Abstract
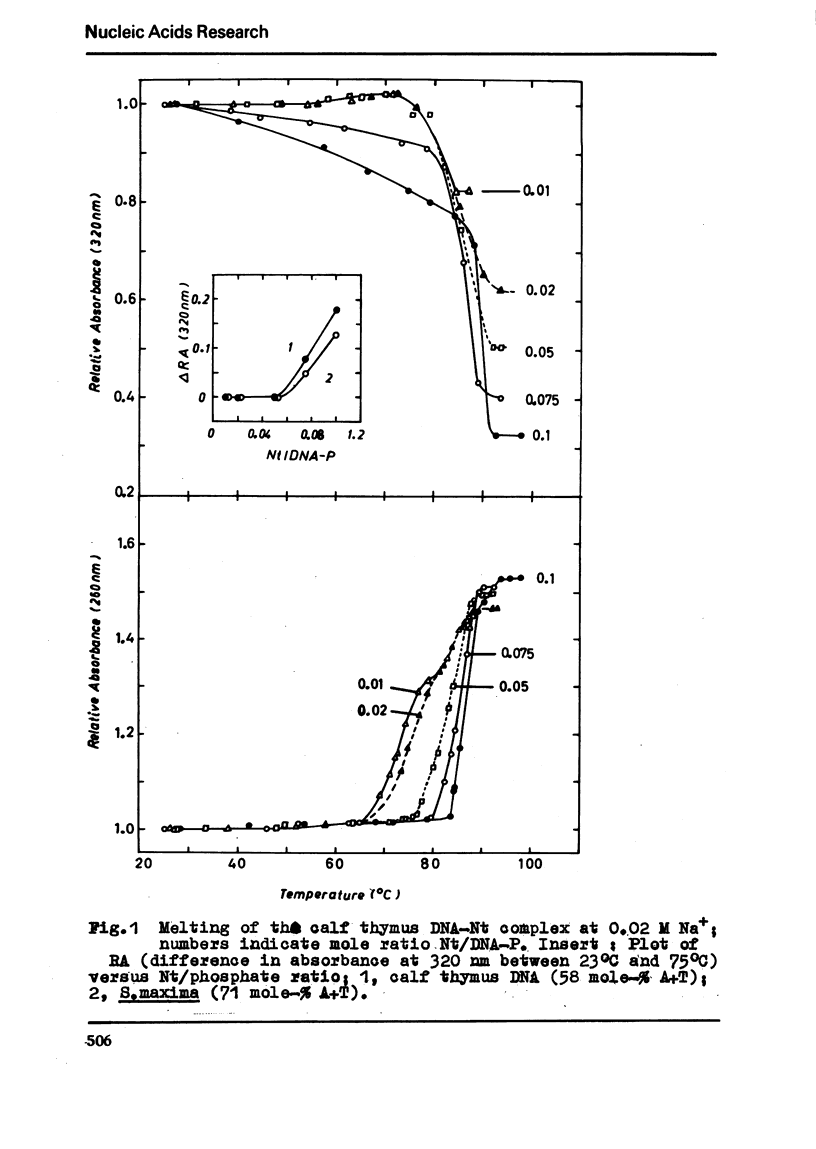
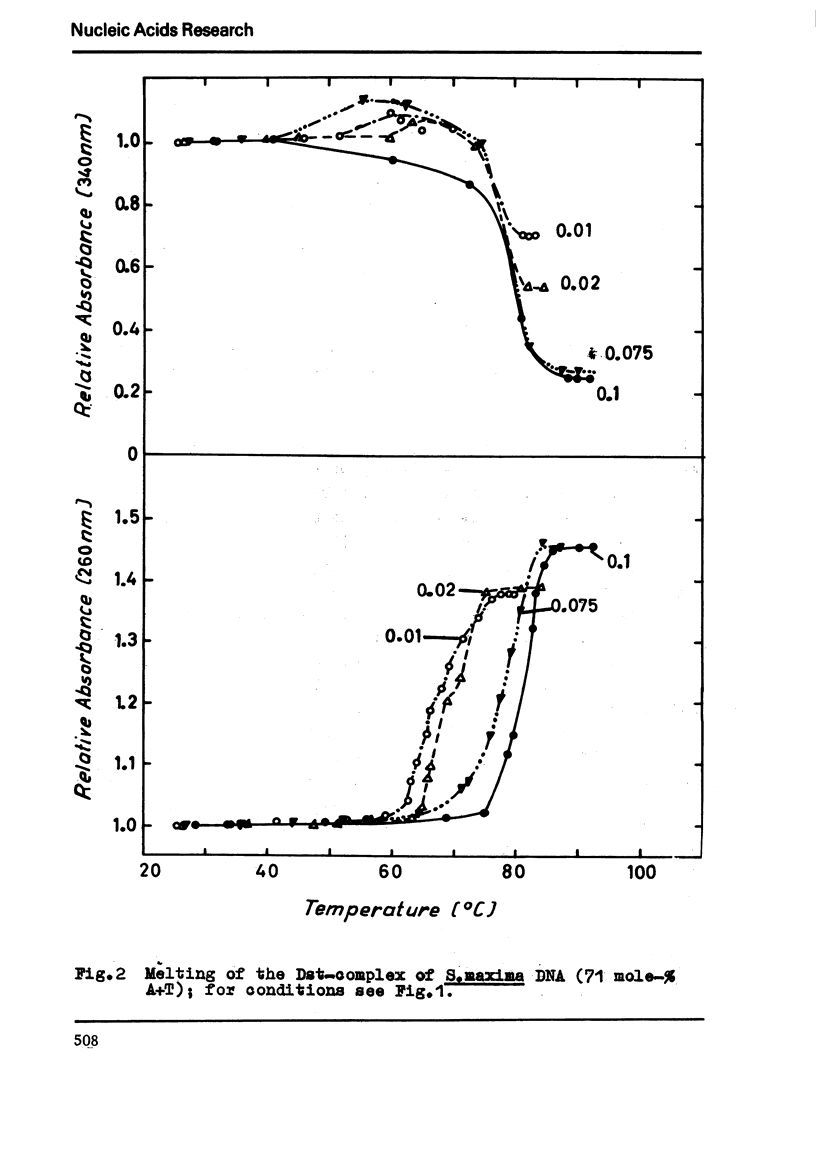
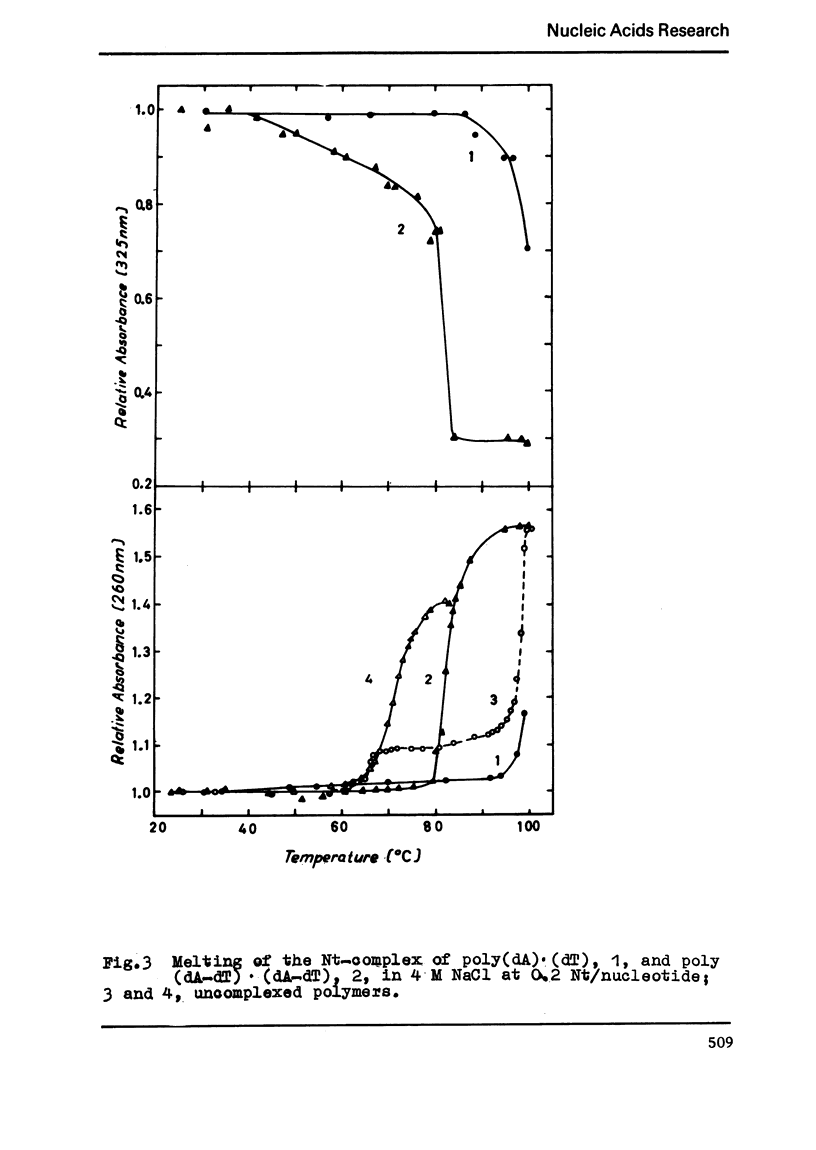
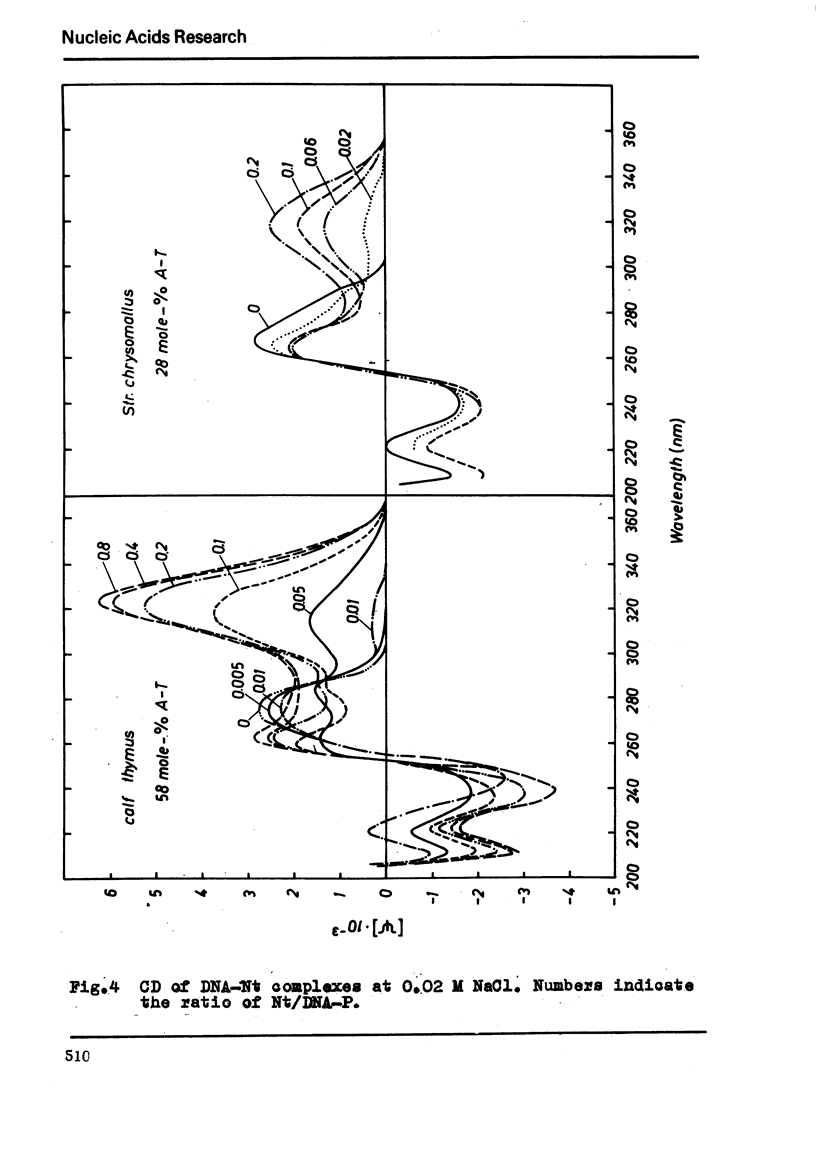
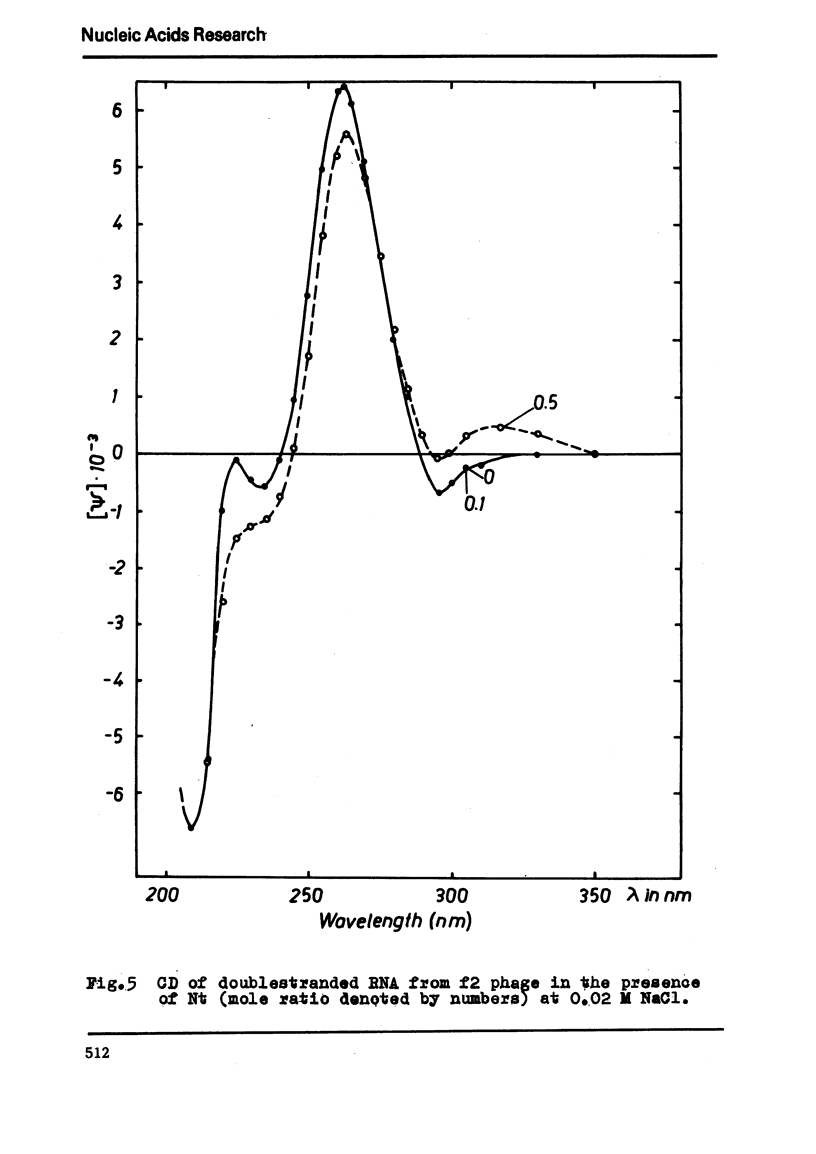
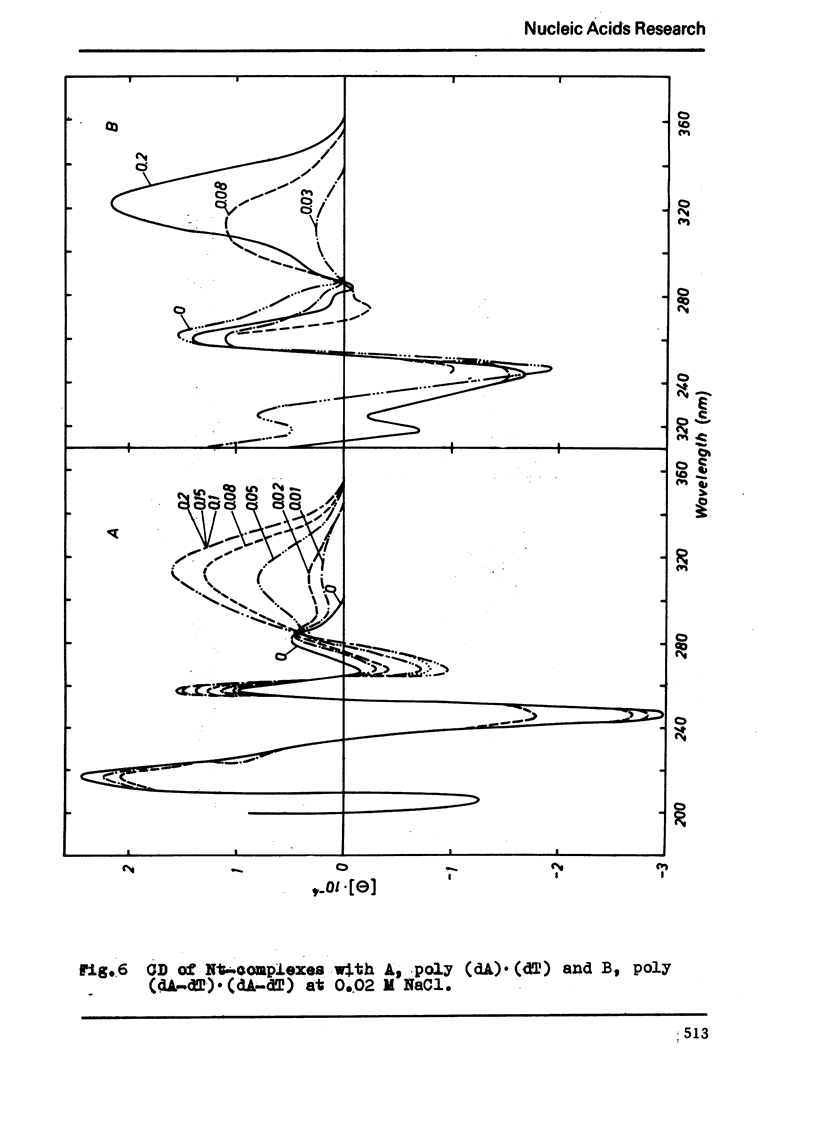
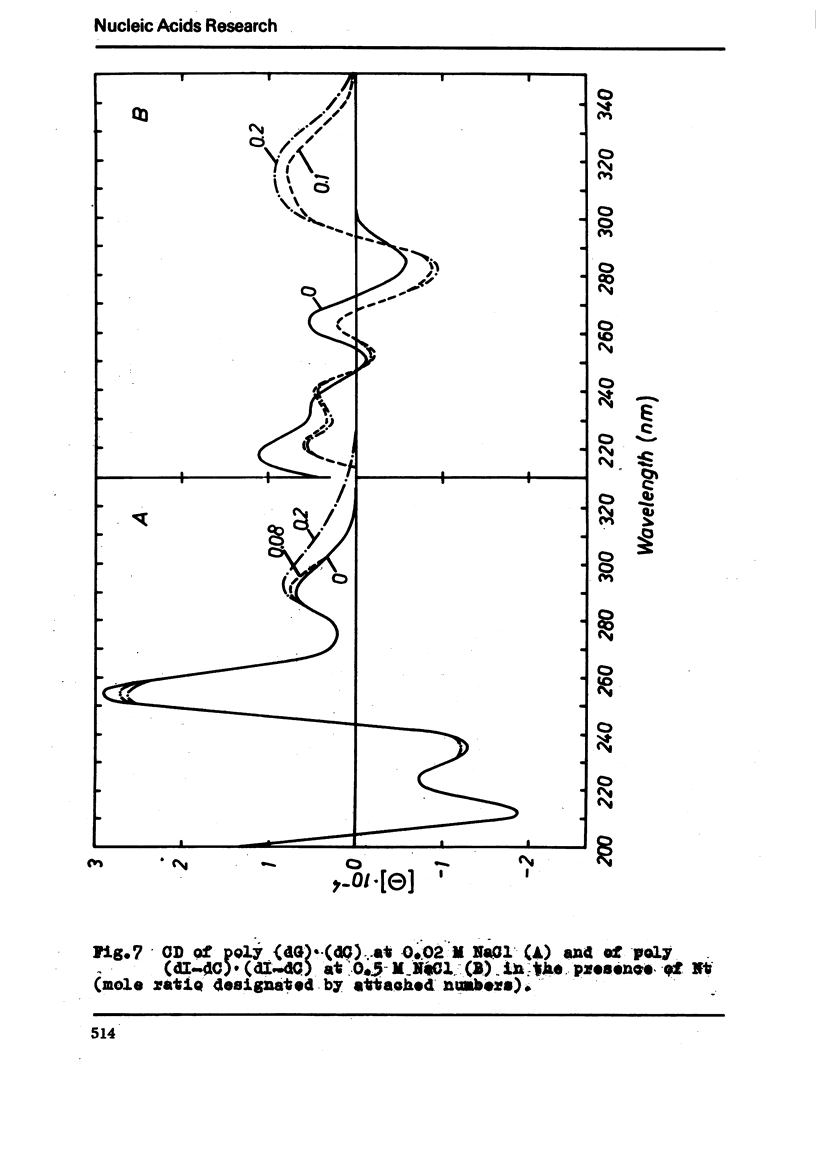
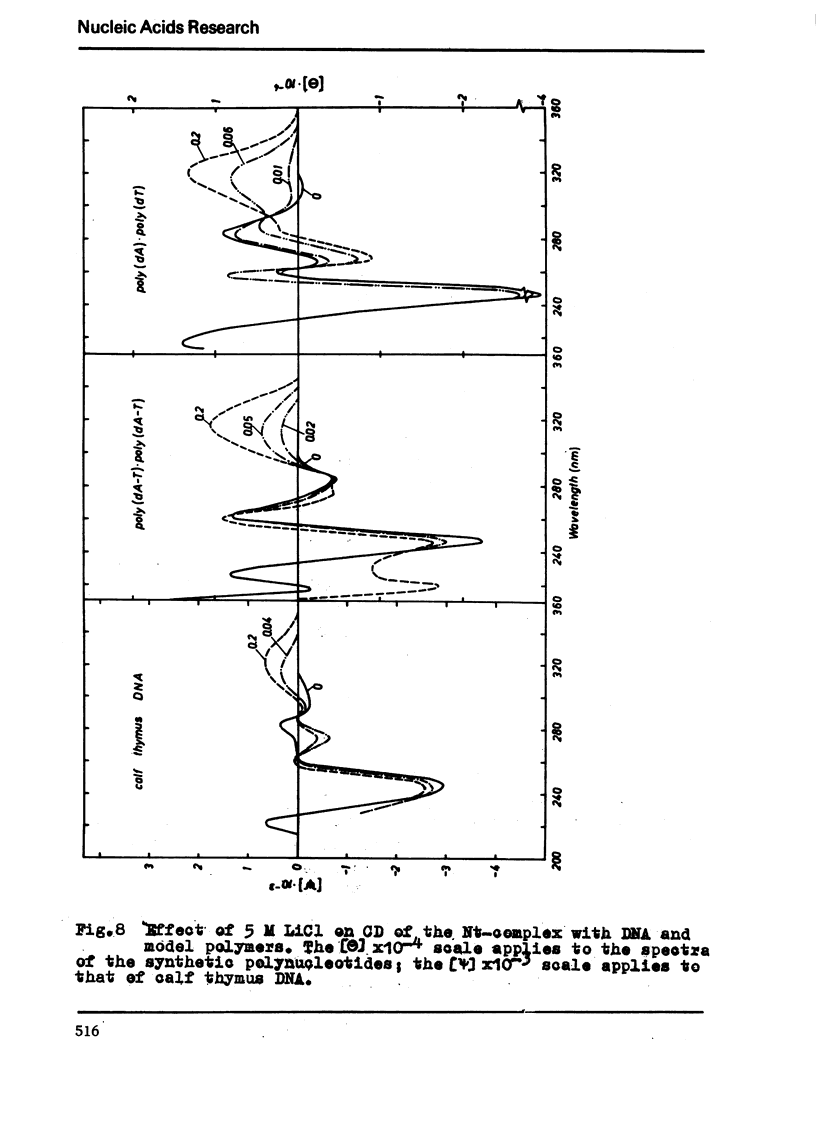
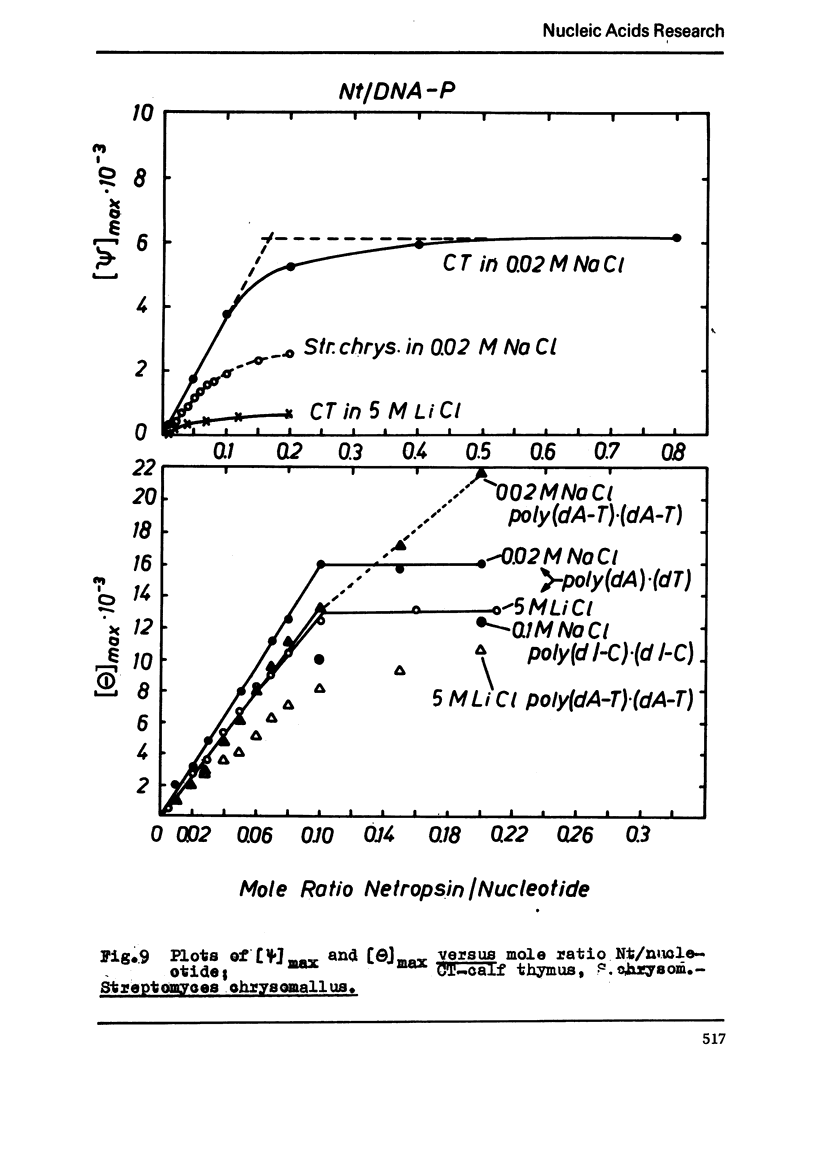
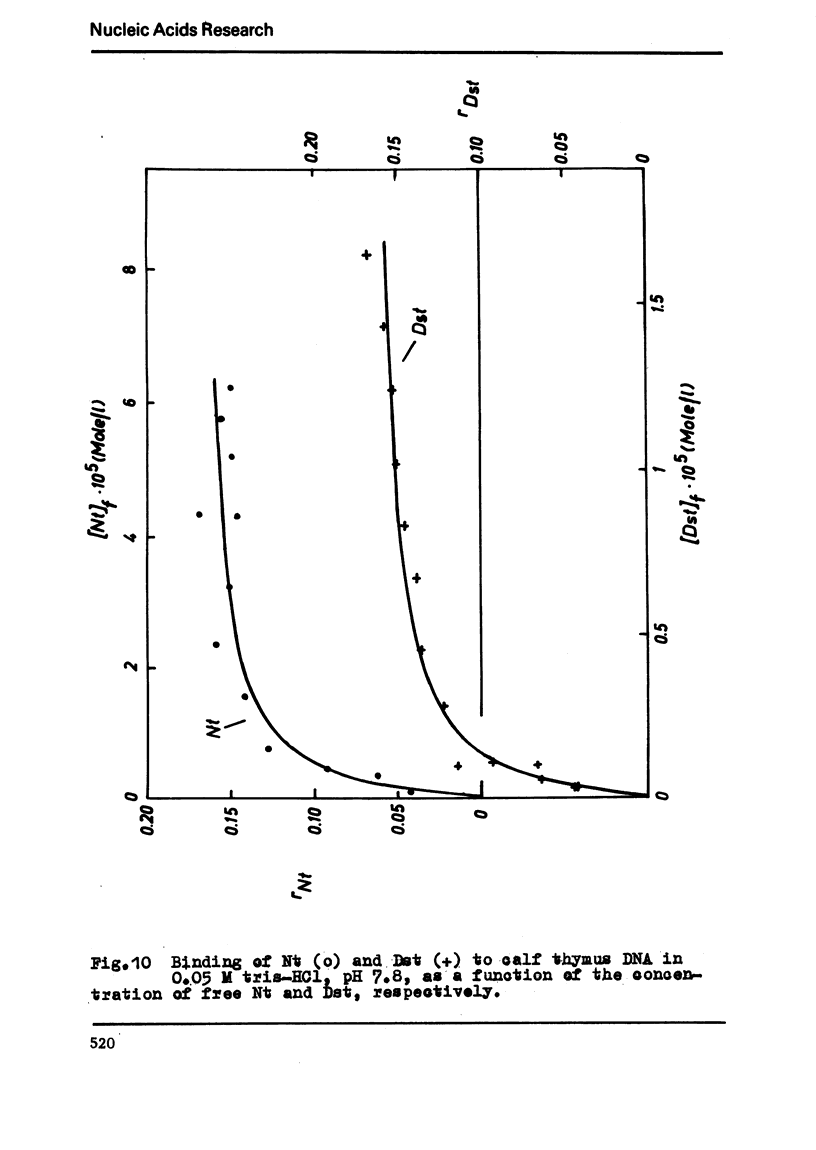
