Abstract
Background
Hormonal contraceptive (HC) use may increase cardiometabolic risk; however, the effect of HC on emerging cardiometabolic and other disease risk factors is not clear.
Objectives
To determine the association between HC use and plasma proteins involved in established and emerging disease risk pathways.
Method
Concentrations of 54 high-abundance plasma proteins were measured simultaneously by LC-MRM/MS in 783 women from the Toronto Nutrigenomics and Health Study. C-reactive protein (CRP) was measured separately. ANCOVA was used to test differences in protein concentrations between users and non-users, and among HC users depending on total hormone dose. Linear regression was used to test the association between duration (years) of HC use and plasma protein concentrations. Principal components analysis (PCA) was used to identify plasma proteomic profiles in users and non-users.
Results
After Bonferroni correction, 19 proteins involved in inflammation, innate immunity, coagulation and blood pressure regulation were significantly different between users and non-users (P<0.0009). These differences were replicated across three distinct ethnocultural groups. Traditional markers of glucose and lipid metabolism were also significantly higher among HC users. Neither hormone dose nor duration of use affected protein concentrations. PCA identified 4 distinct proteomic profiles in users and 3 in non-users.
Conclusion
HC use was associated with different concentrations of plasma proteins along various disease-related pathways, and these differences were present across different ethnicities. Aside from the known effect of HC on traditional biomarkers of cardiometabolic risk, HC use also affects numerous proteins that may be biomarkers of dysregulation in inflammation, coagulation and blood pressure.
Introduction
Hormonal contraceptives (HC) have been widely used globally since the middle of the 19th century for reasons including the prevention of unintended pregnancy, the decreased risk of female (i.e. ovarian and endometrial) cancers, regulation of the menstrual cycle, control of acne and relief of pre-menstrual and menstrual symptoms [1]–[3]. Endogenous estrogens may protect against vascular disease and atherosclerosis in young women [4], [5], yet HC have also been linked to a greater risk of weight gain, cardiovascular disease, dyslipidemia, myocardial infarction, venous thromboembolism, and stroke [6]–[9]. Because of this, HC formulations have changed over the years and newer combinations of estrogen and progestin may confer less disease risk [10]. Since these medications remain widely used, and their physiological effects are widespread, it is important to further investigate how HC alter emerging disease risk pathways using both well known and novel clinical biomarkers.
Most studies of HC use have been conducted in young women and have assessed the effects of HC use on cardiovascular or cardiometabolic endpoints [9]–[15]. All of these studies found some evidence of increased risk among HC users [9]–[15]. Ethnocultural differences may also influence the effect of HC use on physiological pathways that are dysregulated during disease progression, and this may partly explain observed differences in rates of chronic disease across ethnocultural groups [16], [17]. Most [9], [10], [13]–[15], but not all [11], [12] of the studies mentioned were carried out in whites or mixed ethnic groups, and none compared the effects between ethnicities.
Novel technologies in the field of proteomics allow for the measurement of multiple high-abundance plasma proteins involved in several disease processes simultaneously [18], [19]. Such an approach yields a more comprehensive assessment of the plasma proteome, which may help to identify individuals who are at increased risk of disease, and define clinically relevant disease phenotypes [20], [21]. Proteomics may also be a useful tool to explore and identify new associations between plasma proteins and disease risk. There are more than 4000 proteins in human plasma, and they vary widely in concentration (e.g. albumin at ∼800 μmol/L, to Von Willebrand factor at ∼0.05 μmol/L) [22], [23]. Some of these proteins are established biomarkers of disease risk and have important roles in clinical diagnosis [24]. However, the individual functions of many other potentially important plasma proteins, including those involved in acute phase anti/pro inflammatory or anti/pro coagulatory processes, are less well defined, and their potential roles as putative biomarkers of disease risk are less well understood [25], [26].
Recent data from the Women's Health Initiative (WHI) suggested widespread effects of hormone replacement therapy (HRT) on the serum proteome of postmenopausal women [27], [28]. Use of these medications was shown to affect numerous proteins involved in physiologically important pathways, such as coagulation, inflammation, immunity and metabolism. These findings support a potential impact of HRT on disease risk through different mechanisms. However, the effect of HC, which differ in formulation from HRT, on the proteome in young women remains unknown. Given that HC use has been shown to increase the risk of cardiometabolic [9]–[15] and vascular disease (thromboembolism and stroke) [6], [7] and decrease the risk of certain cancers [2], [3], it is important to explore potential links between new and emerging disease risk biomarkers and HC use.
The objectives of this study were: 1) to determine whether HC use affects the concentrations of 55 high-abundance plasma proteins in an ethnoculturally diverse population of healthy young women, and 2) to investigate the effect of hormone dose and duration of hormone exposure on the same high-abundance proteins of the plasma proteome.
Materials and Methods
Ethics Statement
Participants gave written informed consent, and the protocol was approved by the Ethics Review Board of the University of Toronto, and conformed to standards for the use of human subjects in research as outlined in the Declaration of Helsinki. http://www.wma.net/en/30publications/10policies/b3/index.html
Study population
Subjects were participants of the Toronto Nutrigenomics and Health study, a cross-sectional examination of men (n = 518) and women (n = 1,112) aged 20–29 years. Recruitment occurred between the fall of 2004 and the fall of 2010. Participants completed a general health and lifestyle questionnaire (GHLQ), a physical activity questionnaire, and a food frequency questionnaire, and gave a fasting blood sample. We excluded pregnant or breastfeeding women and individuals who were unable to provide a blood sample.
Of the initial 1,112 women recruited, 786 non-smokers had available proteomics data at the time this study was conducted. Of those 786, we excluded one individual with diabetes and two individuals with missing data on the variables included in the analyses. After exclusions, 783 individuals remained. Based on self-reported ancestry, women participating in the study were grouped into one of four ethnocultural groups: whites (n = 375), defined as those of European, Middle Eastern or Hispanic descent; East Asians (n = 282), defined as individuals of Chinese, Japanese, Korean, Filipino, Vietnamese, Thai or Cambodian descent; South Asians (n = 70), defined as those whose ancestors originated from the Indian subcontinent (India, Pakistan, Sri Lanka and Bangladesh); and others (n = 56), who included Aboriginal Canadian, Afro-Caribbean, or mixed-descent individuals. In this study, to ensure adequate sample sizes, the South Asian and Other categories were grouped together (n = 126).
Hormonal contraceptive (HC) use
The use of HC was self-reported in the GHLQ, which included questions on type (e.g. the brand, and/or whether they were oral, trans-dermal or injected) and duration in years of HC use. Based on their responses, subjects were categorized as HC users (n = 240) or non-users (n = 543). Based on the type of medication used, users were further categorized into those taking HC with <1 mg or ≥1 mg of total hormone (estrogen + progesterone derived ingredients) per day, to ascertain whether different doses affected plasma proteomic biomarkers. Lastly, we assessed whether the duration of HC use in years affected the concentration of plasma proteins in the users.
