Abstract
Cellular communication is at the heart of animal development, and guides the specification of cell fates, the movement of cells within and between tissues, and the coordinated arrangement of different body parts. During organ and tissue growth, cell-cell communication plays a critical role in decisions that determine whether cells survive to contribute to the organism. In this review, we discuss recent insights into cell competition, a social cellular phenomenon that selects the fittest cells in a tissue, and as such potentially contributes to the regulation of its growth and final size. The field of cell competition has seen a huge explosion in its study in the last several years, facilitated by the increasingly sophisticated genetic and molecular technology available in Drosophila and driven by its relevance to stem cell biology and human cancer.
INTRODUCTION
Minute genes and the discovery of cell competition
Cell competition was discovered in Drosophila about thirty-five years ago, when Gines Morata and Pedro Ripoll were studying the phenotype of a class of mutations called Minute. These mutations, later found to affect ribosomal protein-encoding (Rp) genes (Kongsuwan et al., 1985), are homozygous lethal, but yield viable flies that are slow to develop when heterozygous (Lambertsson, 1998; Marygold et al., 2007). In a seminal paper, Morata and Ripoll showed that cell-autonomous proliferation defects of Minute/+ (M/+) mutant cells explained the observed developmental delay. More surprisingly, they observed that clones of M/+ mutant cells are not recovered in adult fly wings that are otherwise wildtype for these genes unless the mosaics are induced very late in development (Morata and Ripoll, 1975). They concluded that, although the M/+ cells are viable on their own, they are actively eliminated when growing in the milieu of wildtype cells, a phenomenon they named cell competition.
This model was confirmed and elaborated in studies that quickly followed. Using starvation to slow growth, Pat Simpson found that competition between M/+ mutant and wildtype cells is reduced and the frequency of M/+ clones recovered in mosaic adult wings is increased. This observation demonstrated that competition between the M/+ and wildtype cells was dependent upon the difference in their growth rates, so that when the growth and protein synthesis of wildtype cells is crippled by starvation, their competitive advantage is also compromised (Simpson, 1979). Moreover, she found that competition is more severe in the center of a developmental compartment than near its boundaries (Simpson, 1979), and that competition between M/+ and wildtype cells stops after completion of growth (Simpson, 1981). Altogether, these results showed that cell competition is dependent on growth and cell proliferation.
The discovery of cell competition coincided with the discovery of the developmental compartmentalization of organs, since both were made possible by the use of Minute mutants (Garcia-Bellido et al., 1973). Intriguingly, the final size of wings is unaffected by competition between wildtype and M/+ cells, because although the latter are ultimately eliminated, the wildtype cells proliferate in compensation, often taking over whole compartments. This observation suggested that the faster-growing cells grow at the expense of the slower ones, thereby eliminating them and eventually filling the space, and raised the important idea that cell competition is involved in organ growth and size control. A striking and still poorly understood feature of cell competition is that not only do cells fare better near compartment boundaries, competition does not cross these boundaries (Simpson and Morata, 1981). As several lines of evidence suggested that compartments represent size-control units during development (Crick and Lawrence, 1975; Simpson, 1976), it reinforced the idea that cell competition is a mechanism involved in the regulation of organ growth and size.
Myc and super-competition
Another paradigmatic example of cell competition is attributable to Drosophila Myc (dMyc), the unique homolog of the human Myc family of proto-oncogenes (Johnston et al., 1999). Myc is a transcription factor that regulates the expression of numerous genes affecting cellular growth, proliferation, and ribosome biogenesis (reviewed in de la Cova and Johnston, 2006). Many of the “rules” established in experiments with M/+-induced cell competition hold in dMyc-induced cell competition. For instance, individuals carrying hypomorphic alleles of the gene encoding dMyc, called diminutive (dm), grow slower and are smaller than wildtype flies but are viable (Johnston et al., 1999). When surrounded by wildtype cells in mosaics, however, dm mutant cells are outcompeted (Johnston et al., 1999; Moreno and Basler, 2004; Senoo-Matsuda and Johnston, 2007). This was shown to be due to the induction of apoptosis that specifically requires the function of the pro-death factor, Hid (de la Cova et al., 2004). The Jun N-terminal kinase (JNK) signaling pathway is also activated in both winner and loser cells during competition (de la Cova et al., 2004), and its activity can lead to apoptosis. However JNK accounts only partially for competitive cell death, since loser cells continue to die when it is genetically abolished (de la Cova et al., 2004; Moreno and Basler, 2004; Tyler et al., 2007). Like in M/+ mosaics, competition induced by dMyc is restricted by compartmental boundaries (de la Cova et al., 2004). Importantly, competition induced by dMyc is exceptionally context specific: cells with physiological dMyc levels can display “winner” status when flanked by dm mutant cells (Johnston et al., 1999; Wu and Johnston, 2010), but can be turned into “losers” when flanked by dMyc-overexpressing cells (de la Cova et al.; Moreno and Basler, 2004). This remarkable flexibility indicates that the relative level of dMyc between cells is a source of competitiveness. For this reason dMyc perfectly matches the notion of a super-competitor (Abrams, 2002).
Competition between growing cells that express different levels of dMyc has also been demonstrated in Drosophila S2 cells. The outcome of competitive interactions in the S2 cell cultures is identical to that in vivo: the winner cells proliferate more, while the loser cells die by Hid-induced apoptosis (Senoo-Matsuda and Johnston, 2007). Co-culture experiments between wildtype S2 cells and those that contain an inducible dMyc transgene indicate that super-competition induced by dMyc does not require direct contact between winner and loser cells and is mediated by diffusible factors whose production requires participation of both populations (Senoo-Matsuda and Johnston, 2007). Whereas conditioned medium (CM) from co-cultures of dMyc-expressing and wildtype S2 cells is sufficient to kill naïve S2 cells and accelerate the proliferation of naïve dMyc-expressing S2 cells, CM provided by single cultures of either population is not. The nature of the soluble factors involved is still unknown, but winner and loser cells need to grow in close proximity and “sense” each other to trigger the outcome (Senoo-Matsuda and Johnston, 2007). This illustrates an important and universal attribute of cell competition: it depends upon reciprocal communication of “fitness” status by each population of cells.
Many questions, new insights
The systematic study of cell competition is still in its infancy, and there are numerous unanswered questions regarding how it occurs. How do cells measure their relative fitness so that they recognize neighbors that have a different fitness level? What is it about the confrontation of cells expressing different levels of dMyc or Rp proteins that transforms them into winners and losers? One simple mechanism that provides a solution to both questions is inter-cellular competition for limiting growth or survival factors in the tissue. In this case, more robust cells would be expected to win and proliferate at the expense of the less fit cells. However, this type of mechanism falls short of explaining the fact that differences in growth rates do not always lead to competition, nor the observation that CM from competing co-cultures of cells is sufficient to impart a competitive outcome on completely naïve cells (de la Cova et al., 2004; Senoo-Matsuda and Johnston, 2007).
