Abstract
Objectives
The objectives of this study were to describe sleep quality and evaluate the association of sleep quality with delirium onset amongst patients enrolled in hospice.
Design
The study utilized secondary data from a prospective, observational, longitudinal study.
Setting
Veterans enrolled in hospice were recruited from the Portland Veterans Affairs Medical Center, Portland, Oregon.
Participants
The cohort consisted of 105 patients, of whom 73% had at least one sleep measurement.
Measurements
Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI). Delirium was measured with the Confusion Assessment Method. Other important variables were recorded from the medical record and/or longitudinal interviews with patients and their caregivers. Cox regression was used to estimate hazard ratios (HR) to measure the association between sleep quality and delirium onset.
Results
Of the patients who could be assessed, 44% had poor average sleep quality and 58% reported at least one episode of poor sleep. Overall, sleep quality did not appear to worsen as patients neared death although an increasing number of patients were unable to report on sleep quality. Poor sleep quality was associated with an increased risk of developing delirium, with a HR of 2.37 (95% CI 1.50 – 3.74), for every one point worsening in the sleep quality score on a four point scale.
Conclusions
Poor sleep quality was common amongst Veteran patients enrolled in hospice. These findings may help guide decision making between clinicians, patients, and families regarding the likely impact of sleep disturbance and may help identify patients at higher risk of developing delirium.
Introduction
Patients enrolled in hospice experience many neuropsychiatric symptoms, including problems with sleep (1). Sleep disturbance is common among patients with advanced cancer (2–5) and negatively impacts patients’ and family members’ quality of life (2, 4–8). Less is known about the burden of sleep disturbance among patients with other types of terminal illness (9, 10). In particular, the trajectory of sleep disturbance throughout hospice enrollment is not well described.
In addition to the direct decrement in quality of life from poor sleep, sleep disturbances may predispose patients to delirium (11, 12). Delirium occurs in up to 90% of cancer patients at the end of life (11), and is disturbing for patients, families, and caregivers (13–16). Interventions to improve sleep quality may reduce the incidence of delirium in hospitalized patients (17). Hippocrates noted that sleep disturbance and delirium were linked, noting, “When a delirium or raving is appeased by sleep, it is a good sign” (18). However, the causal nature of the relationship between sleep quality and delirium onset has not been established and the mechanisms linking sleep disturbance to delirium are not well understood (19, 20). Knowledge of the relationship between these two syndromes may lead to earlier identification of delirium and interventions to reduce its impact.
We conducted a secondary analysis of prospectively collected data from a longitudinal study of neuropsychiatric syndromes among hospice patients which utilized validated instruments to measure sleep quality and delirium. The goals of this report were to characterize sleep quality and disturbances in hospice patients, describe characteristics of patients who reported good versus poor sleep quality, describe the trajectory and prevalence of sleep disturbance as patients neared death and particularly, evaluate the association of sleep quality with delirium. Given the expertise of geriatric psychiatrists in these domains, focused attention on sleep quality amongst hospice patients may lead to substantial improvements in quality of care.
Methods
Setting & Subjects
The methods used in this study have been described (1). Eligible participants were Veterans referred for hospice care who survived long enough to be contacted (within two weeks of study referral), lived within a 50-mile radius of the Portland Veterans Affairs Medical Center (PVAMC), Portland, Oregon, and had speech and hearing adequate to complete a structured clinical interview.
All participants with a Mini Mental State Examination (MMSE) score greater than 23 gave written informed consent; those with scores of 23 or less gave consent by proxy through their primary caregivers, usually a family member, and additionally gave their assent to participate. The institutional review board of the PVAMC approved the protocol and recruitment methods.
Subjects were seen on average 6 days after referral to hospice, for three weekly visits and then every other week until either death, withdrawal from the study, or discharge from hospice because of improved medical or functional status. A psychologist or psychology doctoral candidate trained by a psychologist repeatedly visited each subject in his/her primary residence. The interviewers attempted to complete multiple measures with the participant and his/her primary caregiver at each visit. The individual measures that we report in this analysis, and their timing, are described below. Of note, subjects’ illness severity occasionally prevented complete ascertainment of all measures at every visit.
Sleep Measurement
Sleep quality was assessed with the widely used and validated Pittsburgh Sleep Quality Index (PSQI) (21). The primary measure for this study was sleep quality over the previous month, measured on a four-point integer scale (1–4) from “very good” to “very bad” (indicating worse sleep quality as the score increases). This specific question has been shown to be highly correlated with the global PSQI scores (r=0.79–0.80) (22). The complete PSQI has items regarding typical sleep latency and sleep duration as well as items regarding the frequency of specific symptoms interfering with sleep and how often medications were taken for sleep in the prior month (the list of items are included in Figure 1). The study was designed to record the sleep quality item of the PSQI at every visit and the complete PSQI at every other visit (usually monthly). The interviewers preferentially completed the PSQI with the individual subject and would request the caregiver’s rating on the sleep quality item only if the subject could not complete the instrument. If neither could adequately complete the sleep quality item, it was recorded as missing. If a participant was delirious or had an MMSE score below 18, the complete PSQI was not performed on that particular visit only; if the delirium had resolved or the MMSE was ≥ 18 on a subsequent visit, the complete PSQI was then completed.