Anthropometric and physical activity measures
Anthropometric measurements, including height, weight, body mass index (BMI), systolic and diastolic blood pressure, were taken with the participant dressed in light clothing and no shoes, as described previously [19]. Physical activity, which was measured by questionnaire, was expressed as metabolic equivalent task (MET)-hours per week [19].
Biochemical measurements
Blood samples were obtained from participants after a minimum 12-hour overnight fast. Subjects with temporary inflammatory conditions were asked to wait two weeks to provide blood. Samples were collected at LifeLabs medical laboratory services (Toronto, ON, Canada), and measurements of biomarkers of glycemic control, lipid metabolism, and the systemic inflammatory marker C-reactive protein (CRP) were performed on-site using standard procedures as described previously [29]. We calculated insulin resistance and β cell function using the homeostasis model assessment (HOMA) method [30].
Plasma proteomic measurements
The concentrations of 63 high-abundance plasma proteins were measured at the University of Victoria – Genome British Columbia Proteomics Centre (Victoria, BC, Canada), using a multiple reaction monitoring (LC-MRM/MS) assay as described elsewhere [18], [19]. Of the 63 proteins measured, the intra-assay coefficients of variation (CV) for 9 of them were ≥15% [19]. Therefore, only 54 proteins with CVs <15% were included in this study.
Statistical analysis
All statistical analyses were carried out using SAS (version 9.2; SAS Institute Inc, Cary, NC, USA). The α error was set at 0.05 and all reported p-values are two-sided. The Bonferroni correction for multiple testing was applied as necessary (55 tests, α = 0.05: p<0.0009). Subject characteristics were compared between HC users and non-users across all ethnicities using χ2 tests for categorical variables and t-tests for continuous variables. We explored the individual associations between HC use and each of the 54 plasma proteomic biomarkers and CRP using general linear models (GLMs; ANCOVA) stratified by ethnicity and adjusted for age, waist circumference and physical activity. We assessed the distribution of continuous variables prior to analysis and loge- or square root-transformed those that were not normally distributed. In such cases, the p-values from models using transformed values are reported, but untransformed means and measures of spread are reported to facilitate interpretation. Biomarkers of glycemic control and lipid metabolism were compared between users and non-users using ANCOVA adjusted for age, waist circumference, physical activity and ethnocultural group. ANCOVA was also used (adjusted for age, waist circumference and physical activity) to determine the effect of total hormone dose (<1 mg versus ≥1 mg) on the 54 plasma proteins and CRP. We then used linear regression adjusted for the same covariates to assess, in users only and across 3 levels of use (non-users, <1 mg, and ≥1 mg), whether the duration of use of HC affected the plasma protein levels and whether a dose-dependent relationship was present, respectively.
Lastly, we used principal components analysis (PCA) to explore the relationship between HC use and the 54 plasma proteomic biomarkers and CRP. Using PCA, we identified plasma proteomic groups based on the concentrations of the measured proteins in HC users, as well as non-users. We obtained the principal components representative of the proteomic groups for both HC users and non-users through an orthogonal Varimax rotation that yielded independent principal components [31]. We used the Scree test and the Kaiser criterion of eigenvalues >1 to determine the individual principal components. For each principal component, inclusion of a particular protein in that component was determined based on a loading score criterion of ≥0.5. If one protein had a loading score of ≥0.5 for two principal components, the protein was included in both profiles.
Results
Study Population
Our study population was divided into 3 groups based on subjects' self-reported ethnocultural ancestry. In the white group, 166 subjects were HC users and 209 were non-users; in the East Asian group, 29 subjects were HC users and 243 were non-users; and in the South Asian/Other group, 35 subjects were users and 91 were non-users. Table 1 shows subject characteristics stratified by HC use across all ethnicities. Age and physical activity levels were significantly higher in users ( Table 1 ). HC use differed across ethnic groups, with nearly 45% of white women using HC, in contrast to 14% of East Asians and 28% of South Asians/Other. Plasma glucose, HOMA-β, triglycerides, free fatty acids, total cholesterol and high density lipoprotein (HDL) cholesterol were significantly higher in users versus non-users ( Table 2 ).
Table 1. Study participant characteristics stratified by hormonal contraceptive use.
| Non-users (n = 543) | Users (n = 240) | p-value | |
| Age (years) | 22.4±2.5 | 23.0±2.4 | 0.0030 |
| Ethnicity | |||
| White | 209 (55.7) | 166 (44.3) | <0.0001 |
| East Asian | 243 (86.2) | 39 (13.8) | |
| South Asian/Other | 91 (72.2) | 35 (27.8) | |
| Body mass index (kg/m2)* | 22.3±3.4 | 22.6±3.3 | 0.1665 |
| Waist circumference (cm)* | 70.9±7.3 | 71.7±7.4 | 0.1240 |
| Physical activity (met-h/wk) | 7.4±3.1 | 8.0±2.7 | 0.0116 |
P-values are from t-tests for continuous variables and χ2-square tests for categorical variables.
Shown are untransformed means and standard deviations for continuous variables, and n(%) for categorical variables.
Indicates variables that were log-transformed prior to statistical test.
Table 2. Biomarkers of glycemic control and lipid metabolism stratified by hormonal contraceptive use.
| Non-users (n = 543) | Users (n = 240) | p-value | |
| Glucose (mmol/L) | 4.7±0.4 | 4.7±0.3 | 0.0173 |
| Fasting insulin (pmol/L)* | 48.6±32.4 | 49.6±26.3 | 0.1436 |
| HOMA-IR* | 1.5±1 | 1.4±0.8 | 0.2677 |
| HOMA-β* | 113.7±80.0 | 123.7±70.3 | 0.0105 |
| Triglycerides (mmol/L)* | 0.9±0.5 | 1.2±0.4 | <0.0001 |
| Free fatty acids (umol/L)* | 489.0±243.3 | 522.6±237.3 | 0.0101 |
| Total cholesterol (mmol/L) | 4.2±0.7 | 4.6±0.8 | <0.0001 |
| HDL cholesterol (mmol/L)* | 1.6±0.4 | 1.8±0.4 | <0.0001 |
| LDL cholesterol (mmol/L) | 2.2±0.6 | 2.3±0.7 | 0.0914 |
| Total: HDL cholesterol ratio* | 2.7±0.6 | 2.7±0.6 | 0.9059 |
P-values were adjusted for age, waist circumference, physical activity and ethnocultural group.
Shown are untransformed means and standard deviations.
Indicates variables that were log-transformed prior to statistical test.