Another idea is that cells exchange information about fitness via factors whose synthesis depends upon efficient protein synthesis. Myc is an evolutionarily conserved regulator of ribosomal activity and biogenesis (Boon et al., 2001; Eisenman, 2001; Gomez-Roman et al., 2003; Orian et al., 2003; Grewal et al., 2005), and regulates expression of several of the Rp genes that fall into the M class of mutations (Hulf et al., 2005). As ribosomes are composed of stoichiometric components, the appropriate level of each Rp is critical for their assembly and for efficient translation of proteins. As it does to the winner wildtype cells in competition with M/+ cells (Simpson, 1979), starvation diminishes dMyc’s competitive properties (de la Cova and Johnston, unpublished), and genetic impairment of ribosomal activity in dMyc expressing cells has the same effect (Moreno and Basler, 2004). Thus, local differences in ribosome biogenesis or function within a tissue may be an important part of the mechanism that leads to cell competition.
The many similarities between the M/+ and dMyc paradigms of competition has led to the notion that cell competition can be defined by a common set of molecular mechanisms that function in a variety of contexts. However, there are notable differences. Competition between wildtype and M/+ cells is extremely local and primarily affects cells in contact with each other (Martin et al., 2009). Wildtype winner cells are thought to engulf M/+ loser cells in a way that requires the draper, psr or Wasp engulfment genes (Li and Baker, 2007). Interestingly, during the compensatory proliferation the cell division plane is re-oriented in the wildtype winner cells (Li et al., 2009). By contrast, although still a local phenomenon, dMyc-induced cell competition exerts its effect across longer distances (6 to 10 cells away), consistent with sensing and signaling via diffusible factors (de la Cova et al., 2004; Senoo-Matsuda and Johnston, 2007). Although there are clearly many shared features of cell competition, recent studies that demonstrate competition that can occur in the absence of overt differences in ribosomal biogenesis or Myc activity argue against a single, universal mechanism (described below).
Although a relatively young field of investigation, the study of cell competition has accelerated during the last decade and has been covered in several reviews (Diaz and Moreno, 2005; Morata and Martin, 2007; Johnston, 2009; Baker, 2011). Here, we focus specifically on the most recent work emerging in the literature. The new work suggests that tissues use different processes to cope with the presence of disadvantaged cells, and includes examples of competition that do not require Myc, do not require death or elimination of the loser cells, and suggest the presence of diverse mechanisms that regulate competitive cellular interactions. The new work demands that a broader view of the definition of cell competition be accepted, that encompasses numerous signaling pathways and independent, context dependent mechanisms.
RECENT DISCOVERIES IN CELL COMPETITION
The Flower code
A major goal for understanding the mechanism of cell competition is the identification of the molecules used by cells in the recognition of differences in cellular fitness. One approach toward this goal, the use of gene expression arrays, led to the recent identification of genes expressed in cells during competition. An exciting finding from these experiments was that loser cells express a specific isoform of an integral membrane protein called Flower (Fwe) (Rhiner et al., 2010). First identified as a Ca+2 channel associated with synaptic vesicles of photoreceptor nerve terminals, Fwe was given its name due to the small and clustered, flower-like appearance of the synaptic boutons in mutant nerve termini (Yao et al., 2009). Fwe contains three to four transmembrane domains and is expressed as three different isoforms. One isoform is prominent and can be detected in the plasma membrane, probably in a channel-forming homo-multimeric complex (Yao et al., 2009). Loss of Fwe decreases the number of SVs and impairs endocytosis, and also affects resting Ca2+ levels in presynaptic terminals (Yao et al., 2009).
Rhiner et al. discovered that the prominent Fwe isoform (FweUbi) is also expressed in wildtype wing and eye disc cells. Strikingly, induction of wildtype loser clones in a background of wing disc cells that over-express dMyc leads to the expression of two other isoforms, FweLose-A and FweLose-B, in the disadvantaged cells (Rhiner et al., 2010). FweLose-A and FweLose-B are also expressed in the loser cells of other competitive situations including M/+ mosaics, raising the possibility that the FweLose forms function specifically in loser cells to cause their death. This idea was tested by forced expression of either FweLose isoform in wild-type cells. Interestingly, when expressed in whole discs or compartments the FweLose isoforms had no effect, but when expressed in mosaics - FweLose-expressing clones surrounded by cells expressing FweUbi - they were potent, cell-autonomous inducers of apoptosis. A similar effect was observed by simply reducing the expression of FweUbi in clones. Together, these results suggest that the FweLose forms promote cell death only in the context of a cell-cell difference in the composition of Fwe isoforms.
These tantalizing results point to the existence of a “code” whereby cells identify their relative fitness by displaying different forms of Fwe: losers express FweLoseA or B, and distinguish themselves from winners, which express FweUbi. A Fwe code may also be necessary for the growth of winner cells, because knock-down of all three isoforms using RNAi in posterior wing disc cells reduces the ability of dMyc-expressing winner cells to grow (Rhiner et al., 2010). Non-competitive (and non-mosaic) disc growth is not affected by loss of fwe. Rhiner et al. propose that the Fwe code acts as a downstream signaling event that labels cells as losers, leading to their elimination and to over-proliferation of the winner cells (Figure 1A, B). This suggests that in outcompeted cells fwe mRNA is alternatively spliced to favor the FweLose isoform at the expense of Fweubi (Rhiner et al., 2010), and an important goal will be to determine the upstream events that trigger and regulate this splicing decision.
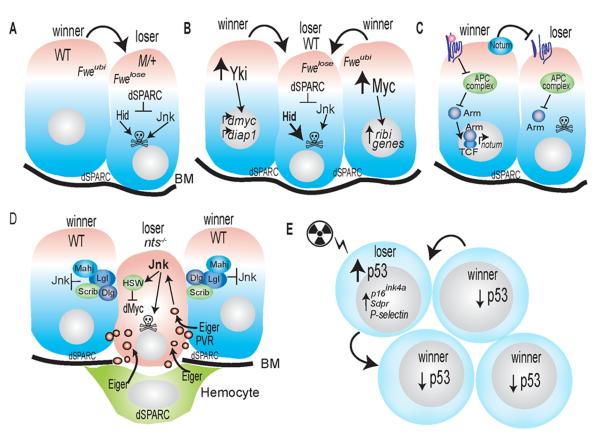
Figure 1. Modes and mechanisms of cell competition.
A. Cell competition between Minute/+ and wildtype cells. Wildtype cells (winners) outcompete M/+ cells (losers). Confrontation leads to alternative splicing of Fwe in the loser cells, and expression of the Fwelose isoform. dSparc is expressed temporarily in loser cells, transiently protecting the cells from apoptosis. Sparc is also expressed constitutively in the basement membrane (BM) of discs and in hemocytes. Neither loss of NST genes and basal membrane disruption is required to trigger M/+ induced cell competition. JNK is activated and contributes to death of loser cells, but inhibition of JNK signaling is not sufficient to prevent their elimination.