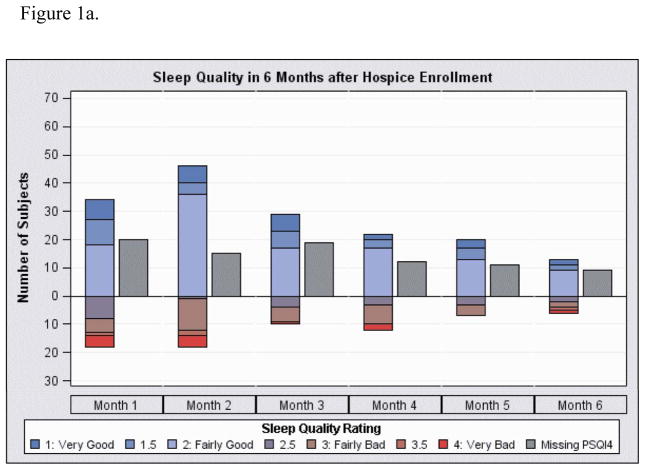
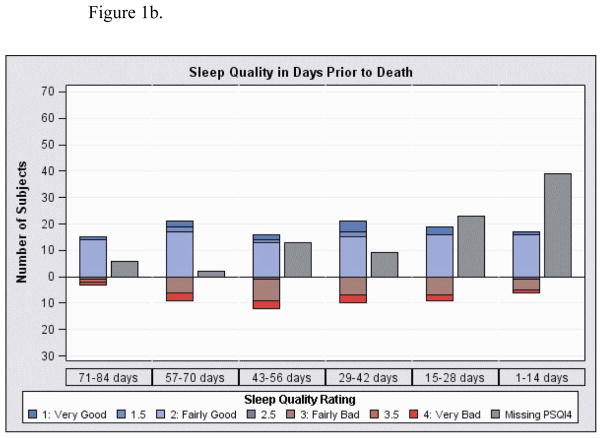
Figure 1.
Sleep quality represents the average response for PSQI4 (overall sleep quality) for all measurements in time period. Missing indicates patients with at least 1 visit in the time interval but with no sleep quality data in the interval. Of note, the number of subjects in each interval differs because each had different time periods in the study until death.
Figure 1a. Sleep quality in 4 week intervals from first visit through 6 months
Figure 1b. Sleep quality in 2 week intervals prior to death
Delirium Measurement
To measure delirium, interviewers completed the observer-rated Confusion Assessment Method (CAM) (23) at every visit. The CAM is an extensively used and validated instrument which documents presence or absence of hallmark symptoms of delirium and results in a positive score for delirium if both acute onset and inattention are observed and either disorganized thinking or altered level of consciousness is present.
Other Measures
We obtained baseline information on all subjects, regardless of delirium, through interview and electronic medical record review, including the participant’s age, sex, educational attainment, race/ethnicity, marital status, medical reason for hospice referral, past mental illness, and physician-diagnosed dementia. Designation of the subject’s primary caregiver was made at the first study visit (spouse/partner, family, friend, staff, guardian, other). Mental state was evaluated using the MMSE at each visit. Prorated MMSE scores were calculated when the participant was physically unable to complete one or more items. Functional status, based on the interviewer’s observations and participant/caregiver report, was recorded at baseline using the 5-point scaled Eastern Cooperative Oncology Group (ECOG) performance status (fully active to completely disabled) (24). At each visit, subjects were asked to rate suffering (where “I am suffering greatly” equals 0 and “I am not suffering” equals 100), and quality of life (QOL) (where “My quality of life is terrible” equals 0 and “My quality of life is as good as it can be” equals 100).
Additional measures were completed at baseline in participants whose CAM was negative and MMSE > 18. A Structured Clinical Interview for DSM-IV (SCID) was conducted at the first visit to document a history of depression and alcohol abuse/dependence. The severity of depression and anxiety was also assessed with the 14-item Hospital Anxiety and Depression Scale (HADS) (25).
Analysis
Demographic variables are reported as frequencies with proportions or means with standard deviations. Good sleep quality was defined as a score of “very good” or “fairly good” on the overall sleep quality component. We evaluated both pre-delirium sleep measures and sleep measures recorded during the entire study, noting in the results which aspect is reported. For the descriptions of sleep quality over time, the average sleep quality score (on a 1 to 4 point scale) was recorded for each time interval. We also evaluated the most often reported score (mode) and the worst score for each participant during the entire study for each item of the PSQI. Given the descriptive nature of these portions of the analysis, comparative statistical tests were not performed. In order to characterize sleep quality, it was characterized as the mean overall sleep quality rating for the entire study (patients without delirium) or until the first delirium episode. Sleep quality was not assessed in 30 subjects because the patient was delirious, had impaired cognition, and/or was too ill to complete the question. We utilized the z approximation from the Wilcoxon signed rank test (with continuity correction), Fisher’s exact test, and chi-squared tests to evaluate differences.