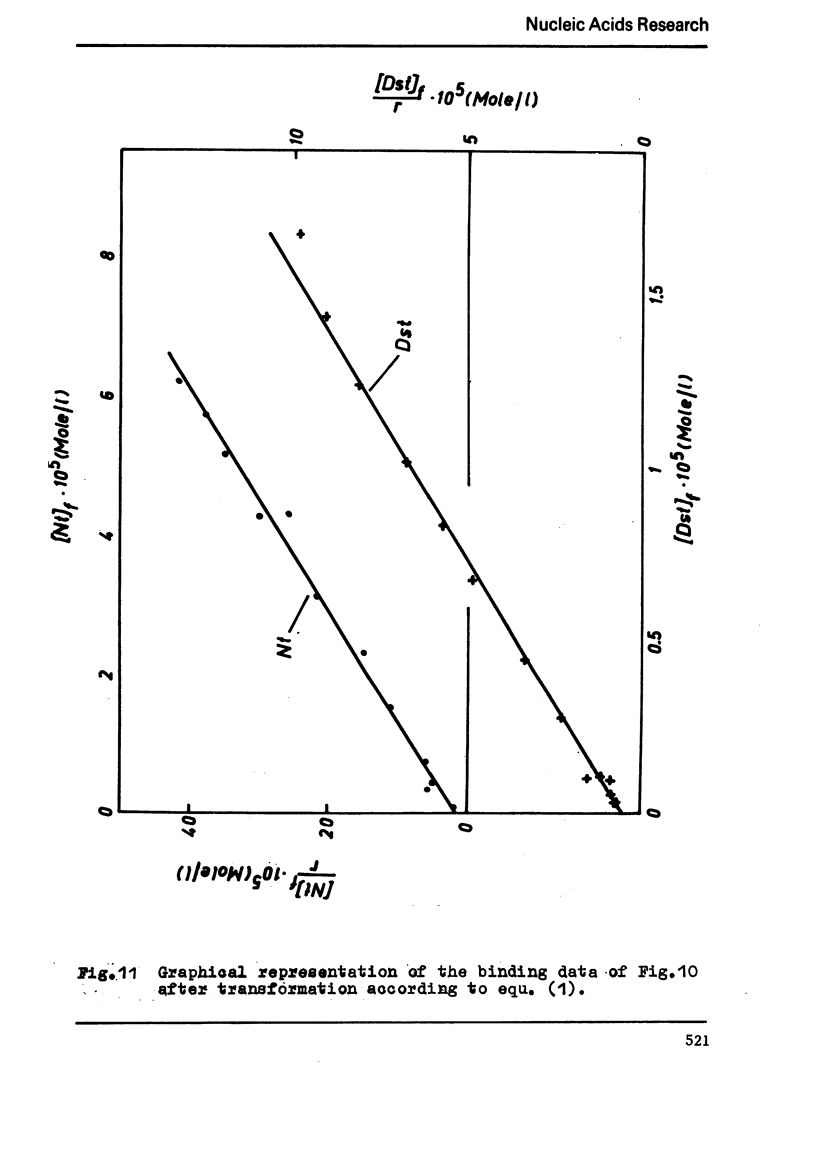
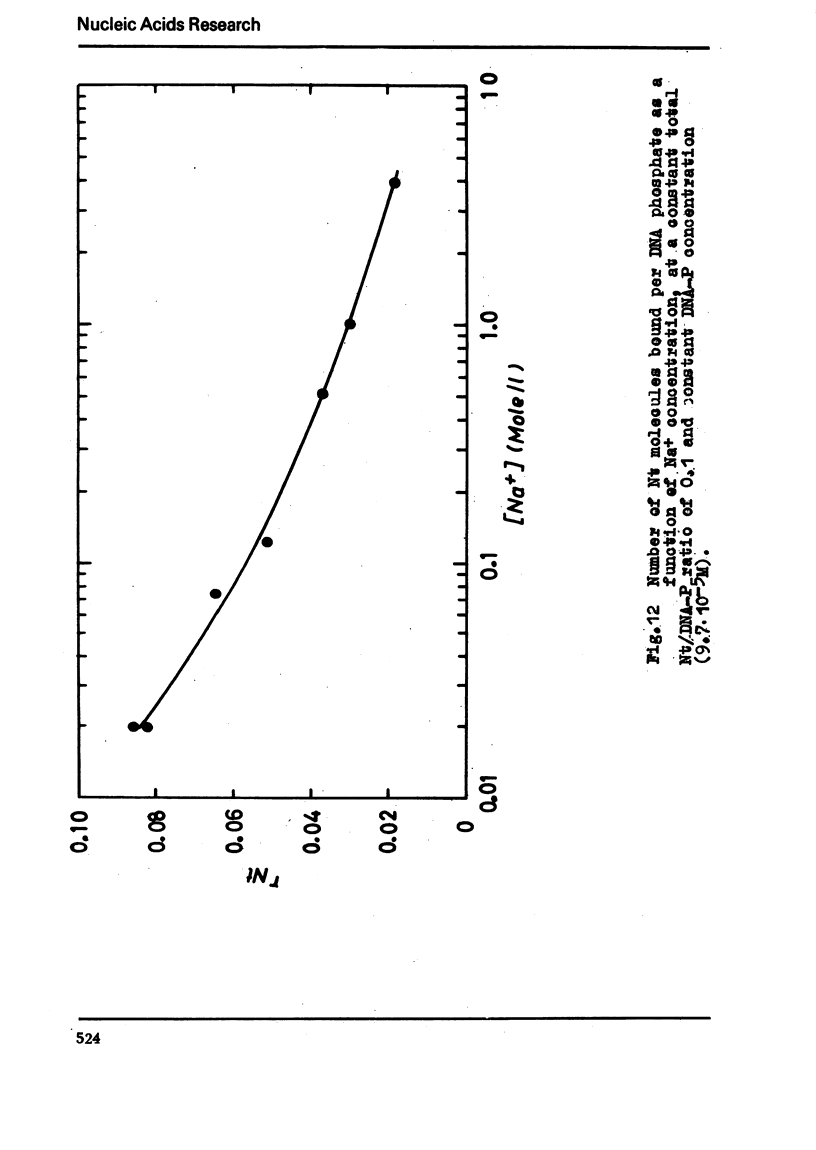
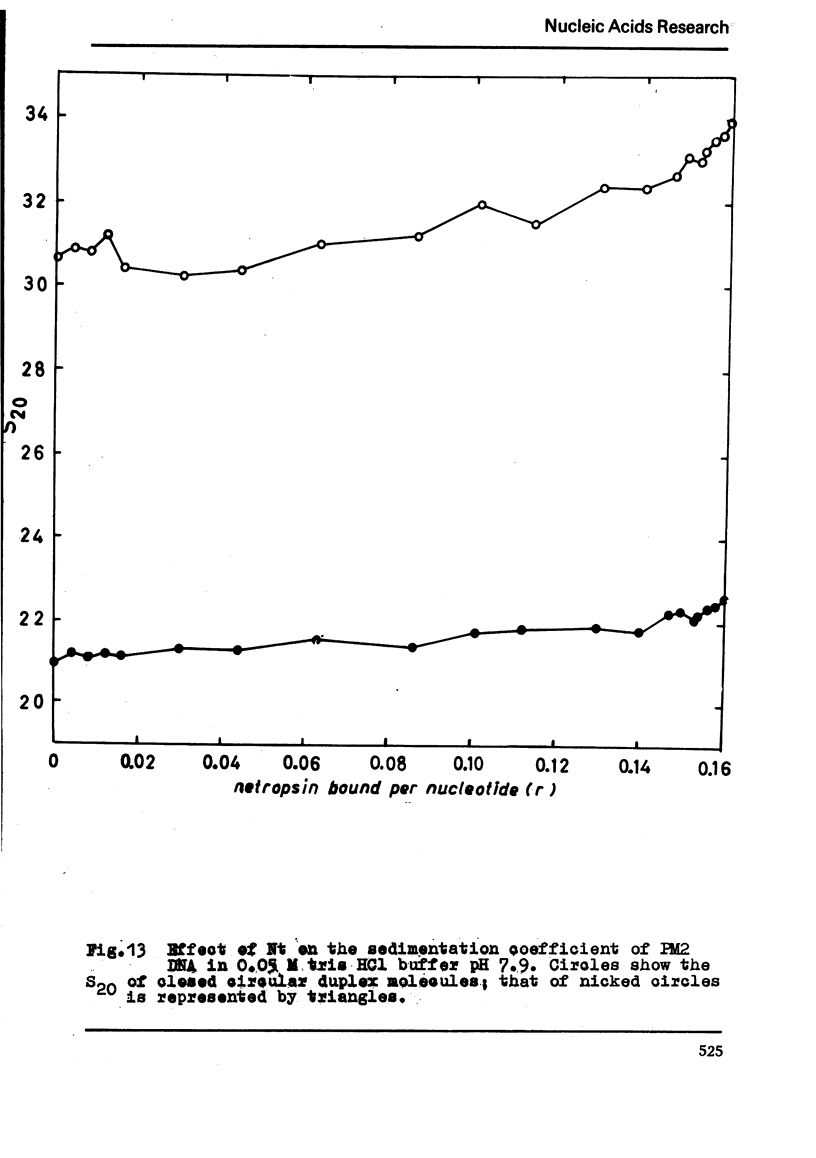
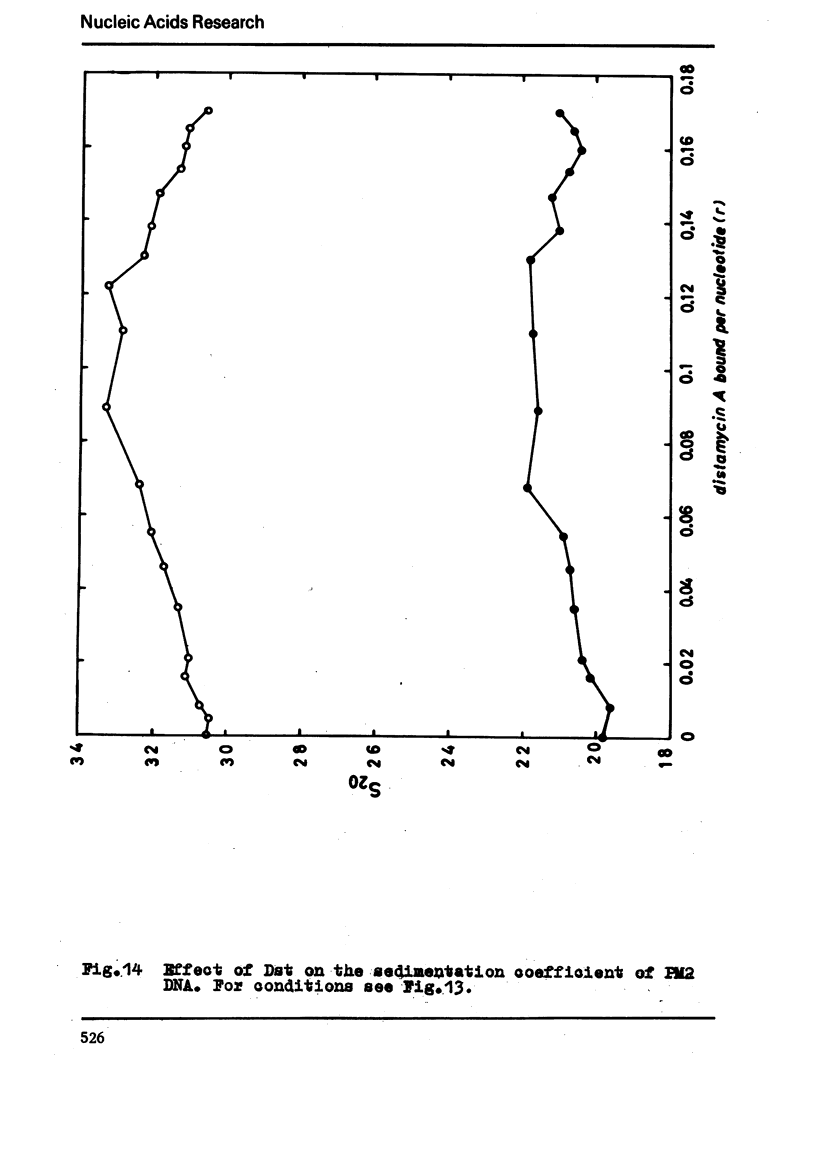
The binding of the antibiotics netropsin and distamycin A to DNA has been studied by thermal melting, CD and sedimentation analysis. Netropsin binds strongly at antibiotic/nucleotide ratios up to at least 0.05. CD spectra obtained using DNA model polymers reveal that