2;4 expression. This would have resulted in absence of Ca2+ permeability in oocytes expressing OsHKT2;4, and thereby also in absence of endogenous Ca2+-activated anion channel activity. This hypothesis reconciles the observations reported by Lan et al. (2010) and Horie et al. (2011) and the present observations, which are more in line with the functional analyses performed so far in other HKT transporters (for review, see Corratgé-Faillie et al., 2010) as well as with first structural data obtained in the Trk/Ktr/HKT superfamily (Cao et al., 2011). Indeed, the observation of an instantaneously activating non-time-dependent current kinetics is in line with what has been so far observed in HKT/Trk transporters of plant or fungal origin when expressed in Xenopus oocytes (Fairbairn et al., 2000; Corratgé et al., 2007; Jabnoune et al., 2009). Furthermore, the fact that the instantaneously activating conductance observed in our OsHKT2;4-expressing oocytes was not permeable to Ca2+ is in agreement with the statement of Lan et al. (2010) that monovalent cations and not Ca2+ carried the ohmic component of the current they observed upon OsHKT2;4 expression in oocytes.
OsHKT2;4 Defines a New Functional Type of Transporter within the HKT Family
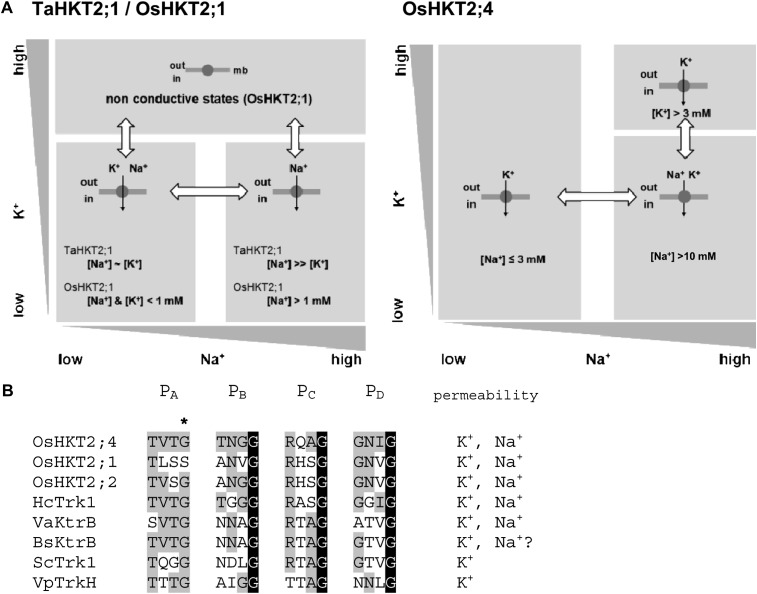
HKT transporters have been sorted out into two subfamilies based on phylogenetic analyses and functional differences observed in heterologous systems (Platten et al., 2006): Subfamily 1 HKT transporters are thought to be Na+ selective whereas subfamily 2 transporters are expected to transport both Na+ and K+ (Rubio et al., 1995; Uozumi et al., 2000; Haro et al., 2005; Ren et al., 2005; Jabnoune et al., 2009). Apart from OsHKT2;4, all the members of subfamily 2 transporters that have been characterized so far are close homologs of TaHKT2;1 (Platten et al., 2006; Hauser and Horie, 2010), the first subfamily 2 transporter characterized (Rubio et al., 1995). Expressed in heterologous systems, these HKT2;1 subgroup transporters display a complex behavior compared with the transporters from subfamily 1, since they are endowed with different conduction modes (Rubio et al., 1995; Gassmann et al., 1996; Haro et al., 2005; Jabnoune et al., 2009). They work as Na+-K+ symporters at low Na+ and K+ concentrations, when Na+ and K+ are in the submillimolar range, and eventually at higher (millimolar) concentrations when Na+ and K+ concentrations are balanced (Fig. 6A). They switch to a Na+ uniport mode when Na+ is in the millimolar range and in excess over K+ (Fig. 6A). A K+ uniport mode has also been proposed for these HKT2;1 subgroup transporters when K+ is in the millimolar range and/or in excess over Na+, but this K+ uniport mode has been described as weakly or nonconductive (Haro et al., 2005; Jabnoune et al., 2009).
Figure 6.