To investigate the association of sleep quality with delirium onset, Cox regression models were used with sleep quality as a time-dependent covariate. For each observation time and subject, the 28-day time-dependent sleep quality covariate was calculated as the average of the sleep quality measures observed in the previous 28 days (Model 1). Sleep quality assessments were limited to measurements obtained prior to the first delirium episode in a patient. As there are no previous data on the time interval between sleep quality and delirium onset, we assessed the sensitivity of the time window for measuring sleep quality by conducting separate analyses using sleep quality measures obtained during the previous 14 days, as well as during all previous study days. In secondary analyses using the 28-day time window, we adjusted one at a time (due to the relatively small number of events) for additional covariates. These covariates included caregiver status (professional caregiver vs. other), history of mental illness (yes vs. no), and hospice diagnosis (dichotomized as cancer vs. other given the multiple other non-cancer hospice diagnoses), respectively. Because sleep quality measures were obtained at different times for different subjects, not all subjects had sleep quality measures available for each time window. Hence, the sample sizes differ in the three analyses. Standard graphical techniques were used to assess the proportional hazards assumption for categorical covariates (caregiver status, mental illness history, cancer diagnosis); we did not assume proportional hazards for sleep quality, using, instead, time-dependent sleep quality measures, as described above. All tests were two-tailed and a P value of less than 0.05 was considered statistically significant. Analyses were conducted using SAS statistical software version 9.2 (SAS Institute, Inc, Cary NC).
Results
We enrolled 105 patients in the study who were followed in hospice for varying lengths of time, from 1 to 1203 days, with a median of 70 days from the first study visit to death. Consistent with a Veteran population with advanced illness from the Pacific Northwest, patients were mainly older, white men with 55% enrolled in hospice because of cancer. Sleep quality was assessed at 1 to 48 visits per patient and delirium assessments were made at 1 to 54 visits per patient. The mean number of sleep assessments per patient was 7.4 (SD 8.6) and the median was 4 for those who completed at least one assessment. For the 103 patients with a known death date while in hospice, the median time from the last visit to death was 11 days and the mean was 66 days (SD 152). Delirium assessments were made at 97% of the visits and sleep quality assessments were made at 71% of the visits. Seventy-seven subjects (73%) had at least one sleep quality measure recorded and of the 566 total sleep quality assessments, 20% indicated sleep quality was “fairly bad” or “very bad”.
Characteristics of the cohort, categorized by average pre-delirium sleep quality (good and poor) during the study, are shown in Table 1. In general, subjects with a spouse/partner caregiver had better sleep quality and those without depression tended to have better sleep quality (p=0.042 and 0.062 for caregiver and depression, respectively). Patients with worse baseline QOL scores, but not suffering scores, also reported worse average sleep quality (p=0.049 and 0.730 for QOL and suffering scores, respectively). Patients with dementia more often had missing sleep quality data (50% of patients with dementia were missing sleep quality ratings compared to 9% in patients without dementia, Fisher’s Exact table probability <0.0001).
Table 1.
Characteristics of cohort categorized by average pre-delirium sleep quality.