B, C. Super-competitors versus wildtype cells. Activation of dMyc, Yki (B) or Wg/Wnt (C) signaling leads to super-competitive behavior. The Fwelose isoform and dSparc are expressed in loser cells in response to dMyc super-competition. Activation of Yki confers super-competitive properties to cells largely due to dMyc upregulation.
C. Binding of Wg to its receptors inhibits the APC complex, resulting in nuclear translocation of Armadillo/β-Catenin (Arm) and target gene expression. Loss of components of the APC complex hyper-activates Wg signaling. Competition induced by Wg occurs independently of dMyc, but requires production of Notum (Nt), a secreted hydrolase that modifies heparan sulfate proteoglycans.
D. Neoplastic Tumor Suppressors. Lethal Giant Larvae (Lgl), Scribble (Scrib) and Disc-large (Dlg) are NTSs and exist in a complex. Mahjong (Mahj) is a binding partner of Lgl. Nts mutant cells are at a disadvantage in mosaic tissues. Reduction of dMyc expression occurs in lgl mutant cells and contributes to their death. Elimination of nts mutant cells also occurs through Eiger-mediated activation of JNK signaling and apoptosis. Loss of apical-basal polarity damages the basement membrane (BM) and attracts hemocytes. A non-autonomous source of Eiger/TNF is required to activate JNK; the relative contribution of surrounding wildtype disc cells versus local hemocytes is unclear. Eiger is taken up via endocytosis in the mutant cells (red bubbles). PVR expression in wildtype cells promotes engulfment of the nts mutant cells.
E. Cell competition in murine HSPCs. In stressed mouse HSPCs, the relative level of p53 determines whether cells are winners or losers. Cells with less or mutant p53 become winners and proliferate more. Cells with activated p53 status are losers, but these cells acquire a senescent state and express markers such as p16, Sdpr, and P-selectin, rather than die by apoptosis. The presence of each competitor cell type is required for the altered gene expression profiles and behaviors (arrows).
How is the Fwe code interpreted as a death signal by loser cells? Several scenarios can be imagined. Fwe forms multimers in the plasma membrane and functions as a Ca+2 channel to promote endocytosis in neuronal cells (Yao et al., 2009), thus it is possible that Ca+2 uptake, which can activate specific signaling pathways, plays a role. Perhaps cells lacking Fweubi have perturbed Ca+2 signaling and endocytosis and are more sensitive to stress. Alternatively, endocytosis of specific extracellular factors could be affected. Other possibilities are that Fwe associates with other cell-surface proteins that induce apoptosis or proliferation, or that trans-cellular binding of different Fwe isoforms triggers distinct intracellular signals, which could depend upon the fitness level of the cells. The identification of Fwe as mediator of cell status recognition is clearly one that provides fuel for future study.
The extracellular matrix protein Sparc
In the same gene expression profiling experiments that identified Fwe, the Drosophila homolog of the SPARC/Osteonectin protein family, dSparc, was also up-regulated (Figure 1A, B) (Portela et al., 2010). The authors found dSparc expression in loser cells of cell competition induced by several different means, including clonal differences in expression of dMyc, Rp genes, or the cell polarity genes lethal giant larvae, discs large, and scribble. dSparc increased as early as 12 hours after induction of loser clones. In Drosophila, dSparc is a component of the extracellular matrix (ECM) and found in the basement membrane (BM) of both epithelial and endothelial internal organs and tissues. It is also associated with the surface of hemocytes, the blood cells of the fly, and the fat body, the storage and secretion organ, both of which participate in secretion of the BM (Martinek et al., 2002). dSparc is also found in the BM of imaginal discs and on adepithelial cells (the muscle precursors) of the wing disc (our unpublished results). The data of Portela et al. suggest that dSparc is transiently expressed in loser cells of the wing disc during competition and thereby protects loser cells from apoptosis. This could allow partially damaged cells to recover before they can be eliminated by their neighbors (Portela et al., 2010). Since dSparc is a component of the ECM it could feasibly facilitate remodeling of the BM, preventing soluble factors to reach loser cells, or blocking delamination and making them more resistant to apoptosis. Such remodeling of ECM by SPARC has been described (Sangaletti et al., 2008).
However, because dSparc’s effect is only temporary, the loser cells still die and are ultimately eliminated. In vivo, hemocytes express dSparc (Martinek et al., 2002), and hemocytes invade tissues after injury and stress and have been detected at sites of dying cells (Srivastava et al., 2007; Cordero et al., 2010). It is also expressed upon serum-deprivation in S2 cells in the absence of competition (Portela et al., 2010), suggesting that dSparc expression is part of a general cellular stress response program rather than a specific marker of loser status conferred by competition. Interestingly, dSparc is also expressed at high levels in S2 cells under robust growth conditions (Cherbas et al., 2011). Additional studies that further probe dSparc’s role in cell competition and other forms of cellular stress will therefore be interesting and informative.
SIGNALING PATHWAYS THAT REGULATE CELL COMPETITION
An important unanswered question regarding the mechanism of cell competition is the identity of the signals that allow cells to perceive differences in fitness – be they regulated by dMyc, Rp gene differences, or some other parameter - and lead to changes in expression of potential biomarkers of competition such as Fwe and dSparc. Two highly conserved signaling pathways have been recently implicated in cell competition, one that appears to regulate competition independently of dMyc or differences in ribosome function, and another that directly regulates expression of dMyc.
The Wingless/Wnt Pathway
A stimulating new study found a direct role in cell competition of the vital and conserved Wnt pathway (Figure 1C). The Wnt pathway is a major regulator of cell survival, cell fate decisions, and tissue growth and is a critical force in some human cancers (Coombs et al., 2008). Wingless (Wg), the primary Wnt protein in Drosophila, is particularly well studied in the developing wing disc, where its targets include the wing selector Vestigial (Vg). Wg promotes expansion of the wing pouch during disc development by supporting a Vg feed forward mechanism (Zecca and Struhl 2007a; Zecca and Struhl 2007b; Zecca and Struhl 2010). Cells deficient in transduction of the Wg pathway do not survive well in the wing disc, especially in mosaics within the region where Vg is expressed, and are eliminated by apoptosis (Giraldez and Cohen, 2003; Johnston and Sanders, 2003).
The new work reveals that the function of Wg as a survival factor might not be intrinsic. Rather, relative differences of signal activation between neighboring cells in the wing disc determine which cells survive (Vincent et al., 2011). When whole compartments lack Wg or its receptors, cells survive. Mutant cells only die at high frequency when surrounded by actively proliferating wildtype cells, obeying a major rule of cell competition (Vincent et al., 2011). Conversely, cell clones mutant for APC or axin, both negative regulators of Wg signaling and conserved tumor suppressors, hyperactivate Wg and over-proliferate, at the same time inducing a non-cell autonomous increase in apoptosis in wildtype cells surrounding the clones, behaviors that evoke super-competition (Abrams, 2002).
