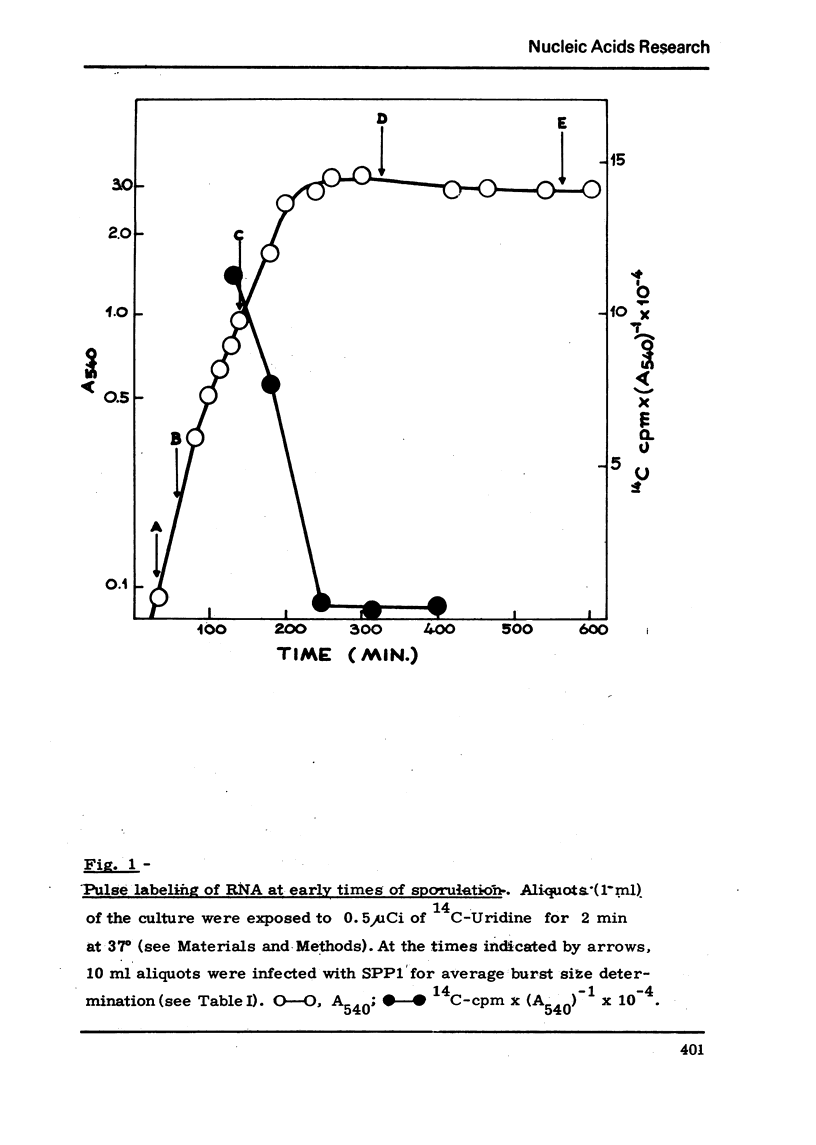
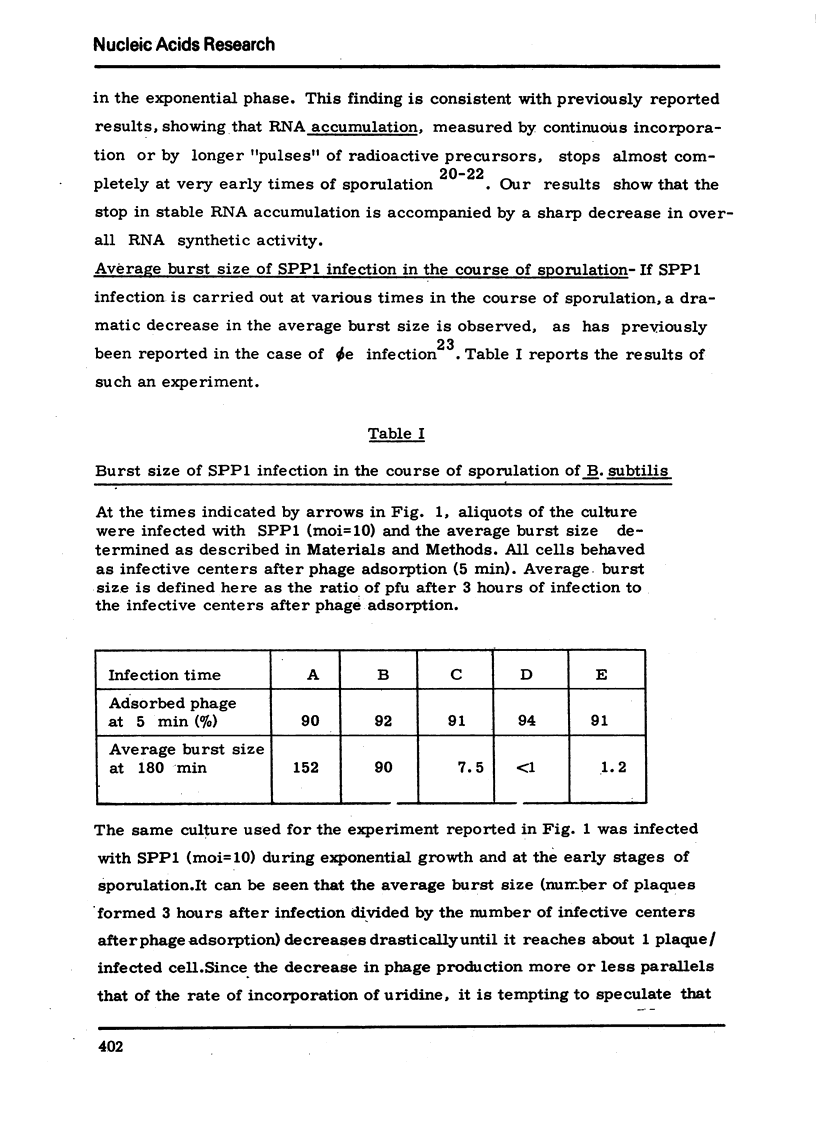
Abstract
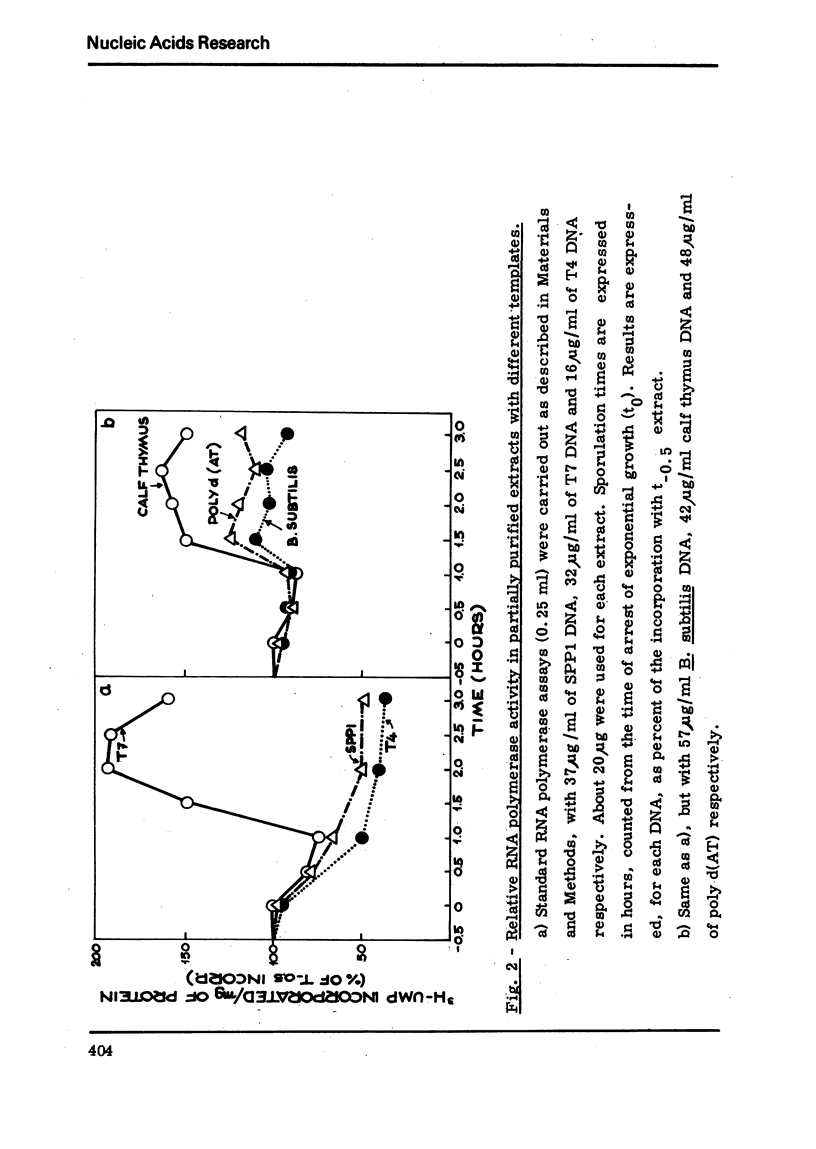
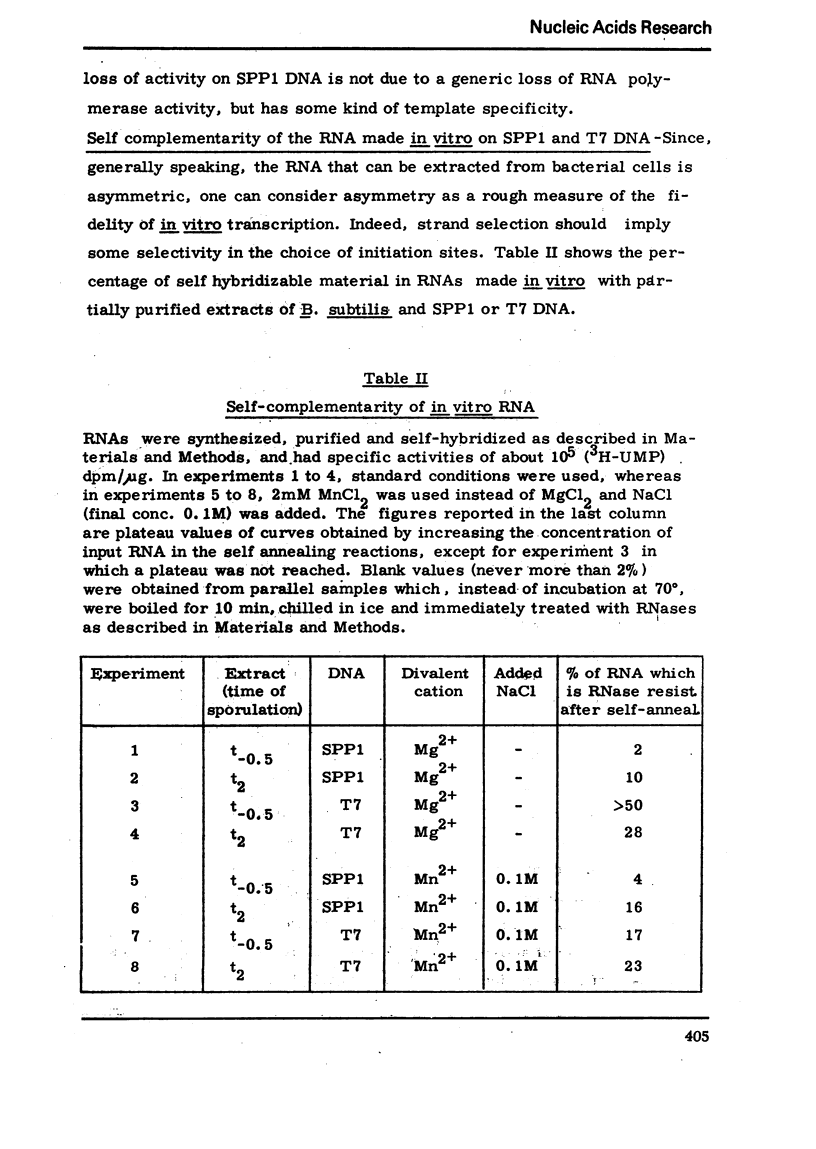
Partially purified extracts from sporulating Bacillussubtilis cultures transcribed different natural DNAs with different efficiencies. This template specificity results in an increased or a decreased synthetic activity with respect to extracts from vegetative cells, depending on the template used. With SPP1 DNA a decrease in activity occurs, whereas with T7 DNA an increased activity was observed, which is due to a higher efficiency of initiation. This is not an intrinsic property of RNA polymerase, but is due to some fraction(s) which can be separated from the enzyme. Together with invivo experiments on transcription and SPP1 phage production