Proteomics and CRP Analyses
Out of the 55 plasma proteins analyzed, 19 had significantly different concentrations at the Bonferroni level (p<0.0009) between users and non-users ( Table 3 ). These differences were consistent across ethnic groups. Of the 19 proteins, 16 had consistently higher concentrations in users (α1-Antitrypsin, Angiotensinogen, α2-HS-Glycoprotein, Apolipoprotein A-I, Apolipoprotein A-II Precursor, Apolipoprotein L1, CRP, Ceruloplasmin, Vitamin D Binding Protein, Coagulation Factor XIIa HC, Heparin Cofactor II, Kininogen-1, Plasminogen, Retinol-Binding Protein, Serum Amyloid P-Component, and Vitronectin) and three had consistently lower concentrations in users (Apolipoprotein E, Complement C1 Inactivator, Histidine-rich Glycoprotein). In addition to these 19 proteins, among whites, 16 other proteins had significantly different concentrations between users and non-users after Bonferroni correction, for a total of 35 out of 55. The majority of these proteins were elevated in users. We observed similar but less robust trends among the East Asians (21 out of 55 proteins were significantly different) and South Asians/Other (26 out of 55 proteins were significantly different) groups.
Table 3. Plasma proteomic and C-reactive protein (CRP) analyses stratified by users and non-users of hormonal contraception in three ethnocultural groups (Whites, East Asians and South Asians/Other).
| WHITES | EAST ASIANS | SOUTH ASIANS + OTHER | ||||||||
| Non-Users (n = 209) | Users (n = 166) | Non-Users (n = 243) | Users (n = 39) | Non-Users (n = 91) | Users(n = 35) | |||||
| Plasma Protein (μmol/L) | Mean±SE‡ | Mean±SE‡ | p-value† * | Mean±SE‡ | Mean±SE‡ | p-value† * | Mean±SE‡ | Mean±SE‡ | p-value† * | DIRECTION relative to users |
| α1-Antitrypsin | 10.58±0.17 | 14.52±0.27 | <.0001 | 10.04±0.13 | 12.78±0.42 | <.0001 | 11.15±0.25 | 14.08±0.48 | <.0001 | higher in users |
| Angiotensinogen | 0.71±0.02 | 2.13±0.07 | <.0001 | 0.67±0.01 | 1.76±0.13 | <.0001 | 0.69±0.03 | 2.15±0.15 | <.0001 | higher in users |
| α2-HS-Glycoprotein | 8.39±0.15 | 10.32±0.18 | <.0001 | 8.31±0.1 | 9.56±0.32 | 0.0003 | 8.62±0.20 | 10.63±0.37 | <.0001 | higher in users |
| Apolipoprotein A-I | 43.41±0.63 | 50.57±0.81 | <.0001 | 43.99±0.62 | 50.66±1.61 | 0.0004 | 41.2±0.95 | 51.89±1.96 | <.0001 | higher in users |
| Apolipoprotein A-II Precursor | 23.66±0.36 | 30.19±0.45 | <.0001 | 23.61±0.31 | 29.35±0.98 | <.0001 | 23.10±0.51 | 31.31±1.25 | <.0001 | higher in users |
| Apolipoprotein L1 | 0.36±0.01 | 0.6±0.02 | <.0001 | 0.34±0.01 | 0.53±0.03 | <.0001 | 0.41±0.02 | 0.58±0.03 | <.0001 | higher in users |
| C-Reactive Protein | 0.92±0.17 | 2.69±0.26 | <.0001 | 0.49±0.09 | 2.40±0.58 | <.0001 | 1.36±0.29 | 2.25±0.38 | <.0001 | higher in users |
| Ceruloplasmin | 2.12±0.05 | 3.69±0.09 | <.0001 | 1.89±0.03 | 3.16±0.11 | <.0001 | 2.26±0.07 | 3.82±0.21 | <.0001 | higher in users |
| D Vitamin Binding Protein | 2.69±0.04 | 3.7±0.07 | <.0001 | 2.56±0.03 | 3.38±0.11 | <.0001 | 2.68±0.05 | 3.63±0.13 | <.0001 | higher in users |
| Coagulation Factor XIIa HC | 0.28±0.01 | 0.40±0.01 | <.0001 | 0.19±0.01 | 0.27±0.01 | <.0001 | 0.22±0.01 | 0.39±0.02 | <.0001 | higher in users |
| Heparin Cofactor II | 0.67±0.01 | 0.88±0.02 | <.0001 | 0.63±0.01 | 0.76±0.03 | <.0001 | 0.72±0.02 | 0.86±0.04 | 0.0006 | higher in users |
| Kininogen-1 | 2.05±0.03 | 2.79±0.05 | <.0001 | 1.94±0.02 | 2.46±0.07 | <.0001 | 2.00±0.05 | 2.76±0.13 | <.0001 | higher in users |
| Plasminogen | 1.15±0.02 | 1.50±0.02 | <.0001 | 1.17±0.01 | 1.47±0.04 | <.0001 | 1.21±0.02 | 1.54±0.06 | <.0001 | higher in users |
| Retinol-Binding Protein | 0.86±0.02 | 1.17±0.02 | <.0001 | 0.79±0.01 | 1.08±0.04 | <.0001 | 0.77±0.02 | 1.13±0.05 | <.0001 | higher in users |
| Serum Amyloid P-Component | 0.38±0.01 | 0.51±0.01 | <.0001 | 0.35±0.01 | 0.56±0.03 | <.0001 | 0.43±0.02 | 0.53±0.03 | 0.0002 | higher in users |
| Vitronectin | 3.53±0.05 | 4.65±0.08 | <.0001 | 3.46±0.04 | 4.32±0.11 | <.0001 | 3.70±0.08 | 4.69±0.17 | <.0001 | higher in users |
| Apolipoprotein E | 0.5±0.01 | 0.43±0.01 | <.0001 | 0.58±0.01 | 0.47±0.02 | 0.0005 | 0.57±0.02 | 0.41±0.02 | <.0001 | lower in users |
| Complement C1 Inactivator | 4.73±0.08 | 3.74±0.1 | <.0001 | 4.89±0.07 | 4.24±0.17 | 0.0006 | 5.04±0.12 | 3.92±0.24 | <.0001 | lower in users |
| Histidine-rich Glycoprotein | 1.37±0.03 | 1.03±0.03 | <.0001 | 1.43±0.03 | 1.14±0.07 | <.0001 | 1.39±0.04 | 0.97±0.05 | <.0001 | lower in users |