A key finding of this work is that the competitive behavior induced by hyperactive Wg signaling operates independently of dMyc, since high levels of Wg activity actually repress expression of dMyc (Johnston et al., 1999; Duman-Scheel et al., 2004; Vincent et al., 2011). Thus in a notable reversal of the existing paradigm, the winner cells of competition induced by cells with high Wg activity are those with low dMyc levels. In fact, neither reduction of dMyc expression nor its over-expression alters the competitive ability of cells with hyperactive Wg signaling (Vincent et al., 2011). Instead, the non-autonomous cell death induced in wildtype cells surrounding cells mutant for APC or axin requires the glypican phospholipase Notum, itself a target of high Wg activity. Expression and secretion of Notum in response to Wg suppresses the response of surrounding cells to Wg (Vincent et al., 2011), and loss of notum prevents axin mutant cells from functioning as super-competitors (Vincent et al., 2011). Interestingly, loss of notum does not prevent competition between wildtype and M/+ cells (Vincent et al., 2011). These are provocative results that suggest that cells with high Wg activity compete by synthesizing and secreting Notum, which then inhibit Wg activity in neighboring cells and leads to their death (Figure 1C). The Wg/Wnt pathway therefore appears to function as an independent and novel regulator of cell competition.
The Hippo-Salvador-Warts pathway: super-competition via dMyc
An important advance within the last few years was the realization that the conserved Hippo-Salvador-Warts (HSW) signaling pathway plays a role in regulating cell competition. This pathway was initially implicated in cell competition by a genetic screen for mutations that inhibit cell competition in M/+ mosaics (Tyler et al., 2007). Mutations in several components of the pathway, including expanded, fat, salvador, hippo (hpo) or warts (wts), were identified as suppressors of the competitive disadvantage of M/+ loser cells when surrounded by wildtype cells. Further examination revealed that cells mutant for any one of these genes in a wildtype background led to an increase in the death of surrounding wild-type cells, suggesting that the mutant cells possessed super-competitive properties (Tyler et al., 2007). These were exciting findings because the HSW pathway has only recently emerged as a major and highly conserved growth regulatory pathway that also has critical tumor suppressor functions (Pan, 2010; Zhao et al., 2011). Linking HSW signaling to cell competition therefore presented an opportunity to solidify a role of cell competition in growth regulation in development and in tumor formation.
Two recent studies addressed this challenge and demonstrated that differences in expression of the downstream effector of the pathway, the transcription co-factor Yki, also led to cell competition. Unleashed Yki activity, through either its over-expression or loss of its negative regulators wts or hpo, functions cell-autonomously to promote extra growth, but it was also found to induce non-autonomous differences in cell proliferation and apoptosis in neighboring wildtype cells, two hallmarks of super-competition (Neto-Silva et al., 2010; Ziosi et al., 2010). These studies identified a linear relationship between HSW activity and expression of the proto-oncogene, dMyc. Yki’s regulation of dMyc is transcriptional and probably direct, because Yki and its DNA-binding partner, the TEAD protein Scalloped, bind to several regions in the dm locus (Neto-Silva et al., 2010). The bound regions include a putative enhancer in the second intron that is regulated by Yki and Sd in tissue culture assays (Ziosi et al., 2010).
In the complete absence of dMyc, Yki is incapable of promoting tissue growth, indicating that dMyc is not only a target of Yki but also an obligate effector of its control of growth (Neto-Silva et al., 2010). In addition, much, if not all, of HSW pathway-dependent cell competition appears to be dependent upon its regulation of dMyc expression. This was shown most dramatically in experiments in which dMyc expression was reduced in clones of cells heterozygous for a weak allele of dm (low Myc cells). When the dm/+ clones were generated in a background of cells with constitutive expression of Myc (high-Myc cells) at less than two times the endogenous level (Wu and Johnston, 2010), the local differences in dMyc levels created strong cell competition that led to elimination of most of the low dMyc cells. When expressed in the dm/+ cells growth driven by Yki was substantially diminished, consistent with its dMyc dependency. Even more strikingly, the dm/+, Yki-expressing cells remained at a strong competitive disadvantage (Ziosi et al., 2010). This observation suggests that when dMyc is limiting, Yki-expressing cells are no longer super-competitive, and raises the intriguing possibility that the dramatic tissue expansion promoted by Yki expression depends not only on the inherent growth regulatory mechanisms controlled by dMyc, but also on its super-competitive properties. These results imply that dMyc is the driving force for Yki-induced super-competition, and that relaxation of the HSW pathway does induce cell-competition per se, but acts through direct regulation of dMyc expression.
The relationship between dMyc and Yki becomes even more interesting in light of the observation that dMyc also controls Yki expression, thereby linking the two oncogenic growth promoters in a feedback loop. Neto-Silva et al. showed that dMyc regulates Yki expression through transcriptional and post-transcriptional mechanisms, though the molecular details are still unknown (Neto-Silva et al., 2010). Negative feedback between Yki and Myc keeps the activity of each in balance and thus could limit their respective oncogenic potential. An intriguing idea suggested by this relationship is that feedback-generated fluctuations in dMyc and Yki levels buffer the growth of cell populations with either high or low dMyc levels, limiting or improving their growth, respectively. In fact, biochemical noise in genetic networks is predicted to optimize overall growth properties of cellular ensembles (Tănase-Nicola and ten Wolde, 2008).
The feedback mechanism between dMyc and Yki also has the potential to regulate the outcome of cell competition. For example, negative feedback between dMyc and Yki could mitigate potential cellular conflicts arising from the dynamic shifts in dMyc expression that occur during wing disc development. Although early in development dMyc is expressed in all wing disc cells, later its expression becomes regional and increases in the wing pouch while plummeting in surrounding cells (Wu and Johnston, 2010). The high level of dMyc expression in the wing pouch attenuates Yki expression in those cells, resulting in even expression of Yki across the wing disc (Neto-Silva et al., 2010). Interestingly, the negative feedback between dMyc and Yki becomes an important factor in cell competition between cells that are mutant for the scribble neoplastic tumor suppressor gene, discussed below.
CELL COMPETITION INDUCED BY LOSS OF EPITHELIAL INTEGRITY
Imaginal disc cells are epithelial and polarized along the apico-basal axis, due to specialized protein complexes along their apical and basolateral membrane that are vital for cellular integrity and allow adhesion and communication between cells. When small clones of disc cells lose factors in these complexes, such as the conserved proteins Scribble (Scrib), Discs large (Dlg) or Lethal giant larvae (Lgl) (Figure 1D), they are often at a disadvantage and can be eliminated (Gateff, 1978; Woods and Bryant, 1991; Agrawal et al., 1995). The elimination of these mutant cells requires the presence of nearby wildtype cells, suggesting that they are subject to cell competition (Brumby and Richardson, 2003). In contrast, cells in whole tissues that are mutant for lgl, dlg, and scrib survive, eventually lose their epithelial integrity and can grow in a neoplastic, unrestrained manner during a prolonged larval growth period. These genes have therefore been classified as neoplastic tumor suppressor (herein called NTS) genes (Hariharan and Bilder, 2006). This type of growth contrasts with the hyperplastic growth induced by the mutations in the HSW pathway, which do not disrupt epithelial integrity.
