Abstract
Several studies support an association between the chronic inflammatory diseases periodontitis and atherosclerosis with a crucial role for the periodontal pathogen Porphyromonas gingivalis. However, the interplay between this pathogen and the adaptive immune system, including T-cells, is sparsely investigated. Here we used Jurkat T-cells to determine the effects of P. gingivalis on T-cell-mediated adaptive immune responses. We show that viable P. gingivalis targets IL-2 expression at the protein level. Initial cellular events, including ROS production and [Ca2+]i, were elevated in response to P. gingivalis, but AP-1 and NF-κB activity dropped below basal levels and T-cells were unable to sustain stable IL-2 accumulation. IL-2 was partially restored by Leupeptin, but not by Cathepsin B Inhibitor, indicating an involvement of Rgp proteinases in the suppression of IL-2 accumulation. This was further confirmed by purified Rgp that caused a dose-dependent decrease in IL-2 levels. These results provide new insights of how this periodontal pathogen evades the host adaptive immune system by inhibiting IL-2 accumulation and thus attenuating T-cell proliferation and cellular communication.
Introduction
Accumulating amount of data during recent years support an association between periodontitis and atherosclerosis, which both are inflammatory conditions involving different kinds of immune cells [1]. The role of T-cells in atherosclerosis is well established [2], [3], however the involvement of T-cells in the pathogenesis and progression of periodontal disease is not fully elucidated [4]. T-cell activation and subsequent IL-2 secretion, which acts to further promote T-cell proliferation, play an important immune regulatory role. Disruption of IL-2 transcription and expression leads to T-cell anergy [5] and as a consequence this would result in an alteration of the antibody-based immunity by B-cells [6]. Andrukhov and colleagues [7] reported a significant decrease in serum IL-2 levels in patients suffering from periodontitis compared to healthy controls. Furthermore, regulatory T-cells and their release of the anti-inflammatory cytokine IL-10 have, in response to IL-2, been shown to exert anti-atherogenic effects [3], [8], [9]. Both CD4+ and CD8+ T-cells are present in atherosclerotic lesions [10], and may upon activation amplify the inflammatory condition in the atherosclerotic plaque through secretion of cytokines. Sasaki and colleagues [11] suggested the use of anti-CD3 to prevent atherosclerosis development and progression. They showed that administration of anti-CD3 activated regulatory T-cells and reduced atherosclerotic lesions and accumulation of other immune cells. Unravelling the effects of the periodontal pathogen Porphyromonas gingivalis on T-cells will contribute to the clarification of the mechanisms applied by this pathogen to evade host immune responses and cause disease.
P. gingivalis is an anaerobic, gram-negative rod associated with periodontal disease progression including bone and tissue destruction [12]. Lamont and colleagues [13] showed that P. gingivalis could invade and translocate into the cytosol within gingival epithelial cells, demonstrating a possible mechanism for its establishment, replication and subsequent pathogenesis by evading the host immune system. Similar results were observed in heart and aortic endothelial cells [14], indicating an association between P. gingivalis-dependent periodontitis and cardiovascular disease. Another mechanism used by P. gingivalis to evade the immune system is through its ability to inhibit CXCL-8 expression [15], and as a consequence impair immune cell recruitment.
Several factors contribute to the pathogenesis of P. gingivalis, including LPS and cysteine proteinases. Establishment and growth of P. gingivalis has been associated with its production and secretion of proteinases. These enzymes are divided into arginine-specific (Rgp) and lysine-specific (Kgp) gingipains [16]. RgpA-Kgp complexes have been reported to inactivate the T-lymphocyte-derived cytokines IL-4 and IL-5 [17] that are important for the activation and proliferation of B-lymphocytes. Even though cytokines and chemokines are expressed in response to P. gingivalis, their release and subsequent action on leukocyte migration is thus modulated due to the enzymatic activity of proteinases that cleave and inhibit the biological properties of different cytokines, including CXCL-8 [18] and TNF [19]. It is therefore important to determine the interactions between P. gingivalis and different host immune cells, as well as possible alterations in inflammatory gene regulation.
It is important to analyse T-cell responses to P. gingivalis infection, since this periodontal pathogen has been shown to be translocated with T-cells in atherosclerotic plaques. We hypothesize that P. gingivalis is able to suppress T-cell-derived responses, which benefits the pathogen to establish itself and proliferate. The aim of the present study was to characterize the effects of P. gingivalis on T-cell-mediated inflammatory responses and gene regulation.
Materials and Methods
Cell culture conditions
Jurkat T-cells cells (E6-1, ATCC) were maintained in 90% RPMI 1640 medium (Fisher scientific, Austria) with 1.5 mM L-glutamine (Invitrogen, USA) and supplemented with 10% fetal bovine serum (Invitrogen). The cells were incubated in a stable environment at 95% air, 5% CO2 and 37°C.
Bacterial culture conditions and preparation
Porphyromonas gingivalis ATCC 33277 (American Type Culture Collection, Manassas, VA) was grown under anaerobic conditions (80% N2, 10% CO2, and 10% H2) at 37°C in an anaerobic chamber (Concept 400 Anaerobic Workstation; Ruskinn Technology Ltd., Leeds, United Kingdom). The bacteria were cultured for 3 days in fastidious anaerobe broth (29.7 g/liter, pH 7.2) before being washed and resuspended in Krebs-Ringer glucose buffer (KRG) (120 mM NaCl, 4.9 mM KCl, 1.2 mM MgSO4, 1.7 mM KH2PO4, 8.3 mM Na2HPO4, and 10 mM glucose, pH 7.3). The bacterial concentration was adjusted to correlate with approximately 109 CFU/ml, which was determined by viable count where the bacteria were grown on fastidious anaerobe agar (46.0 g/liter supplemented with L-tryptophan 0.1 g/liter, pH 7.2; Lab M, Lancashire, United Kingdom) for 5 days.