Comparison of conduction modes and pore sequence between HKT2;4 from rice and HKT2;1 subgroup transporters. A, Dependency of the conduction modes on the external Na+ and K+ concentrations. Three types of conduction modes have been proposed to be encountered by TaHKT2;1 and OsHKT2;1 expressed in Xenopus oocytes, depending on the external Na+ and K+ concentrations (Rubio et al., 1995; Gassmann et al., 1996; Jabnoune et al., 2009): Na+-K+ symport at lower Na+ and K+ concentrations, and Na+ uniport, at higher Na+ concentrations ([Na+] >> [K+] in TaHKT2;1; [Na+] > 1 mm in OsHKT2;1). At high external K+ concentrations (K+ ≥ 10 mm), OsHKT2;1 has been proposed to be in a nonconductive state. In contrast, based on data from Figures 4 and 5, OsHKT2;4 is proposed to switch between a K+ uniport mode at low Na+ concentrations (lower than a few mm), and a Na+-K+ symport mode, at high Na+ concentrations (>10 mm), and external K+ below a few mm. Note that a single arrow is present in the diagrammatic representation of the cotransport mode since Na+ and K+ in these transporters likely move through a unique central pore (Cao et al., 2011). B, Alignment of expected selectivity filter sequences (Cao et al., 2011) in the four pore domains (PA, PB, PC, PD) of different Na+-K+ cotransporters and K+ transporters from the HKT/Trk/Ktr superfamily (Corratgé-Faillie et al., 2010; Cao et al., 2011). The star above the last residue of the putative selectivity filter in PA domain indicates the position of a reported determinant of K+ permeability: A Gly is present at this position in transporters highly permeable to K+, while a Ser is present in transporters weakly or not permeable to this cation (Mäser et al., 2002). Accession numbers of the transporter sequences: BsKtrB NP_390988; HcTrk1 CAL36606.1; OsHKT2;1 Q0D9S3.1; OsHKT2;2 BAB61791.1; OsHKT2;4 Q8L4K5.1; ScTrk1 NP_012406.1; VaKtrB ZP_01261970.1. Bs, Bacillus subtilis; Hc, Hebeloma cylindrosporum; Sc, yeast; Va, Vibrio alginolyticus; Vp, V. parahaemolyticus.
In this study, OsHKT2;4 is shown to display functional similarity to other subfamily 2 members, being able to transport both Na+ and K+ (Figs. 4 and 5). Like HKT2;1-type transporters, OsHKT2;4 can also be proposed to be endowed with different conduction modes depending on the external Na+ concentration (Figs. 4, 5, and 6A). Our experiments, however, suggest that strong differences exist between the transport modes displayed by OsHKT2;4 and HKT2;1 subgroup transporters in the same ionic conditions. Indeed, in the presence of low (submillimolar) concentrations of Na+ and K+, whereas HKT2;1 subgroup transporters behave as Na+-K+ symporters, OsHKT2;4 does not transport Na+ and essentially transports K+ (Figs. 4, 5, and 6A). Furthermore, whereas HKT2;1 subgroup transporters have been described as displaying no, or a weakly conductive, K+ uniport mode of transport, this mode is conductive in OsHKT2;4. Finally, at high Na+ concentrations (>10 mm), when Na+ is in excess over K+, whereas HKT2;1 subgroup transporters are in the Na+ uniport mode, OsHKT2;4 would then transport both Na+ and K+, possibly by mediating Na+-K+ symport (Figs. 4, 5, and 6A).
OsHKT2;4 therefore appears as a new functional type within the HKT family, endowed with high K+ permeability and a particularly low Na+ permeability compared with that in the other HKT transporters so far characterized in both subfamily 1 and subfamily 2 (Rodríguez-Navarro and Rubio, 2006; Corratgé-Faillie et al., 2010; Hauser and Horie, 2010). It should be noted that although OsHKT2;4 is the first member from the plant HKT family reported as displaying such high K+ versus Na+ permeability, some fungal and bacterial HKT homologs from the Trk family have been described as K+-selective transporters (Rodríguez-Navarro, 2000; Corratgé-Faillie et al., 2010). Based on the structural data obtained for the prokaryotic VpTrkH K+ transporter, expected sequences of selectivity filter were aligned in OsHKT2;4 and in different K+-permeable transporters from the HKT/Trk/Ktr superfamily (Fig. 6B). The sequence alignments (Fig. 6B) do not provide obvious clues about the particularly high K+ versus Na+ permeability of OsHKT2;4 within the Na+-K+ cotransporter subfamily.
