Abstract
The intestine secretes a range of hormones with important local and distant actions, including the control of insulin secretion and appetite. A number of enteroendocrine cell types have been described, each characterized by a distinct hormonal signature, such as K-cells producing glucose-dependent insulinotropic polypeptide (GIP), L-cells producing glucagon-like peptide-1 (GLP-1), and I-cells producing cholecystokinin (CCK). To evaluate similarities between L-, K-, and other enteroendocrine cells, primary murine L- and K-cells, and pancreatic α- and β-cells, were purified and analyzed by flow cytometry and microarray-based transcriptomics. By microarray expression profiling, L cells from the upper small intestinal (SI) more closely resembled upper SI K-cells than colonic L-cells. Upper SI L-cell populations expressed message for hormones classically localized to different enteroendocrine cell types, including GIP, CCK, secretin, and neurotensin. By immunostaining and fluorescence-activated cell sorting analysis, most colonic L-cells contained GLP-1 and PeptideYY In the upper SI, most L-cells contained CCK, approximately 10% were GIP positive, and about 20% were PeptideYY positive. Upper SI K-cells exhibited approximately 10% overlap with GLP-1 and 6% overlap with somatostatin. Enteroendocrine-specific transcription factors were identified from the microarrays, of which very few differed between the enteroendocrine cell populations. Etv1, Prox1, and Pax4 were significantly enriched in L-cells vs. K cells by quantitative RT-PCR. In summary, our data indicate a strong overlap between upper SI L-, K-, and I-cells and suggest they may rather comprise a single cell type, within which individual cells exhibit a hormonal spectrum that may reflect factors such as location along the intestine and exposure to dietary nutrients.
The gut endocrine system produces a range of hormones that not only modulate intestinal motility and secretion but are also involved more widely in the control of metabolism. The incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are well-recognized stimuli of pancreatic β-cells and together account for more than 50% of the normal insulin-secretory response to oral glucose (1, 2). Gut-derived cholecystokinin (CCK), PeptideYY (PYY), and GLP-1 have been implicated in the central control of food intake (1, 3, 4). Because GLP-1-based therapies are now extensively used for the treatment of type 2 diabetes, offering the combined benefit of reducing both blood glucose and appetite (5), there is considerable interest in whether it would be a feasible therapeutic strategy to target specific populations of enteroendocrine cells that release peptides involved in appetite and blood glucose control. There is therefore currently a great need for a better understanding of the gut endocrine system.
Enteroendocrine cells are traditionally classified into at least 10 different cell types, based on their morphology, distribution, and principal hormonal product(s) (6). They are produced continuously by the division and differentiation of multipotent crypt stem cells, from which are derived all the different cell types of the intestinal epithelium, including the four secretory cells types: the goblet cells, Paneth cells, enteroendocrine cells, and tuft cells. Lineage-tracing experiments have identified a sequence of transcription factor (TF) expression that determines entry into the enteroendocrine lineage. Expression of Math1/Atoh1 appears to direct cells initially into the secretory cell line, and neurogenin3 (Neurog3) subsequently determines which cells become enteroendocrine cells (7, 8). It is not clear, however, which factors specify how the lineages diverge after this and result in the generation of different members of the enteroendocrine cell family. Knockout of NeuroD1 resulted in loss of CCK- and secretin (Sct)-producing cells but did not reduce the numbers of cells expressing serotonin, GLP-1, PYY, GIP, or neurotensin (Nts) (9). Induced ablation of Sct-positive cells in vivo, however, resulted in more than 80% loss of small intestinal (SI) cells containing CCK, proglucagon derived peptides, and PYY, and 30–50% loss of cells staining for substance P, somatostatin (Sst), and serotonin (10), indicating a greater overlap between enteroendocrine cell types than had been hitherto suspected.
GIP is produced by intestinal K-cells, located primarily in proximal regions of the SI (11), whereas GLP-1 originates from a separate cell type, the L-cell, which additionally produces PYY, GLP-2, and oxyntomodulin (12). L-cells can be found in the duodenum but occur at higher numbers in the ileum and colon. The recent generation of mice carrying fluorescent reporters in L- or K-cells has enabled these cells to be identified and purified without prior fixation and immunostaining (13, 14). When used in combination with protocols for culturing mixed intestinal epithelial cells, it is now possible to study the cellular physiology of GLP-1- and GIP-secreting cells in primary culture as an alternative to using the previously established cell lines such as GLUTag and STC-1.