| Afamin | 0.24±0 | 0.29±0.01 | <.0001 | 0.25±0.01 | 0.27±0.01 | 0.1485 | 0.25±0.01 | 0.30±0.01 | <.0001 | |
| α1-Acid Glycoprotein 1 | 1.97±0.05 | 1.59±0.04 | <.0001 | 1.55±0.03 | 1.42±0.09 | 0.1009 | 1.97±0.07 | 1.48±0.08 | <.0001 | |
| Albumin | 965.89±11.35 | 890.33±10.53 | <.0001 | 976.09±9.52 | 896.57±12.8 | 0.0019 | 933.32±15.04 | 863.39±19.03 | 0.0157 | |
| Apolipoprotein B-100 | 0.77 ± 0.02 | 0.93±0.02 | <.0001 | 0.75±0.01 | 0.80±0.03 | 0.1799 | 0.77±0.03 | 0.95±0.04 | 0.0006 | |
| Apolipoprotein C-III | 2.28±0.05 | 3.05±0.07 | <.0001 | 2.32±0.05 | 2.80±0.13 | 0.0011 | 2.16±0.09 | 2.89±0.15 | <.0001 | |
| Complement C3 | 18.98±0.32 | 21.86±0.35 | <.0001 | 17.75±0.24 | 20.13±0.56 | 0.0011 | 21.10±0.59 | 22.44±0.85 | 0.1815 | |
| Complement Factor B | 1.41±0.03 | 1.57±0.03 | <.0001 | 1.3±0.02 | 1.56±0.07 | 0.0002 | 1.66±0.05 | 1.66±0.06 | 0.8341 | |
| Complement Factor H | 0.60±0.01 | 0.65±0.01 | <.0001 | 0.53±0.01 | 0.57±0.02 | 0.0418 | 0.66±0.02 | 0.66±0.02 | 0.6789 | |
| Clusterin | 1.5±0.02 | 1.64±0.03 | <.0001 | 1.5±0.02 | 1.63±0.04 | 0.0117 | 1.52±0.03 | 1.68±0.06 | 0.0188 | |
| Hemopexin | 10.44±0.15 | 11.44±0.16 | <.0001 | 9.53±0.13 | 10.71±0.24 | 0.0014 | 10.61±0.24 | 11.25±0.41 | 0.1937 | |
| Inter- α-Trypsin Inhibitor HC | 0.60±0.01 | 0.67±0.01 | <.0001 | 0.62±0.01 | 0.68±0.02 | 0.0059 | 0.62±0.01 | 0.69±0.02 | 0.0028 | |
| Prothrombin | 0.56±0.01 | 0.62±0.01 | <.0001 | 0.57±0.01 | 0.61±0.01 | 0.0641 | 0.58±0.01 | 0.60±0.02 | 0.6427 | |
| Transferrin | 12.41±0.21 | 14.57±0.25 | <.0001 | 11.99±0.18 | 13.18±0.39 | 0.008 | 13.01±0.35 | 15.39±0.55 | 0.0003 | |
| Transthyretin | 5.38±0.09 | 5.97±0.09 | <.0001 | 5.41±0.08 | 5.98±0.17 | 0.0064 | 4.97±0.11 | 5.84±0.21 | 0.0003 | |
| Apolipoprotein A-IV | 1.49±0.03 | 1.34±0.03 | 0.0001 | 1.32±0.02 | 1.29±0.05 | 0.7936 | 1.41±0.05 | 1.26±0.07 | 0.0649 | |
| Antithrombin-III | 3.59±0.05 | 3.38±0.04 | 0.0008 | 3.61±0.04 | 3.31±0.07 | 0.0098 | 3.54±0.06 | 3.30±0.09 | 0.0292 | |
| α1B-Glycoprotein | 1.72±0.03 | 1.89±0.05 | 0.002 | 1.62±0.03 | 1.78±0.09 | 0.0864 | 1.64±0.06 | 1.84 ± 0.12 | 0.122 | |
| Apolipoprotein C-I | 3.31±0.07 | 3.51±0.06 | 0.0074 | 3.18±0.05 | 3.43±0.14 | 0.1587 | 3.13±0.10 | 3.36±0.13 | 0.1626 | |
| L-Selectin | 0.08±0 | 0.07±0 | 0.0089 | 0.07±0.01 | 0.06±0.01 | 0.0059 | 0.07±0.01 | 0.07±0.01 | 0.0537 | |
| Fibrinogen γ Chain | 9.54±0.3 | 10.27±0.34 | 0.0139 | 9.46±0.24 | 11.09±1.23 | 0.1462 | 10.13±0.38 | 10.61±0.5 | 0.3454 | |
| Fibrinogen β Chain | 9.59±0.26 | 10.27±0.33 | 0.0192 | 9.49±0.23 | 10.71±0.97 | 0.1513 | 10.06±0.33 | 11.1±0.58 | 0.1364 | |
| Fibrinogen α Chain | 11.99±0.38 | 12.94±0.49 | 0.0205 | 12.01±0.33 | 14.25±1.88 | 0.1225 | 12.36±0.44 | 13.9±0.72 | 0.0979 | |
| Fibrinopeptide A | 7.17±0.19 | 7.56±0.21 | 0.0305 | 6.99±0.16 | 8.07±0.85 | 0.1418 | 7.60±0.24 | 8.09±0.38 | 0.3526 | |
| Gelsolin, isoform 1 | 1.19±0.02 | 1.11±0.02 | 0.0343 | 1.17±0.02 | 1.05±0.04 | 0.0005 | 1.21±0.03 | 1.18±0.05 | 0.6169 | |
| α2-Antiplasmin | 1.92±0.03 | 1.99±0.03 | 0.0344 | 1.91±0.03 | 1.95±0.04 | 0.369 | 1.93±0.04 | 2.03±0.07 | 0.2039 | |
| Zinc- α2-Glycoprotein | 1.02±0.03 | 1.08±0.03 | 0.0561 | 0.96±0.02 | 1.04±0.05 | 0.233 | 1.01±0.04 | 1.09±0.06 | 0.2064 | |
| α2-Macroglobulin | 6.1±0.12 | 5.92±0.15 | 0.0972 | 6.19±0.1 | 5.79±0.19 | 0.2542 | 6.25±0.18 | 6.03±0.22 | 0.7205 | |
| α1-Antichymotrypsin | 3.5±0.06 | 3.35±0.06 | 0.1129 | 3.22±0.05 | 3.19±0.1 | 0.8063 | 3.63±0.09 | 3.17±0.13 | 0.0016 | |
| Apolipoprotein D | 0.35±0.01 | 0.34±0.01 | 0.1283 | 0.33±0.01 | 0.32±0.01 | 0.4027 | 0.36±0.01 | 0.33±0.01 | 0.0988 | |
| Complement C9 | 2.8±0.05 | 2.69±0.06 | 0.1585 | 2.71±0.05 | 2.84±0.16 | 0.6286 | 3.17±0.10 | 2.60±0.10 | 0.0017 | |
| Complement C4 β Chain | 1.36±0.03 | 1.4±0.04 | 0.1646 | 1.36±0.03 | 1.38±0.08 | 0.9182 | 1.66±0.07 | 1.65±0.12 | 0.9227 | |
| Complement C4 γ Chain | 1.49±0.03 | 1.53±0.04 | 0.2421 | 1.51±0.04 | 1.51±0.08 | 0.7504 | 1.83±0.08 | 1.85±0.13 | 0.904 | |
| Adiponectin | 0.08±0 | 0.07±0 | 0.2474 | 0.06±0.01 | 0.06±0.01 | 0.6549 | 0.06±0.01 | 0.06±0.01 | 0.5314 | |
| Fibronectin | 0.65±0.08 | 0.62±0.1 | 0.4056 | 0.59±0.07 | 0.73±0.22 | 0.8263 | 0.50±0.04 | 0.65±0.10 | 0.5252 | |
| Haptoglobin β Chain | 11±0.35 | 10.45±0.36 | 0.6736 | 9.86±0.32 | 9.21±0.91 | 0.3018 | 13.38±0.70 | 9.48±1.09 | <.0001 | |
| β2-Glycoprotein I | 2.83±0.05 | 2.80±0.05 | 0.8658 | 2.62±0.03 | 2.61±0.06 | 0.9237 | 2.77±0.06 | 2.73±0.14 | 0.5768 | |
Bold and Italic = Significant at the Bonferroni level; Italic = significant but not at the Bonferroni level.