| Pre-Delirium Sleep Quality2 | ||||
|---|---|---|---|---|
|
| ||||
| Baseline Measures Mean ± St. Dev or N (Column %)1 | Good Sleep Quality (Average PSQI4 <=2) | Poor Sleep Quality (Average PSQI4 > 2) | ||
|
| ||||
| 42 | 33 | Test Value3 | p | |
|
| ||||
| Age at baseline (years) | 66.5 ± 12.9 | 70.5 ± 10.9 | z=1.52 | 0.128 |
|
| ||||
| Men | 39 (93%) | 32 (97%) | Fisher’s Exact | 0.626 |
|
| ||||
| Education (years) | 12.4 ± 3.4 (N missing=3) | 12.8 ± 2.3 (N missing=2) | z=0.48 | 0.631 |
|
| ||||
| Race/ethnicity | ||||
| White non-Hispanic | 39 (93%) | 30 (91%) | Fisher’s Exact | 0.804 |
|
| ||||
| Marital Status | ||||
| Married | 20 (48%) | 13 (39%) | Fisher’s Exact | 0.303 |
|
| ||||
| Hospice Diagnosis | ||||
| Cancer | 29 (69%) | 26 (79%) | χ2 0.90, 1 d.f. | 0.344 |
|
| ||||
| Past Mental Illness | 25 (60%) | 18 (55%) | χ2 0.19, 1 d.f. | 0.665 |
|
| ||||
| Dementia | 3 (7%) | 4 (12%) | Fisher’s Exact | 0.692 |
|
| ||||
| Caregiver | ||||
| Spouse/partner | 23 (55%) | 13 (39%) | ||
| Family or friend | 9 (21%) | 16 (48%) | ||
| Staff/guardian/other | 10 (24%) | 4 (12%) | χ2 6.32, 2 d.f. | 0.042 |
|
| ||||
| ECOG Performance Status | 2.4 ± 1.0 (N missing=1) | 2.5 ± 0.8 (N missing=0) | z=0.60 | 0.550 |
|
| ||||
| Depression | 4 (10%) | 9 (27%) (N missing=1) | Fisher’s Exact | 0.062 |
|
| ||||
| Alcohol Abuse | 2 (5%) (N missing=1) | 4 (12%) (N missing=1) | Fisher’s Exact | 0.394 |
|
| ||||
| MMSE Score | 25.6 ± 4.6 (N missing=2) | 26.1 ± 4.4 (N missing=2) | z=0.26 | 0.798 |
|
| ||||
| Anxiety | 5.8 ± 4.5 (N missing=11) | 6.1 ± 2.4 (N missing=14) | z=0.98 | 0.330 |
|
| ||||
| Suffering Score | 60 ± 35 (N missing=1) | 59 ± 29 (N missing=1) | z=−0.34 | 0.730 |
|
| ||||
| Quality of Life Score | 78 ± 25 (N missing=1) | 63 ± 33 (N missing=1) | z=−1.97 | 0.049 |
|
| ||||
| Time from enrollment to death (days) | 196 ± 207 (N missing=1) | 238 ± 270 (N missing=0) | z=−0.36 | 0.728 |
Percents listed are % of all, including missing as a category.
Sleep Quality is the mean overall sleep quality rating for the entire study (patients with no delirium) or until the first delirium episode. Number of sleep quality assessments per patient ranged from 1–39, (median = 4) for those who completed at least one assessment.
zapproximation from Wilcoxon signed rank test (with continuity correction)
- MMSE: 0–30; lower scores indicate poorer mental status
- ECOG Performance Status: 0–4; lower scores indicate better performance status
- Anxiety: 0–21: lower scores indicate less anxiety (measured with the HADS)
- Quality of life score: 0–100; lower scores indicate worse quality of life
- Suffering score: 0–100; lower scores indicate more suffering
Table 2 show the mode and worst scores reported for each subject during the study regarding each aspect of the PSQI (note that these are all sleep measures, not limited to pre-delirium measures). During the study, 45 patients (58% of those with sleep assessments) had at least one visit where they reported fairly bad or very bad quality of sleep. Patients frequently reported sleep disturbances from waking up in the middle of the night and needing to use the bathroom. Forty-eight (69%) patients reported at least once during the study experiencing pain that interfered with sleep more than once per week but only 26 (37%) reported this severity as the mode. For the 71 patients who reported on sleep latency, the average time to fall asleep was 32 minutes (SD 37) and median was 20 minutes. For the 71 patients who reported on the amount of sleep per night, the average was 6.9 hours (SD 2.1) with a median of 7.0 hours.
Table 2.
Overall sleep quality, symptoms that interfered with sleep, and medications taken for sleep in the previous month.
| Overall sleep quality | ||||
| Total Respondents=77 | Very good | Fairly good | Fairly bad | Very bad |
| Mode (N & %) | 13 (17%) | 48 (62%) | 10 (13%) | 6 (8%) |
| Worst Score (N & %) | 3 (4%) | 29 (38%) | 27 (35%) | 18 (23%) |
| PSQI3A: Cannot get to sleep within 30 minutes | ||||
| Total Respondents =70 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 38 (54%) | 9 (14%) | 4 (6%) | 19 (26%) |
| Worst Score (N & %) | 18 (26%) | 13 (19%) | 8 (11%) | 31 (44%) |
| PSQI3B:Wake up in the middle of the night | ||||
| Total Respondents =69 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 5 (7%) | 0 | 8 (12%) | 56 (82%) |
| Worst Score (N & %) | 2 (3%) | 0 | 6 (9%) | 61 (88%) |
| PSQI3C: Have to get up to use the bathroom | ||||