Possible Roles of OsHKT2;4 in Planta
HKT transporters are mostly known for their roles in Na+ transport and plant tolerance to salinity constraint (Munns and Tester, 2008; Horie et al., 2009; Hauser and Horie, 2010). A role for an HKT transporter in K+ transport in planta, conversely to that in Na+ transport, has not been evidenced so far. Indeed, Na+-coupled K+ transport has not been reported in terrestrial higher plants up to now (Maathuis et al., 1996; Walker et al., 1996; Haro et al., 2005). Furthermore, few indications have been obtained that HKT2;1 subgroup transporters, shown to be able to work as Na+-K+ symport when heterologously expressed in yeast and Xenopus oocytes, can transport K+ in planta. Indeed, varying the transcript levels of TaHKT2;1 and OsHKT2;1 genes in planta through genetic engineering did not allow to identify a role in root K+ uptake for these transporters, which are highly expressed in root cortex (Laurie et al., 2002; Horie et al., 2007). These results have led to hypothesize that HKT2;1 subgroup transporters only work as Na+ uniporters in planta (Haro et al., 2005; Horie et al., 2007), and that the Na+-K+ symport mode evidenced in yeast or Xenopus oocytes is artifactual, resulting from the heterologous context, or anecdotal in planta. It should however be noted that the rice OsHKT2;2 transporter has recently been shown to have the capacity to mediate K+ transport in a plant context, when overexpressed in cultured tobacco (Nicotiana tabacum) cells (Yao et al., 2010).
Since OsHKT2;4 appears highly K+ permeable in all its conducting modes and can even mediate pure K+ transport at low external Na+ concentrations when expressed in Xenopus oocytes (Figs. 4 and 5), it can certainly be anticipated, in contrast to other previously characterized HKT transporters, to mediate K+ transport in planta. OsHKT2;4 has been reported to be active at the plasma membrane (Horie et al., 2011) and to display an expression pattern including root peripheral layers and shoot vasculature (Lan et al., 2010), suggesting that it could be involved in both nutritional K+ uptake and long-distance K+ transport.
MATERIALS AND METHODS
Expression in Xenopus laevis Oocytes
OsHKT2;4 complementary DNA was subcloned into the pGEMDG vector (D. Becker, University of Würzburg, Germany) downstream from the T7 promoter and between the 5′ and 3′ untranslated regions of the Xenopus β-globin gene. Capped and polyadenylated cRNA was synthesized in vitro from linearized vector using the mMESSAGE mMACHINE T7 kit (Ambion). Stage V or VI X. laevis oocytes, isolated as previously described (Véry et al., 1995), were injected with either 50 ng of OsHKT2;4 cRNA in 50 nL of diethylpyrocarbonate-treated water or 50 nL of diethylpyrocarbonate-treated water (for control oocytes), and then kept at 19°C in ND96 medium (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 2.5 mm sodium pyruvate, and 5 mm HEPES-NaOH, pH 7.4) supplemented with 0.5 mg L−1 gentamycin. For OsHKT2;1, OsHKT1;3, and HvHKT2;1 cloning and expression, see Jabnoune et al. (2009) and Mian et al. (2011).
Two-Electrode Voltage Clamp
Whole oocyte currents were recorded using the two-electrode voltage-clamp technique (correction being made for voltage drop through the series resistance of the bath and the reference electrode) 1 to 2 d after cRNA injection as described by Mian et al. (2011). The external solution bathing the oocyte was continuously percolated during the voltage-clamp experiment. The bath solutions contained, unless otherwise mentioned, a background of 6 mm MgCl2, 1.8 mm CaCl2, and 10 mm MES-1,3-bis[tris(hydroxymethyl)methylamino]propane, pH 5.5. Monovalent cations were added to the background solution as Glu or chloride salts. The chloride concentration was constant in each set of solutions. d-Mannitol was added when necessary to adjust the osmolarity (same osmolarity in each set of solutions in the range 220–240 mosM). In some experiments, the concentrations of CaCl2 ([CaCl2]) and of MgCl2 ([MgCl2]) were varied in the background solution, the total concentration [CaCl2] + [MgCl2] being kept constant, at 10 mm. Some background solutions contained neither MgCl2 nor CaCl2. In this case, Ca2+ was present as gluconate salt. In experiments where external pH was changed to 7.5, MES was replaced by HEPES in the background solution. The actual concentrations of K+ and Na+ in the bath solutions were systematically measured by flame photometry. To extract OsHKT2;4-mediated currents from total oocyte currents in oocytes expressing OsHKT2;4, mean currents recorded in water-injected control oocytes from the same batch in the same ionic conditions were subtracted from those recorded in the former oocytes.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AJ491855 (OsHKT2;4 mRNA).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Insensitivity of OsHKT2;4 currents to changes in external Ca2+ and/or Mg2+ concentrations: examples of current-voltage relationships derived from current recordings in a single OsHKT2;4-expressing oocyte.