Heat-killed P. gingivalis and heat-inactivated P. gingivalis supernatants were prepared following incubation at 70°C for 1 h. To ensure that the bacteria were killed, 10 µl of the heat-killed suspension was spread on a fastidious anaerobe agar plate and incubated at 37°C for 5 days. The absence of colony formation was used as an indicator that no viable bacteria were present in the suspension. P. gingivalis supernatants were sterile filtered through a 0.2 µm filter before being used. Both P. gingivalis and its supernatant were used fresh for every experiment.
Two selected inhibitors of cysteine proteinases (Leupeptin, Roche Diagnostics Corporation, USA and Cathepsin B Inhibitor II, Calbiochem, Germany) were used to determine the role of Arg- and Lys-gingipain activities. Viable P. gingivalis were incubated with different concentrations of the inhibitors for 1 h prior to stimulation of Jurkat T-cells. To further assess the contribution of gingipains, purified Arg-gingipain B (RgpB, Athens GA, USA) was used.
E. coli MG1655 were grown on Luria-Bertani (LB) plates and incubated at 37°C overnight. Single colony was inoculated into 10 ml LB and the tube was incubated at 37°C overnight on shaker set at 200 rpm. The bacteria were then harvested for 10 min at 3000×g, washed with 3 ml KRG and re-suspended in KRG.
Isolation of primary cells
PBMC were isolated by the density gradient medium Ficoll-Paque™ Plus (Amersham Biosciences, Sweden) according to the manufacturers' instructions. Briefly, freshly collected blood from healthy donors was diluted with an equal volume of PBS, and 4 ml were carefully layered on top of 3 ml Ficoll-Paque Plus. The tubes were centrifuged at room temperature for 30 min at 300×g. PBMC were recovered from the interface and washed twice with PBS to remove excess Ficoll-Paque Plus and platelets. The cells were suspended in RPMI media supplemented with 10% foetal bovine serum and incubated in a stable environment at 95% air, 5% CO2 and 37°C for 2 days. Suspended cells were recovered, washed and cultured in a separate T75 flask for 24 h. The cells were then used to determine IL-2 expression in response to P. gingivalis.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed on supernatants from challenged Jurkat T-cells to quantify IL-2 (BD OptEIA Set Human IL-2, BD Biosciences, USA) according to the manufacturer's instructions. Briefly, Jurkat T-cells were either pre-treated with P. gingivalis or bacterial supernatant for 1 h followed by stimulation with 50 ng/ml PMA and 1 µg/ml Calcium Ionophore (Calcium Ionophore A23187 mixed calcium magnesium salt, Sigma #C5149, USA) or stimulated with PMA and Calcium Ionophore prior to treatment with P. gingivalis or bacterial supernatant. The cells were thereafter centrifuged at 95×g for 5 min and the supernatants were collected and stored at −80°C until use.
Transfection and luciferase measurement
Activator protein (AP)-1 and nuclear factor (NF)-κB activity were measured by using luciferase reporter plasmids. Briefly, reporter plasmid (pAP1-Luc, NF-κB-Luc), internal control plasmid (Renilla) (Promega, USA) and lipofectamine 2000 (Invitrogen, USA) were added to each well at 0.54 µg/well, 0.06 µg/well and 1.5 µl/well, respectively. Initially, reporter plasmid and Renilla were mixed separately with OptiMEM (Gibco, USA). After 5 min of incubation at room temperature, lipofectamine 2000 was added and the mixture was incubated further for 20 min at room temperature. The transfection was allowed to proceed overnight at 37°C, after which, the cells were centrifuged, the media removed and fresh pre-warmed media added. The cells were lysed and luciferase activity was measured using the Dual-Luciferase® reporter assay system (Promega, USA) according to the manufacturer's instructions on a TD 20/20 luminometer (Turner Designs, Sunnyvale, CA).
Measurement of ROS production
ROS production in Jurkat T-cells was analyzed using a lumiaggregometer (Chrono-Log Corp., Havertown, PA). Briefly, Jurkat T-cells (106 cells/ml) were suspended in complete RPMI 1640 media supplemented with 10% FBS containing 50 µM luminol and 4 U/ml HRP. The cells were incubated at 37°C for 15 min, at 800 rpm, before being stimulated with 108 CFU/ml P. gingivalis for 30 min, during which time chemiluminescence was registered.
Measurement of [Ca2+]i
Cytosolic Ca2+ concentration was measured by using the fluorescent indicator Fura-2. Briefly, Jurkat T-cells were washed twice and resuspended in KRG to yield a cell-density of 106 cells/ml. The cells were loaded with 4 µM Fura-2-acetoxymethylester (AM) for 40 min during gentle agitation. The cells were then washed twice. The extracellular Ca2+ concentration was set to 1 mM by addition of CaCl2 and intracellular Ca2+ concentration was determined in 2 ml aliquots at 37°C, 300 rpm using a Hitachi F2000 spectrofluorometer (Hitachi Ltd. Tokyo, Japan). Fluorescence emission and excitation was registered at 510 nm and 340/380 nm, respectively. Maximal and minimal ratios were determined following addition of 0.1% Triton X-100 and 24 mM EGTA. The change in intracellular Ca2+ concentration was calculated by using the equation described by Grynkiewicz and colleagues [20]. Calcium Ionophore was used as a positive control.