| Total Respondents =70 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 7 (10%) | 1 (1%) | 6 (9%) | 56 (80%) |
| Worst Score (N & %) | 5 (7%) | 2 (3%) | 5 (7%) | 58 (83%) |
| PSQI3D: Cannot breathe comfortably | ||||
| Total Respondents =70 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 46 (67%) | 3 (4%) | 11 (15%) | 10 (4%) |
| Worst Score (N & %) | 31 (44%) | 7 (10%) | 12 (17%) | 20 (29%) |
| PSQI3E: Cough or snore loudly | ||||
| Total Respondents =70 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 60 (86%) | 0 | 4 (6%) | 6 (8%) |
| Worst Score (N & %) | 46 (66%) | 3 (4%) | 9 (13%) | 12 (17%) |
| PSQI3F: Feel too cold | ||||
| Total Respondents =70 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 61 (87%) | 5 (7%) | 1 (1%) | 3 (4%) |
| Worst Score (N & %) | 37 (53%) | 8 (11%) | 12 (17%) | 13 (19%) |
| PSQI3G: Feel too hot | ||||
| Total Respondents =69 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 47 (68%) | 6 (9%) | 12 (18%) | 4 (5%) |
| Worst Score (N & %) | 24 (35%) | 11 (16%) | 17 (25%) | 17 (25%) |
| PSQI3H: Had bad dreams | ||||
| Total Respondents =69 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 55 (79%) | 6 (9%) | 4 (6%) | 4 (6%) |
| Worst Score (N & %) | 38 (55%) | 10 (14%) | 10 (14%) | 11 (16%) |
| PSQI3I: Have pain | ||||
| Total Respondents =70 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 38 (54%) | 6 (9%) | 11 (16%) | 15 (21%) |
| Worst Score (N & %) | 17 (24%) | 5 (7%) | 18 (26%) | 30 (43%) |
| PSQI5:Taken medication to help you sleep | ||||
| Total Respondents =70 | 0 in last month | < 1 time per week | 1–2 times per week | ≥ 3 times per week |
| Mode (N & %) | 29 (41%) | 1 (1%) | 0 | 40 (57%) |
| Worst Score (N & %) | 21 (30%) | 2 (3%) | 0 | 47 (67%) |
Sleep assessment measures are summaries of all measurements available for each subject.
Average sleep quality for each subject as recorded in each duration from hospice enrollment is shown in Figure 1a and prior to death in Figure 1b. These measures are not limited to pre-delirium sleep quality and represent the average of all sleep measurements for the subjects in the time intervals. The number of subjects with missing sleep quality data is shown as well and increased as they neared death. At the first visit, the average sleep quality score (on a four-point scale) was 2.1 (SD 0.9, N=66 measures) and was 2.0 (SD 0.7, N=39) and 2.2 (SD 0.8, N=19) at 3 and 6 months, respectively, for those still alive at those time points (higher scores indicate worse quality). At the last visit prior to death where sleep quality was recorded, the average sleep quality was 2.3 (SD 0.8, N=77). At 1 month and 3 months prior to death, average sleep quality was 2.3 (SD 0.8, N=28) and 2.2 (SD 0.7, N=28), respectively. Of the 35 subjects with sleep measured within 28 days prior to death, those with poor sleep quality tended to be older (average 72 (SD 12) compared to 64 (SD 13), Wilcoxon rank sum test z=1.81, p=0.07) and were more likely to have concomitant depression (62% vs. 23%, Fisher’s Exact p=0.03).
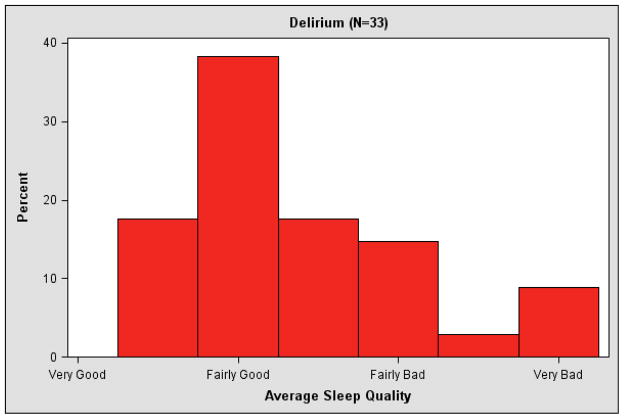
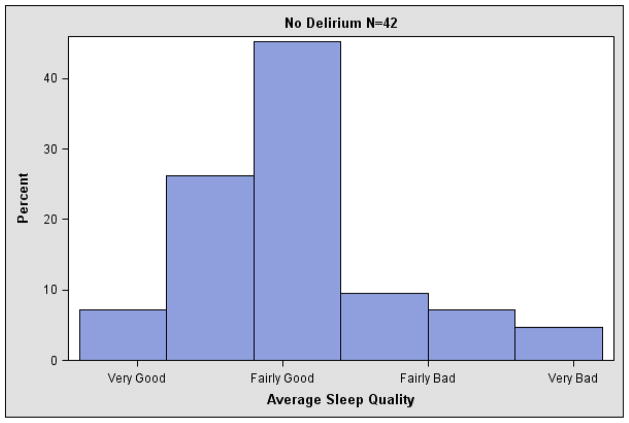
Of the 105 subjects, 54 experienced at least one episode of delirium during the study period and 71 had a sleep quality rating prior to a delirium measure. Average pre-delirium sleep quality was 2.4 (SD 0.7) for those who experienced delirium and 2.1 (SD 0.7) for those without delirium (Wilcoxon rank sum test z 1.97, p=0.049). Figure 2 displays the average pre-delirium sleep quality during the study for patients with and without delirium onset during the study. Pre-delirium sleep quality is the mean overall sleep quality rating for the entire study (patients without delirium) or until the first delirium episode. Poor sleep quality prior to delirium onset was associated with delirium onset, as shown in Table 3. The hazard ratio for developing delirium was 2.37 (95% CI 1.50–3.74) for each one point worsening of sleep quality in the previous 28 days. In sensitivity analyses, this association between worse sleep quality and delirium was similar for sleep quality assessed in the previous 14 days and from the first visit to delirium onset (data not shown). Similarly, this association was similar when individually adjusted for caregiver status (professional caregiver vs. other), history of mental illness (yes vs. no), and hospice diagnosis (cancer vs. other), respectively.
