Abstract
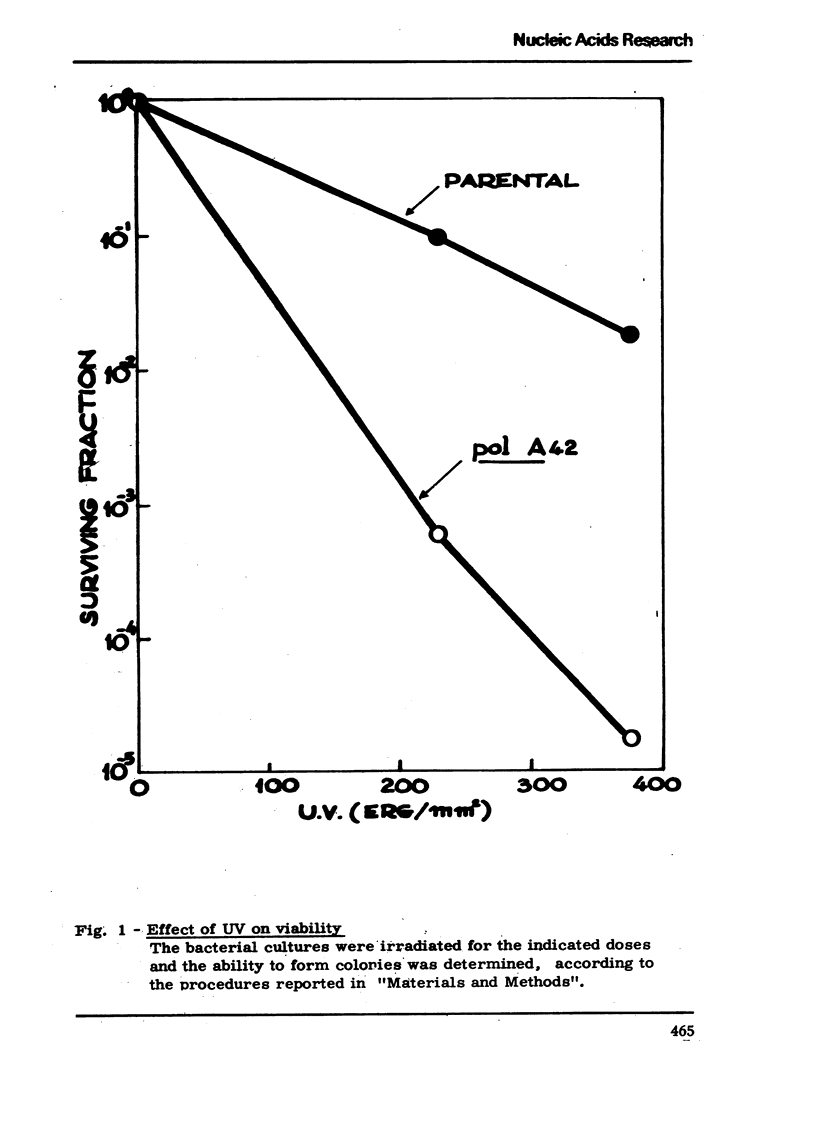
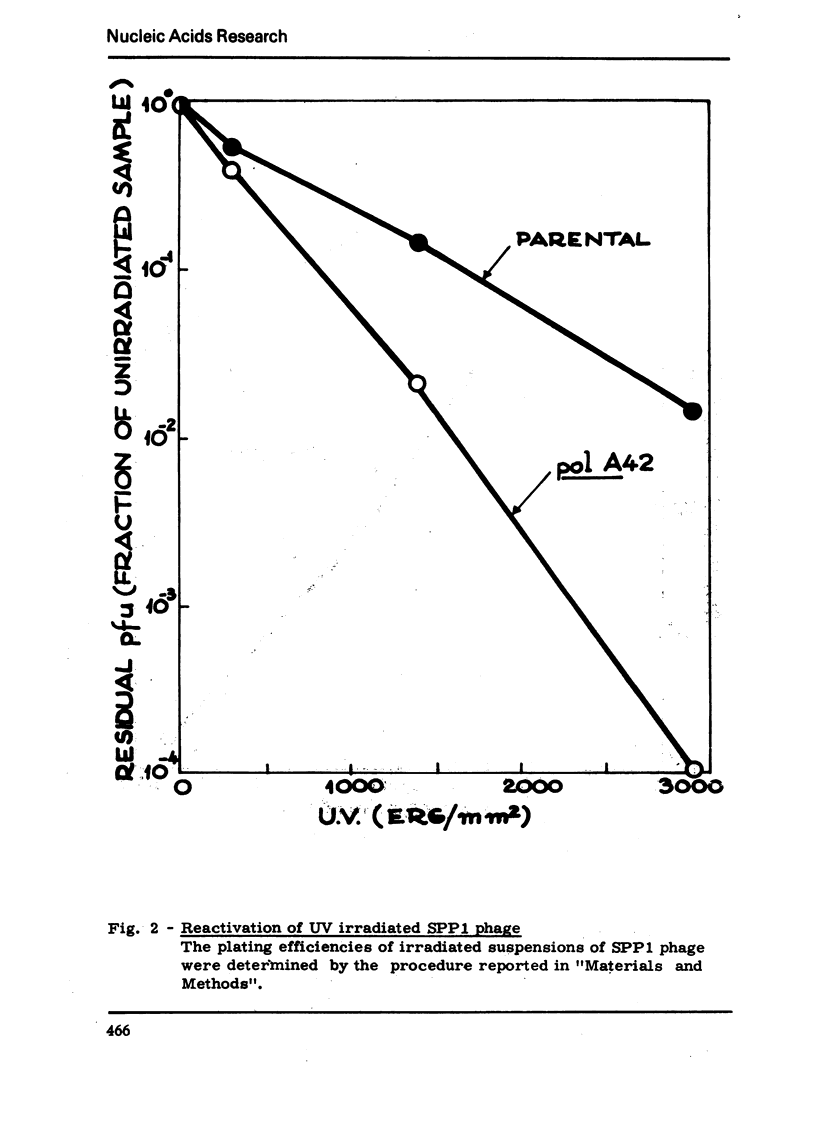
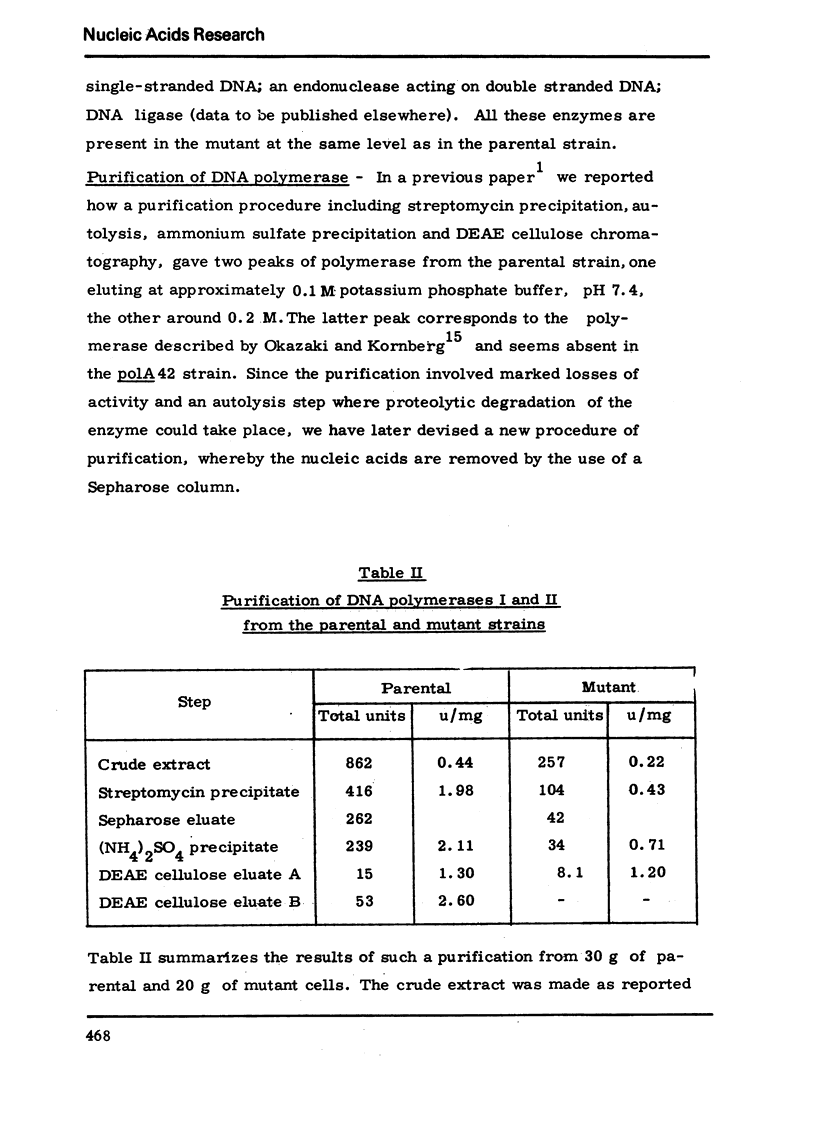
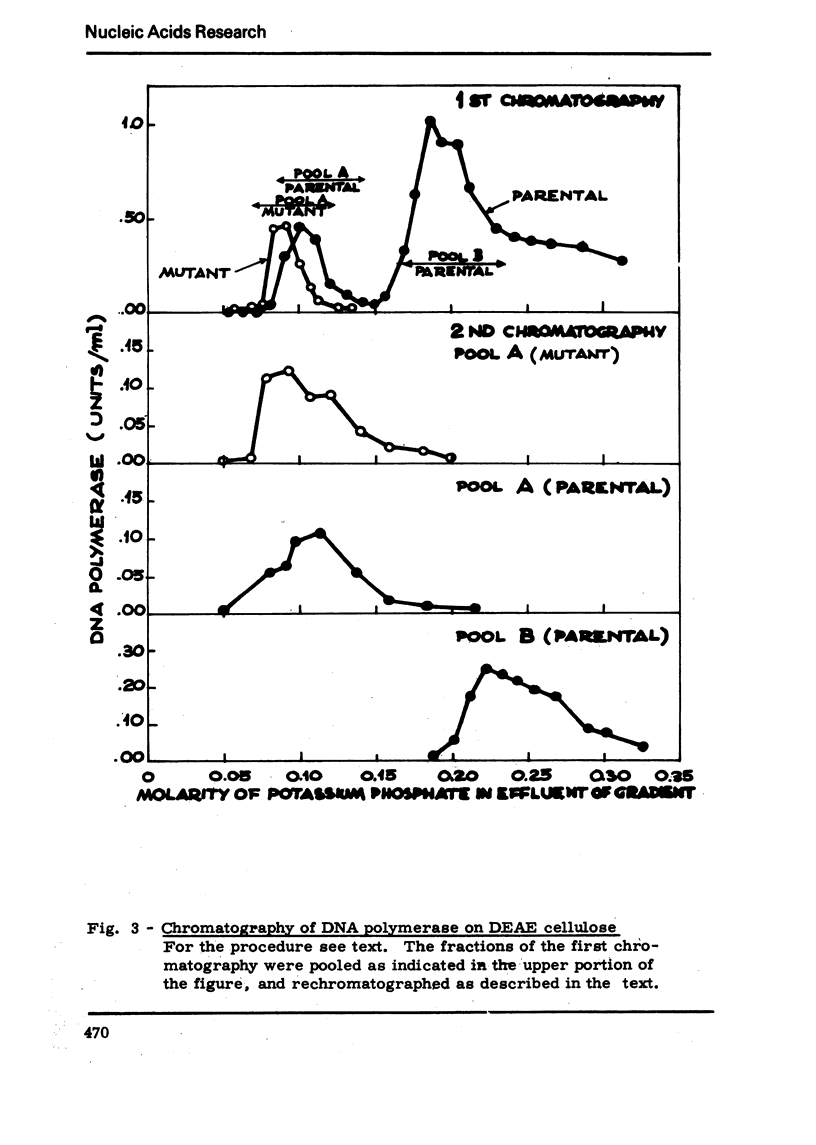
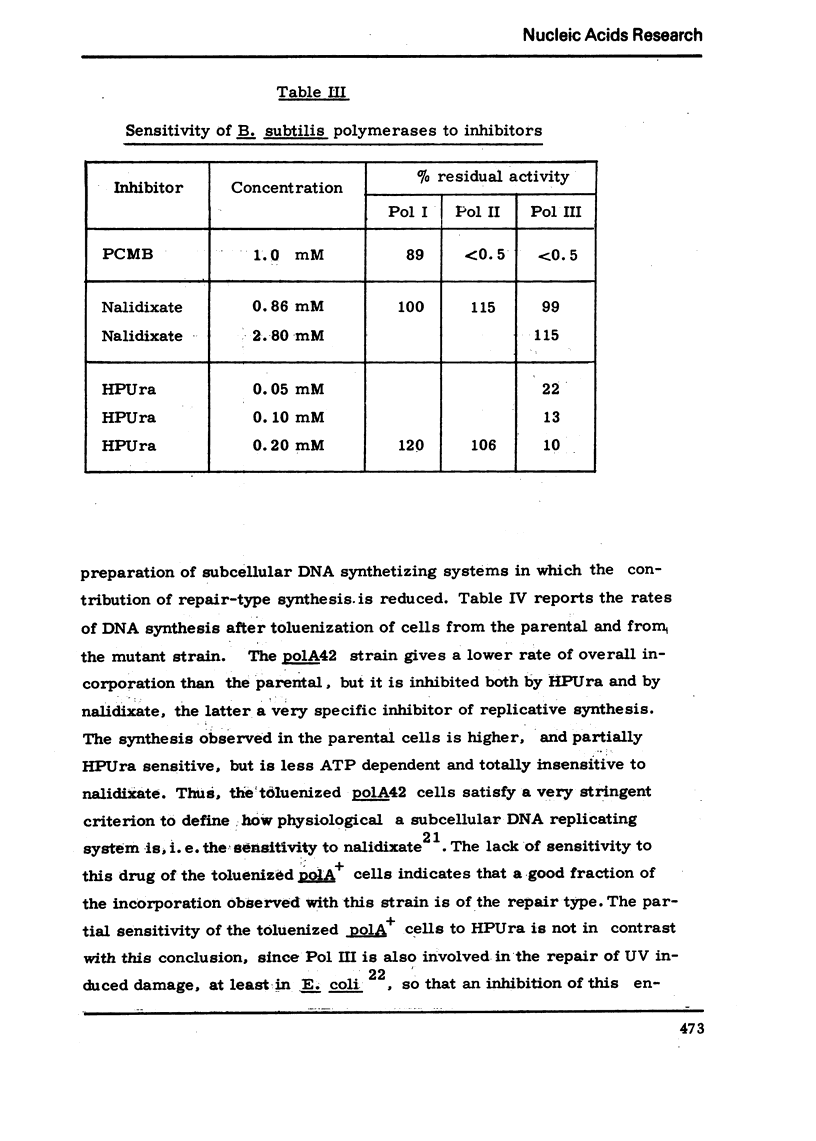
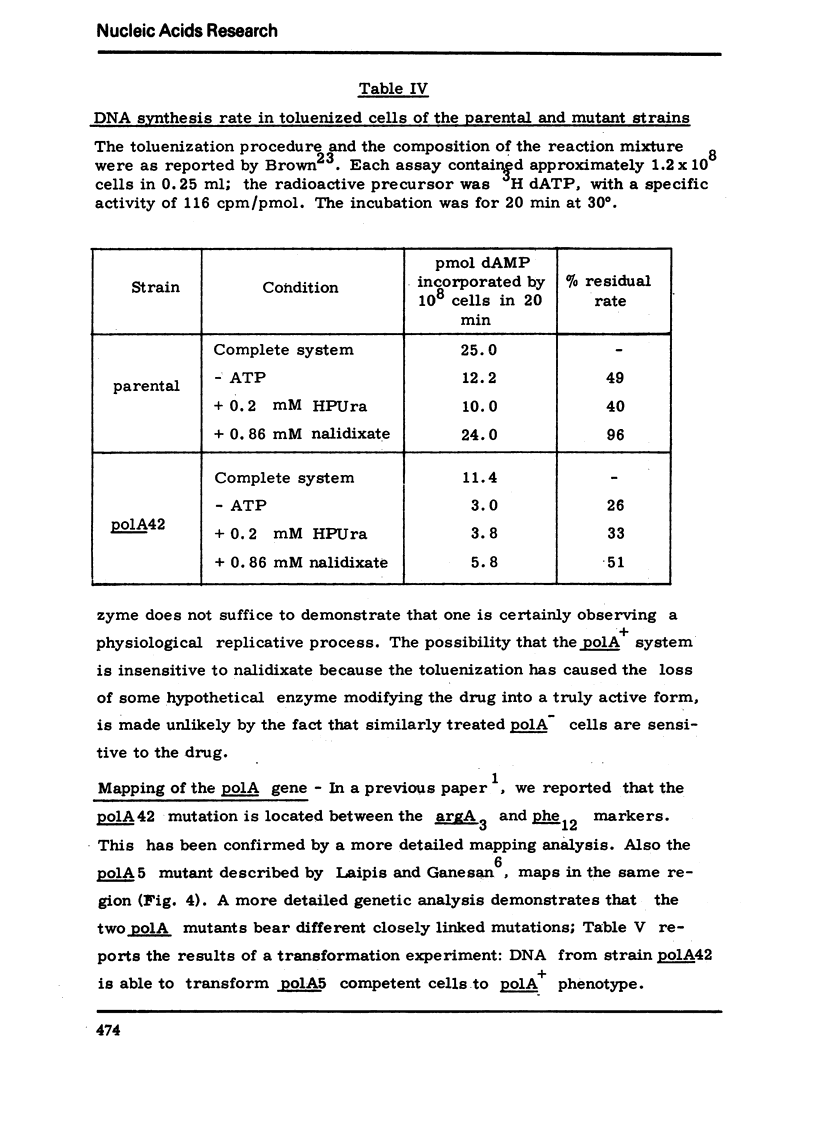
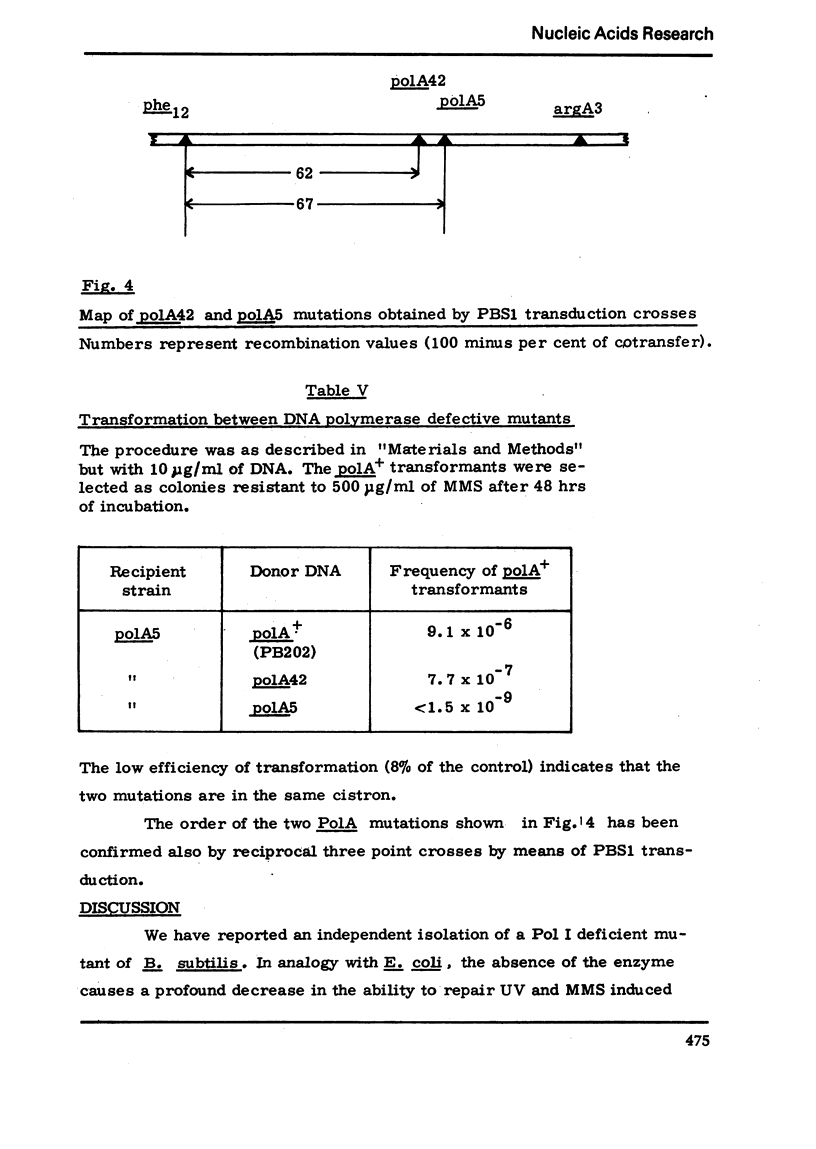
We have isolated a mutant of Bacillussubtilis deficient in DNA polymerase I, denominated polA42, which shows a reduced ability to repair the damage to DNA by UV radiation, MMS and mitomycin C;the ability to perform recombination is not appreciably impaired.DEAE cellulose chromatography allows the separation of polymerases I and II from the parental strain;a simple procedure is also described which allows to