In many respects, L-cell stimulus secretion-coupling pathways overlap with those found in pancreatic β-cells. Both cell types exhibit glucose-dependent electrical activity and voltage-gated Ca2+ entry, synergizing with G protein-dependent signaling pathways such as release of stored Ca2+ and elevated cAMP concentrations (15, 16). A primary difference between L- and β-cells, however, is that L-cells additionally employ classical brush border transporters as nutrient sensors, coupling small nutrient-dependent transporter currents to electrical excitability and secretion (17, 18). In general, the properties of primary L-cells are mirrored in GLUTag cells, although differences have been observed, for example, in the dependence of secretion on L-type Ca2+ channels and different phosphodiesterases (19–21).
In the current study, we have investigated the overlap between different gut endocrine cell populations using a combination of transcriptomic profiling and fluorescence-activated cell sorting (FACS) analysis. The data surprisingly show that colonic and upper SI L-cells, although traditionally considered as the same cell type, share less in common than the classically distinct populations of upper SI L- and K-cells. In the upper SI, we observed a large degree of overlap between different endocrine cell types.
Materials and Methods
Animal models and cell preparation
Animal procedures were approved by the local ethics committee and conformed to United Kingdom Home Office regulations. GLU-Venus and GIP-Venus mice (2–6 months of age) (13, 14), maintained on a C57BL6 background, were killed by cervical dislocation and the gut was collected into ice-cold Leibovitz-15 (L-15) medium (Sigma, Poole, UK); all chemicals were supplied by the same manufacturer unless otherwise stated). Upper SI (10 cm of gut immediately distal to the stomach), lower SI (10 cm proximal to the cecum) or colon (tissue distal to the ileocolic junction), were opened longitudinally and rinsed in PBS. For purification of cell populations by FACS, but not FACS analysis, intestinal pieces were then stripped of the outer muscle layers. Tissue was chopped into 1- to 2-mm pieces and digested to single cells with 1 mg/ml collagenase in calcium-free Hanks' buffered salt solution (HBSS). Pancreatic islets from GLU-Venus mice were prepared by injecting the pancreas with 0.5 mg/ml collagenase type V in HBSS. Tissue was incubated for 15 min at 37 C and mechanically disrupted by shaking. Islets were hand picked into HBSS containing 10 mm glucose and 0.1% BSA, incubated at 37 C for 1 min in 0.1 × trypsin, and triturated to obtain a single cell suspension, after which fetal bovine serum was added to a final concentration of 10%. Samples were separated immediately by flow cytometry, as described below.
Single cell suspensions were separated by flow cytometry using a MoFlo Beckman Coulter Cytomation sorter (Coulter Corp., Hialeah, FL). Side scatter, forward scatter, and pulse-width gates were used to exclude debris and aggregates and Venus-positive cells were collected at approximately 95% purity, alongside negative (control) cells that comprised a mixed population dominated by other epithelial cell types (13). In islet sorts, α-cells were identified by their Venus fluorescence, and β-cells were identified by their lack of Venus fluorescence and high-side scatter, as described previously (13). L- and islet cell populations were collected from GLU-Venus mice and K-cells were collected from GIP-Venus mice. Cells were sorted into lysis buffer for mRNA extraction. Total RNA from FACS-sorted cells were isolated using a microscale RNA isolation kit (Applied Biosystems, Warrington, UK). RNA from GLUTag and STC-1 cells was extracted using a standard TRI Reagent protocol.