Adjusted for age, waist circumference and physical activity.
Untransformed, log transformed or square route transformed.
Unadjusted and untransformed means and standard errors.
The concentrations of two proteins were significantly different between users and non-users in only one ethnocultural group ( Table 3 ). These were Gelsolin isoform 1 in East Asians and Haptoglobin β Chain in South Asians/Other. Results for plasma protein concentration differences among ethnocultural groups (men and women) not stratified by HC use have been published elsewhere [19].
Duration of Hormonal Contraceptive (HC) Use
We assessed whether the duration of HC use, measured in years, affected plasma protein concentrations in users only across all ethnocultural groups. Six proteins changed significantly (p<0.05) with increasing duration of HC use. Three proteins were significantly lower with increased duration of use: Complement C9 ([β estimate ± SE, r2, p-value] −0.178 ±0.07, 0.06, 0.04), α2-Macroglobulin (−0.32±0.15, 0.07, 0.03), and α1-Antichymotrypsin (−0.15±0.07, 0.11, 0.03). Three others were significantly higher with increased duration of use: Apolipoprotein A-II Precursor (1.31±0.54, 0.07, 0.02), Apolipoprotein C-III (0.14±0.08, 0.04, 0.05), and Transferrin (0.54±0.29, 0.06, 0.05). None of these differences in protein concentration remained significant after Bonferroni correction.
Dose of Hormones in Contraceptives (HC)
We assessed whether the dose of total HC hormone affected plasma protein levels. Subjects reported using twelve different types of HC. We divided them by total hormone concentration into three groups: 0 mg/d (n = 531 non-users), <1 mg/d (n = 141 users) and ≥1 cmg/d (n = 52 users). We excluded subjects (n = 59) from this analysis if they were getting hormone injections every few months since it was difficult to ascertain a daily hormonal exposure, if they were HC users but classified their type of medication as “other”, if they classified themselves as non-users but reported a medication type (i.e. misreporting), or if they provided no information at all on HC use. Seven proteins were significantly different between the two groups of HC users. Four proteins were significantly higher in the <1 mg compared to the ≥ 1 mg group, respectively: Apolipoprotein L1 ([mean ± SE] 0.62±0.02 vs. 0.54±0.03, p<0.003), Albumin (901.40±10.21 vs. 845.57±16.85, p<0.009), Serum Amyloid P-Component (0.55±0.01 vs. 0.50±0.02, p<0.02), α1-Antitrypsin (14.73±0.28 vs. 13.83±0.46, p<0.05). Three were lower in the <1 mg compared to the ≥1 mg group, respectively: Complement C4 γ Chain (1.52±0.05 vs. 1.75±0.08, p<0.03), Complement C4 β Chain (1.40±0.04 vs. 1.58±0.07, p<0.04), Histidine-rich Glycoprotein (0.98±0.03 vs. 1.08±0.05, p<0.05). None of the proteins remained significant after Bonferroni correction. We then examined whether these associations were dose-dependent by comparing the two levels of hormone dose (<1 mg/d and ≥1 mg/d) to no hormone dose (i.e. non-users). No dose-response effects were observed.
Principal Components Analysis
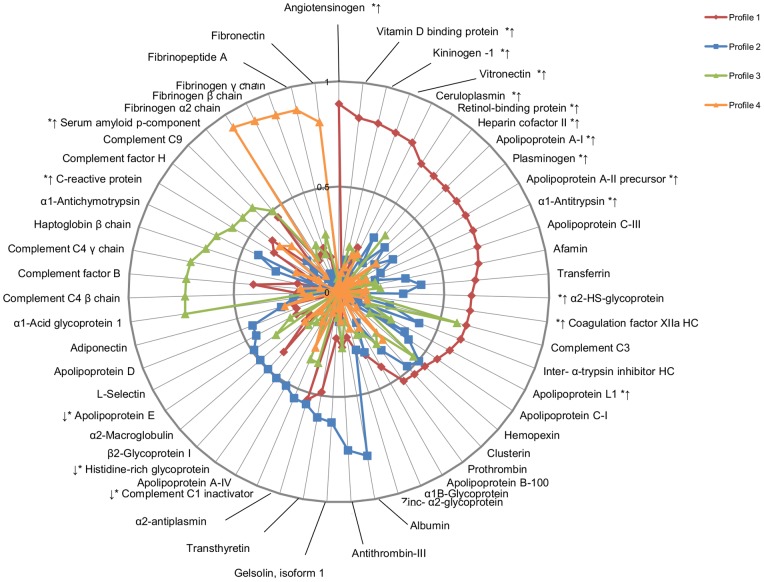
Four distinct proteomic profiles were identified among HC users ( Figure 1 ). Profile 1 included 24 proteins, most of which were positive acute phase reactants involved in inflammatory and blood pressure-related processes, such as Complement C-3, α1-Antitrypsin and Angiotensinogen. Fourteen of these proteins were significantly higher in users than in non-users ( Figure 1 ). Profile 2 was comprised of 10 anti-inflammatory and anti-coagulatory negative acute phase proteins. Two of these proteins were significantly lower in users than non-users (Complement C1 Inactivator, Histidine-rich Glycoprotein). Profile 3 consisted of 11 innate immunity-related complement proteins, adaptive immunity-related acute phase proteins and CRP. Two of these proteins (CRP and Serum Amyloid P-Component) were significantly higher in users than non-users. Profile 4 consisted of 5 proteins that were exclusively related to coagulation, and none were significantly different between users and non-users. The variance explained by profiles 1-4 in HC users was 13.4%, 6.9%, 6.6% and 5.0%, respectively, and together, these principal components explained 32% of the observed variance in the data set. Three proteins (Complement C3, Hemopexin and α2-Antiplasmin) had loading scores of >0.5 for two principal components ( Figure 1 ) and were, therefore, included in both components. Eight proteins (Apolipoprotein B-100, α1B-Glycoprotein, Zinc-α2-Glycoprotein, L-Selectin, Adiponectin, Apolipoprotein D, α2-Macroglobulin, Apolipoprotein E) had loading scores <0.5 for each principal component and, therefore, were not included in any profile.
