Abstract
The antiphospholipid syndrome (APS), as both a primary syndrome and a syndrome in association with systemic lupus erythematosus (SLE), can be a devastating disease. It is unclear what factors (genetic and/or environmental) lead to the generation of antiphospholipid antibodies (aPL). It is equally unclear why only certain individuals with aPL develop clinical events. We hypothesize that innate immune activation plays a critical role at two distinct stages of APS, namely, the initiation phase, in which aPL first appear, and the effector phase, in which aPL precipitate a thrombotic event. According to the model we propose, aPL alone are insufficient to cause thrombosis and a concomitant trigger of innate immunity, e.g. a toll-like receptor (TLR) ligand, must be present for thrombosis to occur. Here, we discuss our findings that mice immunized with β2-glycoprotein I (β2GPI) and lipopolysaccharide (LPS), a TLR ligand, produce high levels of aPL and other SLE-associated autoantibodies, and develop lupus-like glomerulonephritis. We also discuss our data showing that autoantibodies to heat shock protein 60 (HSP60), an ‘endogenous TLR ligand’, promote thrombus generation in a murine model of arterial injury. Thus, both pathogen-derived TLR ligands (e.g. LPS) and endogenous TLR ligands (e.g. HSP60) may contribute to the pathogenesis of APS. This putative dual role of innate immunity provides new insight into the generation of aPL as well as the enigma of why some individuals with aPL develop APS, while others do not.
Keywords: antiphospholipid syndrome, β2-glycoprotein I, heat shock protein 60, innate immunity, lipopolysaccharide, toll-like receptor ligands
Introduction
The antiphospholipid syndrome (APS) is classified clinically by the presence of: (1) a laboratory abnormality (antiphospholipid antibodies, aPL); and (2) a clinical event (vascular thrombosis and/or complication of pregnancy).1 aPL are found in approximately 20–30% of patients with systemic lupus erythematosus (SLE), and are the major autoantibodies in patients with primary APS.2,3 APS, both as a primary syndrome and as a syndrome secondary to SLE, can be a devastating disease with significant morbidity and mortality. It remains unclear what factors (genetic and/or environmental) lead to the generation of aPL. Equally unclear is the reason only certain individuals with aPL develop clinical events.
The major evidence that aPL are pathogenic comes from murine models in which passive transfer of aPL results in an adverse event, such as fetal resorption.4 These models, however, often lack physiological relevance and fail to address the question whether aPL are the sole etiological factor in causing the clinical event. Moreover, most current APS models provide little insight as to how and why aPL arise in the first place. We have recently developed a murine model of SLE, in which mice produce high levels of aPL and other SLE-associated autoantibodies.5 These mice develop lupus-like glomerulonephritis, but, despite high aPL titers, do not develop overt thrombotic events. This SLE-like model not only replicates the sequential emergence of autoantibodies observed in human SLE,6 but also approximates the prevalent clinical scenario of an SLE patient who manifests high-titer aPL without thrombotic events.2,3 This scenario (in both human and murine disease) begs the question of what additional factors are necessary to induce thrombotic events.
Several studies support an important role for innate immune activation in the pathogenesis of APS. In vitro, antibodies to β2-glycoprotein I (β2GPI) were shown to bind to endothelial cells and induce MyD88-dependent nuclear factor-κB (NF-κB) translocation, resulting in an endothelial cell phenotype similar to that elicited by lipopolysaccharide (LPS).7 Similarly, in monocytes, anti-β2GPI antibodies triggered NF-κB translocation, leading to a proinflammatory and procoagulant monocyte phenotype.8 β2GPI has been shown to interact with annexin A2 on both endothelial cells7,9 and monocytes.8,10 In the case of monocytes, at least, membrane association of β2GPI is with both annexin A2 and toll-like receptor 4 (TLR4), and it appears to occur within lipid rafts.8 In vivo, in a rat model of mesenteric arterial thrombosis, human polyclonal anti-β2GPI antibodies lacked a procoagulant effect, while anti-β2GPI antibodies combined with LPS resulted in thrombotic occlusion.11 The latter findings are consistent with another in vivo model of thrombosis, which showed that mice receiving aPL antibodies produced thrombi only if subjected to a physical ‘pinch injury’. Together, these data suggest that innate immune activation may be required for aPL to exert their pathogenic effects.12
Based on the literature and our own data, we hypothesize that innate immune activation plays a dual role in the pathophysiology of APS, and that innate immune activation is necessary both for initiating the production of aPL and for precipitating a thrombotic event. According to the model we propose, aPL alone are insufficient to cause thrombosis and, for a thrombotic event to occur, there must be a concomitant trigger of innate immunity (e.g. a TLR ligand). This review will address the dual role of innate immune activation in both the ‘initiation’ and ‘effector’ phases of APS.
