Abstract
We coordinated biogeographical comparisons of the impacts of an exotic invasive tree in its native and non-native ranges with a congeneric comparison in the non-native range. Prosopis juliflora is taxonomically complicated and with P. pallida forms the P. juliflora complex. Thus we sampled P. juliflora in its native Venezuela, and also located two field sites in Peru, the native range of Prosopis pallida. Canopies of Prosopis juliflora, a native of the New World but an invader in many other regions, had facilitative effects on the diversity of other species in its native Venezuela, and P. pallida had both negative and positive effects depending on the year, (overall neutral effects) in its native Peru. However, in India and Hawaii, USA, where P. juliflora is an aggressive invader, canopy effects were consistently and strongly negative on species richness. Prosopis cineraria, a native to India, had much weaker effects on species richness in India than P. juliflora. We carried out multiple congeneric comparisons between P. juliflora and P. cineraria, and found that soil from the rhizosphere of P. juliflora had higher extractable phosphorus, soluble salts and total phenolics than P. cineraria rhizosphere soils. Experimentally applied P. juliflora litter caused far greater mortality of native Indian species than litter from P. cineraria. Prosopis juliflora leaf leachate had neutral to negative effects on root growth of three common crop species of north-west India whereas P. cineraria leaf leachate had positive effects. Prosopis juliflora leaf leachate also had higher concentrations of total phenolics and L-tryptophan than P. cineraria, suggesting a potential allelopathic mechanism for the congeneric differences. Our results also suggest the possibility of regional evolutionary trajectories among competitors and that recent mixing of species from different trajectories has the potential to disrupt evolved interactions among native species.
Introduction
Why some exotic plants, when introduced to a new part of the world, become far more abundant and have greater impact than in their native range is one of the most puzzling questions in ecology [1], [2]. Prosopis juliflora (Swartz) DC appears to be one of these species. Several species of Prosopis have been introduced to different parts of the world and four – P. glandulosa, P. velutina, P. juliflora and P. pallida – have become invasive [3]. Prosopis juliflora and P. pallida are tropical species and have become serious invasives in many parts of Africa, Middle East and Indian subcontinent. Prosopis juliflora is native to Central America, northern South America and the Caribbean islands [4] (Figure 1A), and P. pallida is native to northern South America. In their native ranges both species appear to coexist with large numbers of other native species. A significant amount of confusion exists in the identification of P. juliflora and P. pallida [3]. For example, a species previously identified as P. juliflora in Peruvian-Ecuadorian coast (one of the native ranges) is now identified as P. pallida or P. limensis [5]. We used morphological characters to confirm identity of P. juliflora [3] and treated both P. juliflora and P. pallida as a complex, “P. juliflora s. lat. (incl. P. pallida)” and recognize this as the P. juliflora complex.
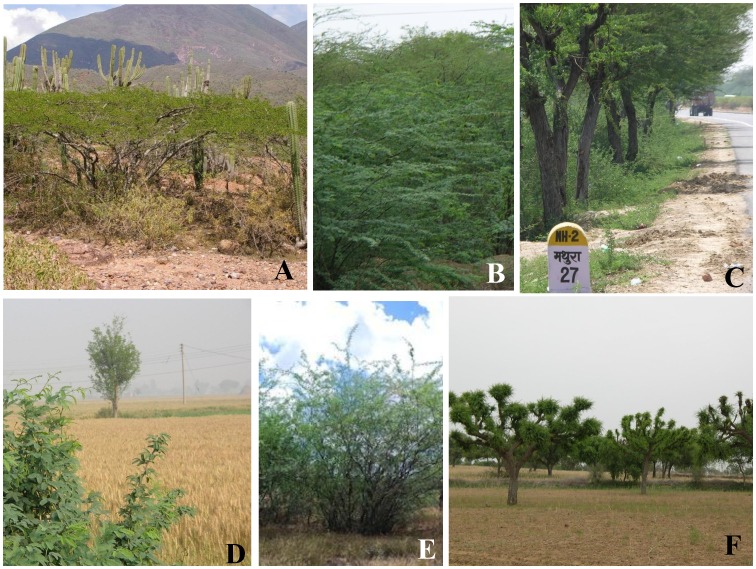
Figure 1. Prosopis juliflora in its native range of Venezuela (A); the invaded range of Haryana, India (B), along the National Highway to Rajasthan (C), at the boundaries of an agricultural field in India (D), and in Hawaii, USA (E); Prosopis cineraria in its native range, Rajasthan, India (F).
Photo credits: Pascual J. Soriano (A); Inderjit (B, C, D and F) and Timothy J. Gallaher (E).
P. juliflora is a major invasive species in India, and has also invaded other regions throughout the world including Saharan and southern Africa, the Middle East, Pakistan, India, and Hawaii (USA) [3] where it appears to strongly suppress species native to those regions. Prosopis juliflora forms pure stands in its invaded range in India, and occurs in forests, wastelands and at the boundaries of crop fields (Figure 1B, C, D). Prosopis juliflora also occurs in saline habitats in Hawaii USA (Figure 1E).
In its native range, densities of P. juliflora can be high relative to other leguminous shrubs and trees, but its canopies can have much stronger facilitative effects on neighbors than other leguminous tree species [6]. Many other Prosopis species, in their native ranges, create “resource islands” with higher concentrations of organic matter, nitrogen, phosphorus and potassium beneath their canopies and behave as strong facilitators of other species [7]–[17]. Accordingly, Prosopis cineraria, indigenous to North-Western India, can facilitate native species [18]. Farmers keep P. cineraria in their fields because their crops grow better under the trees than in the open fields [19] (Figure 1F). Aggarwal et al. [19] also found that soil nitrogen, phosphorus, and potassium were higher under P. cineraria canopies than in open fields, and that the biomass of Pennesetum typhoides was three times higher when grown in P. cineraria soil than in open soil. Adding nutrients to non-P. cineraria soil reduced these differences, but P. typhoides grown in P. cineraria soils was always at least two times larger than when grown in soil from outside of P. cineraria canopies regardless of nutrient additions. These studies suggest that in their native ranges, Prosopis species often have neutral to positive effects on the species beneath them.
Prosopis juliflora was first introduced to India in 1877 where it has become invasive. However it is also a source of fuel wood, fodder, charcoal and timber [3]. In India, Aggarwal et al. [18] found that the canopies of the invasive P. juliflora had far fewer understory species than any of four other species measured, whereas the native congener, P. cineraria, was associated with higher subcanopy diversity than any other species. In the Arabian Peninsula where P. juliflora is invasive has strong negative impacts on native species despite increases in the concentrations of some nutrients in subcanopy soil [20].
Exotic plants can also have different impacts on nutrient cycling than native species through nutrient release and biochemical effects [21]–[24]; suggesting the potential importance of comparisons of the effects of P. juliflora to its native congener, P. cineraria, on the chemical characteristics of soil. Inderjit et al. [25] compared the effects of soils from the rhizospheres of P. juliflora and P. cineraria and found that soil under P. juliflora had higher concentrations of total phenolics and inhibited total biomass of Bambusa arundinacea more than soil from under the native congener. Goel et al. [26] reported allelopathic potential of P. juliflora leaf leachate prepared in hot water and decomposing litter residues. The amino acid L-tryptophan has been isolated from foliage leachate of P. juliflora and has been shown to have allelopathic potential on Echinochloa crus-galli in filter paper bioassays [27].
The apparent contrasts in the ecology of P. juliflora in its native and non-native ranges, and between the effects of P. juliflora and P. cineraria in the latter’s native range, is curious. This biogeographical contrast parallels those exhibited by many other invasive species and raises questions about interactions among species from different parts of the world might be affected by evolutionary mismatches [1], [28]. We hypothesized that P. juliflora in its invaded ranges has (i) greater negative impacts on species richness compared to its native range and compared to a congeneric species in its invaded range, (ii) more pronounced effects on soil chemical characteristics and allelochemical pools than a congener, and (iii) greater inhibition of species native to the invaded ranges in soil and leaf leachate experiments than a congener. We tackled this general issue by comparing (1) subcanopy species richness under P. pallida canopies with species richness in open areas, in its native range Peru and P. juliflora in its native range Venezuela, and two parts of its non-native range, India and Hawaii, (2) subcanopy species richness under P. juliflora and the Indian native P. cineraria in India, (3) the chemical characteristics (inorganic ions and total phenolics) of the soil from the rhizosphere of the two congeners, (4) the effects of litter from the two congeners on a suite of native Indian species, and (5) the effect of soil amended with leaf leachate of the two congeners on local crop species in India.