separate rapidly the polymerases II and III of the mutant strain. The three separated polymerases have similar catalytic properties but they can be distinguished for their sensitivity to inhibitors: PCMB inhibits polymerases II and III but not polymerase I; HPUra inhibits only polymerase III. All three enzymes are unaffected by nalidixate. The DNA synthesis occurring in cells of the polA42 strain permeabilized with toluene is inhibited by nalidixate, whereas the synthesis occurring in polA+ toluenized cells is unaffected by the drug. The polA gene has been mapped by transduction and localized between the phe12 and argA3 genes.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazill G. W., Gross J. D. Effect of 6-(p-hydroxyphenyl)-azouracil on B. subtilis DNA polymerases. Nat New Biol. 1972 Nov 15;240(98):82–83. doi: 10.1038/newbio240082a0. [DOI] [PubMed] [Google Scholar]
- Bazill G. W., Gross J. D. Mutagenic DNA polymerase in B. subtilis. Nat New Biol. 1973 Jun 20;243(129):241–243. doi: 10.1038/newbio243241a0. [DOI] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication in vitro by 6-(p-hydroxyphenylazo)-uracil: selective interference with the function of deoxyguanosine nucleotides. Biochim Biophys Acta. 1972 Oct 11;281(2):202–215. [PubMed] [Google Scholar]
- Ganesan A. T., Yehle C. O., Yu C. C. DNA replication in a polymerase I deficient mutant and the identification of DNA polymerases II and 3 in Bacillus subtilis. Biochem Biophys Res Commun. 