Figure 2.
Histogram of average sleep quality for patients with no delirium and average pre-delirium sleep quality for patients with delirium during hospice care.
Table 3.
Cox Regression Analysis of Onset of Delirium by Average Sleep Quality in Prior 28 Days
| Cox Regression Analysis of Onset of Delirium by Average Sleep Quality in Prior 28 Days
| |||||
|---|---|---|---|---|---|
| Predictor Variables | Hazard Ratio | 95% Profile Likelihood Confidence Limits for Hazard Ratio | Wald χ2 (1 df) | p | |
| Analysis 1 | |||||
| Sleep quality rating in last 28 days1, unadjusted | 2.37 | 1.50 | 3.74 | 13.64 | 0.0002 |
|
| |||||
| Analysis 2 | |||||
| Sleep quality rating in last 28 days | 2.45 | 1.54 | 3.89 | 14.59 | 0.0001 |
| Caregiver other than family or friend | 2.01 | 0.78 | 4.66 | 2.40 | 0.1210 |
|
| |||||
| Analysis 3 | |||||
| Sleep quality rating in last 28 days, | 2.29 | 1.45 | 3.59 | 3.59 | 0.0003 |
| Any Psychiatric Diagnosis | 1.90 | 0.84 | 4.83 | 4.83 | 0.1446 |
|
| |||||
| Analysis 4 | |||||
| Sleep quality rating in last 28 days, | 2.46 | 1.52 | 3.94 | 13.93 | 0.0002 |
| Cancer Diagnosis | 0.65 | 0.31 | 1.40 | 1.25 | 0.2640 |
Test: Cox regression models were used with sleep quality as a time-dependent covariate
Sleep quality rating is the average PSQI 4 (Overall Sleep rating) obtained in the 28 days prior to the event time. Sleep quality ratings after the onset of delirium are not included in the analysis.
N= 70 subjects, 29 subjects had delirium onset with data on sleep quality obtained in the 28 day interval prior to onset.
Analyses 2 – 4 are individually-adjusted analyses for the second variable listed and show the adjusted HR for sleep quality in the last 28 days and the HR for the second variable
Discussion
In this study of Veterans enrolled in hospice, we found that poor sleep quality was common and the majority reported poor sleep on at least one occasion. In general, older patients, those with depression, and those with a caregiver other than a spouse/partner reported poorer sleep quality. While the quality of life score we used is not validated, patients who had poor average pre-delirium sleep quality also had worse overall quality of life. Sleep quality could not be assessed in a third of patients because of illness, dementia, and/or delirium. Among patients able to report on sleep, average sleep quality remained fairly constant and did not appear to substantially worsen as time elapsed from hospice enrollment or as death neared. Patients who developed delirium averaged poorer sleep quality than those who did not in the time period preceding delirium onset, after adjusting for potential confounders.
Sleep quality has been reported as an important determinant of quality of life (2, 4–8). We found that poor sleep was common as 44% of patients had poor average sleep quality and 58% had reported at least one episode of poor sleep among those who could be assessed. While not limited to patients in hospice, it is widely recommended that patients with advanced illness be queried for sleep problems and evaluated for treatment (26–29). Identifying risks for poor sleep amongst hospice patients may prove beneficial, especially since non-medication treatments may improve sleep quality (30). For instance, subjects in our study with non-spouse caregivers had worse sleep quality, a result echoed in a study of patients with dementia admitted to a hospital in order to provide respite for their caregivers (31). While the choice of caregiver is often not modifiable, these results highlight the need for clinicians to be vigilant regarding sleep disturbances amongst groups of hospice patients that may be at higher risk.
Interestingly, at least among hospice patients who could be assessed, sleep quality appeared to be fairly static both during hospice enrollment and before death. We did not collect information on the dose or duration of medications that impact sleep. However, the mode and worst score regarding self-reported sleep medication use were similar, indicating that most patients likely remained on stable doses of these medications throughout the study. Thus, we cannot determine if worsening sleep quality is counterbalanced by increases in these medications though it seems unlikely this possibility is the sole explanation for our finding. Alternatively this finding may reflect that subjects who were becoming delirious could no longer validly complete the sleep scale.