Reverse transcription quantitative PCR (RT-qPCR)
RT-qPCR was used to determine gene expression levels of il-2 in response to viable and heat-killed P. gingivalis. Briefly, Jurkat T-cells were pre-treated with P. gingivalis for 1 h, followed by stimulation with PMA/Ionophore for 24 h. RNA was extracted using RNeasy® Plus Micro Kit (Qiagen, USA) according to the manufacturer's recommendations. Reverse transcription was performed using Maxima® First Strand cDNA Synthesis Kit (Fermentas, Sweden). The following primer sequences were used: forward- ACCTCAACTCCTGCCACAATGTAC, reverse- TCAGTTCTGTGGCCTTCTTGGGCA. Thermal cycling conditions for SYBR Green (Fermentas) consisted of a denaturation step at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Gene expression was analyzed using a 7900 HT real-time PCR instrument (Applied Biosystems). The obtained Ct values were normalized against GAPDH. Relative quantification of gene-expression was determined by using the ΔΔCt method. The ΔCt was calculated by subtracting the Ct of GAPDH from the Ct of il-2 for each sample. The ΔΔCt was calculated by subtracting the ΔCt of the control sample from the ΔCt of each treated sample. Fold change was generated by using the equation 2−ΔΔCt.
Fluorescence microscopy
Jurkat T-cells were stimulated with FITC-labeled P. gingivalis (108 CFU/ml, MOI:100) for 24 h and fixation overnight in 4% paraformaldehyde (PFA) at 4°C. F-actin was visualized by incubating the cells with 2 units Alexa Fluor® 594 phalloidin (Invitrogen) and 100 µg/ml lysophosphatidylcholine in darkness for 1 h at room temperature. The cells were washed and mounted on a coverslip. Adherence of P. gingivalis to Jurkat T-cells was analyzed by confocal microscopy (Leica Microsystems, Heidelberg, Germany).
Statistical analysis
Statistical significant differences were determined using two-tailed Student's t-test (*p<0.05; **p<0.01; ***p<0.001).
Results
P. gingivalis triggers [Ca2+]i changes
Changes in intracellular free calcium concentration [Ca2+]i is an important action for the establishment of a proper response to foreign pathogens. This prompted us to determine the levels of [Ca2+]i in Jurkat T-cells in response to viable- and heat-killed P. gingivalis. The basal [Ca2+]i levels of unstimulated cells was around 45±14 nM. Viable P. gingivalis increased [Ca2+]i by 5.1, 4.5 and 8.3 fold in response to 107, 5×107 and 108 CFU/ml, respectively (figure 1a). However, T-cells did not respond when P. gingivalis were heat-killed (HK, figure 1a). Representative traces of the calcium changes following treatment of Jurkat T-cells with viable E. coli MG1655, viable P. gingivalis and heat-killed P. gingivalis are shown in figure 1b. As a positive control we used the calcium ionophore ionomycin, which caused a significant increase in [Ca2+]i by ∼78 fold (data not shown). Viable E. coli MG1655 (5×107 CFU/ml) was used as a control, and resulted in minor changes in [Ca2+]i.
Figure 1. Viable P. gingivalis increased [Ca2+]i concentration in Jurkat T-cells.
[Ca2+]i was measured by loading Jurkat T-cells (106 cells/ml) with the fluorescent indicator Fura-2. A- Cells were exposed to the indicated concentrations of either viable (black bars)- or heat-killed (HK, grey bars) P. gingivalis or Viable E. coli MG1655 (5×107 CFU/ml, MOI:50). Ca2+ release was induced in response to viable, but not heat-killed bacteria (MOI:10, 50 and 100, respectively). B- Representative curves of the excitation wavelengths 340 nm and 380 nm as well as the calculated Ca2+ concentrations following treatment of Jurkat T-cells with viable E. coli MG1655, viable P. gingivalis and heat-killed P. gingivalis, respectively. The arrows indicate the starting point of stimulation. Data shown are the mean±SD of three independent experiments. *-p<0.05; ***-p<0.001 (Student's t-test).
P. gingivalis binds to T-cells and induces ROS production
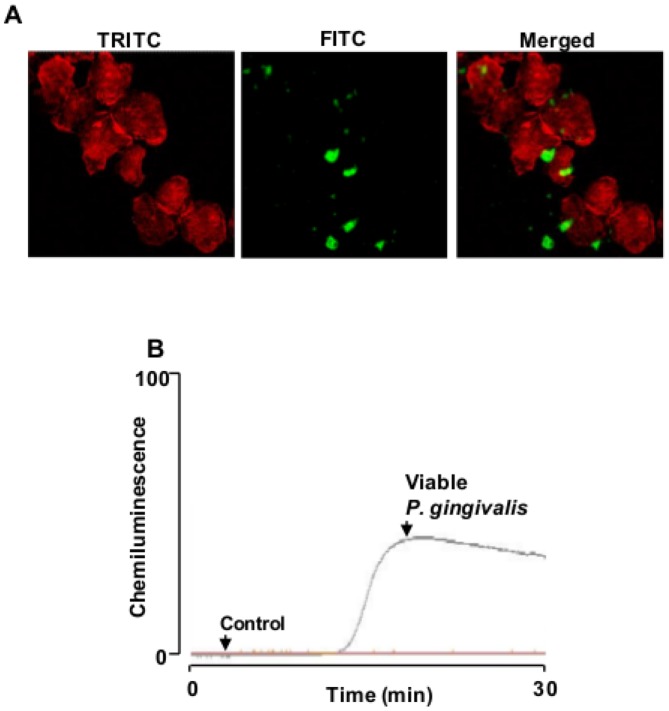
The ability of P. gingivalis to attach to Jurkat T-cells and cause cell aggregation was shown by labeling the bacteria with FITC and stain the actin cytoskeleton with Alexa Fluor 594 phalloidin (figure 2a). We found that P. gingivalis efficiently binds to Jurkat T-cells and stimulate morphological changes and aggregate formation. As a consequence, bactericidal molecules, such as reactive oxygen species (ROS) may be released of P. gingivalis- T-cells interaction. ROS may function either, at low concentrations, as signaling molecules derived from different metabolic processes or, at high concentrations, as bactericidal toxic molecules produced by NADPH-oxidases. By using luminol-dependent chemiluminescence, changes in ROS production over time was analyzed in Jurkat T-cells following exposure to P. gingivalis. The cells where either left untreated, or exposed to viable P. gingivalis for 30 min. After a lag phase of around 12–14 min, viable P. gingivalis caused an extensive and long-lasting ROS production reaching maximum after 20 min (figure 2b).