Overview of innate immunity
TLRs and TLR ligands
The discovery of TLRs and their respective ligands has been critical in elucidating the mechanisms by which microbes and their products activate innate immunity.13 Ten (human) and 13 (murine) TLRs have been identified, along with their respective ligands. TLRs recognize their ligands by binding to a number of highly conserved pathogen-associated molecular patterns (PAMPs). These include a variety of microbial peptides, LPS, lipoteichoic acids, bacterial DNA, and viral single- and double-stranded RNA. TLR recognition of PAMPs triggers intracellular signaling pathways that result in activation of several key transcription factors, especially NF-κB, activator protein 1 (AP-1), and members of the interferon regulatory factor (IRF) family. Much signaling occurs through the adapter protein MyD88, but some TLRs (e.g. TLR4) also trigger MyD88-independent pathways.13
TLR signaling leads to innate immune activation, which can, in turn, result in an inflammatory response. Upon activation, tissue-resident macrophages release proinflammatory cytokines (tumor necrosis factor-alpha [TNF-α], interleukin-1 beta [IL-1β], and interleukin-6 [IL-6]) that coordinate both local and systemic inflammatory responses.14 TNF-α and IL-1β activate the local endothelium, inducing vasodilation and increased vascular permeability. The activated endothelium expresses increased levels of tissue factor, leading to local activation of the coagulation cascade. Together, IL-1β and IL-6 activate hepatocytes to produce a number of acute phase proteins, including complement, that further amplify the innate immune response.
Endogenous TLR ligands
There is increasing evidence that TLRs may also be involved in sterile injury (i.e. injury in the absence of infection). Certain TLRs, especially TLR4, respond to endogenous molecules that are released from injured or stressed tissues.15 Moreover, TLR4-deficient animals exhibit reduced inflammation in in vivo models of injury, suggesting that TLR4-mediated signaling results from sterile injury.15 Endogenous TLR4 ligands that could result from tissue injury include heat shock proteins (HSPs), fibrinogen, and heparan sulfate.15,16 Together, these data indicate that TLR activation may occur in the absence of overt infection and support an emerging paradigm for TLR4 as a sentinel to detect tissue damage.15
Link between innate and adaptive immunity
TLRs are expressed on multiple cells of the innate immune system, including macrophages, dendritic cells (DCs), neutrophils, mucosal epithelial cells, and endothelial cells. These receptors not only alert the immune system to infection but also initiate and facilitate adaptive immune responses. Among its many effects, TLR signaling induces up-regulation of both major histocompatibility complex class II (MHC II) and costimulatory (CD80/CD86) molecules on DCs, and promotes migration of DCs to the nearest lymph node. Through these and other activities, the innate immune response provides a link between innate and adaptive immunity.