Numerous studies over the last decade have demonstrated that the death and elimination of nts mutant cells in mosaic tissues requires JNK signaling (Brumby and Richardson, 2003; Pagliarini and Xu, 2003; Uhlirova and Bohmann, 2006). JNK also appears to play a role in restraining the proliferation of cells lacking NTSs, since inhibiting JNK function in nts mutant cell clones led to considerably larger clones than inhibition of apoptosis in the presence of active JNK (Brumby and Richardson, 2003). More recent work demonstrated that Eiger, the Drosophila tumor necrosis factor (TNF), is produced by surrounding wildtype cells and taken up by the mutant cells via endocytosis. Endocytic activation of Eiger within the mutant cells then triggers proapoptotic JNK activity and leads to their death (Igaki et al., 2009; Ohsawa et al., 2011). Interestingly, JNK activity was also found in the wildtype cells surrounding nts mutant cells. In these cells its role was non-apoptotic, and induced expression of PVR, the Drosophila PDGF/VEGF receptor. Expression of PVR led to engagement of a phagocytic engulfment pathway and helped eliminate the nts mutant cells from the epithelium (Ohsawa et al., 2011). However, other recent experiments suggest that circulating phagocytic hemocytes carry out the engulfment of polarity mutant cells, prompted by hemocyte-intrinsic expression of Eiger (Cordero et al., 2010), a mechanism incompatible with cell competition. Additional studies will be important to clarify the relative role of each cell type in the engulfment process.
Three exciting papers have now implicated dMyc and the HSW pathway in the competitive elimination of cells mutant for lgl or scrib (Froldi et al., 2010; Menendez et al., 2010; Chen et al., 2012). Grifoni and colleagues noticed that clones of lgl− null mutant cells generated within the central, wing pouch region of the wing disc had reduced dMyc expression relative to surrounding wildtype cells, suggesting that this contributed to their competitive disadvantage (Froldi et al., 2010). Consistent with this idea, expression of dMyc in wing pouch lgl− clones prevented their elimination, while its expression in lgl− clones outside of the wing pouch drove the cells into a neoplastic state and they formed tumor-like masses (Froldi et al., 2010). Competitive elimination of scrib mutant clones can also be rescued by expression of dMyc (Chen et al., 2012). Strikingly, although dMyc expression in scrib mutant clones rescued their growth, the increase in dMyc as such was unable to explain their ability to survive, because if scrib mutant cells and surrounding non-mutant cells express equal levels of dMyc, the scrib mutant clones are still eliminated (Chen et al., 2012). Likewise, when grown in a background of M/+ cells with wildtype lgl genes, lgl mutant cell clones were able to survive and proliferate; moreover, they successfully competed against the M/+ cells (Froldi et al., 2010). These observations lead to the important conclusion that lgl or scrib mutant cells are only eliminated when surrounded by cells with greater fitness. In other words, the defining feature of nts mutant cells leading to competitive elimination is loss of a critical component of cellular fitness, not nts gene function per se.
Morata and colleagues found that expression of Yki also prevented elimination of lgl− cell clones and led to neoplastic growth (Menendez et al., 2010). It has been known for some time that expression of an activated form of Ras (RasV12) in nts mutant cells promotes their survival and neoplastic growth (Brumby and Richardson, 2003; Pagliarini and Xu, 2003), but Menendez et al found that RasV12 expression in lgl− or scrib− cells led to nuclear localization of Yki and increased expression of Yki target genes (Menendez et al., 2010). As the dm gene, encoding dMyc, is a target of Yki (Neto-Silva et al., 2010; Ziosi et al., 2010), its expression was presumably increased in these cells, and RasV12 also promotes the stabilization of dMyc (Prober and Edgar, 2000). The effect of RasV12 on the nts mutant cells thus appears to include super-competitive properties imparted by a cell-autonomous increase in dMyc expression.
These new papers thus implicate regulation of HSW activity and its downstream effector, dMyc, as critical players in whether nts− cells live and proliferate or die from competitive interactions. Accordingly, sustained activation of Yki targets would promote cell survival and proliferation while dMyc would confer its super-competitive power. In wildtype cells this behavior would be kept in check by the negative feedback regulation of Yki by dMyc (Neto-Silva et al., 2010). However, in intriguing contrast to wildtype cells, Yki activity was not suppressed in scrib mutant cells. Expression of dMyc specifically within scrib− clones prevented their loss through competition and also resulted in increased expression of Expanded, a Yki target (Chen et al., 2012). This finding reinforces the notion that if nts mutant cells evade competition, their transition into a neoplastic state is fueled by the activity of Yki target genes, combined with eventual loss of cell polarity. Thus, limiting the activity of Yki through dMyc-mediated feedback is an important mechanism for restricting the oncogenic potential of cells by preventing both super-competitive behavior and sustained proliferative signaling.
Still, JNK activation dominates the death of nts mutant cells, in contrast to its relatively minor role in competition induced directly by dMyc differences. Where does JNK activity fit into this scenario? As mentioned, JNK had been noted to inhibit growth of nts mutant cells as well as promote their death (Brumby and Richardson, 2003). Interestingly, careful measurement of clone sizes in wing discs revealed that this effect is context dependent and correlates with the endogenous expression pattern of dMyc in the mature wing disc: high in the wing pouch, lower in the surrounding cells (Johnston et al., 1999; Wu and Johnston, 2010). Outside of the wing pouch, lgl− clones in which JNK activity was inhibited expressed dMyc at increased levels and grew to large sizes, and the cells lost apical-basal polarity (Froldi et al., 2010). Within the wing pouch, however, lgl− clones were still at a considerable growth disadvantage when JNK was inhibited, and grew to only half the size of their wildtype counterparts; in these clones dMyc expression was actually diminished relative to surrounding cells (Froldi et al., 2010). In general, clonal expansion of lgl− clones was favored in regions of the wing disc where endogenous dMyc is low (Froldi et al., 2010).
JNK activation promotes activity from the HSW pathway in several different contexts (Grzeschik et al., 2010a; Parsons et al., 2010; Doggett et al., 2011; Sun and Irvine, 2011). In addition, recent studies have demonstrated a tight link between HSW activity and the status of the polarity protein complexes (reviewed in (Grzeschik et al., 2010b; Enomoto and Igaki, 2011; Genevet and Tapon, 2011). Thus, a picture that emerges from all of these studies is that when surrounded by non-mutant cells, JNK activation in nts mutant cells enhances HSW activity, thereby blocking Yki activity (Chen et al., 2012). As a result, dMyc expression is repressed in the mutant cells (Froldi et al., 2010), encouraging their competitive elimination. The key event appears to be Eiger-mediated JNK activation in the nts mutant cells, thus explaining the absolute requirement for this pathway in the elimination of the mutant cells in mosaic tissues. When nts mutant cells are not subject to competition – as when surrounded by themselves, or the less fit M/+ cells – JNK is not activated, HSW activity is relaxed, and Yki directs expression of dMyc and other target genes that promote growth and super-competitive behavior.