Materials and Methods
Prosopis juliflora and Species Richness in Native and Non-native Ranges
Prosopis juliflora is part of the Prosopis juliflora-pallida complex, a taxonomically complicated, interrelated, and controversial group both in the native and non-native ranges of species in this complex [5]. Because of this pervasive taxonomic confusion, we took a conservative approach to sampling in Venezuela, native range of P. juliflora, and in Peru, the native range of what is most likely to be Prosopis pallida [5]. Our intent was to make a broader measurement of the impacts of the Prosopis juliflora complex in the native ranges by using both ends of the species that make up the P. juliflora-pallida complex. We located two field sites in Peru, (i) Chulucanas (S 05°11′ 49.4″; W 080°16′ 53.3″; 174 m) and (ii) Catacaos (S 05°27′ 06.5″; W 80°14′ 37.2″; 253 m). We located two field sites in Venezuela, in the native range of Prosopis juliflora [5], (i) Mucumi (N 08°30′ 10.0″; W 71°21′ 53.0″; 1000 m) and (ii) Puente Real (N 08°28′ 41.0″; W 71°24′ 38.0″; 650 m). Both sites are within the Lagunillas Semi-arid Enclave, which is an inter-Andean enclave, the largest in Venezuela with an area of 262 km2. The vegetation at both study sites is classified as thorn scrub. In these sites the dominant elements in the upper stratum were the thorny shrubs Prosopis juliflora and Acacia macracantha, which form an open and discontinuous canopy up to 3 m in height. The columnar cacti Stenocereus griseus, Pilosocereus tillianus and Cereus repandus are emergent elements (up to 5–7 m in height), which tend to be spatially associated with these leguminous shrubs. The intermediate stratum (up to 50 cm) is formed by shrubs of Croton spp., Capparis odoratissima, herb Hibiscus phoeniceus, cacti Opuntia caribea, Opuntia depauperata, Opuntia aff. elatior and Solanum spp. A bromeliacea of the Pitcairnia genus, the herbs Jatropha gossypifolia, Cnidosculus urens and Evolvulus sp., and annual plant species were also present. Three field sites were located in India and two sites were located in Honolulu, Hawaii, non-native ranges of P. juliflora. The sites located in India were: (i) Delhi (N 28°35′ 58.6″; E 077°10′ 15.6″; 233 m), (ii) Kutch, Gujarat (N 23°55′ 58.2″; E 069°48′ 50.4″; 407 m), and (iii) Jaipur, Rajasthan (N 27°01′ 00.3″; E 075°46′ 12.9″; 484 m). The three Indian sites were chosen to represent a range of climates, with Delhi, Gujarat and Rajasthan having humid subtropical, tropical monsoon and tropical arid climates, respectively. The two sites in Hawaii were: (i) Sand Island (N 21° 18′ 12.5″ W 157° 52′ 51.7″ and (ii) Iroquois Point-Hau Bush (N 21° 19′ 24.9″; W 157° 58′ 16.7″ and N 21° 18′ 20.9″; W 157° 1′ 52.5″), both at sea level.
At each site in Peru, we randomly placed 1 m2 quadrats in areas of large patches of P. pallida and where P. pallida was not present, what we refer to as ‘open areas’. We recorded the number of species in each plot. In 2008, we sampled 96 pairs of plots at Chulucanas and 92 pairs at Catacaos. In 2009, we sampled 72 pairs in Chulucanas and 120 pairs at Catacaos. At each site in Venezuela in July 2012, we randomly placed one 1 m2 quadrat under each of 15 different P. juliflora trees and in open grassland adjacent to each tree where P. juliflora was not present and recorded the number of species in each plot. At the Delhi site P. juliflora primarily occurs in dense stands (3.6 trees per 25 m2). Here in May 2010 we established 10 randomly placed 5×5 m2 quadrats under P. juliflora, with each plot representing the understory of a different set of several trees, and 10 5×5 m2 quadrats in open area outside of the P. juliflora stands. At Gujarat, the site was a scrub forest of P. juliflora (1.4 trees per 25 m2) in which 7 randomly placed 5×5 m2 plots were located under P. juliflora and 7 in open areas away from P. juliflora canopy, during June 2008. At Rajasthan, P. juliflora tended to be smaller and occur in very large closed canopy patches precluding pairing local sub canopies and open patches (4.4 trees per 25 m2). Thus 10 randomly placed 5×5 m2 quadrats were located under P. juliflora roughly in the centre of a typical stand that was several kilometres in diameter, with control quadrats (no P. juliflora) approximately 2 km away, from the quadrats with P. juliflora but in what appeared to be very similar environmental conditions. Sampling was in June 2010. At Delhi and Rajasthan, open areas were selected outside P. juliflora stands because these stands were closed-canopy woodlands rather than savanna-like vegetation. At Delhi, the open area was characterized by few far-separated trees of Albizzia lebbeck, Balanites roxburghii with interspersed patches of shrub species including Grewia sp., Capparis sepiaria, Capparis decidua and Zizyphus nummularia, and grasses including Cenchrus ciliaris, Chrysopogon fulvus and Heteropogon sp. At Rajasthan also, open area consisted of far-separated tree species (Dalbergia sissoo and Azadirachta indica ) with interspersed patches of shrubs (Zizyphus sp., Lantana camara, Morus sp.) and grasses (Saccharum sp. and Cenchrus ciliaris). However at Gujarat, open areas and P. juliflora subcanopy quadrats were interspersed within the P. juliflora stand due to non-overlapping canopies and open areas included tree species (Acacia senegal and Wrightia tinctoria), shrub species (Euphorbia caducifolia, Commiphora wightii and Grewia sp.) and herb species (Tephrosia sp., Rhynchosia sp. and Cenchrus sp.).
Prosopis juliflora was first recorded at the Sand Island site in Hawaii in the early 1970’s and today this area holds the largest population of Prosopis juliflora and P. juliflora x P. pallida hybrids in Hawaii. The second site, Iroquois Point-Hau Bush, is within a small stretch of undeveloped coastal area. Its location is approximately 10 km to the west of the Sand Island site with a very similar climate to that of Sand Island. Within this area, there is a large established population of P. pallida with P. juliflora and hybrids found near the coast. In October-November 2011, 5×5 m2 quadrats were placed at each site in areas of dense infestation of P. juliflora and areas where it was not present. The number of plant species present in each quadrat was counted.
Our initial plan was to use 1 m2 plots in the two non-native ranges, India and Hawaii, as we did in the native range, of P. juliflora, Venezuela and of P. pallida, Peru. However, the number of species present was much lower under canopies of P. juliflora in the non-native ranges, India and Hawaii. We therefore used larger plots to avoid large numbers of zeros in these plots. We did intra-site species richness comparisons under P. juliflora canopies and nearby open areas where we used identical sampling methods, at each site in native and invaded range and then compared the effect of P. juliflora on species richness observed in its invaded ranges to those observed in its native range.
Species richness in the open and under P. juliflora canopies at each site was compared using independent samples t-tests, one-way ANOVA or Mann-Whitney U tests [29].
No specific permits were required for the field studies carried out in Venezuela, India or Hawaii, USA; and field studies in these areas did not involve endangered or protected species. For the field studies carried out in Peru, permission was obtained from the Agriculture Ministry – Perú (N°145-2008-INRENA-IFFS-DCB). The field study did not involve endangered or protected species.
Effects of Prosopis Congeners on Species Richness
We compared species richness beneath P. juliflora canopies to that beneath canopies of the native congener, P. cineraria, at Deer Park at Patiala, Punjab, India (N 30°17′ 00.2″; E 076°23′ 07.5″; 239 m). The area has a sub-tropical climate and total annual rainfall during the last 5 years of 703.6 mm. The selected site has been invaded by P. juliflora that reach heights of 12 to 14 m and densities of 1.1 trees per 25 m2. Prosopis cineraria occurs there as scattered individuals reaching heights of 4 to 9 m and densities of less than 0.4 trees per 25 m2. The canopy areas occupied by individual P. juliflora and P. cineraria trees were 139.9 and 20.8 m2, respectively. We used slightly smaller quadrats (4×4 m2) for measurements at this site so that a single quadrat represented a single tree either of P. juliflora or P. cineraria. In October 2010, we randomly selected 10 trees of P. juliflora or P. cineraria of similar sizes and laid a 16 m2 quadrat under the canopy of each tree keeping the trunk in the centre, and recorded the number of plant species in each quadrat. We also located 10 randomly located 16 m2 quadrats in open areas where neither P. juliflora nor P. cineraria was present. Differences in species richness in open, under P. juliflora and P. cineraria were tested using one-way ANOVA and post-hoc Tukey’s test [30].
Effects of Congeners on Soil Chemistry
We also compared soils from the rhizospheres of P. juliflora and P. cineraria at Deer Park. We did sampling at this site during two time periods, November 2009 and March 2011. During November 2009, soil was collected from rhizosphere of 4 individual trees of each Prosopis species and from 4 nearby open locations (n = 4 for each of the three treatments). Soil was collected near tree trunk to a depth of 30 cm and presence of a mesh of horizontal and vertical roots in the pit confirmed that it was rhizosphere soil. In March 2011, soil was collected from rhizosphere of 6 individual trees of each Prosopis species and from 6 nearby open locations (n = 6 for each of the three treatments). Soil was collected from 3 points around the tree trunk of each tree selected (no. of subsamples per tree = 3). The soil was then air-dried, sieved (2 mm), and stored in paper bags for later analyses. Five g soil from each sample was shaken with 25 mL water for 1 h followed by filtration through Whatman # 1 filter papers. pH and electrical conductivity were measured in soil extracts using a digital meter (Metrex Sci. Instr. Pvt. Ltd., India). Organic carbon was estimated following methods of Walkley and Black [31]. Total organic nitrogen was estimated using semi-micro Kjeldahl digestion [32]. We estimated PO4 3–P in 2.5% acetic acid soil extracts through molybdenum blue method [32]. Total phenolic content of soils was measured with the Folin-Ciocalteu method [33].