Figure 2. P. gingivalis is able to attach and induce ROS production in Jurkat T-cells.
A- Jurkat T-cells cells were stimulated with FITC-labeled P. gingivalis (108 CFU/ml, MOI:100) for 24 h and analyzed by confocal microscopy. Magnification is ×63, with a 2× digital zoom. B- ROS production in Jurkat T-cells was detected by luminol-amplified chemiluminescence following treatment with 108 CFU/ml of viable P. gingivalis (MOI:100) for 30 min. Shown is a representative graph of five independent experiments.
Viable P. gingivalis inhibits IL-2 expression and accumulation
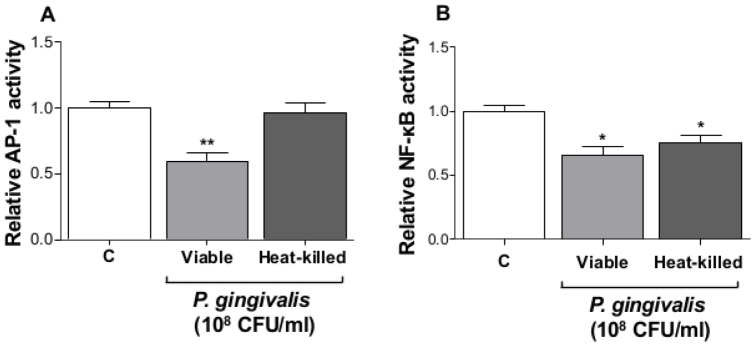
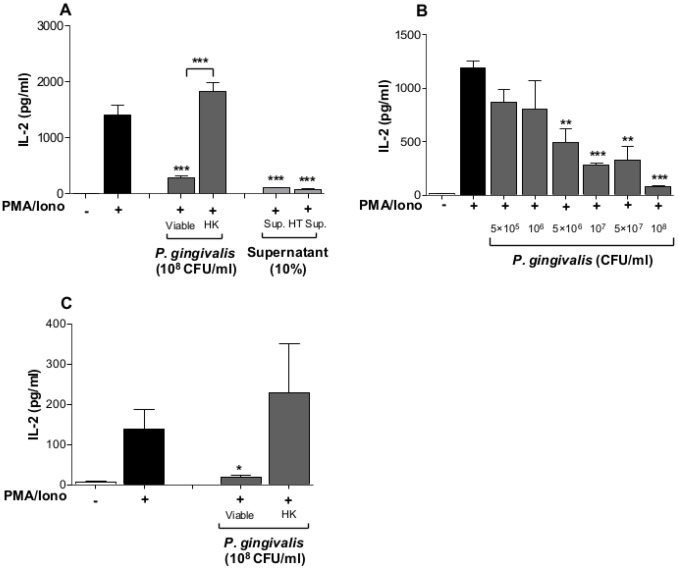
Alteration in the levels of intracellular calcium and ROS prompted us to determine AP-1 activity in response to P. gingivalis. Jurkat T-cells were transfected with luciferase-reporter plasmids containing cis-acting elements for AP-1 or NF-κB, followed by exposure to viable or heat-killed P. gingivalis. Viable, but not heat-killed, P. gingivalis caused a significant inhibition of AP-1 activity (figure 3a), while both viable and heat-killed P. gingivalis reduced basal level of NF-κB activity (figure 3b). Considering the apparent effects on Jurkat T-cell signaling involving calcium, ROS and the transcription factors AP-1 and NF-κB, we then determined whether P. gingivalis and its supernatant affected accumulation of IL-2. The PMA/Ionophore-induced IL-2 accumulation after 24 h was significantly decreased by pre-treatment with viable bacteria, while exposure to heat-killed bacteria resulted in increased IL-2 levels, compared to the positive control (figure 4a). Pre-exposure of Jurkat T-cells to either untreated or heat-treated P. gingivalis supernatant resulted in a significant reduction in IL-2 levels. A viability assay showed that the observed inhibition of IL-2 accumulation by viable P. gingivalis was not due to cell death. Cell-density increased by 20% after stimulation of Jurkat T-cells with viable P. gingivalis for 24 h, compared to 50% increase in the control and cells treated with heat-killed P. gingivalis (data not shown). Furthermore, treatment of Jurkat T-cells with either viable or heat-killed P. gingivalis, without PMA/Ionophore, did not alter IL-2 levels compared to the untreated negative control (data not shown). Inhibition of IL-2 accumulation by bacterial supernatants was shown to be due to small molecular weight compounds present in the fastidious anaerobe broth, as determined by molecular mass fractionation, rather than any bacterial-derived compound(s) (data not shown).
Figure 3. P. gingivalis suppressed AP-1 and NF-κB activity.
Jurkat T-cells (106 cells/ml) were transfected with either AP-1 (A) or NF-κB (B) luciferase reporter plasmids. The cells were treated with viable or heat-killed P. gingivalis (108 CFU/ml, MOI:100) for 24 h. AP-1 and NF-κB activation were determined by measuring luciferase activity, which was normalized against the internal control Renilla. *-p<0.05; **-p<0.01 (Student's t-test).
Figure 4. IL-2 accumulation decreases in response to viable P. gingivalis and its derived supernatant.