RNA amplification and microarray hybridization
Before use in downstream applications, RNA isolated from FACS-sorted cells was amplified using two rounds of T7-based in vitro transcription, as previously described (22). Briefly, RNA was primed with a T7 promoter-oligo (dT) primer and reverse transcribed to generate first-strand cDNA, which was used as a template to synthesize second-strand cDNA by DNA polymerase (Two-cycles cDNA Synthesis Kit, Affymetrix, UK Ltd., High Wycombe, UK). T7 polymerase was used to transcribe antisense amplified RNA (aRNA; MEGAscript T7 kit, Ambion, Austin, TX; Applied Biosystems, Foster City, CA). The aRNA was then randomly primed to make single-strand cDNA, which in turn served as the template for second-strand cDNA synthesis, primed, as in the first round, with a T7 promoter-oligo dT primer to make double-stranded cDNA containing a T7 promoter site. A second T7 in vitro transcription step produced the second round of aRNA with biotin-labeled ribonucleotide (GeneChip IVT labeling Kit, Affymetrix). The biotin-labeled cRNA was fragmented and hybridized to Affymetrix Murine 430 2.0 GeneChips. The hybridized arrays were stained with streptavidin phycoerythrin conjugate and scanned on an Affymetrix GeneChip 7G scanner.
Microarray analysis
Raw image data were converted to CEL files using Affymetrix GeneChip Operating Software. Downstream analysis of microarray data was performed using GeneSpring GX 10 (Agilent, Edinburgh, Scotland, UK). After the data were imported, each chip was normalized to the 50th centile of the measurements taken from that chip, and gene expression data are reported based on Robust Multi-chip Average (RMA) analyses, providing an intensity value that reflects the relative expression level of each probe. Our experience is that RMA values greater than 100 represent expression levels that can be robustly verified by quantitative RT-PCR. Hierarchical clustering analyses were performed by clustering on cell-type, using Pearson-centered similarity measures and a centroid linkage rule. Further data analysis was performed using Microsoft Excel.
Quantitative RT-PCR
Total RNA, collected as above, was treated with DNase (Applied Biosystems), and reverse-transcribed according to standard protocols. Quantitative RT-PCR was performed with 7900 HT Fast Real-Time PCR system (Applied Biosystems), using primers/probes (Applied Biosystems) for: Actb (Mm00607939_s1), Gip (Mm00433601_m1), Cck (Mm00446170_m1), Gcg (Mm01269055_m1), Sct (Mm00441235_g1), Nts (Mm00481140_m1), Pyy (Mm00520716_g1), Sst (Mm00436671_m1), Ppy (Mm01250509_g1), Etv1 (Mm00514804_m1), Pax4 (Mm01159036_m1), Prox1 (Mm00435969_m1). The PCR mix consisted of first-strand cDNA template, primer pairs, 6-carboxyfluorescein/quencher probes, and PCR Master mix (Applied Biosystems). Expression was compared with that of β-actin measured on the same sample, giving a CT difference (ΔCT) for β-actin minus the test gene. Mean, se, and statistics were calculated from the ΔCT data. Data were analyzed using Microsoft Excel, and statistics were performed using GraphPad Prism 5.01 software (GraphPad Software, San Diego, CA).
Immunocytochemistry and FACS analysis
Single-cell suspensions were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature and stored in 0.01% sodium azide PBS at 4 C (usually overnight). Cells were permeabilized with 0.1% vol/vol Triton X-100 for 30 min at room temperature, blocked with 10% goat serum in PBS for 15 min, centrifuged at 800 × g for 5 min, and then incubated with primary antibody in PBS-10% goat serum at 4 C overnight on a rotator. The primary antibodies used were: anti-GLP-1 [that detects different processed forms of proglucagon; Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); sc-13091; 1:200), anti-GIP (gifted by Professor J. J. Holst; 1:1000), anti-CCK (gifted by Professor G. J. Dockray; 1:500), anti-PYY (Progen, London, UK; 16066; 1:100), anti-Chromogranin A (Abcam, Cambridge, UK; antibody 15160; 1:200) and anti-Sst (DAKO Corp., Carpinteria, CA; A0566; 1:1000]. Cells were rinsed three times in PBS and then incubated with secondary antibody (Alexa-Fluor 633, Invitrogen, Carlsbad, CA; A-21070 or A-21105, both at 1:300]. For negative controls, primary antibody was substituted with PBS-10% goat serum. After three washes with PBS, cells were analyzed using CyAn advance digital processing Flow Cytometer (DAKO Cytomation, Carpinteria, CA). Samples were excited at 488 nm (emission 530 ± 40 nm) to detect Venus, and at 635 nm (emission 665 ± 20 nm) to detect the red fluorescent secondary antibody. Events with very low side and forward scatter were excluded because these are likely to represent debris, and events with a high pulse width were excluded to eliminate cell aggregates. Flow Cytometer analysis was based on approximately 0.5–1 × 106 events per sample, using FlowJo 7.6 software.