Data from collection during two time periods were combined (n = 10 for each treatment) and, tested for the differences among open, P. juliflora and P. cineraria soils for each soil chemical property using one-way ANOVA and post-hoc Tukey’s tests [29].
Effects of Leaf Litter from Prosopis Congeners on Other Species
We first tested the effects of leaf litter at The University of Montana in a greenhouse experiment. We compared the effects of litter of P. juliflora and P. cineraria on the mortality of six native Indian species (Acacia nilotica, Brassica campestris, Brassica juncea, Chloris dolichostachya, Dalbergia sissoo and Prosopis cineraria). Litter from the Prosopis congeners were collected from three different trees in Aravalli Biodiversity Park, Delhi, India (N 28° 33′ 18.9″; E 077° 08′ 56.8″; 247 m), mixed, and then air dried. Rocket pots (200 cm3, 3 cm diam) were filled with 20/30 grit silica sand, and 5–10 seeds were planted 5 mm below the surface of the sand in each pot. We put 1.0 gm of litter from either P. juliflora or P. cineraria on the surface of the substrate, so that the seeds would have to germinate and grow through the leaves as they would through litter in the field. As seeds germinated they were thinned continually so as to keep only the largest seedling in each pot. The experiment started on 8 January 2010 and ended on 9 February 2010. We summed the total final mortality for all of the remaining largest seedlings in all pots for each species, and then compared treatments using the means of mortality for the six species as replicates in an ANOVA testing treatments (control, P. juliflora, P. cineraria) and followed by Tukey HSD tests [29].
Effects of Leaf Leachate from Prosopis Congeners on Other Species
We also tested the effects of soil amended with leachate from leaves of the two congeners on three crop species, B. campestris, B. juncea and Sorghum bicolor, at the University of Delhi. We used these three crop species because these are common crop species in north-west India where P. juliflora has invaded agricultural fields or is present at the boundaries. Prosopis cineraria is often present in crop fields (Figure 1F). Leaves of the two Prosopis species were collected from the same sites as in the previous experiment and air dried prior to use. Ten g of air dried leaves of each were soaked separately in 100 mL distilled water for 14 h in the dark followed by filtration through Whatman # 1 filter paper. Soil (sandy loam) for the bioassay was collected from an area not occupied by P. juliflora, and at the same site where leaves were collected. 50g air-dried soil was weighed into a 9 cm Petri dish and treated with 15 mL distilled water, 5x, 2x, or 1x diluted P. juliflora or P. cineraria leaf leachate which yielded concentrations of leaf leachate in soil at 0, 60, 150 or 300 µL of leaf leachate/g soil. Brassica campestris and B. juncea seeds were washed with distilled water before use. Sorghum bicolor seeds were surface-sterilized by soaking in 1% sodium hypochlorite for 10 min and then rinsing in distilled water 20 times before use. For each of the seven treatments and three test species, we used six Petri dishes. Ten seeds of B. campestris, B. juncea, or S. bicolor were placed in each Petri dish and kept at 20–23°C. Seven days after treatment application we measured the shoot and root lengths of the germinants. Two-way ANOVA was used to test the effect of leachate source and concentration as fixed factors on root length. We first compared the effect of P. juliflora leachate or P. cineraria leachate treatment (each concentration) with control and then the effect of P. cineraria leachate with P. juliflora leachate applied at identical concentrations, using independent samples t-tests [30].We also correlated leachate concentration with root length for each bioassay species [30].
Effects of Microbes in Litter of Congeners on Other Species
The biochemical effects of litter from different species or different regions could be confounded by different effects of the pathogens in the litter. Therefore we cultivated bacteria and fungi from the leaves of the two Prosopis congeners and applied them to two species native to India, Brassica juncea and Chloris dolichostachya. Cultivation of bacterial and fungal communities of Prosopis leaves was achieved by streaking the leaves of each congener (in triplicate) across the surface of several standard microbiological media agar plates. Plates were incubated at 37°C for 1 week in order to observe any potential bacterial or fungal growth. Five different media were used to cultivate microbial growth. The media used to select for bacteria were R2 agar [34], Minimal Agar, and Nutrient Agar [35] treated with 300 mg L−1of chloramphenicol to inhibit fungal growth. The media used to select for fungi were Mycobiotic Agar and Potato Dextrose Agar [35]. After the incubation period, individual colonies were selected from the media plates and were used to make separate bacterial and fungal community inocula from each Prosopis congener. Each inoculum (either bacterial or fungal) was prepared by aseptically transferring individual microbial colonies into sterile Eppendorf tubes (2 mL volume) filled with 1.5 mL sterile Milli-Q water, and gently vortexed. Mixing 0.5 mL of the bacterial community inoculum with 0.5 mL of the fungal community inoculum made a third composite microbial inoculum. Individual seedlings of B. juncea and C. dolichostachya were germinated in 200 cm3 rocket pots filled with 20/30 grit silica sand, and after two weeks were inoculated with the bacterial, fungal, or composite microbial inocula, with n = 15 for each species and treatment combination. We measured survival of these seedlings 20 days after inoculation.
Chemical Characteristics of Soil Amended with Leaf Leachate of Two Congeners
To determine the effect of leaf leachate treatment on soil chemistry, 50g soil in a 9 cm Petri dish was amended with 15 mL distilled water (control) and 1x diluted P. juliflora or P. cineraria leaf leachate (0 and 300 µL P. juliflora or P. cineraria leaf leachate/g soil) as described above. Six replicate Petri dishes were used per treatment. Immediately after treatment, lids of Petri dishes were removed and soil was allowed to air-dry for 48 h. Soil was then analysed for pH, electrical conductivity, organic carbon, PO4 3–P, and total organic N as described above. We tested the difference among P. juliflora, P. cineraria leaf leachate amended and unamended soil for each soil variable using one-way ANOVA and post-hoc Tukey’s test [30].
Effects of Leaf Leachate and Leaf Litter of Prosopis Congeners on Soil Phenolic Content
Soil amended with leaf leachates of P. juliflora or P. cineraria, as described above, was also analyzed for total phenolic concentration in addition to other chemical properties. In a separate experiment, we added 60 mg of air dried leaves of P. juliflora or P. cineraria to 5 g soil and moistened the soil with 2 ml distilled water. Litter-amended soil was incubated at 30–34°C for 0, 1, 2, 3, 4, 6, 8, 10 or 14 d. Each treatment and the control (unamended soil) were replicated four times. Measurement of total phenolics in soils from two experiments was performed as described above. We tested the differences among P. juliflora litter, P. cineraria litter, leaf leachate amendments, and untreated soil for total phenolics using one-way ANOVA and post-hoc Tukey’s test [30].
L-tryptophan Recovery from Leaf Leachate Amended Soil
Nakano et al. [27] reported that the allelopathic effects of P. juliflora foliage appeared to be caused by L-tryptophan, thus we suspected it might play a role in the effects we measured for litter and leaf leachates. For this, we quantified L-tryptophan in leaf leachates from P. juliflora or P. cineraria and in soil amended with these leachates. Two ml of leaf leachate from P. juliflora or P. cineraria was added to 10 mL methanol, and L-tryptophan was quantified with high-performance liquid chromatography (HPLC). Five g soil was treated with 2 mL leaf leachate from P. juliflora, P. cineraria or with distilled water and then immediately extracted in 10 mL methanol by shaking for 20 min followed by centrifugation at 4500 rpm for 10 min. The supernatant was filtered through 0.2 µm PES syringe filters directly into HPLC vials.
L-tryptophan level in methanol extract of each soil and leachate sample was determined by using HPLC (Waters Corp., Milford, U.S.A), employing a Waters Spherisorb 5 µm ODS2 column (4.6×250 mm Analytical Column). The mobile phase consisted of 50 mM sodiumphosphate buffer, pH 3.5 containing 20% methanol (v/v) and flow rate was 1 mL/min. L-tryptophan was detected at 214 nm using a photodiode array detector. 10–20 µL of sample was injected in the partial loop needle overfill mode, run time for each sample was 20 min, and the retention time of L-tryptophan was 8.17 min with 0.27% RSD. L-tryptophan concentration in leaf leachates from two congeners was compared using independent samples t-test [30].