netropsin binds tightly to poly (dA) · poly (dT), poly (dA-dT) · poly(dA-dT) and poly (dI-dC) · poly (dI-dC) but poorly, if at all, to poly (dG) · poly (dC). Binding curves obtained with calf thymus DNA reveal one netropsin-binding site per 6.0 nucleotides (Ka=2.9 · 105 M−1); corresponding values for distamycin A are one site per 6.1 nucleotides with Ka= 11.6 · 105 M−1. Binding sites apparently involve predominantly A·T-rich sequences whose specific conformation determines their high affinity for the two antibiotics. It is suggested that the binding is stabilized primarily by hydrogen bonding and electrostatic interactions probably in the narrow groove of the DNA helix, but without intercalation. Any local structural deformation of the helix does not involve unwinding greater than approximately 3° per bound antibiotic molecule.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bram S. Variation of type-B DNA x-ray fiber diagrams with base composition. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2167–2170. doi: 10.1073/pnas.70.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEUGHELMAN M., LANGRIDGE R., SEEDS W. E., STOKES A. R., WILSON H. R., HOOPER C. W., WILKINS M. H., BARCLAY R. K., HAMILTON L. D. Molecular structure of deoxyribose nucleic acid and nucleoprotein. Nature. 1955 May 14;175(4463):834–838. [PubMed] [Google Scholar]
- Müller W., Crothers D. M., Waring M. J. A non-intercalating proflavine derivative. Eur J Biochem. 1973 Nov 1;39(1):223–234. doi: 10.1111/j.1432-1033.1973.tb03120.x. [DOI] [PubMed] [Google Scholar]
- Studdert D. S., Patroni M., Davis R. C. Circular dichroism of DNA: temperature and salt dependence. Biopolymers. 1972;11(4):761–779. doi: 10.1002/bip.1972.360110404. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G. Conformation and reactivity of DNA. 3. Circular dichroism studies of the effects of aqueous concentrated univalent salt solutions upon helix conformation. Biochim Biophys Acta. 1973 Jun 23;312(2):215–227. [PubMed] [Google Scholar]
- Zimmer C., Puschendorf B., Grunicke H., Chandra P., Venner H. Influence of netropsin and distamycin A on the secondary structure and template activity of DNA. Eur J Biochem. 1971 Jul 29;21(2):269–278. doi: 10.1111/j.1432-1033.1971.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Reinert K. E., Luck G., Wähnert U., Löber G., Thrum H. Interaction of the oligopeptide antibiotics netropsin and distamycin A with nucleic acids. J Mol Biol. 1971 May 28;58(1):329–348. doi: 10.1016/0022-2836(71)90250-6. [DOI] [PubMed] [Google Scholar]