Hypothesis: dual role of innate immunity in APS
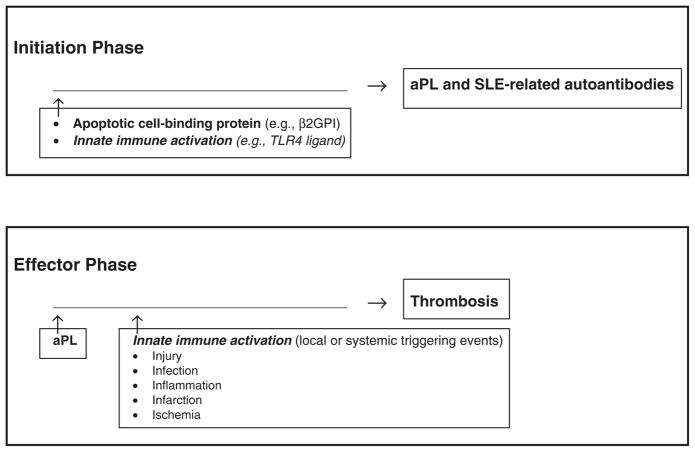
We hypothesize that innate immunity contributes critically to the pathogenesis of APS in two distinct phases: (1) an ‘initiation (or immunologic) phase’ and (2) an ‘effector (or pathologic) phase’ (Figure 1). During the ‘initiation phase’, the role of innate immunity is to amplify the adaptive immune response (e.g. to phospholipid-binding [PL-binding] proteins such as β2GPI), resulting in the long-lived production of aPL and other SLE autoantibodies. Subsequently, during the ‘effector (or pathologic) phase’, the role of innate immunity is to enhance the prothrombotic effects of aPL via priming of the vascular endothelium (e.g. cellular activation and/or disruption) at the site of eventual thrombosis. During both phases, innate immunity may be triggered by events such as injury, infection, inflammation, infarction, or ischemia.
Figure 1.
Proposed dual role of innate immunity in the antiphospholipid syndrome (APS). The model proposes two phases in the development of APS, both dependent on innate immune activation: (1) an ‘initiation (or immunologic) phase’, in which the role of innate immunity is to amplify the adaptive immune response (e.g. to a phospholipid/apoptotic cell-binding protein such as β2-glycoprotein I [β2GPI]), leading to the long-lived production of antiphospholipid antibodies (aPL) and other SLE-related autoantibodies; and (2) an ‘effector (or pathologic) phase’, in which the role of innate immunity is to enhance the prothrombotic effects of aPL via priming of the vascular endothelium (e.g. activation and/or disruption) at the site of eventual thrombosis. Innate immunity may be triggered by local or systemic events, such as injury, infection, inflammation, infarction, or ischemia. TLR4: toll-like receptor 4.
Innate immunity in the ‘initiation phase’ of APS
We have recently reported a murine model of SLE that produces high levels of aPL and other SLE autoantibodies, and develops lupus-like glomerulonephritis. 5 Healthy non-autoimmune mice (C57BL/6 or BALB/c) were immunized with human β2GPI in the presence of the TLR4 ligand LPS. In addition to being a PL-binding protein and major target of aPL, β2GPI binds readily to the surface of apoptotic cells17 and appears to be one of the first autoantigens targeted in SLE.6 Mice developed a long-lived, potent immune response to β2GPI, which, over time, led to epitope spread to multiple SLE autoantigens. Remarkably, autoantibodies appeared in a sequential manner that recapitulates the order seen in human SLE.6 These findings demonstrate that immunization with an apoptotic cell-binding protein (β2GPI), together with a TLR4 ligand (LPS) that triggers innate immune activation, can induce aPL and SLE-like disease in mice.