Several medication classes that are widely used in the care of terminally ill patients, including those that significantly impact sleep, such as opioids and benzodiazepines, cause delirium (32). Our finding that poor sleep was associated with the development of delirium supports the practice of providing interventions that can ameliorate sleep disturbances without causing delirium. Melatonin may be useful in this setting since it can be useful to improve sleep quality (33) and a systematic review found that melatonin was helpful in decreasing agitated behaviors in patients with dementia (34). However, the use of melatonin to improve sleep quality is not well studied amongst elderly patients (35) and it is not known if melatonin decreases delirium onsets. Thus, while our study offers a potential mechanism for how melatonin’s effect on sleep quality might decrease the incidence of delirium, trials should be performed prior to widespread adoption amongst hospice patients.
The association between poor sleep quality and delirium is intriguing but more research is required before establishing this relationship as causal. For instance, it is not known if sleep disturbances lead to delirium or if they are simply early manifestations of the same syndrome (20). However, our finding that poor sleep quality precedes delirium onset has potentially important clinical ramifications. For example, noting changes in sleep quality may prompt earlier recognition and treatment of other causes of delirium, such as evaluating the patient for other causes of delirium such as dehydration, infection, pain, respiratory insufficiency, or adverse reactions to medications (11, 12), and more attention and intervention for improving sleep quality. Also, witnessing a loved one with delirium is very stressful for caregivers (15). Utilizing a change in sleep quality as an opportunity to counsel patients and caregivers on the potential development of delirium in the subsequent weeks may reduce caregiver stress.
There are several strengths of this study. First, it is longitudinal and utilizes a repeated measures design which enables the evaluation of sleep quality prior to the development of delirium. Second, we used validated and widely used measures of delirium and sleep quality. Third, as compared to many studies of hospice patients, the subjects were not limited to those with cancer.
Despite these strengths, the study also has several limitations. While the amount of missing data was comparable to other studies (36), it is very difficult to conduct studies among hospice patients. While we structured our reporting and analyses to account for missing data on sleep quality, this problem, likely biases our results in an unpredictable manner. Second, the number of subjects was relatively small so our ability to adjust for confounding is limited. Finally, the subjects were mainly older, male Veterans whose results may be less generalizable to other populations.
In conclusion, sleep quality is poor for a large number of hospice patients though it appears to be relatively stable during enrollment in hospice and even in the time before death. In addition, poor sleep quality was associated with delirium onset. These findings may help guide decision making between clinicians, patients, and families regarding sleep hygiene, sleep medications, and prognosis of sleep disturbance. Furthermore, these results may be useful in identifying patients at higher risk of developing delirium. Geriatric psychiatrists are well poised to assist hospice clinicians with detection and treatment of both sleep disturbances and delirium. Finally, our results provide evidence for Hippocrates’s observation linking sleep quality with delirium and may be useful for future research into the mechanisms that cause delirium.
Acknowledgments
The study was funded by a Merit Review Award from the Clinical Sciences Research and Development Program, Department of Veterans Affairs (Dr. Ganzini). Drs. Slatore and Goy are recipients of VA HSRD Career Development Awards. Drs. Slatore, Goy, O’Hearn, Boudreau, and Ganzini are supported by resources from the Portland VA Medical Center. Dr. Dawn Peters and Jean O’Malley are supported by resources from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 01 from the National Center for Research Resources (NCRR). The Department of Veterans Affairs did not have a role in the conduct of the study, in the collection, management, analysis, or interpretation of data, or in the preparation of the manuscript.
Footnotes
Note: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
Previous Presentations: The results reported in this paper have not previously been presented.
Conflicts of Interest: The authors have no disclosures to report.