Results
Prosopis juliflora and Species Richness in Native and Non-native Ranges
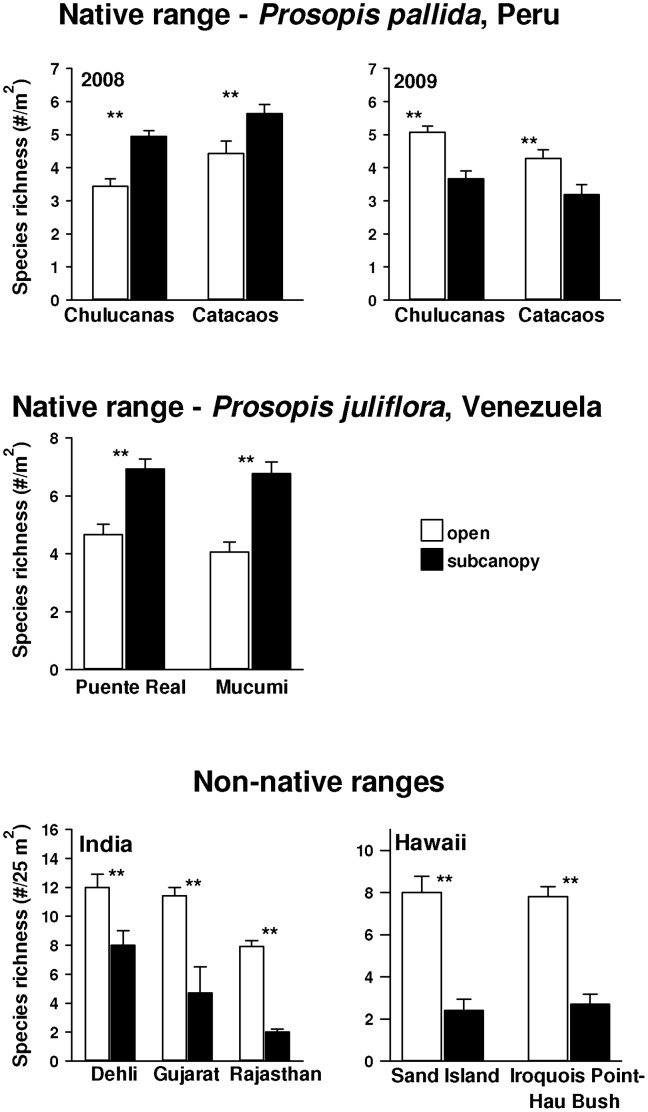
Plant species richness in the understory of P. juliflora in its native range of Venezuela was 48% and 63% higher than in nearby open areas, at Puente Real and Mucumi, respectively (Figure 2, middle panel; Funderstory vs. open = 324.0; df = 1,56; p = 0.035). Plant species richness in the understory of P. pallida in its native range of Peru was 27% and 44% higher than in nearby open areas, at Chulucanas and Catacaos, respectively in 2008 (Figure 2, upper panel; t Chulucanas = 5.13; df = 1,94; p<0.001; t Catacaos = = 2.67; df = 1,92; p = 0.011). However, in 2009 richness was 28% and 25% lower than in the open at those same sites (Figure 2, upper panel; t Chulucanas = 2.70; df = 1,70; p = 0.009; t Catacaos = = 4.678, df = 1, 118, p<0.0001). Analyzed over both years P. pallida had no effect on understory richness in its native range in Peru (Fcanopy = 0.79; df = 1,374; P = 0.778).
Figure 2. Plant species richness (mean + SE) under canopies of Prosopis pallida and nearby open areas in Chulucanas and Catacaos in its native Peru (upper panel); and under canopies of Prosopis juliflora and nearby open areas in its native range of Venezuela (middle panel), and non-native ranges of Delhi, Gujarat and Rajasthan in India, and Honolulu in Hawaii, USA two of its non-native ranges (lower panel).
Asterisks above paired bars were derived from independent t tests except Rajasthan where Mann-Whitney U test (p<0.05) was used.
In the non-native range of India, species richness in P. juliflora invaded areas was 32%, 54% and 78% lower at Delhi, Gujarat, and Rajasthan, respectively, compared to areas without P. juliflora (Figure 2, lower panel: tDelhi = 2.72; df = 1,18, p = 0.014; tGujarat = 3.37; df = 1,12; p = 0.006, and for Rajasthan, Mann-Whitney U test, p = 0.012). The mean effect in India over all three sites was a decrease in species richness of 56%. In habitats invaded by P. juliflora in India native species accounted for 92%, 67% and 100% of the total number of species present below P. juliflora canopies at sites Delhi, Rajasthan and Gujarat, respectively. In Honolulu, Hawaii species richness in P. juliflora invaded areas was 70% and 65.3% lower at Sand Island and Iroquis point, respectively (Figure 2, lower panel: tSand Island = 5.93, df = 1,18, p<0.001; tIroquis point = 7.49; df = 1,18, p<0.001). In Hawaii, however, more than 90% of understory species were non-native.
Effects of Prosopis Congeners on Species Richness
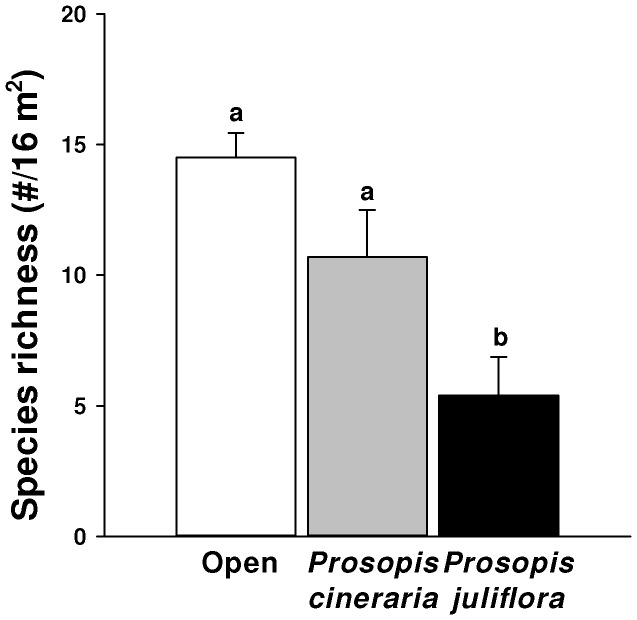
At a fourth site in India, species richness did not differ beneath canopies of P. cineraria relative to open areas, but species richness was 63% lower under P. juliflora canopies than in the open and 50% lower than under P. cineraria canopies (Figure 3; F = 10.018; df = 2,27; P<0.001).
Figure 3. Plant species richness (mean + SE) beneath canopies of P. juliflora, its native congener P. cineraria, and from open areas.
Differences in plant species richness beneath P. cineraria and P. juliflora canopies and open areas were tested using one way-ANOVA and post-hoc Tukey’s test (p<0.05).
Effects of Congeners on Soil Chemistry
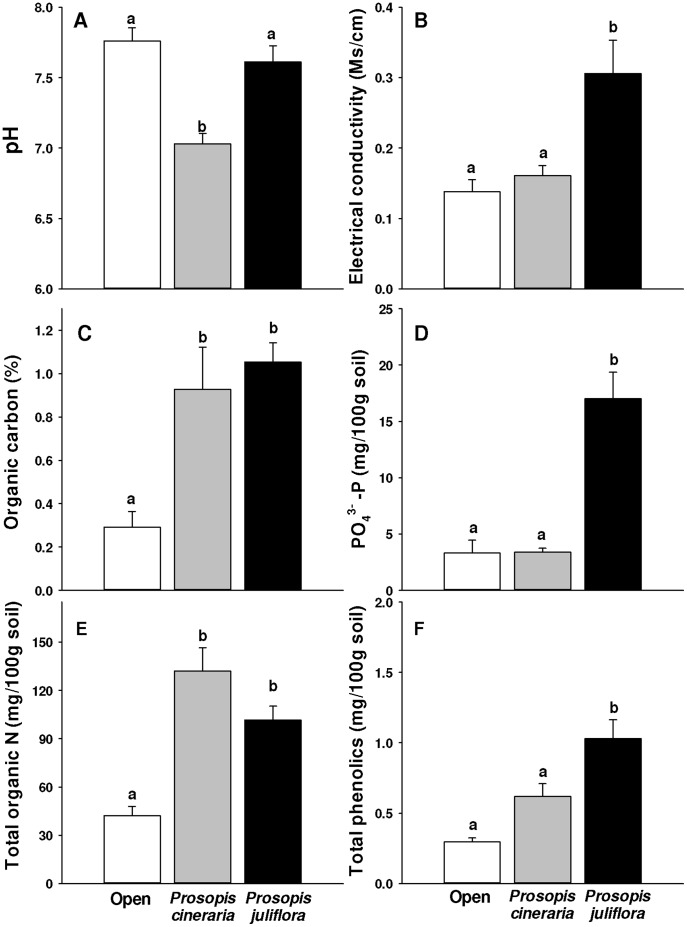
The pH of P. cineraria soil was significantly lower than that of soil in the open (Figure 4A: FpH = 15.914; df = 2,27; p<0.0001, Tukey, p<0.0001) and P. juliflora soil (Tukey, p = 0.001) but that of P. juliflora soil did not differ from soil in open (Tukey, p = 0.525). As compared to soil in open, organic carbon and nitrogen were significantly higher in P. juliflora (Figure 4C; FOrganic carbon = 9.798; df = 2,27; p = 0.001, Tukey, p = 0.001; Figure 4E: FNitrogen = 19.897; df = 2,27; p<0.0001, Tukey, p = 0.001) and P. cineraria soils (organic carbon: Tukey, p = 0.005; nitrogen: Tukey’s test, p<0.0001) but did not differ significantly between the two congeners (organic carbon: Tukey, p = 0.775; nitrogen: Tukey, p = 0.109). Electrical conductivity, exchangeable PO4 3–P and total phenolic content were significantly higher in soil from under P. juliflora than soil from under P. cineraria (Figure 4B: FElectrical conductivity = 9.105; df = 2,27, p = 0.001, Tukey, p = 0.006; Figure 4D: FPhosphorus = 26.574; df = 2,27, p<0.0001, Tukey, p<0.0001: Figure 4F: FPhenolics = 13.711; df = 2,25, p<0.0001, Tukey, p = 0.020) or from the open (electrical conductivity: Tukey, p = 0.001; exchangeable PO4 3–P: Tukey, p<0.0001; total phenolic content: Tukey, p<0.0001). These factors did not differ significantly between P. cineraria soil and soil from the open (electrical conductivity: Tukey, p = 0.847; exchangeable PO4 3–P: Tukey, p = 1.000; total phenolic content: Tukey, p = 0.084).