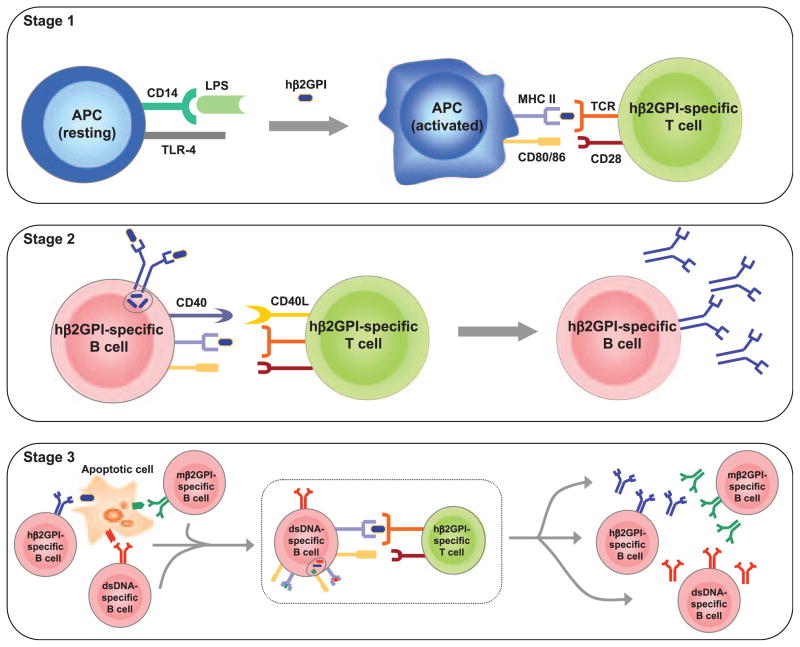
Our studies were motivated by the following considerations (Figure 2). First, we required an immunogen that could take advantage of apoptotic cells as a scaffold for epitope spread, but avoid the immunosuppression associated with administration of apoptotic cells. Most SLE- and APS-associated autoantigens are found on the surface of apoptotic cells, suggesting that these cells may constitute the cellular platform or ‘scaffold’ upon which epitope spread occurs in this disease.5,18,19 In SLE, epitope spread is defined as a sequential spread of the autoimmune response, leading to the ordered emergence of autoreactivity to multiple autoantigens. Epitope spread can be both intermolecular (epitopes on different molecules that are physically associated) and intramolecular (epitopes on the same molecule). In general, intermolecular epitope spread occurs between molecules whose physical association permits their simultaneous uptake by an antigenpresenting cell (APC). This type of epitope spread provides an elegant explanation for how autoantibodies to non-protein antigens (e.g. DNA and PL) can occur. For example, uptake of an apoptotic cell by an APC should result in presentation of all antigens derived from that apoptotic cell. In this way, the apoptotic cell serves as a ‘scaffold’ physically linking multiple autoantigens that are individually targeted by the autoimmune response. Consistent with epitope spread, autoantibodies in human SLE appear in a remarkably consistent order, and precede the development of clinical disease by up to 9 years.6 As aPL are one of the earliest autoantibody specificities to emerge in SLE,6 we chose β2GPI, a prominent APS-associated autoantigen, as our immunogen. Importantly, to minimize the well-described, potent anti-inflammatory effects of apoptotic cells, we used soluble β2GPI, which should interact with endogenous apoptotic cells.
Figure 2.
Role of innate immunity in the ‘initiation phase’ of antiphospholipid syndrome (APS) and systemic lupus erythematosus (SLE). This figure outlines a minimal, but sufficient, model by which immunization of mice with human β2-glycoprotein I (β2GPI) (a phospholipid-binding protein and target of antiphospholipid antibodies [aPL]) and lipopolysaccharide (LPS) (a toll-like receptor [TLR4] ligand) leads to aPL production, followed by a break in tolerance and epitope spread to multiple SLE autoantigens. We propose 3 stages: (1) activation of antigen-presenting cells (APCs) and human β2GPI-specific T-cells; (2) activation of human β2GPI-specific B-cells; and (3) generation of B-cell autoimmunity. In this setting, the human β2GPI-specific T-cell can provide help to any B-cell that internalizes an apoptotic cell having human β2GPI bound to its surface. In this manner, multiple autoreactive B-cells can be activated by a single human β2GPI-specific T-cell and lead to multiple SLE autoantibodies and SLE-like disease. hβ2GPI: human β2GPI; mβ2GPI: murine β2GPI. Source: reproduced with permission from Levine et al.5 (copyright 2006. The American Association of Immunologists, Inc.)