Results
Microarray analysis was used to compare the transcriptional profiles of K-cells from the duodenum (K+), L cells from the upper 10 cm of the SI (L+) or colon (LC+), control (nonfluorescent) intestinal cell populations (K−, L−, and LC−), and pancreatic α- and β-cells, using mRNA from FACS-sorted cell populations from GIP-Venus or GLU-Venus mice (13, 14). Expression profiles of the different cell types are depicted in Fig. 1A–I as scatter plots, showing the relative intensities of all probes represented on the microarrays. Because the plots and associated correlation coefficients suggested a high level of similarity between upper SI L-cells and K-cells, the relationships between different enteroendocrine cell types and cell lines were further examined by hierarchical clustering analysis (Fig. 1J). This confirmed that upper SI L-cells are more closely related to upper SI K-cells than to colonic L-cells. Neither GLUTag nor STC-1 cells faithfully reproduced the expression profiles of primary L-cells. Although α- and L-cells are the two main gcg-expressing cell types in the gastrointestinal tract, α-cells appeared more similar to β-cells than to upper SI L-cells.
Fig. 1.
Microarray expression profile comparisons between intestinal and endocrine cell populations. A—I, RMA intensities of individual microarray probes are compared between different cell types and plotted on a log (10) scale: Upper SI L-cells and their controls (L+, L−), K-cells and controls (K+, K−), colonic L-cells and controls (LC+, LC−), pancreatic α- and β-cells, and STC1 and GLUTag cells. Solid and dashed lines represent 2- and 10-fold differences, respectively, between the populations. Data points represent the mean of two or three replicates. Correlation coefficients (ρ) were determined from the mean data. J, Dendrogram showing the relationships between K+, L+, LC+, GLUTag, and STC-1 cells as determined by Hierarchical Cluster Analysis.
Cell-specific hormone expression
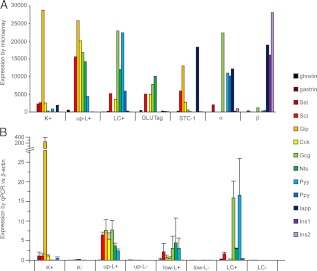
Because gut endocrine cells are classically defined by the hormones they contain, we examined the intensities of probes against different gut hormones (Fig. 2A). Interestingly, upper SI L-cells expressed message for a range of gut hormones, including gip, cck, gcg, sct, nts, and pyy. In addition, they showed a low level of expression of ghrl (ghrelin), but were negative for gast (gastrin) and sst. Colonic L-cells differed from upper SI L-cells in that they lacked gip and had slightly lower levels of cck and sct, but expressed detectable levels of ppy (pancreatic polypeptide). Upper SI K-cells expressed very high levels of gip, lower levels of cck, sct, sst, iapp (islet amyloid polypeptide), pyy, and gcg, and lacked message for ghrl, gast, and nts. GLUTag cells expressed sct, cck, and gcg, as described previously (23), and also gave a strong signal for nts. They did not contain detectable message for gip or pyy. Hormonal signals in STC-1 cells were dominated by sct, gip, cck, and iapp, with a smaller expression of gcg (Fig. 2A).
Fig. 2.
Expression of hormonal transcripts. A, Mean microarray RMA intensities for probes against known intestinal and pancreatic hormones in K-cells (K+), upper SI L-cells (L+), colonic L-cells (LC+), model cell lines GLUTag and STC-1, and in pancreatic α- and β-cells (n = 2–3 each). B, Expression of gut hormones measured by quantitative RT-PCR. Data are expressed relative to that of β-actin measured in parallel in the same samples. SI L-cells were collected separately from the upper third (up L+) and lower third (low L+) of the SI, together with nonfluorescent control cells from the same region. Columns represent means and se values (n = 3–6 per sample).