Figure 4. Chemical properties – pH (A), electrical conductivity (B), organic carbon (C), PO4 3–Phosphorus (D), total organic nitrogen (E) and total phenolic content (F) of soil from the rhizosphere of Prosopis juliflora, Prosopis cineraria and soil from adjacent open area in Punjab, India.
Error bars represent +1SE of mean. Different letters above bars indicate significant differences (one way ANOVA, post-ANOVA Tukey’s test; p<0.05).
Effects of Leaf Litter from Prosopis Congeners on Other Species
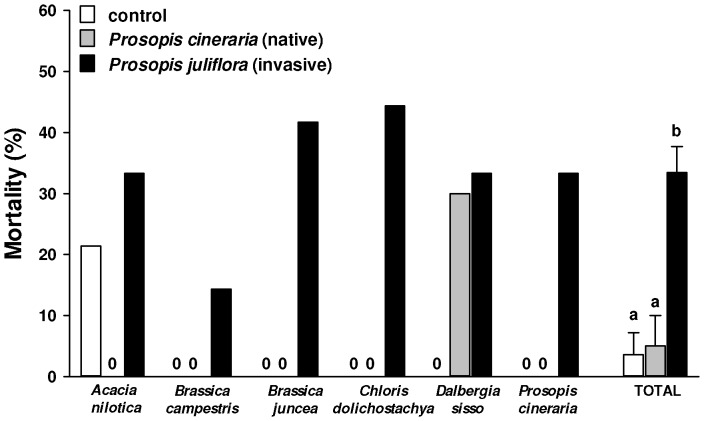
Leaf litter from the Indian native P. cineraria increased the mortality of Dalbergia sissoo, but had no effect on any other native Indian species (Figure 5). In contrast, leaf litter from the invasive P. juliflora increased the mortality of all six native Indian species from 14–44%. The mean mortality of Indian species when exposed to litter from P. cineraria (5±5%) was not significantly different than that in the control (ANOVA, Ftreatment = 15.09; df = 2,15; P<0.001; Tukey P = 0.970), but much lower than the mean mortality (33±4%) of plants treated with P. juliflora litter (Tukey P = 0.001).
Figure 5. Mortality (mean + SE) of different species - Acacia nilotica, Brassica campestris, Brassica juncea, Chloris dolichostachya, Dalbergia sissoo and Prosopis cineraria - when grown in soil amended with leaf litter of Prosopis juliflora (black box), P. cineraria (gray box) or without litter (white box).
Different letters above bars indicate significant differences (one way ANOVA, post-ANOVA Tukey’s test; p<0.05).
Effects of Leaf Leachate from Prosopis Congeners on Other Species
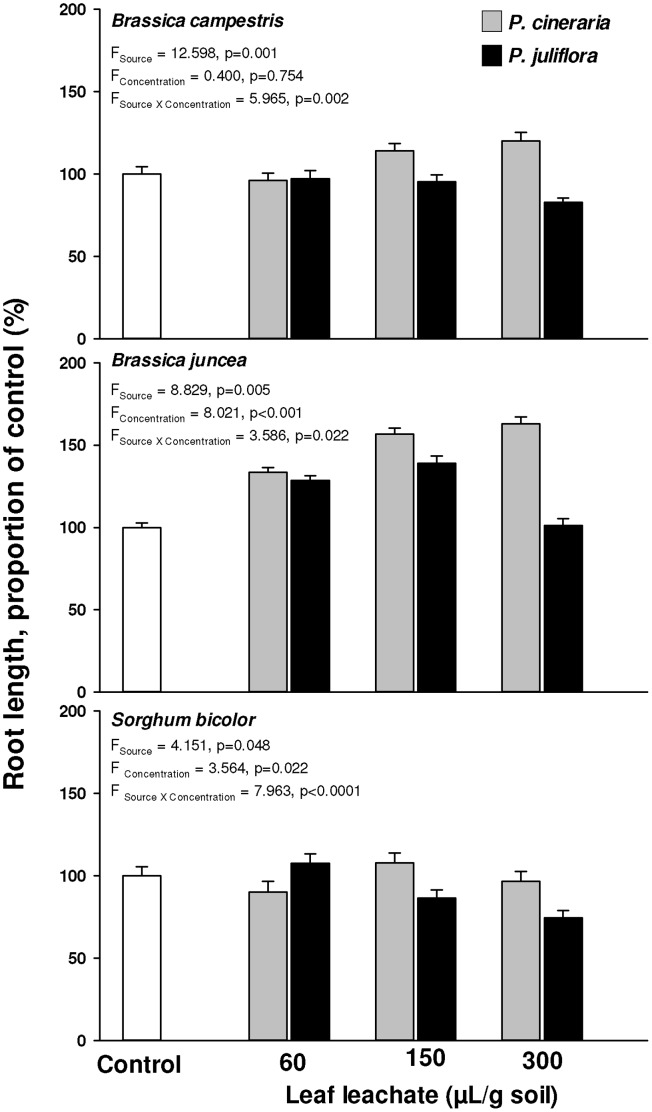
As compared to control, leachate from the leaves of the native P. cineraria significantly enhanced the root growth of B. campestris at 150 (Figure 6 upper panel; t0 vs. 150 = −2.561; df = 1,10; p = 0.028;) and 300 µL/g soil concentrations (t0 vs. 300 = −2.955; df = 1,10; p = 0.014), and of B. juncea at 60 (Figure 6 middle panel; t0 vs. 60 = −3.506; df = 1,10; p = 0.006), 150 (t0 vs. 150 = −4.759, df = 1,6; p = 0.003), and 300 µL/g soil concentrations (t0 vs. 300 = −3.067; df = 1,6; p = 0.025). The root length of S. bicolor seedlings grown in P. cineraria leaf leachate amended soil was not significantly different from control at any tested concentration (Figure 6 lower panel; t0 vs. 60 = 1.475; df = 1,10; p = 0.171; t0 vs. 150 = −1.370, df = 1,10; p = 0.201; t0 vs. 300 = 0.496; df = 1,10; p = 0.631). In contrast, leachate from the leaves of the invasive P. juliflora significantly reduced the root growth of B. campestris as compared to control at the highest concentration (Figure 6 upper panel, t0 vs. 300 = 3.893, df = 1,10, p = 0.003) and of S. bicolor at 150 (Figure 6 lower panel, t0 vs. 150 = 3.006, df = 1,10, p = 0.013) and 300 µL/g soil concentrations (t0 vs. 300 = 6.226, df = 1,10, p<0.0001). Brassica juncea root growth increased in soil treated with 60 (Figure 5 middle panel; t0 vs. 60 = −2.477, df = 1,10; p = 0.033) and 150 µL/g soil concentrations (t0 vs. 150 = −3.693, df = 1,10, p = 0.004) of P. juliflora leachate and was not affected at highest concentration (t0 vs. 300 = 0.002, df = 1,10, p = 0.999).
Figure 6. Root length (proportion of control, %) of Brassica campestris (upper panel), B. juncea (middle panel) and Sorghum bicolor (lower panel) seedlings grown in soil treated with different amounts (60, 150 and 300 µL/g soil) of Prosopis cineraria (gray bars) or Prosopis juliflora (black bars) leaf leachate.
Soil treated with distilled water served as untreated control (white bars). Error bars represent +1SE of mean. F and P values are shown for two way ANOVAs.
Root length of seedlings of all 3 species grown in soil amended with leaf leachate from P. juliflora was significantly lower than that of seedlings grown in P. cineraria leaf leachate-amended soil at similar concentrations i.e. 150 (Figure 6 upper panel; tB. campestris = 2.764; df = 1,10; p = 0.020; tS. bicolor = 3.116; df = 1,10, p = 0.011) and 300 µL/g soil (tB. campestris = 5.573; df = 1,10; p<0.001; tB. juncea = 2.936; df = 1,10; p = 0.015; tS. bicolor = 4.963; df = 1,10; p = 0.001), with the exception of B. juncea at 150 µL/g soil (tB. juncea = 1.151; df = 1,10; p = 0.277). However at 60 µL/g soil concentration, there was no significant difference in root growth of all 3 species grown in P. juliflora leaf leachate-amended soil compared to those in P. cineraria leaf leachate-amended soil.