References
- 1.Goy ER, Ganzini L. Prevalence and Natural History of Neuropsychiatric Syndromes in Veteran Hospice Patients. J Pain Symptom Manage. 2011;41:394–401. doi: 10.1016/j.jpainsymman.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Vena C, Parker K, Allen R, et al. Sleep-wake disturbances and quality of life in patients with advanced lung cancer. Oncol Nurs Forum. 2006;33:761–769. doi: 10.1188/06.ONF.761-769. [DOI] [PubMed] [Google Scholar]
- 3.Parker KP, Bliwise DL, Ribeiro M, et al. Sleep/Wake Patterns of Individuals With Advanced Cancer Measured by Ambulatory Polysomnography. Journal of Clinical Oncology. 2008;26:2464–2472. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 4.Gibbins J, McCoubrie R, Kendrick AH, et al. Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Manage. 2009;38:860–870. doi: 10.1016/j.jpainsymman.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Mystakidou K, Parpa E, Tsilika E, et al. Palliat Med. England: 2009. How is sleep quality affected by the psychological and symptom distress of advanced cancer patients? pp. 46–53. [DOI] [PubMed] [Google Scholar]
- 6.Mystakidou K, Parpa E, Tsilika E, et al. The relationship of subjective sleep quality, pain, and quality of life in advanced cancer patients. Sleep. 2007;30:737–742. doi: 10.1093/sleep/30.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mystakidou K, Parpa E, Tsilika E, et al. Depression, hopelessness, and sleep in cancer patients’ desire for death. Int J Psychiatry Med. 2007;37:201–211. doi: 10.2190/0509-7332-388N-566W. [DOI] [PubMed] [Google Scholar]
- 8.Mystakidou K, Parpa E, Tsilika E, et al. Sleep quality in advanced cancer patients. J Psychosom Res. 2007;62:527–533. doi: 10.1016/j.jpsychores.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson E, Gaston-Johansson F, Ohlen J, et al. Scand J Public Health. Sweden: 2008. Clinical problems at the end of life in a Swedish population, including the role of advancing age and physical and cognitive function; pp. 177–182. [DOI] [PubMed] [Google Scholar]
- 10.Gibson J, Grealish L. Relating palliative care principles to the promotion of undisturbed sleep in a hospice setting. Int J Palliat Nurs. 2001;7:140–145. doi: 10.12968/ijpn.2001.7.3.8912. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK. N Engl J Med. United States: 2006. Delirium in older persons; pp. 1157–1165. [DOI] [PubMed] [Google Scholar]
- 12.Young J, Inouye SK. Delirium in older people. BMJ. 2007;334:842–846. doi: 10.1136/bmj.39169.706574.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitbart W, Gibson C, Tremblay A. The Delirium Experience: Delirium Recall and Delirium-Related Distress in Hospitalized Patients With Cancer, Their Spouses/Caregivers, and Their Nurses. Psychosomatics. 2002;43:183–194. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 14.Buss MK, Vanderwerker LC, Inouye SK, et al. Associations between caregiver-perceived delirium in patients with cancer and generalized anxiety in their caregivers. J Palliat Med. 2007;10:1083–1092. doi: 10.1089/jpm.2006.0253. [DOI] [PubMed] [Google Scholar]
- 15.Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him”. JAMA. 2008;300:2898–2910. E2891. doi: 10.1001/jama.2008.885. [DOI] [PubMed] [Google Scholar]
- 16.Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115:2004–2012. doi: 10.1002/cncr.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inouye SK, Bogardus ST, Charpentier PA, et al. A Multicomponent Intervention to Prevent Delirium in Hospitalized Older Patients. New England Journal of Medicine. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 18.Lipowski ZJ. Delirium: Acute Confusional States. New York: Oxford University Press, Inc; [Google Scholar]
- 19.Hshieh TT, Fong TG, Marcantonio ER, et al. J Gerontol A Biol Sci Med Sci. United States: 2008. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence; pp. 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinhouse GL, Schwab RJ, Watson PL, et al. Crit Care. England: 2009. Bench-to-bedside review: delirium in ICU patients - importance of sleep deprivation; p. 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 23.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Berger AM, Parker KP, Young-McCaughan S, et al. Oncol Nurs Forum. United States: 2005. Sleep wake disturbances in people with cancer and their caregivers: state of the science; pp. E98–126. [DOI] [PubMed] [Google Scholar]
- 27.Berger AM. Oncol Nurs Forum. United States: 2009. Update on the state of the science: sleep-wake disturbances in adult patients with cancer; pp. E165–177. [DOI] [PubMed] [Google Scholar]
- 28.Kvale EA, Shuster JL. Sleep disturbance in supportive care of cancer: a review. J Palliat Med. 2006;9:437–450. doi: 10.1089/jpm.2006.9.437. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Sloan JA. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J Pain Symptom Manage; United States. 2010; U.S. Cancer Pain Relief Committee; Elsevier Inc; 2010. pp. 1077–1085. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery P, Dennis J. Sleep Med Rev. England: 2004. A systematic review of non-pharmacological therapies for sleep problems in later life; pp. 47–62. [DOI] [PubMed] [Google Scholar]
- 31.Lee D, Morgan K, Lindesay J. J Am Geriatr Soc. United States: 2007. Effect of institutional respite care on the sleep of people with dementia and their primary caregivers; pp. 252–258. [DOI] [PubMed] [Google Scholar]
- 32.Clegg A, Young JB. Age Ageing. England: 2011. Which medications to avoid in people at risk of delirium: a systematic review; pp. 23–29. [DOI] [PubMed] [Google Scholar]
- 33.Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 34.de Jonghe A, Korevaar JC, van Munster BC, et al. Effectiveness of melatonin treatment on circadian rhythm disturbances in dementia. Are there implications for delirium? A systematic review. Int J Geriatr Psychiatry. 2010;25:1201–1208. doi: 10.1002/gps.2454. [DOI] [PubMed] [Google Scholar]
- 35.Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57:761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diehr P, Lafferty WE, Patrick DL, et al. Health Qual Life Outcomes. England: 2007. Quality of life at the end of life; p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]