Microarray analysis of pancreatic islet cells showed good separation of insulin and glucagon in the β- and α-cell populations, respectively (Fig. 2A), with only minor contamination by sst, but additionally suggested that murine α-cells express relatively high levels of message for iapp, ppy, and pyy. Contrary to a previous report (24), we did not detect gip signal in pancreatic α-cells, nor did we observe Venus fluorescent cells in the islets of GIP-Venus mice (data not shown). The low insulin signal in α-cells argues against the idea that the iapp signal arose from contamination by neighboring β-cells. None of the other gut hormones examined was identified in either pancreatic islet cell type.
Expression of key hormones from the microarray data were examined by quantitative RT-PCR in K-cells and L-cells from the upper SI, L-cells from the lower SI or colon, and control nonfluorescent cells (Fig. 2B). Mirroring the microarray findings, the hormonal message in upper SI K-cells was dominated by gip, but the cells additionally contained detectable mRNA for sst, sct, cck, gcg, and pyy. The gcg signal was low, but elevated compared with the nonfluorescent control cells (K-). Upper SI L-cells, by contrast, expressed high levels of mRNA for sct, gip, cck, gcg, nts, and pyy. We also measured the hormonal expression profile in L- cells from the lower third of the SI, which had not been examined by microarray analysis. Levels of mRNA for gcg, nts, and pyy were similar to those found in the upper SI, but these more distally located L-cells contained substantially less mRNA for gip and cck. Indeed, the hormonal profile of L-cells from the lower SI was more similar to that of the colonic L-cells.
FACS analysis of colonic L-cells
The microarray data are derived from cell populations and do not show whether the different hormone mRNA are translated, and whether they are expressed homogeneously in all K- or L-cells, or occur only in subgroups of cells. To distinguish between these possibilities, we performed FACS analysis of cell suspensions from GIP-Venus and GLU-Venus mice, immunostained against different hormones.
Colonic digests from GLU-Venus mice were stained with red secondary antibody alone (Fig. 3A) or with secondary plus a primary anti-GLP-1 antibody (Fig. 3B). Strongly stained cells exhibit a high red fluorescence and therefore appear in region R2 of Fig. 3B. Background staining, detected in the absence of primary antibody, is shown in the equivalent region of Fig. 3A. When we examined only the Venus fluorescent population (identified by the R1 gate in Fig. 3C and plotted on different axes in Fig. 3D) we observed that the majority of Venus-positive cells stained strongly for GLP-1, as evident from their high red fluorescence. This is shown also in histogram form (Fig. 3E), in which the red immunofluorescence of the entire Venus-positive cell population is plotted together with that of all strongly immunopositive cells in the same sample (identified from R2 in Fig. 3B). From the histogram, it is evident that the majority of cells that exhibit high red fluorescence are accounted for by the Venus-positive population. To combine data from different experiments, however, we calculated the proportion of Venus-positive cells that were strongly GLP-1 immunofluorescent, i.e. the number of cells appearing in R3 vs. the number in R4, plotted as a percentage in Fig. 3G. We also calculated the proportion of immunofluorescent cells that were also Venus positive, determined by whether they also appeared in R1 when visualized on yellow/green axes as in Fig. 3C. This is plotted in Fig. 3H and approximates the number of cells in R3 vs. R2. As evident from Fig. 3, G and H, approximately 80% of colonic Venus-positive cells were strongly immunofluorescent for GLP-1, and about 80% of immunofluorescent cells expressed Venus. Because the Venus transgene was driven by the gcg promoter in these mice, we would have expected close to 100% overlap in these graphs. Factors that may contribute to the percentages being lower than expected include the small proportion of background-stained cells (evident in Fig. 3A) that were not subtracted in these calculations, incomplete antibody labeling, or Venus fluorescence that was too low in some cells to distinguish from background autofluorescence.
Fig. 3.