There was a significant interaction effect between leachate source and concentration on root growth for all 3 species (Figure 6). We also found significantly negative correlations between P. juliflora leachate concentration and root growth of B. campestris (r = −0.681, p<0.001) and S. bicolor (r = −0.476, p = 0.019), however no significant correlation was observed in case of B. juncea (r = −0.060, p = 0.779). In contrast, significant positive correlations between P. cineraria leachate concentration and root growth of B. campestris (r = 0.594, p = 0.002) and B. juncea (r = 0.589, p = 0.002) were observed, however no significant correlation was observed in case of S. bicolor (r = 0.084, p = 0.695).
Effect of Microbes in Litter of Prosopis Congeners on Other Species
Bacteria and fungi isolated from the leaves of P. julifora and P. cineraria, when applied directly to other species, killed almost all B. juncea and C. dolichostachya seedlings precluding statistical analysis, but there were no discernible patterns in mortality for isolates from the two congeners. Fungal isolates from P. juliflora, applied as a group, killed 97% of B. juncea and 98% of C. dolichostachya seedlings (data not shown). Bacterial isolates from P. juliflora, applied as a group, killed 100% of B. juncea and 94% of C. dolichostachya. Fungal isolates from P. cineraria, applied as a group, killed 98% of B. juncea and 100% of C. dolichostachya (data not shown). Bacterial isolates from P. cineraria, applied as a group, killed 94% of B. juncea and 99% of C. dolichostachya.
Chemical Characteristics of Soil Amended with Leaf Leachates of Two Congeners
Both treatments, P. juliflora leaf leachate and P. cineraria leaf leachate, resulted in a significant decrease in pH and an increase in EC, OC, PO4 3–P, and total organic N of soil with the effect of former treatment being significantly higher than latter (Table S1). Prosopis cineraria leaf leachate treated soil did not differ significantly for PO4 3–P from untreated soil (Table S1).
Effects of Leaf Leachate and Leaf Litter on Soil Phenolic Content
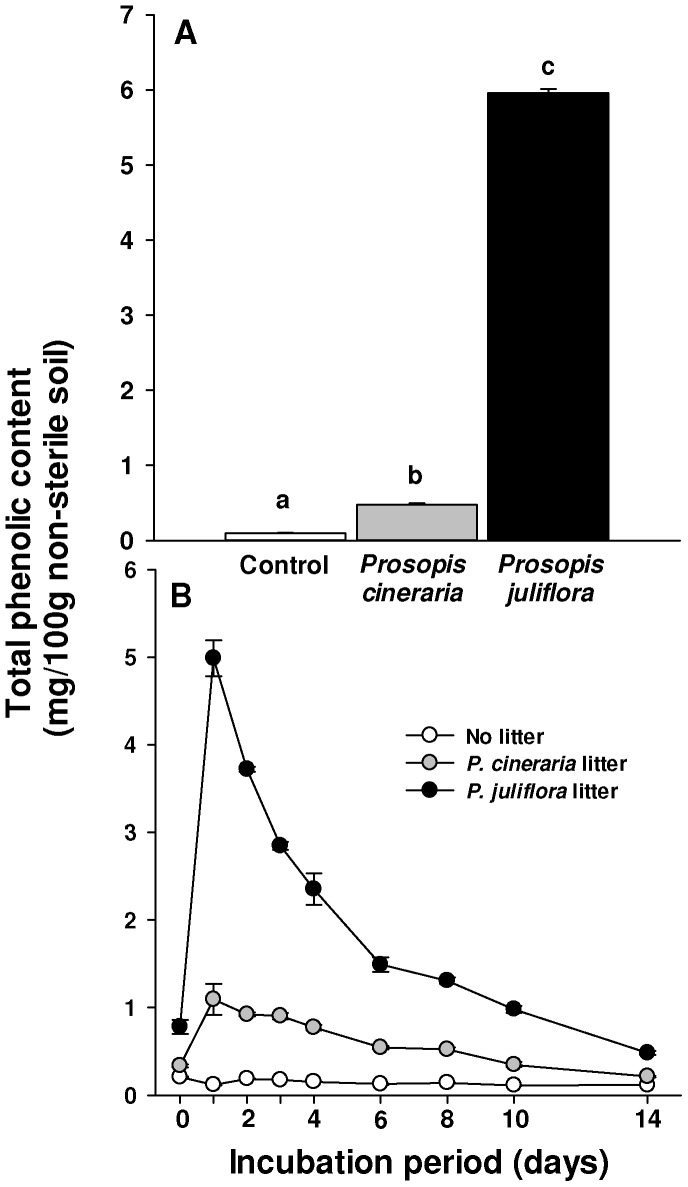
Soil amended with leaf leachate of P. juliflora had 12.4 times higher values of total phenolics (5.956±0.054 mg/100 g soil) than soil amended with P. cineraria (0.479±0.019 mg/100 g soil) and 60.2 times higher concentrations than unamended soil (0.099±0.004 mg/100 g soil; F = 9734.99; df = 2,15; p<0.015; Figure 7A). The total phenolic content of soil amended with leaf litter from P. juliflora was significantly higher than soil amended with P. cineraria litter and unamended soil, from 0 d to 14 d of incubation (Figure 7B, Table S2).
Figure 7. Total phenolic content (mean + SE) of soil amended with Prosopis cineraria (gray bar) and P. juliflora (black bar) leaf leachates and unamended soil (control, white bar). (A).
Different letters above bars indicate significant differences (ANOVA, post-ANOVA Tukey’s test; p<0.05). (B) Total phenolic content (mean ± SE) of soil treated with no litter (white circles), P. cineraria (gray circles) or P. juliflora leaf litter (black circles) at rate of 12 mg/g soil, and incubated at 30–34°C under 12 h/12 h light/dark cycle for 0, 1, 2, 3, 4, 6, 8, 10 and14 days.
L-tryptophan Recovery from Leaf Leachate Amended Soil
L-tryptophan concentrations in the leachates of leaves of P. juliflora (4.92±0.05 µg/ml leaf leachate) was 73.1% greater than that of P. cineraria (1.32±0.014 µg/ml leaf leachate) (t = 70.509, df = 4, p<0.0001). Over 40% (0.79±0.006 µg/g soil) of the originally added 1.968 µg/g soil of L-tryptophan from P. juliflora leachate, was recovered from soil immediately after application, but we could not detect L-tryptophan in soil amended with P. cineraria leachates.
Discussion
Prosopis juliflora is among the most invasive species in hot semiarid and arid regions of the world, and our results indicate that this invader has substantially stronger impacts on native diversity in two non-native ranges than in its native range of Venezuela where P. juliflora had facilitative effects and in the native range of Peru where P. pallida (often misidentified as P. juliflora [5]) had overall neutral effects. Despite the widely documented and very strong impacts of invasive species on natives [36], [37], to our knowledge only two other studies have quantified the impact of an invasive species on the productivity or diversity of its neighbors in the field in both its native and non-native ranges. Inderjit et al. [38] found that the canopies of Ageratina adenophora, a widespread and aggressive subtropical invader, had facilitative effects on other species in its native Mexico but highly inhibitory effects in its non-native ranges in China and India. These differences were correlated with differences in the allelopathic effects of volatile organic compounds on species native to the different ranges. Callaway et al. [2] found that the biomass of native species in Acroptilon repens stands was 25–30 times lower in the non-native range than in the native range. It is important to note that lower plant species richness in plots invaded by P. juliflora compared to plots not yet invaded by it, could be due to the negative impact of the invader or colonization of sites that inherently have low species richness; mechanisms that cannot be separated through measurements of correlative patterns. However, consistently stronger negative canopy effects of P. juliflora in two non-native ranges compared to its native range, differences in canopy effects between congeners in the non-native range, and direct comparisons of litter from the congeners support the hypothesis that P. juliflora is at least in part a “driver” of decreased diversity rather than a “passenger” responding to other factors that also decrease diversity [39]. Strong negative impacts of P. juliflora on the richness, evenness and densities of other plant species have also been reported in the United Arab Emirates where it is also invasive [20]. The observed effects of P. juliflora canopies on species richness are less likely to be due to higher stand densities because similar negative canopy effects of P. juliflora on species richness have also been observed at lower stand densities (unpublished work).
Congeneric comparisons demonstrated stronger impacts of the invasive P. juliflora than the native P. cineraria on plant diversity. Prosopis cineraria, a native Indian species, has been reported to facilitate growth of crop species and is an important species in agroforestry [40]; however, we measured its neutral effects on native diversity. Although biogeographic comparisons have been performed for other invasive plants such as Ageratina adenophora [38], Alliaria petiolata [41], Centaurea stoebe [42], Acroptilon repens [2] and C. diffusa [43], to our knowledge, this congeneric comparison is unique. Since P. cineraria is not an invasive species, we did not study, reciprocally, its positive or negative effects on species exotic to its native range India.