A- Jurkat T-cells (106 cells/ml) were pre-treated with 108 CFU/ml of viable or heat-killed (HK) P. gingivalis (MOI:100) as well as 10% untreated- or heat-treated (HT) supernatant from P. gingivalis broth cultures for 1 h. The cells were then stimulated with 50 ng/ml PMA and 1 µg/ml Calcium ionophore for 24 h. IL-2 accumulation was significantly reduced by viable, but not heat-killed P. gingivalis in Jurkat T-cells, while both untreated and heat-treated bacterial supernatant resulted in a significant IL-2 reduction. B- T-cells were pre-treated with the indicated concentrations of viable P. gingivalis (MOI:0.5, 1, 5, 10, 50 and 100, respectively) for 1 h followed by stimulation with 50 ng/ml PMA and 1 µg/ml Calcium ionophore for 24 h. IL-2 accumulation was reduced in a dose-dependent manner. C- Primary cells were isolated as described in materials and methods. Cells were pre-treated with viable or heat-killed P. gingivalis for 1 h, followed by stimulation with 50 ng/ml PMA and 1 µg/ml Calcium ionophore for 24 h. Viable, but not heat-killed P. gingivalis (MOI:100) resulted in a significant reduction in IL-2 accumulation. *-p<0.05; **-p<0.01; ***-p<0.001 (Statistical significance between different treatments and the positive control PMA/Iono, Student's t-test).
Furthermore, pre-incubation of Jurkat T-cells with different concentrations of viable P. gingivalis showed that inhibition of IL-2 accumulation was dose-dependent (figure 4b). A final concentration of 5×106 CFU/ml (MOI:5) of viable bacteria was sufficient to significantly reduce IL-2 accumulation by ∼2.4 fold, compared to the positive control PMA/Ionophore, while the highest concentration 108 CFU/ml reduced IL-2 accumulation by ∼14 fold. We thereafter aimed to confirm these results by using primary cells isolated from healthy volunteers. Viable, but not heat-killed P. gingivalis caused a significant reduction in IL-2 accumulation (figure 4c).
Viable P. gingivalis targets IL-2 at the protein level
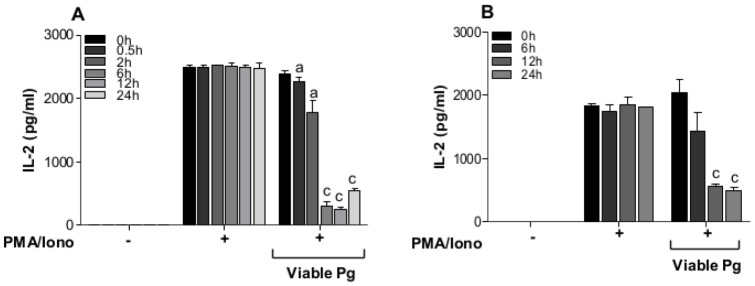
In the experiments so far, the cells have been pre-exposed with either viable or heat-killed P. gingivalis to determine whether this treatment can alter intracellular mechanisms involved in IL-2 transcription and expression. In order to determine whether viable P. gingivalis affect produced and accumulated IL-2, Jurkat T-cells were first stimulated with PMA/Ionophore for 24 h, and then exposed to viable P. gingivalis for the indicated times (figure 5). IL-2 levels remained constant over time (0.5–24 h) in the untreated and the PMA/Ionophore-stimulated groups, while treatment with viable P. gingivalis resulted in a significant reduction in IL-2 levels already after 30 min and the levels continued to decrease over time (figure 5a). A second set of experiment was performed to determine whether the bacteria can alter the expression and subsequent release of IL-2. Jurkat T-cells were stimulated for IL-2 production as mentioned above, followed by centrifugation to exclude the cells. P. gingivalis was then added to the cell-free, IL-2 containing media for the indicated times (figure 5b). A similar trend was observed where P. gingivalis caused a significant reduction in IL-2 levels.
Figure 5. Viable P. gingivalis cleaves and prevents IL-2 accumulation.
A- Jurkat T-cells (106 cells/ml) were stimulated with 50 ng/ml PMA and 1 µg/ml Calcium ionophore for 24 h followed by exposure to viable P. gingivalis (Viable Pg, 108 CFU/ml, MOI:100) for the indicated times. IL-2 accumulation was significantly decreased by P. gingivalis over time. B- Jurkat T-cells (106 cells/ml) were stimulated with 50 ng/ml PMA and 1 µg/ml Calcium ionophore for 24 h. The cells were then removed and viable P. gingivalis (Viable Pg, 108 CFU/ml, MOI:100) were added to cell-culture supernatants, containing secreted IL-2, for the indicated times. P. gingivalis is involved in cleaving and de-activating IL-2 proteins. The letters indicate significant differences compared to their respective positive control PMA/Iono at each specific time point. a-p<0.05; c-p<0.001 (Student's t-test).
We then aimed to determine whether the observed inhibition of the extracellular IL-2 accumulation was affected at the transcript level. Jurkat T-cells were pre-treated with viable- or heat-killed P. gingivalis, followed by induction with PMA/Ionophore. RT-qPCR analysis showed that il-2 mRNA levels, induced by PMA/Ionophore, remained elevated in response to both viable- and heat-killed P. gingivalis treatment (figure 6).
Figure 6. RT-qPCR analysis of il-2 gene-expression in response to P. gingivalis.

Jurkat T-cells (106 cells/ml) were pre-treated with 108 CFU/ml viable- or heat-killed (HK) P. gingivalis (MOI:100) for 1 h followed by stimulation with 50 ng/ml PMA and 1 µg/ml Calcium ionophore for 24 h. Il-2 mRNA levels were not affected by viable or heat-killed P. gingivalis. ***-p<0.001 (Statistical significance between different treatments and the positive control PMA/Iono, Student's t-test).