Mahjong
An Lgl binding protein has also been newly implicated in cell competition. The mammalian Viral protein r Binding Protein (VprBP) directly binds Lgl2, a mammalian Lgl, and its Drosophila counterpart, Mahjong, was recently identified based on homolgy (Tamori et al., 2010). mahj mutant cells grow well when in a homotypic environment, but undergo apoptosis when in mosaics, consistent with cell competition. Interestingly, overexpression of Mahj in lgl− clones suppresses their elimination by inhibiting JNK activation and preventing basal extrusion of the mutant cells, thereby increasing their viability (Figure 1D) (Tamori et al., 2010). Mahj did not rescue the elimination of scrib mutant cells in mosaics, indicating an exclusive interaction with Lgl, but importantly suggesting that JNK is activated upon loss of different polarity genes by distinct mechanisms. Since Mahj expression essentially rescues the death of cell clones that lack lgl it must operate downstream, but how it functions in this capacity is not known.
Overexpression of the mammalian protein, VprBP, in mammalian cell cultures triggers cell elimination, but only when co-cultured with non-expressing cells, in a phenocopy of competition against mahj- clones in the wing (Tamori et al., 2010). Additional work will be important to elucidate the relationship between Mahj and the rest of the apical-basal complex, and whether a connection exists between Mahj and the HSW pathway.
CELL COMPETITION IN MAMMALS: INCREASING EVIDENCE FOR PHYSIOLOGICAL FUNCTION
The question of conservation of cell competition in evolution is an important one, and cellular behaviors in mammals akin to cell competition in Drosophila have been reported (Oliver et al., 2004; Oertel et al., 2006). Recent findings add to this list and highlight the relevance of cell competition for normal mammalian development as well as diseases. Tumor-suppressors and proto-oncogenes play central roles in cell competition in Drosophila (de la Cova et al., 2004; Moreno and Basler, 2004; Tyler et al., 2007), and have now been shown to also mediate competitive interactions in mammalian cells.
p53 as a sensor of cellular fitness
p53 is a critical tumor suppressor in mammals is that induces apoptosis and growth arrest after DNA damage or a wide variety of metabolic stresses (Horn and Vousden, 2007). A particularly intriguing recent paper demonstrated cell competition between stem cells of the murine hematopoietic system (Bondar and Medzhitov, 2010). Using a xenograft model and low-dose ionizing radiation as a mild stress, Bondar and Medzhitov mixed populations of irradiated and non-irradiated bone marrow cells and studied competitive interactions between them. They found that populations of HSPCs (hematopoietic stem and progenitor cells) subjected to different levels of stress compete to reconstitute the hematopoietic compartment in a manner that is dependent on their p53 status (Figure 1E). In the mixed populations, the irradiated cells activate p53 and are at a growth disadvantage due to its brake on cell division. The non-stressed cells continue to proliferate and outcompete these loser cells. Even in the absence of irradiation cells that lack one copy of MDM2, a p53 inhibitor can be out-competed by wildtype cells, suggesting that a cell’s ability to report its fitness is governed by its capacity to de-regulate p53. Indeed, the relative, not absolute, level of p53 was demonstrated to determine winner and loser status in chimeras (Bondar and Medzhitov, 2010).
The data of Bondar and Medzhitov suggest that when p53 is mutant or absent, cells are unable to recognize and report their fitness if damaged, and thus elude the mechanisms in place to eliminate them. Indeed, when mixed with irradiated wildtype cells in the in vivo assay, cells with mutant p53 have an advantage and become the winners (Bondar and Medzhitov, 2010). In principle, fitness-sensing and competition that depends upon p53 status should benefit the development of the animal by ridding tissues of damaged cells, which will have higher p53 levels than undamaged cells. However, Bondar and Medzhitov discovered that in p53-mediated HSPC competition, the loser cells don’t actually die; instead they express genes characteristic of a growth-arrested, quasi-inert state called senescence. Importantly, this loser cell profile and a corresponding proliferation-rich gene expression profile in the winner cells was only induced in the presence of competitors, highlighting the non-cell autonomous nature of the process (Figure 1E). In addition, the competition led to appropriate size of the relevant hematopoietic stem cell compartment, indicating that the lack of cell death, and the continued presence of the senescent loser cells did not interfere with size control (Bondar and Medzhitov, 2010).
The Ras and Src oncogenes
Oncogenes have also been implicated recently in cell competition in mammalian cell culture experiments. Canine MDCK cells harboring a mutation that provides a proliferative advantage (RasV12 or a temperature sensitive mutant of v-Src) are extruded from the cell monolayer and eliminated when cultured with wild-type cells, but not when cultured alone (Hogan et al., 2009; Kajita et al., 2010). The mechanism by which this occurs involves reorganization of the adhesion proteins, E-cadherin and β-catenin, and remodeling of the cytoskeleton through activation of small GTPases, the focal adhesion associated kinase, FAK, the MAPK pathway, and myosin activity. However, it is not clear whether these observations correspond to cell competition, since they do not clearly involve differences in proliferation rates, apoptosis and growth.
Very similar observations have been reported in the Drosophila wing disc: dSrc-transformed cells (using a mutant of an inhibitory kinase of dSrc, Csk) re-localize E-cadherin and the associated p120-catenin to the outside of adherens junctions, but this occurs exclusively at boundaries with wildtype cells. The relocalization is accompanied by cell extrusion, JNK-mediated apoptosis, tissue invasion and MMP-dependent cell-migration (Vidal et al., 2006; Vidal et al., 2010). Cells with increased dSrc activity, although normally viable in whole eye and wing discs, are eliminated in mosaics with wildtype cells. This is highly reminiscent of cell competition, and suggests that similar pathways regulate cell competition in Drosophila and mammals, perhaps through evolutionarily conserved mechanisms.
CONCLUSIONS AND PERSPECTIVES
The recent discoveries of new pathways that regulate competitive interactions between growing cells are exciting and raise numerous questions for future work. Although epithelial cells are particularly susceptible to competition, the studies in the non-epithelial S2 cells and in mouse hematopoietic cells indicate that competition is not restricted to this cell type. The recent work also makes it clear that the mechanism of loser cell death is not identical, nor is elimination of loser cells required in all situations. A burning question that still remains is the identity of additional sensor molecules that allow recognition of differences between cells, and whether they are the same, or different, in these different contexts and animals.