Supplemental Figure S2. Cation selectivity of OsHKT2;4 compared with that of HKT transporters from subfamily 1 or 2.
Supplementary Material
Acknowledgments
We are grateful to A. Rodríguez-Navarro (Madrid, Spain) for providing us with OsHKT2;4 complementary DNA.
Glossary
- MPM
transMembrane segment, Pore loop, transMembrane segment
- cRNA
complementary RNA
References
- Albright RA, Joh K, Morais-Cabral JH. (2007) Probing the structure of the dimeric KtrB membrane protein. J Biol Chem 282: 35046–35055 [DOI] [PubMed] [Google Scholar]
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. (2007) Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19: 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish ME. (1983) A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol 342: 309–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthomieu P, Conéjéro G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al. (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22: 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R. (2007) HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 143: 1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Jin X, Huang H, Derebe MG, Levin EJ, Kabaleeswaran V, Pan Y, Punta M, Love J, Weng J, et al. (2011) Crystal structure of a potassium ion transporter, TrkH. Nature 471: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Kim SA, Murata Y, Lee S, Jae S-K, Nam HG, Kwak JM. (2009) De-regulated expression of the plant glutamate receptor homolog AtGLR3.1 impairs long-term Ca2+-programmed stomatal closure. Plant J 58: 437–449 [DOI] [PubMed] [Google Scholar]
- Corratgé C, Zimmermann S, Lambilliotte R, Plassard C, Marmeisse R, Thibaud J-B, Lacombe B, Sentenac H. (2007) Molecular and functional characterization of a Na+-K+ transporter from the Trk family in the ectomycorrhizal fungus Hebeloma cylindrosporum. J Biol Chem 282: 26057–26066 [DOI] [PubMed] [Google Scholar]
- Corratgé-Faillie C, Jabnoune M, Zimmermann S, Véry A-A, Fizames C, Sentenac H. (2010) Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell Mol Life Sci 67: 2511–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. (1987) The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem 22: 317–387 [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77 [DOI] [PubMed] [Google Scholar]
- Durell SR, Guy HR. (1999) Structural models of the KtrB, TrkH, and Trk1,2 symporters based on the structure of the KcsA K+ channel. Biophys J 77: 789–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell SR, Hao Y, Nakamura T, Bakker EP, Guy HR. (1999) Evolutionary relationship between K+ channels and symporters. Biophys J 77: 775–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn DJ, Liu W, Schachtman DP, Gomez-Gallego S, Day SR, Teasdale RD. (2000) Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Mol Biol 43: 515–525 [DOI] [PubMed] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A. (2003) Sodium transport and HKT transporters: the rice model. Plant J 34: 788–801 [DOI] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI. (1996) Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J 10: 852–869 [DOI] [PubMed] [Google Scholar]
- Haro R, Bañuelos MA, Senn ME, Barrero-Gil J, Rodríguez-Navarro A. (2005) HKT1 mediates sodium uniport in roots: pitfalls in the expression of HKT1 in yeast. Plant Physiol 139: 1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. (2005) Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758 [DOI] [PubMed] [Google Scholar]
- Hauser F, Horie T. (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33: 552–565 [DOI] [PubMed] [Google Scholar]
- Hille B. (1992) Ionic Channels of Excitable Membranes, Ed 2. Sinauer Associates, Sunderland, MA, pp 341. [Google Scholar]
- Horie T, Brodsky DE, Costa A, Kaneko T, Lo Schiavo F, Katsuhara M, Schroeder JI. (2011) K+ transport by the OsHKT2;4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions. Plant Physiol 156: 1493–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI. (2007) Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 26: 3003–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Hauser F, Schroeder JI. (2009) HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 14: 660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A. (2001) Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J 27: 129–138 [DOI] [PubMed] [Google Scholar]
- Huang S, Spielmeyer W, Lagudah ES, James RA, Platten JD, Dennis ES, Munns R. (2006) A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol 142: 1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Spielmeyer W, Lagudah ES, Munns R. (2008) Comparative mapping of HKT genes in wheat, barley, and rice, key determinants of Na+ transport, and salt tolerance. J Exp Bot 59: 927–937 [DOI] [PubMed] [Google Scholar]