Degradation of IL-2 by arginine gingipains
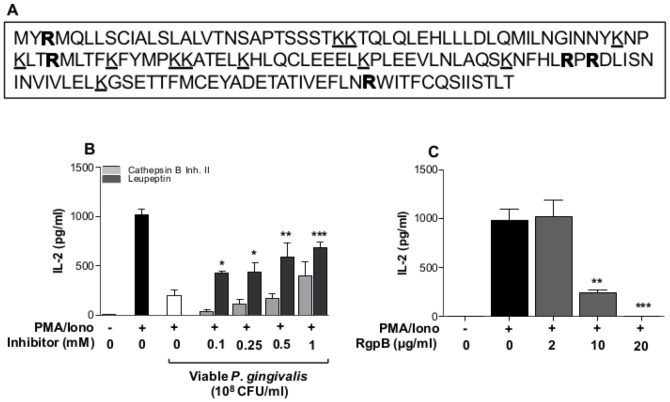
The ability of viable P. gingivalis to cleave or in-activate the pre-accumulated IL-2 led us hypothesize that P. gingivalis-derived proteinases are involved in the inhibition of IL-2 accumulation. Analysis of the IL-2 amino acid sequence revealed 5 arginine cleavage sites and 11 lysine cleavage sites (figure 7a). In order to determine the involvement of these specific proteinases, viable bacteria were incubated with the selected arginine-specific and lysine-specific proteinase inhibitors Leupeptin and Cathepsin B inhibitor II, respectively, prior to exposure of Jurkat T-cells. The results showed that inhibition of IL-2 accumulation was partially dependent on the action of Rgp proteinases, either released and free in suspension or bound to the bacteria (figure 7b). Furthermore, treatment of PMA/Ionophore stimulated Jurkat T-cells with purified RgpB resulted in a dose-dependent inhibition of IL-2 accumulation (figure 7c).
Figure 7. The proteinase inhibitor Leupeptin partially restored IL-2 accumulation.
A- IL-2 amino acid sequence with predicted Rgp (bold)- and Kgp (underlined) cleavage sites. Five Rgp sites and 11 Kgp sites were found, source: http://www.ncbi.nlm.nih.gov/CCDS/CcdsBrowse.cgi?REQUEST=CCDS&DATA=CCDS3726. B- The involvement of Rgp and Kgp in IL-2 cleavage was determined by using Cathepsin B inhibitor II and Leupeptin. Viable P. gingivalis were incubated with the indicated concentration for 1 h prior to exposure of cells. Jurkat T-cells (106 cells/ml) were pre-treated with 108 CFU/ml of viable P. gingivalis (MOI:100) for 1 h followed by stimulated with 50 ng/ml PMA and 1 µg/ml Calcium ionophore for 24 h. IL-2 accumulation was partially restored by Leupeptin, but not by Cathepsin B inhibitor. The asterisks indicate significant differences compared to their respective control (Viable Pg, without Leupeptin). C- Purified RgpB resulted in a dose-dependent inhibition of IL-2 accumulation. The asterisks indicate significant differences compared to the positive control PMA/Iono. *-p<0.05; **-p<0.01 (Student's t-test).
Discussion
T-cells are found in atherosclerotic plaques and their contribution to the progression of this inflammatory condition is well established [2]. Proliferation of effector T-cells in plaques requires IL-2, and the activity of effector T-cells is strictly controlled by regulatory T-cells [3]. Intracellular free Ca2+ is an important signaling molecule that is required for the activation of T-cells upon recognition of a foreign antigen [21]. Activation of the transcription factors AP-1, NFAT and NF-κB, and subsequent gene-expression and cytokine release requires a sustained Ca2+ influx [22]. These transcription factors are activated through Ca2+-dependent signaling proteins, including PKC [23] and calmodulin/calcineurin complex [24]. We found that intracellular Ca2+ levels increased in response to viable, but not heat-killed, P. gingivalis. The fact that only viable bacteria were able to elevate [Ca2+]i may be due to that they possess intact fimbriae, which has been reported as an important feature, enabling the bacteria to attach, invade and induce an inflammatory response [25]. Furthermore, heat-sensitive bacterial proteinases have been reported to cleave and thus activate proteinase-activated receptor-2 (PAR-2), which is expressed on T-cells [26], leading to an increase in [Ca2+]i [27].
Intracellular ROS production is important for elimination of invading pathogens and has been reported to influence T-cell activation [28]. In this study, we found that P. gingivalis binds to and aggregate T-cells and induces an extensive ROS production, which may reflect an ability of this pathogen to recognize specific cell surface receptors and induce intracellular signaling cascades involving ROS. P. gingivalis has previously been shown to affect the activity and/or expression of several cell surface receptors that are expressed on a variety of cells, including T-cells. These receptors include proteinase-activated receptors that are activated by P. gingivalis- derived proteinases [29]. However, microarray analysis showed an inhibitory effect of TCR expression by this periodontal pathogen [4]. Furthermore, Kitamura and colleagues reported that P. gingivalis was able to cleave CD4 and CD8 and thus impair T-cell activation [30]. P. gingivalis probably utilizes such a strategy to evade the host immune system, which benefits its establishment and proliferation. Several studies have reported the ability of P. gingivalis to invade host cells, including gingival epithelial cells [13] and heart endothelial cells [14] and that expression of fimbriae enables pathogen attachment and invasion [31]. Other strategies utilized by this pathogen to evade the host immune system are by affecting cells of the innate immune system. These effects are mainly due to the action P. gingivalis-derived proteinases and include proteolysis of CD14 on monocytes [32] and C5a receptor on neutrophils [33]. This would, as a consequence, lead to reduced bacterial recognition by monocytes and neutrophil migration, respectively.