Figure 1. Principal components analysis (PCA) in users of HC.
Four independent proteomic profiles were identified, based on a loading score criterion of >0.5. Profile 1 consisted of primarily positive acute phase reactants, while profile 2 comprised mainly negative acute phase reactants. Profile 3 consisted of complement system components and acute phase proteins, and Profile 4 represented primarily proteins involved in coagulation. * designates the 19 proteins that were significantly different between users and non-users in the whole study population. The arrows designate the direction of the difference (i.e. higher or lower levels of the protein) with respect to non-users.
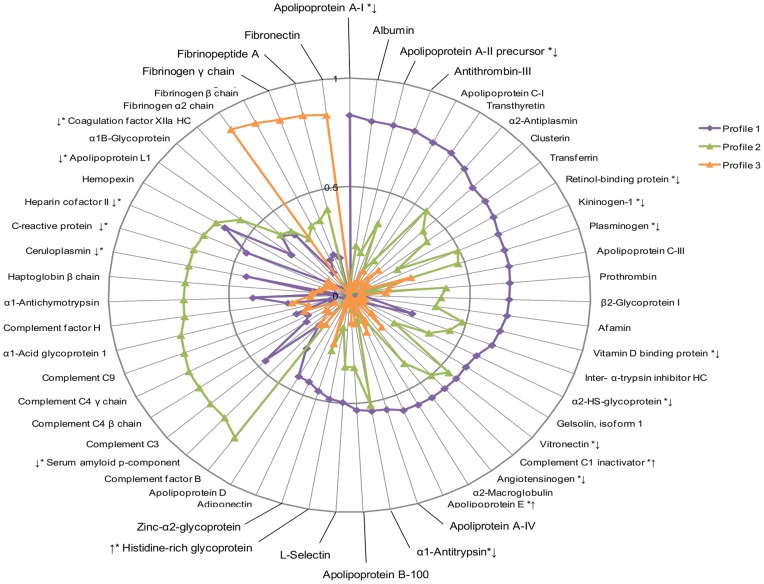
Three distinct proteomic profiles were identified among non-users of HC ( Figure 2 ). Profile 1 included 29 proteins from several physiologic pathways including positive acute phase proteins involved in inflammation and blood pressure (e.g. Plasminogen, Complement C4 and Angiotensinogen), as well as negative acute phase proteins involved in downregulating inflammation and coagulation (e.g. Transthyretin and Kininogen-1). Ten of these proteins were significantly lower in non-users than in users, and two were significantly higher in non-users than in users. Profile 2 was comprised of 19 innate immunity-related complement proteins, innate and adaptive immunity-related acute phase proteins, and CRP. Five of these proteins were significantly lower in non-users than in users. Profile 3, like profile 4 in the users, contained 5 proteins exclusively related to coagulation, and none were significantly different between users and non-users. The variance explained by profiles 1–3 in HC non-users was 15.8%, 12.1% and 4.5%, respectively, and together, these principal components explained 32% of the observed variance in the data set. Five proteins (Hemopexin, α2-Antiplasmin, Vitronectin, Complement C1 Inactivator and α1-Antitrypsin) had loading scores of >0.5 for two principal components ( Figure 2 ) and were, therefore, included in both components. Seven proteins (L-Selectin, Histidine-rich Glycoprotein, Zinc- α2-Glycoprotein, Adiponectin, Apolipoprotein D, α1B-Glycoprotein, and Coagulation Factor XIIa HC) had loading scores <0.5 for each principal component and were, therefore, were not included in any profile.
Figure 2. Principal components analysis (PCA) in non-users of HC.
In contrast to HC users, only three independent proteomic profiles were identified among non-users of HC, based on a loading score criterion of >0.5. No profile was characteristically representative of a negative or positive acute phase response. * designates the 19 proteins that were significantly different between users and nonhyphen;users in the whole study population. The arrows designate the direction of the difference (i.e. higher or lower levels of the protein) with respect to users.
Discussion
This study assessed the effects of HC use on both novel plasma proteomic biomarkers and traditional cardiometabolic risk factors in an ethnoculturally diverse population of young women. Our results suggest that HC use modulates the levels of multiple high abundance plasma proteins belonging to pathways that become dysregulated during disease progression. Indeed, 19 of the 55 plasma proteins measured were significantly different between users and non-users, and this effect was observed consistently across three distinct ethnocultural groups. The concentrations of most of these proteins were higher in users, including those that are established biomarkers of cardiovascular disease risk, such as CRP [24] and angiotensinogen [32]. Other proteins involved in (but not limited to) coagulation, such as Coagulation Factor XIIa HC, Heparin Cofactor II, Plasminogen and Vitronectin, were all higher in users, suggesting that HC use may also modulate disease risk via this pathway [33], [34]. While 19 proteins showed consistent differences across all ethnicities, an additional 9 proteins were significantly different only in whites. As well, Gelsolin was only different between users and non-users in East Asians, while Haptoglobin β Chain was only different between users and non-users in South Asians/Other. These results may indicate differential responses to HC use among ethnicities; however, our power to detect differences in some of the other ethnic groups may have been limited due to decreased sample sizes, and in particular, a lower proportion of HC users in East Asians and South Asians/Others.
Previous work examined the effects of HRT consisting of estrogens (conjugated equine estrogens; CEE) or CEE plus progestin (medroxyprogesterone acetate; CEE + P), on the serum proteome in postmenopausal women participating in the WHI [27], [28]. Together, these studies identified approximately 100 proteins whose levels were affected by the use of either type of hormone replacement medication. The proteins identified were involved in coagulation, inflammation, metabolism, the immune response, and other physiologically important pathways. In the present study, of the 19 proteins associated with HC use, 10 proteins (Angiotensinogen, α2-HS-Glycoprotein, Apolipoprotein A-II precursor, Ceruloplasmin, D Vitamin Binding Protein, Coagulation Factor XIIa HC, Kininogen-1, Plasminogen, Retinol-Binding Protein, and Vitronectin) showed similar associations with HRT in the WHI [27], [28]. Several differences exist between the present study and the WHI studies. In addition to fundamental physiological differences between younger and older (postmenopausal) women, as well as ethnic differences in the study samples, the formulation of hormone medications used in each study differed. HC generally consist of a combination of synthetic ethinyl estradiol and progestins [35], while the HRT used in the WHI were equine estrogens (estrone, equilin and equilenin), either alone or in combination with progestin [27], [28]. Furthermore, the WHI studies employed a different proteomic profiling method that allowed for the assessment of a greater number of proteins, but in doing so, prior to analysis, they depleted the samples of some highly abundant proteins, such as Albumin, Haptoglobin, and α1-Antitrypsin. Despite these differences, the considerable overlap in identified proteins and physiologic pathways affected by hormone medications between our study and the WHI studies provides compelling evidence that the use of estrogenic hormones, regardless of formulation or life stage, has marked effects on the plasma/serum proteome.