Second, we needed a potent activator of innate immunity in order to generate a strong and persistent T-cell response to the inciting immunogen. To generate a strong innate immune, and subsequent, T-cell response, we co-immunized with LPS, a known TLR ligand. As described above, activators of the innate immune system, such as LPS, enhance MHC-associated antigen presentation and induce up-regulation of co-stimulatory molecules like CD80 and CD86. LPS also has additional immunogenic effects, such as promoting survival of memory T-cells.20
In accordance with our hypothesis, repeated co-immunization of β2GPI with LPS led to a potent β2GPI-specific T helper cell response, with subsequent production of anti-β2GPI antibodies and other aPL, as well as intermolecular epitope spread to other apoptotic cell-associated autoantigens. Notably, immunized mice not only produced aPL and SLE autoantibodies, but also developed SLE-like glomerulonephritis.5
Innate immunity in the ‘effector phase’ of APS
Murine models of APS
Among the few existing murine models of APS, most have used passive transfer of human polyclonal or murine monoclonal β2GPI-dependent anticardiolipin antibodies (aCL) to produce experimental APS.4 Moreover, these models have evaluated pregnancy loss rather than thrombotic events as their major outcome. Other models have used less physiological immunizations, such as human and murine monoclonal aCL in complete Freund’s adjuvant (CFA), to induce APS. To date, two models have been used to study aPL-associated thrombosis in vivo.11,12,21 In one model, a pinch injury of the femoral vein was required to induce thrombosis.12 Mice injected with aCL (both polyclonal and monoclonal) developed larger clots and more persistent thrombi.21 A role for TLR4 activation in this model was suggested by the finding that aCL-induced thrombi were smaller in LPS-non-responsive (C3H/HeJ) than in LPS-responsive (C3H/HeN), mice.22 In the other model, the effects of aPL, with or without LPS, were studied.11 aPL alone had no effect, while aPL with LPS resulted in platelet–leukocyte aggregates and thrombotic occlusions. It is noteworthy that in our induced model of SLE-like disease, careful histological evaluation of multiple tissues (including kidney, liver, spleen, heart, lung, brain, pancreas, and bone marrow) failed to reveal any thrombotic lesions despite high titers of aPL.5 These findings beg the question of what additional factors are necessary to induce aPL-associated thrombosis. They also suggest that models of the ‘initiation phase’ of APS may not be suitable for investigating the ‘effector phase’ of APS.
Murine model of thrombosis induced with autoantibodies to HSP60, an endogenous TLR ligand
Based on our hypothesis that the presence of aPL alone is insufficient for thrombosis, we and our collaborators (Y Merhi and E Thorin) elected to use a ferric chloride (FeCl3)-induced murine model of carotid artery injury.23 In this model, application of FeCl3 to the outside of the carotid artery leads to endothelial cell injury and resultant thrombus formation. Mice can be treated prior to injury with autoantibodies or other agents that promote or inhibit thrombus formation. Using this model, we have recently shown that autoantibodies to HSP60 (anti-HSP60) promote endothelial dysfunction and thrombus generation in healthy non-autoimmune mice.23 Occlusion of the carotid occurred significantly faster in anti-HSP60-treated than in control IgG-treated mice. The most striking effect of anti-HSP60 IgG was on thrombus stabilization. Pretreatment with anti-HSP60 led to complete and stable occlusion (blood flow = 1.7 ± 0.6%) in all animals by 12 min, while the minimum blood flow achieved in control IgG-treated mice was 34.0 ± 12.6% (p = 0.0157). Furthermore, thrombi were significantly larger in anti-HSP60-treated mice and contained four-fold more inflammatory cells, as compared to controls. Notably, uninjured contralateral arteries in anti-HSP60-treated, but not control IgG-treated, mice were also affected, with abnormal endothelial cell morphology and significantly increased surface expression of von Willebrand factor (vWf) and P-selectin. These data demonstrate that passive transfer of anti-HSP60 not only results in altered endothelial cell morphology and increased vWf and P-selectin expression, consistent with innate immune activation, but also promotes arterial thrombosis and inflammatory cell recruitment.
Mammalian HSP60 is an intracellular protein that is generally thought to be expressed on the surface only of cells that have been activated or stressed. However, endothelial cells in culture have been shown to express surface HSP60 that can be recognized by anti-HSP60.24 It is possible that surface HSP60 expression occurs on morphologically intact endothelial cells during normal turnover. This would explain the effect of anti-HSP60 treatment on uninjured contralateral carotid arteries in our mice. As HSP60 appears to bind to innate immune cells, and through interaction with LPS promotes proinflammatory responses, it has been called an ‘endogenous TLR ligand’.25 Circulating anti-HSP60 may arise during infection or as part of an autoimmune response. In either case, anti-HSP60 could amplify the innate immune response through their ability to induce endothelial dysfunction.