Our results show that like other species of Prosopis, both P. juliflora and P. cineraria form resource islands by accumulating total organic N and organic carbon in their rhizosphere soil. Unlike P. cineraria, P. juliflora also accumulates soluble salts, exchangeable-P and total phenolics in its soil. However, it appears that the presence of high concentrations of total phenolics in P. juliflora soil may override its potential positive effects on soil fertility.
Our study indicates that litter may play at least some role in the impact of P. juliflora on soil nutrients, soil phenolics, and the reduction of native species diversity in India. Leaf litter of P. juliflora killed far more seedlings of native Indian species than litter of P. cineraria. These effects of leaf litter appeared to be through their biochemical effects, results consistent with the Novel Weapons Hypothesis [44]. Bacteria and fungi isolated from P. juliflora and P. cineraria leaf litter and applied to seedlings killed almost all native Indian species, but there was no hint of a difference in effects between the congeners. Also, much higher amounts of total phenolics in soil amended with P. juliflora leaf leachate compared to that amended with P. cineraria leachate suggest that the release of more total phenolics into soil may play an important role in the stronger effects of P. juliflora litter. In a similar comparison of the phytotoxicity of P. juliflora and P. cineraria rhizosphere soils, Inderjit et al. [25] reported that Bambusa arundinacea seedlings grown in P. juliflora soil were smaller than those grown in P. cineraria soils, a result that could be due to a higher total phenolic content of P. juliflora soils than P. cineraria soils, as we measured here. Also, there were much higher amounts of total phenolics leached from P. juliflora litter than P. cineraria litter over a 14 d period (Figure 7B), suggesting a higher and more continuous supply of phenolics to soil from the litter of P. juliflora. Other studies also indicate that P. juliflora has substantial allelopathic potential [26], [45], [46]. Allelochemicals could directly affect plant growth or also impact soil microbes [47]. Our unpublished studies suggest that soils amended with P. juliflora litter have higher microbial activity compared to soil amended with P. cineraria litter. P. juliflora allelochemicals might affect plant growth directly and also by influencing microbial activity or microbial communities.
Higher concentrations of allelochemicals in P. juliflora compared to P. cineraria could be due to higher ploidy levels in P. juliflora. Trenchard et al. [48] studied ploidy of 10 Prosopis species including P. juliflora and found all 9 species to be diploid (2n = 2x = 28) except P. juliflora which was tetraploid (2n = 4x = 56). Invasive species are commonly polyploids [49]–[51] and polyploids may have an advantage over their diploid progenitors in having novel phenotypic variation resulting from increased variation in expression of dosage-regulated genes, altered regulatory interactions, rapid genetic and epigenetic changes [52]. The observed higher levels of phenolics or L-tryptophan from the leaf leachate of P. juliflora than that of P. cineraria could be a novel phenotype resulting from any of these novel gene expression mechanisms following polyploidization.
Although many chemicals or combinations of chemicals could cause the apparent allelopathic effects we measured, we focused on the release of L-tryptophan from litter and in soil because of reports of this chemical in leachates of P. juliflora foliage by Nakano et al. [27]. We detected L-tryptophan in leaf leachates of both P. juliflora and P. cineraria, but the amounts of L-tryptophan in leaf leachate of P. juliflora were 73% higher than that of leachate of P. cineraria. The presence of higher concentrations of L-tryptophan in soil amended with P. juliflora leachate and its rapid disappearance from soil amended with P. cineraria leachate supports the potential for L-tryptophan to affect the phytotoxic nature of P. juliflora litter. In India the annual leaf litterfall of P. juliflora has been reported at 8.1 Mg/ha, and litterfall is highest between February and May [53] when native species (such as Butea monosperma, Holoptelea integrifolia, Carissa spinarum, Capparis sepiaria, C. decidua, Cenchrus ciliaris or Azadirachta indica) germinate and recruit. Also, the evergreen habit of P. juliflora ensures the consistent presence of L-tryptophan in soils. Thus P. juliflora litter possesses large amounts of a chemical which is present at far lower amounts in the leaf litter of the native congener P. cineraria. There are likely to be other allelopathic or antibiotic chemicals in the litter, leachate or root exudates of P. juliflora that we did not measure, but such higher concentrations of a particularly biologically active chemical produced by an invasive species is only partially consistent with the Novel Weapons Hypothesis. The Novel Weapons Hypothesis poses that some successful invaders may possess allelopathic [54], antibiotic [41] or herbivore defense chemicals [55] that are unique in the non-native range, giving the invasive species an advantage against evolutionary naive native species [44]. As reported here, small amounts of L-tryptophan also occur in the litter of the Indian native P. cineraria. However, the striking differences in the inhibitory effects of litter between the two Prosopis species suggest that L-tryptophan is unlikely to be the only active biochemical in the leaves of the invader.
Our results add to a growing body of literature indicating that there is substantial species specificity in the effects of plant-released secondary metabolites [56]. Also, these species-specific interactions suggest that assemblages of plants, or communities, may be less individualistic than often thought (see [57]). Finally, our results suggest that regional evolutionary trajectories exist and that novel competitive mechanisms have the potential to disrupt coevolved interactions among long-associated native species.
Supporting Information
Summary of statistical analysis of pH, electrical conductivity (EC), organic carbon (OC), phosphate-P and total organic N (TON) of soil treated with no leachate (control, C), P. cineraria (PC) or P. juliflora (PJ) leaf leachate (one-way ANOVA and posthoc Tukey’s test at p<0.05).
(DOCX)
Summary of statistical analysis of total phenolic content of soil treated with no litter (control, C), P. cineraria (PC) or P. juliflora (PJ) leaf litter at rate of 12 mg/g soil, and incubated for 0, 1, 2, 3, 4, 6, 8, 10 and 14 days.
(DOCX)
Acknowledgments
We thank Professors C.R. Babu and S. R. Yadav for their help in the identification of plant species. Field work at Hawaii, USA was carried out during 9-month appointment of Professor Inderjit as G.P. Wilder Chair Professor at the University of Hawaii Manoá.
Funding Statement
The research was funded by the University of Delhi. RMC gratefully acknowledges funding from the National Science Foundation, DEB 0614406, and the Rocky Mountain Research Station, United States Department of Agriculture Forest Service. WGL gratefully acknowledges funding from the Programa de Ciencia y Tecnología (FINCyT), Contrato N°011-FINCyT-PIBAP-2007. The funders had no role in study, design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Callaway RM, Maron JL (2006) What have exotic plant invasions taught us over the past 20 years? Trends Ecol Evol 21: 369–374. [DOI] [PubMed] [Google Scholar]
- 2. Callaway RM, Schaffner U, Thelen GC, Khamraev A, Juginisov T, et al. (2012) Impact of Acroptilon repens on co-occurring native plants is greater in the invader’s non-native range. Biol Invas 14: 1143–1155. [Google Scholar]
- 3.Pasiecznik NM, Felker P, Harris PJC, Harsh LN, Cruz G, et al. (2001) The Prosopis juliflora - Prosopis pallida Complex: A Monograph. Coventry, UK: HDRA.
- 4.Burkart A (1976) A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). (Part 1 and 2). Catalogue of the recognized species of Prosopis. J Arnold Arborat 57: 219–249; 450–525.