Furthermore, viable P. gingivalis reduced AP-1 and NF-κB activity below basal levels. Transcriptional regulators, including AP-1 and NF-κB, are important for inflammatory gene-expression, such as CXCL-8 and IL-6 [34]. In addition, AP-1 has been shown to be an important regulator of IL-2 expression, in cooperation with NFAT [35], through PKC [36]. Mutation of the NF-κB site did not affect IL-2 expression, while mutation of the AP-1 site or PKC depletion almost revoked IL-2 release. These observations indicate that the MAPK pathway and the transcription factor AP-1 play an important role in the induction of inflammatory responses in Jurkat T-cells. We therefore aimed to determine IL-2 expression in response to P. gingivalis. Presence of viable P. gingivalis significantly inhibited PMA/Ionophore-induced IL-2 accumulation in Jurkat T-cells suspension. This effect may be due to the action of P. gingivalis-derived proteinases that have been reported to regulate several cytokines and chemokines. Kobayashi-Sakamoto and colleagues [18] reported the involvement of proteinases in degradation of CXCL-8 and MCP-1 and the Th2 cytokine IL-4 and IL-5 have also been shown to be targets for degradation by P. gingivalis proteinases [17]. However, receptor activator of NF-κB ligand (RANKL) is induced in Jurkat T-cells by P. gingivalis secreted compounds [37], while P. gingivalis outer membrane proteins were shown to induce IL-17 rather than RANKL [38]. These observations indicate that different P. gingivalis-derived components can differentially regulate cytokine expression.
We thereafter aimed to determine whether addition of P. gingivalis could alter a pre-stimulated accumulation of IL-2 and observed that only viable bacteria were able to reduce IL-2 levels and did that in a time-dependent manner. IL-2 was shown to be targeted at the protein level, since the transcript levels were not affected by P. gingivalis. This indicates that P. gingivalis regulates T-cell activation and proliferation by targeting the accumulation of IL-2, considering the importance of this cytokine in P. gingivalis-mediated T-cell proliferation [39].
P. gingivalis-derived proteinases have been reported to inhibit cytokine and chemokine expression [40]. This inhibitory effect is dependent on the enzymatic activity of these proteinases that cleave and inactivate several inflammatory markers, including TNF [19] and IL-6 [41]. Yun and colleagues [42] showed that P. gingivalis-derived proteinases activated T-cells through protease-activated receptors, but were also able to degrade CD27, which is a TNF receptor family member, as well as its ligand CD70, present on B-cells. These observations indicate that cellular communication is interrupted by P. gingivalis and may result in an impaired host immune response and less efficient clearance of an infection. The involvement of proteinases in the inhibition of IL-2 accumulation in response to viable P. gingivalis, was shown by the partial restoration of IL-2 in the presence of Leupeptin. Furthermore, purified gingipains completely antagonized IL-2 accumulation. By considering the IL-2 amino acid sequence, the number of arginine and lysine cleavage sites were predicted and corresponded to 5 and 11, respectively. However, IL-2 was shown to be targeted by Rgp rather than Kgp proteinases. This is in accordance with the observations made by Yun and colleagues [42] showing that T-cell activation and subsequent cellular communication are interrupted by arginine-specific cysteine proteinases.
The importance of sustained IL-2 levels for growth of regulatory T-cells has previously been reported [43]. The effects of P. gingivalis and its proteinases on T-cells are evident. We show that IL-2 accumulation is targeted by P. gingivalis at the protein level, and partially through suppression of AP-1, unraveling a mechanism applied by P. gingivalis to benefit its establishment by altering adaptive immune responses. Hence, alteration of IL-2 levels benefits bacterial establishment and may also contribute to progression of the inflammatory state in atherosclerosis, considering that IL-2 play an important role in the clonal expansion of regulatory T-cells. Furthermore, effector T-cells are found in atherosclerotic plaques and are considered to contribute to the progression of the inflammatory process. Identification of immune-regulatory compounds from P. gingivalis and the effects of these compounds on different T-cell subsets may be crucial in the development of new strategies to restrict further progression of atherosclerotic plaque formation and development.
Funding Statement
This work was supported by grants from the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Fund for Research without Animal Experiments, the Swedish Heart and Lung Association and the Foundation of Olle Engkvist. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tonetti MS (2009) Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol 36 Suppl 1015–19. [DOI] [PubMed] [Google Scholar]
- 2. Hansson GK, Jonasson L (2009) The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 29: 1714–1717. [DOI] [PubMed] [Google Scholar]
- 3. Nilsson J, Wigren M, Shah PK (2009) Regulatory T cells and the control of modified lipoprotein autoimmunity-driven atherosclerosis. Trends Cardiovasc Med 19: 272–276. [DOI] [PubMed] [Google Scholar]
- 4. Gemmell E, Yamazaki K, Seymour GJ (2007) The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000 43: 14–40. [DOI] [PubMed] [Google Scholar]
- 5. Macian F, Im SH, Garcia-Cozar FJ, Rao A (2004) T-cell anergy. Curr Opin Immunol 16: 209–216. [DOI] [PubMed] [Google Scholar]
- 6. Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, et al. (2005) Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol 3: e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrukhov O, Ulm C, Reischl H, Nguyen PQ, Matejka M, et al. (2011) Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontol 82: 885–892. [DOI] [PubMed] [Google Scholar]
- 8. Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, et al. (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12: 178–180. [DOI] [PubMed] [Google Scholar]
- 9. Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, et al. (1999) Protective role of interleukin-10 in atherosclerosis. Circ Res 85: e17–24. [DOI] [PubMed] [Google Scholar]