FACS analysis of GLP-1 and PYY staining in colonic cells. A, Single-cell colonic suspensions from GLU-Venus mice were analyzed by FACS. Cells were stained with a red secondary antibody and excited at 488 nm and 561 nm. Green fluorescence from the 488 laser (detecting Venus) is plotted vs. red fluorescence from the 561 laser. B, Cells, as in panel A, were stained with anti-GLP-1 antibody and a red secondary. R2 indicates cells that were positive in the red channel. C, Cells were excited with a 488-nm excitation laser. Green (530) and yellow (580) fluorescence are plotted on logarithmic axes. The gated region R1 outlines Venus-positive cells. D, Cells were stained as in panel B. The plot shows only the Venus-positive cell population, identified from the R1 region of panel C, but now the red fluorescence is plotted against green fluorescence. R4 outlines all Venus-positive cells, and R3 delineates strongly immunopositive cells. E, Frequency histogram of the red fluorescence of all Venus-positive cells (identified from R1 in C; dark shading) and the red fluorescence of all cells appearing in R2 in B (light shading). The y-axis represents an arbitrary scale, determined by the total number of cells analyzed. F, PYY staining, analyzed as in panels A–D and represented as in panel E. G, Mean data (n = 3) from experiments performed as in panels A–F, representing the percentage of Venus-positive cells that stained positive for GLP-1 or PYY, calculated as the number of cells in R3/R4*100%. H, Mean data (n = 3) from experiments as in panel A–F, representing the percentage of strongly red fluorescent cells (R2 in panel B) that were also positive for Venus (determined by whether they also appeared in R1 when visualized as in panel C). Ab, Antibody.
A similar analysis was performed of colonic cells from GLU-Venus mice stained with a primary PYY antibody (Fig. 3, F–H), and showed that most Venus-positive cells contained PYY, and most PYY-positive cells expressed Venus. Overall, 0.46 ± 0.07% of the total colonic cell count were labeled by the GLP-1 antibody, and 0.45 ± 0.01% were labeled by the PYY antibody (n = 3 of each).
FACS analysis of peptide hormones in SI L-cells
In the upper SI of GLU-Venus mice, the proportion of Venus-positive cells staining for GLP-1 was slightly lower than in the colon, averaging approximately 70% (Fig. 4, A and D). GIP antibodies stained only about 15% of Venus-positive cells (Fig. 4, B and D), indicating that the gip message in the FACS-purified L-cells was derived from only a limited subpopulation of these cells. Double GIP/Venus-positive cells accounted for about 15% of all GIP-stained cells in the upper SI (Fig. 4E), indicating that a large number of GIP-positive cells do not express gcg. Using two separate anti-CCK antibodies with different degrees of immunoreactivity against gastrin, almost all Venus-positive cells were immunopositive for CCK, but only approximately 40% of CCK-positive cells contained Venus (Fig. 4, C–E). Indeed, in the total cell populations there were about 2-fold more cells staining positive for CCK than GLP-1, as shown in Fig. 4F. A similar picture was observed in the lower SI, with the majority of Venus-positive cells staining positive for GLP-1 and CCK, but only about 10% staining for GIP (Fig. 4D). In the lower SI it was not possible to enumerate cells that were immunopositive but Venus negative due to a distinct but unidentified population of cells that nonspecifically bound the fluorescent secondary antibody.
Fig. 4.
FACS analysis of SI cells from GLU-Venus mice. A–C, Single-cell upper SI suspensions from GLU-Venus mice were stained with antibodies against GLP-1 (A), GIP (B), or CCK (C), and a red secondary antibody, and analyzed by FACS. Venus-positive cells were identified as in Fig. 3C, and antibody-positive cells were identified as in Fig. 3B. Frequency histograms represent the red fluorescence of all Venus-positive cells (dark shading) or all strongly red fluorescent cells (light shading). Y-axes represent an arbitrary scale. D, Mean data from experiments performed as in panels A–C with cell suspension isolated from the upper or lower small intestine, representing the percentages of Venus-positive cells that stained positive for GLP-1, GIP, CCK, or PYY. Data represent the mean and se of the number of samples indicated. Statistical significance of hormone expression differences between upper and lower small intestine was assessed by Student's t test; *, P < 0.05; ***, P < 0.001. E, Mean data from experiments performed as in panels A–C, representing the percentage of red fluorescent cells that were also positive for Venus. Data represent the mean and se of the number of preparations indicated. F, Percentage of all cells from the upper SI that stained positive for the hormones indicated. Data represent the means and se values of the number of preparations indicated. G and H, Histograms representing GLP-1 (G) and PYY (H) staining of Venus-positive cell populations from the upper (light gray) and lower (mid gray) SI and colon (dark gray). Cells were stained with anti-GLP-1 and anti-PYY antibody and a red secondary and analyzed as in panels A–C. Only cells positive for Venus are shown. The y-axis represents the frequency, scaled according to the same total number of cells counted for each region, so the area under the curve represents the total proportion of Venus-positive cells. Ab, Antibody; a.u., arbitrary units.