- Jabnoune M, Espéout S, Mieulet D, Fizames C, Verdeil J-L, Conéjéro G, Rodríguez-Navarro A, Sentenac H, Guiderdoni E, Abdelly C, et al. (2009) Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol 150: 1955–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Sakaguchi M, Mori Y, Saito K, Nakamura T, Bakker EP, Sato Y, Goshima S, Uozumi N. (2001) Evidence in support of a four transmembrane-pore-transmembrane topology model for the Arabidopsis thaliana Na+/K+ translocating AtHKT1 protein, a member of the superfamily of K+ transporters. Proc Natl Acad Sci USA 98: 6488–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruma A, Hartzell HC. (1999) Dynamics of calcium regulation of chloride currents in Xenopus oocytes. Am J Physiol 276: C161–C175 [DOI] [PubMed] [Google Scholar]
- Lan W-Z, Wang W, Wang S-M, Li L-G, Buchanan BB, Lin HX, Gao J-P, Luan S. (2010) A rice high-affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proc Natl Acad Sci USA 107: 7089–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Brown AT, Cicuta P, Davies JM. (2010) Annexins: components of the calcium and reactive oxygen signaling network. Plant Physiol 152: 1824–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry A-A, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA. (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32: 139–149 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Verlin D, Smith FA, Sanders D, Fernández JA, Walker NA. (1996) The physiological relevance of Na+-coupled K+-transport. Plant Physiol 112: 1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Hosoo Y, Goshima S, Horie T, Eckelman B, Yamada K, Yoshida K, Bakker EP, Shinmyo A, Oiki S, et al. (2002) Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc Natl Acad Sci USA 99: 6428–6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian A, Oomen RJFJ, Isayenkov S, Sentenac H, Maathuis FJM, Véry A-A. (2011) Over-expression of an Na+-and K+-permeable HKT transporter in barley improves salt tolerance. Plant J 68: 468–479 [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Platten JD, Cotsaftis O, Berthomieu P, Bohnert H, Davenport RJ, Fairbairn DJ, Horie T, Leigh RA, Lin HX, Luan S, et al. (2006) Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci 11: 372–374 [DOI] [PubMed] [Google Scholar]
- Ren Z-H, Gao J-P, Li L-G, Cai X-L, Huang W, Chao D-Y, Zhu M-Z, Wang Z-Y, Luan S, Lin H-X. (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37: 1141–1146 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A. (2000) Potassium transport in fungi and plants. Biochim Biophys Acta 1469: 1–30 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A, Rubio F. (2006) High-affinity potassium and sodium transport systems in plants. J Exp Bot 57: 1149–1160 [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270: 1660–1663 [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370: 655–658 [DOI] [PubMed] [Google Scholar]
- Shimbo K, Brassard DL, Lamb RA, Pinto LH. (1995) Viral and cellular small integral membrane proteins can modify ion channels endogenous to Xenopus oocytes. Biophys J 69: 1819–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunarpi, Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, et al. (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J 44: 928–938 [DOI] [PubMed] [Google Scholar]
- Terhag J, Cavara NA, Hollmann M. (2010) Cave Canalem: how endogenous ion channels may interfere with heterologous expression in Xenopus oocytes. Methods 51: 66–74 [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Maylie J, Adelman JP. (1995) Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys J 69: 904–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI. (2000) The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol 122: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A-A, Gaymard F, Bosseux C, Sentenac H, Thibaud J-B. (1995) Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J 7: 321–332 [DOI] [PubMed] [Google Scholar]
- Walker NA, Sanders D, Maathuis FJM. (1996) High-affinity potassium uptake in plants. Science 273: 977–979 [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM. (2002) Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta 1564: 299–309 [DOI] [PubMed] [Google Scholar]
- Yao X, Horie T, Xue S, Leung H-Y, Katsuhara M, Brodsky DE, Wu Y, Schroeder JI. (2010) Differential sodium and potassium transport selectivities of the rice OsHKT2;1 and OsHKT2;2 transporters in plant cells. Plant Physiol 152: 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.