during sporulation, these results suggest a possible role of promoter recognition in sporulation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dausse J. P., Sentenac A., Fromageot P. Interaction of RNA polymerase from Escherichia coli with DNA. Influence of DNA scissions on RNA-polymerase binding and chain initiation. Eur J Biochem. 1972 Dec 4;31(2):394–404. doi: 10.1111/j.1432-1033.1972.tb02546.x. [DOI] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf A. L., Linn T. G., Losick R. Isolation of a new RNA polymerase-binding protein from sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):490–494. doi: 10.1073/pnas.70.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey C., Pero J., Shorenstein R. G., Losick R. In vitro synthesis of ribosomal RNA by Bacillus subtilis RNA polymerase. Proc Natl Acad Sci U S A. 1972 Feb;69(2):407–411. doi: 10.1073/pnas.69.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Maia J. C.C., Kerjan P., Szulmajster J. DNA-dependent RNA polymerase from vegetative cells and from spores of Bacillus subtilis. IV. Subunit composition. FEBS Lett. 1971 Mar 22;13(5):269–274. doi: 10.1016/0014-5793(71)80238-7. [DOI] [PubMed] [Google Scholar]
- Matsukage A. The effects of KC1 concentration on the transcription by E. coli RNA polymerase. II. Effect on the specificity of T7 transcription. Mol Gen Genet. 1972;118(1):23–31. doi: 10.1007/BF02428329. [DOI] [PubMed] [Google Scholar]
- Milanesi G., Cassani G. Transcription after bacteriophage SPP1 infection in Bacillus subtilis. J Virol. 1972 Aug;10(2):187–192. doi: 10.1128/jvi.10.2.187-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