Innate immunity and the ‘effector phase’ of human APS
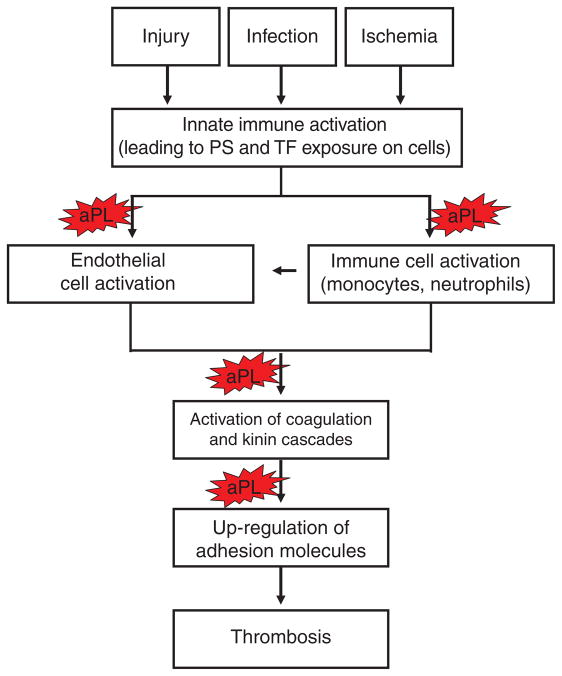
We (with P Fortin and coworkers) have recently completed an epidemiological study of anti-HSP60 in a cohort of SLE and non-SLE patients with and without aPL. Preliminary analyses indicate that anti-HSP60 are an important predictor of vascular events, in particular when found concomitantly with aPL.26 These data are consistent with a broadening role for TLR activation in multiple non-infectious diseases, including autoimmune disease (e.g. APS), cardiovascular disease, allergy, and cancer. Although infection is sometimes associated with APS27 and triggers the disease in as many as 24% of patients with catastrophic APS, it is unlikely that infection initiates APS in most patients. Other factors (including surgical trauma, withdrawal of anticoagulation, and carcinoma) also likely play a role,27 and may, like infection, result in activation of innate immunity. Innate activation appears to be the common denominator across a broad spectrum of triggers to thrombosis, with infection and tissue injury or death at one end of the spectrum, and cellular stress or malfunction at the opposite end.28 Innate immune activation may, in turn, lead to increased expression of tissue factor by the endothelium and peripheral blood cells, with initiation of the coagulation cascade (Figure 3).
Figure 3.
Role of innate immunity in the ‘effector phase’ of APS. This figure outlines the interplay between innate immunity and antiphospholipid antibodies (aPL). A stimulus of innate immunity (e.g. injury, infection, or ischemia) induces the exposure of negatively charged phospholipid (PS), i.e. phosphatidyl-serine, and tissue factor (TF) on the surface of vascular cells, resulting in binding of coagulation proteins and other phospholipid-binding proteins (e.g. β2-glycoprotein I) and subsequent recognition of cells by circulating aPL. aPL have been proposed to affect a number of steps in this process, including cellular activation, activation of the coagulation and kinin cascades, and up-regulation of adhesion molecules, all resulting in enhanced thrombogenicity.
Conclusions and future perspectives
We have discussed how innate immunity can play a role in both the ‘initiation phase’ and the ‘effector phase’ of APS. Pathogen-derived TLR ligands (e.g. LPS) may trigger the initial production of aPL, while endogenous TLR ligands (e.g. HSP60) may be important in local endothelial changes that enable circulating aPL to activate the coagulation cascade. The occurrence of autoantibodies to endogenous ligands, such as HSP60, may contribute to the pathophysiology of APS, not only amplifying the innate immune response, but also modulating endothelial cell morphology and function. This dual role of innate immunity in the pathogenesis of APS, as put forth in this review, provides new insight not only into the generation of aPL but also the enigma of why some individuals with aPL develop APS, while others do not.
Acknowledgments
This work was supported by Canadian Institutes of Health Research operating grants (MOP-42391 [JR], MOP-67101 [JR], and IMHA Priority Announcement MUS-67101 [JR]); a Genzyme Renal Innovations Program Award (Genzyme Corporation) (JSL); and postdoctoral fellowships from the Research Institute of the McGill University Health Centre (MD) and the Department of Medicine of McGill University (MD).