We also investigated whether the dose of daily hormone or the duration of HC use were associated with plasma protein concentrations. Two complement proteins (Complement C4 γ Chain and Complement C4 β Chain) had higher concentrations in those with greater hormone exposure, suggesting that HC dose may affect pathways involved in innate immunity. However, the differences were no longer significant after correcting for multiple comparisons. With regards to duration of HC use, our results suggest that years of exposure to HC may affect cholesterol metabolism, with increased levels of Apolipoprotein A-II Precursor and Apolipoprotein C-III, yet, again, differences were no longer significant after Bonferroni correction. In agreement with our results, a population-based retrospective analysis of a multicultural North American cohort also found no association between long-term oral contraceptive use and the prevalence of metabolic syndrome, measures of glycemic control or lipid metabolism [10]. Finally, our PCA analysis illustrated that plasma proteins clustered differently among users and non-users of HC. Overall, our results show that plasma protein concentrations indeed differed between users and non-users of HC in the direction of increased inflammation and dysregulation of certain pathways involved in coagulation and innate immunity in users. Our findings corroborate previous research on HC use and cardiometabolic disease risk [9]–[15], but also highlight potential novel effects of HC on other disease risk pathways. To our knowledge, the present study is the first to report the widespread effects of HC on the plasma proteome.
We observed a greater number of associations between HC and plasma proteomic biomarkers among whites than the other ethnic groups. We also noted ethnic-specific differences between users and non-users for certain proteins ( Table 3 ). These observations may be partly a result of the difference in sample sizes between the ethnic groups. Nonetheless, this is the first study to assess the association between these proteins and HC use across ethnicities. Gelsolin is a member of the actin scavenging system with anti-apoptotic and anti-inflammatory properties [36]. Low levels of Gelsolin have been identified as a potential colorectal cancer biomarker in a Chinese population [37]. Haptoglobin is a positive acute phase reactant with antioxidant properties that regulates the pro-oxidant activity of hemoglobin [38]. The lower levels of Gelsolin and Haptoglobin among East Asian and South Asian/Other HC users, respectively, support the view that HC may contribute to the dysregulation of physiological processes in these groups. Whether the observed differences translate into ethnic-specific effects of HC on cardiometabolic disease remains to be elucidated. Indeed, a common polymorphism in the Haptoglobin gene has been associated with increased cardiometabolic and autoimmune disease risk [39], and Haptoglobin genotypes have been shown to modify the relationship between dietary intake of vitamin C, an antioxidant, and circulating levels of ascorbic acid [40].
PCA revealed different plasma proteomic profiles among HC users and non-users. In users, four profiles were identified ( Figure 1 ). Profile 1 consisted of primarily positive acute phase reactants, such as Complement C3 and Ceruloplasmin, while profile 2 comprised mainly negative acute phase reactants, such as Transthyretin and Albumin [25], [41]. These distinct groupings suggest a different acute phase response in HC users, which may translate into increased disease risk. In contrast, only three proteomic profiles were identified in non-users ( Figure 2 ), and no profile was characteristically representative of a negative or positive acute phase response. Overall, this indicates a different pattern of plasma protein clustering between users and non-users of HC. Future studies assessing the effects of HC on particular plasma proteins or pathways may shed light on the biological implications of this finding.
This study had some limitations. First, information on type of HC (i.e. different generation formulations) was not assessed. Earlier contraceptive formulations, which contained higher levels of estradiol and no progestins, were associated with a different disease risk profile than later HC formulations [10]. We did not distinguish between HC generation when examining the associations between HC use and individual proteins or proteomic profiles. However, given the younger age of the study participants, and noting the brands of contraceptives they were taking, the majority of users reported taking third generation medications. Another limitation is the cross-sectional nature of the study, which prevents establishing causality for any of the observed associations. In addition, the small sample size of the non-white ethnic groups, particularly the South Asian and Other category, may have led to a lack of sufficient statistical power to adequately assess the effect of HC in these individual groups. Finally, although we adjusted for a number of covariates, residual confounding may have affected some of the observed results.
In summary, we observed associations between HC use and the concentrations of 19 plasma proteomic biomarkers, as well as cardiometabolic biomarkers of disease risk, across distinct ethnocultural groups, in a population of healthy young women. HC use was associated with higher concentrations of numerous proteins along inflammatory, coagulatory and immune related pathways that become dysregulated during disease progression. By examining the plasma proteome, this study sheds some light on possible novel pathophysiologic links between HC use and disease beyond their established effect on the cardiovascular system. In conclusion, HC use has a profound and robust effect on the proteins investigated in this plasma proteomic panel, and hence, may be associated with increased disease risk through novel pathways and mechanisms.