The emergence of new competitive pathways raises questions about their intersection: How much redundancy occurs between the pathways? Do these pathways all intersect or do they function independently? Alternatively, there may be antagonism between them. Do some conditions favor one competitive process over another, and does a hierarchy exist between the pathways? It is tempting to speculate that the existence of several pathways that regulate cell competition enable more stringent control of the outcome of cell-cell interactions. On the other hand, building a common mechanistic framework for cell competition becomes more difficult. It is now clear that several competitive processes or pathways exist with varying degrees of overlap and exclusivity. Although thus far only tested on dMyc-induced competition, CM from co-cultures of other competitive situations could prove useful for determining whether different pathways regulating cell competition converge towards a central mechanism that informs cells about their general fitness in the tissue.
What are the physiological functions of cell competition? The new work illustrates its importance in stem cells, reinforces the analogies of the process with cancer, and also emphasizes its critical function in limiting oncogenesis of cells carrying mutations in cell polarity genes. Most of the current studies suggest a role in the selection for the fittest cells to populate the tissue. It has also been shown that inhibiting cell competition can affect the regulation of growth and impair final tissue size (de la Cova et al., 2004). However, recent studies in M/+ competition suggest that not all competition contributes to size control (Martín et al., 2009), and with the extreme super-competition of Yki or polarity defective cells that express Rasv12, size control can be completely ignored.
Notwithstanding all of these questions, the existence of numerous mechanisms for cell competition provide powerful ways of eliminating cells that are potentially dangerous for the tissue, including those with insufficient growth or aberrant fates, cells with damage, or neoplastic mutations. This suggests that intersection or antagonism between the numerous modes of competition may be beneficial to the organism. Competition has also been proposed to promote field cancerization, whereby clonal expansion of cells expressing a super-competitor favors tumor development (Moreno and Basler, 2004). The increasing evidence that cell competition exists in mammals suggests its conservation and relevance to not only the tumor environment during cancer progression, but also to stress resistance, stem cell expansion, and normal developmental processes. It will be exciting to see expansion of these new frontiers in cell competition in future research.
ACKNOWLEDGEMENTS
We thank our colleagues in the field for numerous discussions over the years. Funding for work in our lab is provided by the FRM and Philippe Foundation (SdB), CIRC “Giorgio Prodi”, University of Bologna (MZ), and the NIH (LAJ).
REFERENCES
- Abrams JM. Competition and compensation: coupled to death in development and cancer. Cell. 2002;110:403–406. doi: 10.1016/s0092-8674(02)00904-2. [DOI] [PubMed] [Google Scholar]
- Agrawal N, Kango M, Mishra A, Sinha P. Neoplastic transformation and aberrant cell-cell interactions in genetic mosaics of lethal(2)giant larvae (lgl), a tumor suppressor gene of Drosophila. Dev Biol. 1995;172:218–229. doi: 10.1006/dbio.1995.0017. [DOI] [PubMed] [Google Scholar]
- Baker NE. Cell competition. Current biology : CB. 2011;21:R11–15. doi: 10.1016/j.cub.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon K, Caron HN, van Asperen R, Valentijn L, Hermus MC, van Sluis P, Roobeek I, Weis I, Voute PA, Schwab M, Versteeg R. N-myc enhances the expression of a large set of genes functioning in ribosome biogenesis and protein synthesis. EMBO J. 2001;20:1383–1393. doi: 10.1093/emboj/20.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:484–489. doi: 10.1073/pnas.1113882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L, Willingham A, Zhang D, Yang L, Zou Y, Eads BD, Carlson JW, Landolin JM, Kapranov P, Dumais J, Samsonova A, Choi JH, Roberts J, Davis CA, Tang H, van Baren MJ, Ghosh S, Dobin A, Bell K, Lin W, Langton L, Duff MO, Tenney AE, Zaleski C, Brent MR, Hoskins RA, Kaufman TC, Andrews J, Graveley BR, Perrimon N, Celniker SE, Gingeras TR, Cherbas P. The transcriptional diversity of 25 Drosophila cell lines. Genome research. 2011;21:301–314. doi: 10.1101/gr.112961.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GS, Covey TM, Virshup DM. Wnt signaling in development, disease and translational medicine. Current drug targets. 2008;9:513–531. doi: 10.2174/138945008784911796. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FH, Lawrence PA. Compartments and polyclones in insect development. Science. 1975;189:340–347. doi: 10.1126/science.806966. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Johnston LA. Myc in model organisms: a view from the flyroom. Semin Cancer Biol. 2006;16:303–312. doi: 10.1016/j.semcancer.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Moreno E. The competitive nature of cells. Experimental cell research. 2005;306:317–322. doi: 10.1016/j.yexcr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Doggett K, Grusche FA, Richardson HE, Brumby AM. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC developmental biology. 2011;11:57. doi: 10.1186/1471-213X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M, Johnston LA, Du W. Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc Natl Acad Sci USA. 2004;101:3857–3862. doi: 10.1073/pnas.0400526101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman RN. Deconstructing myc. Genes Dev. 2001;15:2023–2030. doi: 10.1101/gad928101. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Igaki T. Deciphering tumor-suppressor signlaing in flies: Genetic link between Scribble/Dlg/Lgl and the Hippo pathways. J Genetics and Genomics. 2011;38:461–470. doi: 10.1016/j.jgg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Froldi F, Ziosi M, Garoia F, Pession A, Grzeschik NA, Bellosta P, Strand D, Richardson HE, Grifoni D. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC biology. 2010;8:33. doi: 10.1186/1741-7007-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nature New Biol. 1973;245:251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011;436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Cohen SM. Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development. 2003;130:6533–6543. doi: 10.1242/dev.00904. [DOI] [PubMed] [Google Scholar]
- Gomez-Roman N, Grandori C, Eisenman RN, White RJ. Direct activation of RNA polymerase III transcription by c-Myc. Nature. 2003;421:290–294. doi: 10.1038/nature01327. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Li L, Orian A, Eisenman RN, Edgar BA. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat Cell Biol. 2005;7:295–302. doi: 10.1038/ncb1223. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Current biology : CB. 2010a;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Richardson HE. Lgl, the SWH pathway and tumorigenesis: It’s a matter of context & competition! Cell Cycle. 2010b;9:3202–3212. doi: 10.4161/cc.9.16.12633. [DOI] [PubMed] [Google Scholar]
- Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet. 2006;40:335–361. doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
- Hogan C, Dupré-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-López LA, Vincent JP, Itoh Y, Hosoya H, Pichaud F, Fujita Y. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol. 2009;11:460–467. doi: 10.1038/ncb1853. [DOI] [PubMed] [Google Scholar]
- Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- Hulf T, Bellosta P, Furrer M, Steiger D, Svensson D, Barbour A, Gallant P. Whole-genome analysis reveals a strong positional bias of conserved dMyc-dependent E-boxes. Mol Cell Biol. 2005;25:3401–3410. doi: 10.1128/MCB.25.9.3401-3410.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16:458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA. Competitive interactions between cells: death, growth and geography. Science. 2009;324:1679–1682. doi: 10.1126/science.1163862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat Cell Biol. 2003;5:827–833. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, Charras G, Tada M, Fujita Y. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. Journal of Cell Science. 2010;123:171–180. doi: 10.1242/jcs.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan K, Yu Q, Vincent A, Frisardi MC, Rosbash M, Lengyel JA, Merriam J. A Drosophila Minute gene encodes a ribosomal protein. Nature. 1985;317:555–558. doi: 10.1038/317555a0. [DOI] [PubMed] [Google Scholar]
- Lambertsson A. The Minute genes in Drosophila and their molecular functions. Advances in Genetics. 1998;38:69–134. doi: 10.1016/s0065-2660(08)60142-x. [DOI] [PubMed] [Google Scholar]
- Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Li W, Kale A, Baker NE. Oriented cell division as a response to cell death and cell competition. Current biology : CB. 2009;19:1821–1826. doi: 10.1016/j.cub.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FA, Herrera SC, Morata G. Cell competition, growth and size control in the Drosophila wing imaginal disc. Development. 2009;136:3747–3756. doi: 10.1242/dev.038406. [DOI] [PubMed] [Google Scholar]
- Martín FA, Herrera SC, Morata G. Cell competition, growth and size control in the Drosophila wing imaginal disc. Development. 2009;136:3747–3756. doi: 10.1242/dev.038406. [DOI] [PubMed] [Google Scholar]
- Martinek N, Zou R, Berg M, Sodek J, Ringuette M. Evolutionary conservation and association of SPARC with the basal lamina in Drosophila. Dev Genes Evol. 2002;212:124–133. doi: 10.1007/s00427-002-0220-9. [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Roote J, Reuter G, Lambertsson A, Ashburner M, Millburn GH, Harrison PM, Yu Z, Kenmochi N, Kaufman TC, Leevers SJ, Cook KR. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 2007;8:R216. doi: 10.1186/gb-2007-8-10-r216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J, Perez-Garijo A, Calleja M, Morata G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci U S A. 2010;107:14651–14656. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G, Martin FA. Cell competition: the embrace of death. Dev Cell. 2007;13:1–2. doi: 10.1016/j.devcel.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Neto-Silva RM, de Beco S, Johnston LA. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell. 2010;19:507–520. doi: 10.1016/j.devcel.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Dabeva MD, Shafritz DA. Cell Competition Leads to a High Level of Normal Liver Reconstitution by Transplanted Fetal Liver Stem/Progenitor Cells. Gastroenterology. 2006;130:507–520. doi: 10.1053/j.gastro.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, Igaki T. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev Cell. 2011;20:315–328. doi: 10.1016/j.devcel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Oliver ER, Saunders TL, Tarle SA, Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131:3907–3920. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian A, van Steensel B, Delrow J, Bussemaker HJ, Li L, Sawado T, Williams E, Loo LW, Cowley SM, Yost C, Pierce S, Edgar BA, Parkhurst SM, Eisenman RN. Genomic binding by the Drosophila Myc, Max, Mad/Mnt transcription factor network. Genes Dev. 2003;17:1101–1114. doi: 10.1101/gad.1066903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- Pan D. The Hippo Signaling Pathway in Development and Cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LM, Grzeschik NA, Allott ML, Richardson HE. Lgl/aPKC and Crb regulate the Salvador/Warts/Hippo pathway. Fly. 2010;4:288–293. doi: 10.4161/fly.4.4.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela M, Casas-Tinto S, Rhiner C, López-Gay JM, Domínguez O, Soldini D, Moreno E. Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev Cell. 2010;19:562–573. doi: 10.1016/j.devcel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–446. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Rhiner C, López-Gay JM, Soldini D, Casas-Tinto S, Martín FA, Lombardía L, Moreno E. Flower Forms an Extracellular Code that Reveals the Fitness of a Cell to its Neighbors in Drosophila. Dev Cell. 2010;18:985–998. doi: 10.1016/j.devcel.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Sangaletti S, Di Carlo E, Gariboldi S, Miotti S, Cappetti B, Parenza M, Rumio C, Brekken RA, Chiodoni C, Colombo MP. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Research. 2008;68:9050–9059. doi: 10.1158/0008-5472.CAN-08-1327. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Johnston LA. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc Natl Acad Sci U S A. 2007;104:18543–18548. doi: 10.1073/pnas.0709021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. Analysis of the compartments of the wing of Drosophila melanogaster mosaic for a temperature-sensitive mutation that reduces mitotic rate. Dev Biol. 1976;54:100–115. doi: 10.1016/0012-1606(76)90289-x. [DOI] [PubMed] [Google Scholar]
- Simpson P. Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev Biol. 1979;69:182–193. doi: 10.1016/0012-1606(79)90284-7. [DOI] [PubMed] [Google Scholar]
- Simpson P. Growth and cell competition in Drosophila. J. Embryol. exp. Morph. 1981;65:77–88. [Google Scholar]
- Simpson P, Morata G. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev Biol. 1981;85:299–308. doi: 10.1016/0012-1606(81)90261-x. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci U S A. 2007;104:2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Developmental biology. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Bialucha CU, Tian AG, Kajita M, Huang YC, Norman M, Harrison N, Poulton J, Ivanovitch K, Disch L, Liu T, Deng WM, Fujita Y. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 2010;8:e1000422. doi: 10.1371/journal.pbio.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tănase-Nicola S, ten Wolde PR. Regulatory control and the costs and benefits of biochemical noise. PLoS Comput Biol. 2008;4:e1000125. doi: 10.1371/journal.pcbi.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler DM, Li W, Zhuo N, Pellock B, Baker NE. Genes affecting cell competition in Drosophila. Genetics. 2007;175:643–657. doi: 10.1534/genetics.106.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Vidal M, Salavaggione L, Ylagan L, Wilkins M, Watson M, Weilbaecher K, Cagan R. A Role for the Epithelial Microenvironment at Tumor Boundaries. The American Journal of Pathology. 2010;176:3007–3014. doi: 10.2353/ajpath.2010.090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, Kolahgar G, Gagliardi M, Piddini E. Steep differences in wingless signaling trigger myc-independent competitive cell interactions. Dev Cell. 2011;21:366–374. doi: 10.1016/j.devcel.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Wu D-M, Johnston LA. Control of Wing Size and Proportions by Drosophila Myc. Genetics. 2010;184:199–211. doi: 10.1534/genetics.109.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–960. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Struhl G. Control of Drosophila wing growth by the vestigial quadrant enhancer. Development. 2007a;134:3011–3020. doi: 10.1242/dev.006445. [DOI] [PubMed] [Google Scholar]
- Zecca M, Struhl G. Recruitment of cells into the Drosophila wing primordium by a feed-forward circuit of vestigial autoregulation. Development. 2007b;134:3001–3010. doi: 10.1242/dev.006411. [DOI] [PubMed] [Google Scholar]
- Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8:e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan K. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziosi M, Baena-López LA, Grifoni D, Froldi F, Pession A, Garoia F, Trotta V, Bellosta P, Cavicchi S, Pession A. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]