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 3.Baker WF, Jr, Bick RL. The clinical spectrum of antiphospholipid syndrome. Hematol Oncol Clin North Am. 2008;22:33–52. doi: 10.1016/j.hoc.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Ziporen L, Shoenfeld Y. Anti-phospholipid syndrome: from patient’s bedside to experimental animal models and back to the patient’s bedside. Hematol Cell Ther. 1998;40:175–182. [PubMed] [Google Scholar]
- 5.Levine JS, Subang R, Nasr SH, et al. Immunization with an apoptotic cell-binding protein recapitulates the nephritis and sequential autoantibody emergence of systemic lupus erythematosus. J Immunol. 2006;177:6504–6516. doi: 10.4049/jimmunol.177.9.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 7.Raschi E, Testoni C, Bosisio D, et al. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–3500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 8.Sorice M, Longo A, Capozzi A, et al. Anti-β2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor α and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 9.Ma K, Simantov R, Zhang J-C, et al. High affinity binding of β2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem. 2000;275:15541–15548. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Ling S, Yu Y, et al. Involvement of annexin A2 in anti-β2GPI/β2GPI-induced tissue factor expression on monocytes. Cell Res. 2007;17:737–739. doi: 10.1038/cr.2007.33. [DOI] [PubMed] [Google Scholar]
- 11.Fischetti F, Durigutto P, Pellis V, et al. Thrombus formation induced by antibodies to β2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 12.Pierangeli SS, Barker JH, Stikovac D, et al. Effect of human IgG antiphospholipid antibodies on an in vivo thrombosis model in mice. Thromb Haemost. 1994;71:670–4. [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. Toll-like receptor and RIG-1-like receptor signaling. Ann NY Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 15.Mollen KP, Anand RJ, Tsung A, et al. Emerging paradigm: Toll-like receptor 4 - sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 16.Tsan M-F, Gao B. Endogenous ligands of toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 17.Price BE, Rauch J, Shia MA, et al. Antiphospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a β2-glycoprotein I-dependent manner. J Immunol. 1996;157:2201–2208. [PubMed] [Google Scholar]
- 18.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAleer JP, Zammit DJ, Lefrançois L, et al. The lipopolysaccharide adjuvant effect on T cells relies on nonoverlapping contributions from the MyD88 pathway and CD11c+ cells. J Immunol. 2007;179:6524–6535. doi: 10.4049/jimmunol.179.10.6524. [DOI] [PubMed] [Google Scholar]
- 21.Pierangeli SS, Harris EN. In vivo models of thrombosis for the antiphospholipid syndrome. Lupus. 1996;5:451–455. doi: 10.1177/096120339600500524. [DOI] [PubMed] [Google Scholar]
- 22.Pierangeli SS, Vega-Ostertag ME, Raschi E, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dieudé M, Gillis M-A, Théorêt J-F, et al. Autoantibodies to heat shock protein 60 (HSP60) promote thrombus formation in a murine model of arterial thrombosis. J Thromb Haemost. 2009;7:710–719. doi: 10.1111/j.1538-7836.2009.03305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieudé M, Senécal JL, Raymond Y. Induction of endothelial cell apoptosis by heat-shock protein 60-reactive antibodies from anti-endothelial cell autoantibody-positive systemic lupus erythematosus patients. Arthritis Rheum. 2004;50:3221–3231. doi: 10.1002/art.20564. [DOI] [PubMed] [Google Scholar]
- 25.Habich C, Burkart V. Heat shock protein 60: regulatory role on innate immune cells. Cell Mol Life Sci. 2007;64:742–751. doi: 10.1007/s00018-007-6413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieudé M, Correa J, Neville C, et al. Autoantibodies to heat shock protein 60 are associated with arterial vascular events in patients with anti-phospholipid antibodies. Arthritis Rheum. 2009;60:S766. doi: 10.1002/art.30411. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in catastrophic antiphospholipid syndrome. Causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568–2576. doi: 10.1002/art.22018. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]