1973 Jan 4;50(1):155–163. doi: 10.1016/0006-291x(73)91077-2. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Low R. L., Cozzarelli N. R. Inhibition of a DNA polymerase from Bacillus subtilis by hydroxyphenylazopyrimidines. Proc Natl Acad Sci U S A. 1973 Jan;70(1):103–107. doi: 10.1073/pnas.70.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laipis P. J., Ganesan A. T. A deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis. J Biol Chem. 1972 Sep 25;247(18):5867–5871. [PubMed] [Google Scholar]
- Mazza G., Eisenstark H. M., Serra M. C., Polsinelli M. Effect of caffeine on the recombination process of Bacillus subtilis. Mol Gen Genet. 1972;115(1):73–79. doi: 10.1007/BF00272219. [DOI] [PubMed] [Google Scholar]
- Mazza G., Galizzi A., Minghetti A., Siccardi A. Interaction between deoxyribonucliec acid and distamycin A studied by transformation in Bacillus subtilis. Antimicrob Agents Chemother. 1973 Mar;3(3):384–391. doi: 10.1128/aac.3.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. M., Brown N. C. Inhibition of a discrete bacterial DNA polymerase by 6-(p-hydroxyphenylazo)-uracil and 6-(p-hydroxyphenylazo-)-isocytosine. Nat New Biol. 1972 Nov 15;240(98):80–82. doi: 10.1038/newbio240080a0. [DOI] [PubMed] [Google Scholar]
- OKAZAKI T., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XV. PURIFICATION AND PROPERTIES OF A POLYMERASE FROM BACILLUS SUBTILIS. J Biol Chem. 1964 Jan;239:259–268. [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Physical heterogeneity among Bacillus subtilis deoxyribonucleic acid molecules carrying particular genetic markers. J Bacteriol. 1969 Jun;98(3):1239–1247. doi: 10.1128/jb.98.3.1239-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H., Takahashi I. Transducing particles of PBS 1. Virology. 1968 Dec;36(4):639–645. doi: 10.1016/0042-6822(68)90194-3. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Involvement of DNA polymerase 3 in excision repair after ultraviolet irradiation. Nat New Biol. 1973 Aug 22;244(138):240–241. doi: 10.1038/newbio244240a0. [DOI] [PubMed] [Google Scholar]