Funding Statement
Funding for the research was obtained from the Advanced Food and Materials Network (AFMNet). ARJ is funded by Canadian Institutes of Health Research (CIHR) and Canadian Diabetes Association (CDA) Post-Doctoral fellowships; BGB is funded by an Ontario Graduate Scholarship; AES is supported by a Canada Research Chair in Nutrigenomics. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Haider Z, D'Souza R (2009) Non-contraceptive benefits and risks of contraception. Best Pract Res Clin Obstet Gynaecol 23: 249–262. [DOI] [PubMed] [Google Scholar]
- 2. Collins J, Crosignani PG (2005) Noncontraceptive health benefits of combined oral contraception. Hum Reprod Update 11: 513–525. [DOI] [PubMed] [Google Scholar]
- 3. Huber JC, Bentz EK, Ott J, Tempfer CB (2008) Non-contraceptive benefits of oral contraceptives. Expert Opin Pharmacother 9: 2317–2325. [DOI] [PubMed] [Google Scholar]
- 4. Mendelsohn ME, Karas RH (2005) Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587. [DOI] [PubMed] [Google Scholar]
- 5. Alonso de Lecinana M, Egido JA (2006) Estrogens as neuroprotectants against ischemic stroke. Cerebrovasc Dis 21 Suppl 248–53. [DOI] [PubMed] [Google Scholar]
- 6. Baillargeon JP, McClish DK, Essah PA, Nestler JE (2005) Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: a meta-analysis. J Clin Endocrinol Metab 90: 3863–3870. [DOI] [PubMed] [Google Scholar]
- 7. Khader YS, Rice J, John L, Abueita O (2003) Oral contraceptives use and the risk of myocardial infarction: a meta-analysis. Contraception 68: 11–17. [DOI] [PubMed] [Google Scholar]
- 8. Kluft C (2007) Effects of hormone treatment on hemostasis variables. Climacteric 10 Suppl 232–37. [DOI] [PubMed] [Google Scholar]
- 9. Soska V, Fiala J, Nebeska K, Jarkovsky J, Hruba D (2011) The atherogenic index of plasma is increased by hormonal contraception. Scand J Clin Lab Invest 71: 94–100. [DOI] [PubMed] [Google Scholar]
- 10. Hurwitz BE, Henry N, Goldberg RB (2009) Long-term oral contraceptive treatment, metabolic syndrome and measures of cardiovascular risk in pre-menopausal women: National Health and Nutrition Examination Survey 1999–2004. Gynecol Endocrinol 25: 441–449. [DOI] [PubMed] [Google Scholar]
- 11. Frempong BA, Ricks M, Sen S, Sumner AE (2008) Effect of low-dose oral contraceptives on metabolic risk factors in African-American women. J Clin Endocrinol Metab 93: 2097–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Obisesan KA, Adenaike FA, Okunlola MA, Adenaike AA (2002) Effects of oral contraceptives on total serum proteins, albumin, globulins and cholesterol levels in Ibadan, Nigeria. West Afr J Med 21: 197–199. [DOI] [PubMed] [Google Scholar]
- 13. Du Y, Rosner BM, Knopf H, Schwarz S, Doren M, et al. (2011) Hormonal contraceptive use among adolescent girls in Germany in relation to health behavior and biological cardiovascular risk factors. J Adolesc Health 48: 331–337. [DOI] [PubMed] [Google Scholar]
- 14. Gronich N, Lavi I, Rennert G (2011) Higher risk of venous thrombosis associated with drospirenone-containing oral contraceptives: a population-based cohort study. CMAJ 183: E1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwok S, Canoy D, Ashton WD, Lowe GD, Wood D, et al. (2009) Increased C-reactive protein levels in overweight and obese women taking exogenous hormones: the United Kingdom Women's Heart Study (UKWHS). Clin Endocrinol (Oxf) 71: 727–732. [DOI] [PubMed] [Google Scholar]
- 16.Lear SA, Chockalingam A, Kohli S, Richardson CG, Humphries KH (2012) Elevation in Cardiovascular Disease Risk in South Asians Is Mediated by Differences in Visceral Adipose Tissue. Obesity (Silver Spring). [DOI] [PubMed]
- 17. Foulds HJ, Bredin SS, Warburton DE (2012) The relationship between hypertension and obesity across different ethnicities. J Hypertens 30: 359–367. [DOI] [PubMed] [Google Scholar]
- 18. Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, et al. (2009) Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol Cell Proteomics 8: 1860–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia-Bailo B, Brenner DR, Nielsen D, Lee HJ, Domanski D, et al. (2012) Dietary patterns and ethnicity are associated with distinct plasma proteomic groups. Am J Clin Nutr 95: 352–361. [DOI] [PubMed] [Google Scholar]
- 20. Gerszten RE, Accurso F, Bernard GR, Caprioli RM, Klee EW, et al. (2008) Challenges in translating plasma proteomics from bench to bedside: update from the NHLBI Clinical Proteomics Programs. Am J Physiol Lung Cell Mol Physiol 295: L16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Apweiler R, Aslanidis C, Deufel T, Gerstner A, Hansen J, et al. (2009) Approaching clinical proteomics: current state and future fields of application in fluid proteomics. Clin Chem Lab Med 47: 724–744. [DOI] [PubMed] [Google Scholar]
- 22. Hortin GL, Sviridov D, Anderson NL (2008) High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin Chem 54: 1608–1616. [DOI] [PubMed] [Google Scholar]
- 23. Shen Y, Kim J, Strittmatter EF, Jacobs JM, Camp DG 2nd, et al (2005) Characterization of the human blood plasma proteome. Proteomics 5: 4034–4045. [DOI] [PubMed] [Google Scholar]
- 24. Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, et al. (2010) Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med 7: e1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salgado FJ, Arias P, Canda-Sánchez A, Nogueira M (2011) Acute Phase Proteins as Biomarkers of Disease: From Bench to Clinical Practice. In: Veas F, editor. Acute Phase Proteins as Early non-Specific Biomarkers of Human and Veterinary Diseases. Rijeka, Croatia: InTech.
- 26. Anderson L (2005) Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J Physiol 563: 23–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katayama H, Paczesny S, Prentice R, Aragaki A, Faca VM, et al. (2009) Application of serum proteomics to the Women's Health Initiative conjugated equine estrogens trial reveals a multitude of effects relevant to clinical findings. Genome Med 1: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pitteri SJ, Hanash SM, Aragaki A, Amon LM, Chen L, et al. (2009) Postmenopausal estrogen and progestin effects on the serum proteome. Genome Med 1: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cahill L, Corey PN, El-Sohemy A (2009) Vitamin C deficiency in a population of young Canadian adults. Am J Epidemiol 170: 464–471. [DOI] [PubMed] [Google Scholar]
- 30. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Mueller C (1978) Factor analysis: statistical methods and practical issues. Newbury Park, CA.: Sage Publications, Inc.
- 32. DeClercq V, Taylor C, Zahradka P (2008) Adipose tissue: the link between obesity and cardiovascular disease. Cardiovasc Hematol Disord Drug Targets 8: 228–237. [DOI] [PubMed] [Google Scholar]
- 33. Ekmekci OB, Ekmekci H (2006) Vitronectin in atherosclerotic disease. Clin Chim Acta 368: 77–83. [DOI] [PubMed] [Google Scholar]
- 34. Folsom AR (2001) Hemostatic risk factors for atherothrombotic disease: an epidemiologic view. Thromb Haemost 86: 366–373. [PubMed] [Google Scholar]
- 35. Williams JK (2004) Rationale for new oral contraceptive dosing. Int J Fertil Womens Med 49: 30–35. [PubMed] [Google Scholar]
- 36.Li GH, Arora PD, Chen Y, McCulloch CA, Liu P (2010) Multifunctional roles of gelsolin in health and diseases. Med Res Rev. [DOI] [PubMed]
- 37. Fan NJ, Gao CF, Wang CS, Lv JJ, Zhao G, et al. (2012) Discovery and verification of gelsolin as a potential biomarker of colorectal adenocarcinoma in the Chinese population: Examining differential protein expression using an iTRAQ labelling-based proteomics approach. Can J Gastroenterol 26: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Asleh R, Levy AP (2005) In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag 1: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, et al. (2010) Haptoglobin: basic and clinical aspects. Antioxid Redox Signal 12: 293–304. [DOI] [PubMed] [Google Scholar]
- 40. Cahill LE, El-Sohemy A (2010) Haptoglobin genotype modifies the association between dietary vitamin C and serum ascorbic acid deficiency. Am J Clin Nutr 92: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 41. Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ (2005) Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B 6: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]