- 5. Palacios RA, Burghardt AD, Frŕas-Hernández JT, Olalde-Portugal V, Grados N, et al. (2012) Comparative study (AFLP and morphology) of three species of Prosopis of the Section Algarobia: P. juliflora, P. pallida, and P. limensis. Evidence for resolution of the “P. pallida–P. juliflora complex”. Plant Systemat Evol 298: 165–171. [Google Scholar]
- 6. Larrea-Alcázar DM, Soriano PJ (2008) Columnar cacti-shrub relationships in an Andean semiarid valley in Western Venezuela. Plant Ecol 196: 153–161. [Google Scholar]
- 7. Tiedemann AR, Klemmedson JO (1973) Nutrient availability in desert grassland soils under Mesquite (Prosopis juliflora) trees and adjacent open areas. Soil Sci Soc Am J 37: 107–111. [Google Scholar]
- 8. Tiedemann AR, Klemmedson JO (1977) Effect of mesquite trees on vegetation and soils in the desert grassland. J Range Manage 30: 361–367. [Google Scholar]
- 9. Tiedemann AR, Klemmedson JO (1986) Long-term effects of mesquite removal on soil characteristics: I. Nutrients and bulk density. Soil Sci Soc Am J 50: 472–475. [Google Scholar]
- 10. Virginia RA, Jarrell WM (1983) Soil properties in a mesquite-dominated Sonoran desert ecosystem. Soil Sci Soc Am J 47: 138–144. [Google Scholar]
- 11. Archer S, Scifres CR, Bassham CR, Maggio R (1988) Autogenic succession in a subtropical savanna: conversion of grassland to thorn woodland. Ecol Monogr 58: 111–127. [Google Scholar]
- 12. Franco-Pizaña J, Fulbright TE, Gardiner DT (1995) Spatial relations between shrubs and Prosopis glandulosa canopies. J Veg Sci 6: 73–78. [Google Scholar]
- 13. Franco-Pizaña J, Fulbright TE, Gardiner DT, Tipton AR (1996) Shrub emergence and seedling growth in microenvironments created by Prosopis glandulosa. . J Veg Sci 7: 257–264. [Google Scholar]
- 14. Carillo-Gracia A, Bashan Y, Bethlenfalvay GJ (2000) Resource-island soils and the survival of the giant cactus, carbon, of Baja California Sur. Plant Soil 218: 207–214. [Google Scholar]
- 15. Rossi BE, Villagra PE (2003) Effect of Prosopis flexuosa on soil properties and the spatial pattern of understorey species in arid Ageratina. J Veg Sci 14: 543–550. [Google Scholar]
- 16. Schade JD, Sponseller R, Collins SL, Stiles A (2003) The influence of Prosopis canopies on understorey vegetation: effects of landscape position. J Veg Sci 14: 743–750. [Google Scholar]
- 17. Zou CB, Barnes PW, Archer S, McMurty CR (2005) Soil moisture redistribution as a mechanism of facilitation in savanna tree-shrub clusters. Oecologia 145: 32–40. [DOI] [PubMed] [Google Scholar]
- 18. Aggarwal RK, Gupta JP, Saxena SK, Muthana KD (1976) Studies on soil physico-chemical and ecological changes under twelve years old desert tree species of Western Rajasthan. Indian For 102: 863–872. [Google Scholar]
- 19. Aggarwal RK, Kumar P, Raina P (1993) Nutrient availability from sandy soils underneath Prosopis cineraria (Linn. Macbride) compared to adjacent open site in an arid environment. Indian For 199: 321–325. [Google Scholar]
- 20. El-Keblawy A, Al-Rawai A (2007) Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecol 190: 23–35. [Google Scholar]
- 21. Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6: 503–523. [Google Scholar]
- 22. Allison SD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141: 612–619. [DOI] [PubMed] [Google Scholar]
- 23. Rothstein DE, Vitousek PM, Simmons BL (2004) An Exotic Tree Alters Decomposition and Nutrient Cycling in a Hawaiian Montane Forest. Ecosystems 7: 805–814. [Google Scholar]
- 24. Rout M, Callaway RM (2009) An invasive plant paradox. Science 324: 734–735. [DOI] [PubMed] [Google Scholar]
- 25. Inderjit, Seastedt TR, Callaway RM, Pollock J, Kaur J (2008) Allelopathy and plant invasions: traditional, congeneric, and biogeographical approaches. Biol Invas 10: 875–890. [Google Scholar]
- 26. Goel U, Saxena DB, Kumar B (1989) Comparative study of allelopathy as exhibited by Prosopis juliflora swartz and Prosopis cineraria (L) Druce. J Chem Ecol 15: 591–600. [DOI] [PubMed] [Google Scholar]
- 27. Nakano H, Nakajima E, Fujii Y, Yamada K, Shigemori H, et al. (2003) Leaching of the allelopathic substance, L-tryptophan from the foliage of mesquite (Prosopis juliflora (Sw.) DC.) plants by water spraying. Plant Growth Reg 40: 49–52. [Google Scholar]
- 28. Brooker RW, Callaway RM, Cavieres LA, Kikvidze Z, Lortie CJ, et al. (2009) Don’t diss integration: a comment on Ricklefs’ disintegrating communities. Am Natu 174: 919–927. [DOI] [PubMed] [Google Scholar]
- 29.IBM SPSS (2010) IBM SPSS Statistics 19: A Brief Guide. Chicago IL: SPSS Inc.
- 30.SPSS (2008) SPSS version 16.0.2. Chicago, IL: SPSS Inc.
- 31.Piper CS (1966) Soil and Plant Analysis. Bombay, India: Hans Publishers.
- 32.Allen SE (1989) Chemical Analysis of Ecological Materials, 2nd edn. Cambridge, USA: Blackwell Scientific Publications.
- 33. Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica L. - the quantitative analysis of phenolic constituents. J Sci Food Agric 10: 63–68. [Google Scholar]
- 34. Reasoner DJ, Blannon JC, Geldreich EE (1979) Rapid seven-hour fecal coliform test. Appl Environ Microbiol 38: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atlas RM (2004) Handbook of Microbiological Media. London: CRC Press.
- 36. Wardle D, Bardgett D, Callaway RM, van der Putten W (2011) Terrestrial ecosystem responses to species gains and losses. Science 332: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 37. Simberloff D, Souza L, Nũnez MA, Barrios-Garcia N, Bunn W (2012) The natives are restless, but not often and mostly when disturbed. Ecology 93: 598–607. [DOI] [PubMed] [Google Scholar]
- 38. Inderjit, Evan H, Crocoll C, Bajpai D, Kaur R, et al. (2011) Volatile chemicals from leaf litter are associated with invasiveness of a neotropical weed in Asia. Ecology 92: 316–324. [DOI] [PubMed] [Google Scholar]
- 39. MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86: 42–55. [Google Scholar]
- 40. Kaushik N, Kumar V (2003) Khejri (Prosopis cineraria)-based agroforestry system for arid Haryana, India. J Arid Environ 55: 433–440. [Google Scholar]
- 41. Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, et al. (2008) Novel weapons: invasive plants suppresses fungal mutualists in America but not in its native Europe. Ecology 89: 1043–1055. [DOI] [PubMed] [Google Scholar]
- 42. Thorpe AS, Thelen GC, Diaconu A, Callaway RM (2009) Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97: 641–645. [Google Scholar]
- 43. Callaway RM, Aschehoug ET (2000) Invasive plant versus their new and old neighbors: a mechanism for exotic invasion. Science 290: 521–523. [DOI] [PubMed] [Google Scholar]
- 44. Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2: 436–443. [Google Scholar]
- 45. Al-Humaid AI, Warrag MOA (1998) Allelopathic effects of mesquite (Prosopis juliflora) foliage on seed germination and seedling growth of bermudagrass (Cynodon dactylon). J Arid Environ 38: 237–243. [Google Scholar]
- 46. Noor M, Salam U, Khan MA (1995) Allelopathic effects of Prosopis juliflora Swartz. J Arid Environ 31: 83–90. [Google Scholar]
- 47. Inderjit, van der Putten WH (2010) Impacts of soil microbial communities on exotic plant invasion. Trends Ecol Evol 25: 512–519. [DOI] [PubMed] [Google Scholar]
- 48. Trenchard LJ, Harris PJC, Smith SJ, Pasiecznik NM (2008) A review of ploidy in the genus Prosopis (Leguminosae). Bot J Linn Soc 156: 425–438. [Google Scholar]
- 49. Pandit MK, Pocock MJO, Kunin WE (2011) Ploidy influences rarity and invasiveness in plants. J Ecol 99: 1108–1115. [Google Scholar]
- 50. Treier UA, Broennimann O, Normand S, Guisan A, SchaffnerU, et al (2009) Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa . Ecology 90: 1366–1377. [DOI] [PubMed] [Google Scholar]
- 51. te Beest M, Le Roux JJ, Richardson DM, Suda J, Kubešová M, et al. (2012) The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot 109: 19–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Osborn TC, Pires JC, Birchler JA, Auger DL, Chen ZJ, et al. (2003) Understanding mechanisms of novel gene expression in polyploids. Trends Genet 19: 141–147. [DOI] [PubMed] [Google Scholar]
- 53. Jha P, Mohapatra MP (2010) Leaf litterfall, fine root production and turnover in four major tree species of the semi-arid region of India. Plant Soil 326: 481–491. [Google Scholar]
- 54. Inderjit, Wardle DA, Karban R, Callaway RM (2011) The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26: 655–662. [DOI] [PubMed] [Google Scholar]
- 55. Schaffner U, Ridenour WM, Wolf VC, Bassett T, Muller C, et al. (2011) Plant invasions, generalist herbivores, and novel defense weapons. Ecology 92: 829–835. [DOI] [PubMed] [Google Scholar]
- 56.Callaway RM, Hierro JL, Thorpe AS (2005) Evolutionary trajectories in plant and soil microbial communities: Centaurea invasions and the geographic mosaic of coevolution. In: Sax DF, Gaines SD, Stachowicz JJ. eds. Exotic Species Invasions: Insights into Ecology, Evolution and Biogeography. Sunderland: Sinauer. 341–363.
- 57. Lortie CJ, Brooker RW, Choler P, Kikvidze Z, Michalet R, et al. (2004) Rethinking plant community theory. Oikos 107: 433–438. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of statistical analysis of pH, electrical conductivity (EC), organic carbon (OC), phosphate-P and total organic N (TON) of soil treated with no leachate (control, C), P. cineraria (PC) or P. juliflora (PJ) leaf leachate (one-way ANOVA and posthoc Tukey’s test at p<0.05).
(DOCX)
Summary of statistical analysis of total phenolic content of soil treated with no litter (control, C), P. cineraria (PC) or P. juliflora (PJ) leaf litter at rate of 12 mg/g soil, and incubated for 0, 1, 2, 3, 4, 6, 8, 10 and 14 days.
(DOCX)