- 10. Ross R (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 11. Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, et al. (2009) Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation 120: 1996–2005. [DOI] [PubMed] [Google Scholar]
- 12. Holt SC, Kesavalu L, Walker S, Genco CA (1999) Virulence factors of Porphyromonas gingivalis. Periodontol 2000 20: 168–238. [DOI] [PubMed] [Google Scholar]
- 13. Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, et al. (1995) Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun 63: 3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande RG, Khan MB, Genco CA (1998) Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun 66: 5337–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Darveau RP, Belton CM, Reife RA, Lamont RJ (1998) Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun 66: 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuramitsu HK (1998) Proteases of Porphyromonas gingivalis: what don't they do? Oral Microbiol Immunol 13: 263–270. [DOI] [PubMed] [Google Scholar]
- 17. Tam V, O′Brien-Simpson NM, Chen YY, Sanderson CJ, Kinnear B, et al. (2009) The RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis Inactivate the Th2 cytokines interleukin-4 and interleukin-5. Infect Immun 77: 1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi-Sakamoto M, Isogai E, Hirose K (2003) Porphyromonas gingivalis modulates the production of interleukin 8 and monocyte chemotactic protein 1 in human vascular endothelial cells. Curr Microbiol 46: 109–114. [DOI] [PubMed] [Google Scholar]
- 19. Calkins CC, Platt K, Potempa J, Travis J (1998) Inactivation of tumor necrosis factor-alpha by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. Implications of immune evasion. J Biol Chem 273: 6611–6614. [DOI] [PubMed] [Google Scholar]
- 20. Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450. [PubMed] [Google Scholar]
- 21. Cui J, Bian JS, Kagan A, McDonald TV (2002) CaT1 contributes to the stores-operated calcium current in Jurkat T-lymphocytes. J Biol Chem 277: 47175–47183. [DOI] [PubMed] [Google Scholar]
- 22. Quintana A, Kummerow C, Junker C, Becherer U, Hoth M (2009) Morphological changes of T cells following formation of the immunological synapse modulate intracellular calcium signals. Cell Calcium 45: 109–122. [DOI] [PubMed] [Google Scholar]
- 23. Patrussi L, Baldari CT (2008) Intracellular mediators of CXCR4-dependent signaling in T cells. Immunol Lett 115: 75–82. [DOI] [PubMed] [Google Scholar]
- 24. Pores-Fernando AT, Zweifach A (2009) Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol Rev 231: 160–173. [DOI] [PubMed] [Google Scholar]
- 25. Aoki Y, Tabeta K, Murakami Y, Yoshimura F, Yamazaki K (2010) Analysis of immunostimulatory activity of Porphyromonas gingivalis fimbriae conferred by Toll-like receptor 2. Biochem Biophys Res Commun 398: 86–91. [DOI] [PubMed] [Google Scholar]
- 26. Shpacovitch VM, Brzoska T, Buddenkotte J, Stroh C, Sommerhoff CP, et al. (2002) Agonists of proteinase-activated receptor 2 induce cytokine release and activation of nuclear transcription factor kappaB in human dermal microvascular endothelial cells. J Invest Dermatol 118: 380–385. [DOI] [PubMed] [Google Scholar]
- 27. Lourbakos A, Chinni C, Thompson P, Potempa J, Travis J, et al. (1998) Cleavage and activation of proteinase-activated receptor-2 on human neutrophils by gingipain-R from Porphyromonas gingivalis. FEBS Lett 435: 45–48. [DOI] [PubMed] [Google Scholar]
- 28. Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R (2011) NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15: 2197–2208. [DOI] [PubMed] [Google Scholar]
- 29. Guo Y, Nguyen KA, Potempa J (2010) Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 54: 15–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kitamura Y, Matono S, Aida Y, Hirofuji T, Maeda K (2002) Gingipains in the culture supernatant of Porphyromonas gingivalis cleave CD4 and CD8 on human T cells. J Periodontal Res 37: 464–468. [DOI] [PubMed] [Google Scholar]
- 31. Weinberg A, Belton CM, Park Y, Lamont RJ (1997) Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun 65: 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugawara S, Nemoto E, Tada H, Miyake K, Imamura T, et al. (2000) Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J Immunol 165: 411–418. [DOI] [PubMed] [Google Scholar]
- 33. Jagels MA, Travis J, Potempa J, Pike R, Hugli TE (1996) Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect Immun 64: 1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khalaf H, Jass J, Olsson PE (2010) Differential cytokine regulation by NF-kappaB and AP-1 in Jurkat T-cells. BMC Immunol 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Macian F, Lopez-Rodriguez C, Rao A (2001) Partners in transcription: NFAT and AP-1. Oncogene 20: 2476–2489. [DOI] [PubMed] [Google Scholar]
- 36. Jain J, Valge-Archer VE, Sinskey AJ, Rao A (1992) The AP-1 site at −150 bp, but not the NF-kappa B site, is likely to represent the major target of protein kinase C in the interleukin 2 promoter. J Exp Med 175: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belibasakis GN, Reddi D, Bostanci N (2011) Porphyromonas gingivalis induces RANKL in T-cells. Inflammation 34: 133–138. [DOI] [PubMed] [Google Scholar]
- 38. Oda T, Yoshie H, Yamazaki K (2003) Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol 18: 30–36. [DOI] [PubMed] [Google Scholar]
- 39. Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, et al. (2004) Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol 172: 4744–4751. [DOI] [PubMed] [Google Scholar]
- 40. Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF (2009) The host cytokine response to Porphyromonas gingivalis is modified by gingipains. Oral Microbiol Immunol 24: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kadowaki T, Nakayama K, Okamoto K, Abe N, Baba A, et al. (2000) Porphyromonas gingivalis proteinases as virulence determinants in progression of periodontal diseases. J Biochem 128: 153–159. [DOI] [PubMed] [Google Scholar]
- 42. Yun LW, Decarlo AA, Hunter N (2007) Blockade of protease-activated receptors on T cells correlates with altered proteolysis of CD27 by gingipains of Porphyromonas gingivalis. Clin Exp Immunol 150: 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA (2007) IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 178: 2018–2027. [DOI] [PubMed] [Google Scholar]