Few PYY-positive cells were detected in the upper SI (Fig. 4D), when expressed either as a percentage of the total cell population (0.02%, Fig. 4F) or relative to all Venus-positive cells (21 ± 2%; n = 3). Progressive increases along the length of the gastrointestinal tract were evident, however, in the total number of PYY-positive cells, the total number of Venus-positive cells, and the proportion of Venus-positive cells that expressed PYY (illustrated in Fig. 4, G and H), with 44 ± 1% (n = 3) of Venus-positive cells being PYY positive in the lower SI.
FACS analysis of hormones in K-cells from GIP-Venus mice
To investigate the corresponding relationships in K-cells, upper SI preparations from GIP-Venus mice were stained with antibodies against GIP, GLP-1, CCK, and Sst for FACS analysis (Fig. 5). GIP was detected in about 90% of upper SI K-cells, although only approximately 30% of GIP-immunopositive cells contained detectable Venus fluorescence (Fig. 5, A, E, and F). The latter finding reflects the lower intensity of Venus fluorescence in GIP-Venus mice, perhaps reflecting the low transgene copy number. Of the Venus-labeled upper SI K-cells, about 80% stained positive for CCK, 3% stained positive for GLP-1, and 6% were positive for Sst (Fig. 5, C–F).
Fig. 5.
FACS analysis of upper SI K-cells from GIP-Venus mice. A–D, Single-cell upper SI suspensions from GIP-Venus mice were stained with antibodies against GIP (A), GLP-1 (B), CCK (C), or Sst (D), and a red secondary, and analyzed by FACS. Venus-positive cells were identified as in Fig 3C, and antibody-positive cells were identified as in Fig 3B. Frequency histograms represent the red fluorescence of all Venus-positive cells (dark shading) or all strongly red fluorescent cells (light shading). Y-axes represent an arbitrary scale. E, Mean data from experiments performed as in panels A–D, representing the percentage of Venus-positive cells that also stained positive for GIP, GLP-1, Sst, or CCK. Data represent the mean and se of the number of preparations indicated. F, Mean data from experiments performed as in panels A–D, representing the percentage of red fluorescent cells that were also positive for Venus. Data represent the mean and se of the number of preparations indicated. Ab, Antibody; a.u., arbitrary units.
Chromogranin A (CgA) staining in the SI
CgA is a widely used marker of intestinal endocrine cells, although our microarray data did not detect high cell-specific expression of CgA in K- or L-cells. Both K- and L-cells had higher expression of chromogranin B (CgB) than CgA by microarray, and the secretogranins 2, 3, and 5 were significantly enriched in both enteroendocrine cell types (Fig. 6A). By FACS analysis, strong CgA staining was observed in 0.23 ± 0.07% (n = 4) of upper SI cells. The population of strongly CgA-positive cells (R4 in Fig. 6, C–E) did not, however, overlap with the Venus-positive cells from either GLU-Venus (R3 in Fig. 6, C and D) or GIP-Venus (R3 in Fig. 6E), indicating that the most strongly CgA-positive cells in the upper small intestine are a population distinct from the K- and L-cells. Weak CgA staining in upper SI K- and L-cells is suggested by the small rightward shift of the histograms in Fig. 6, D and E, from R1 in the absence of CgA antibody to R3 in the presence of CgA antibody.
Fig. 6.
Chromogranins and secretogranins in enteroendocrine cells. A, Microarray RMA intensities for probes against chromogranin A (Chga), chromogranin B (Chgb), and secretogranins 2, 3, and 5 (Scg2, 3, 5) in upper SI K-cells (K+), upper SI L-cells (L+), and colonic L-cells (LC+), their respective control populations (K−, L−, LC−), and pancreatic α- and β-cells. B and C, Single upper SI suspensions from GLU-Venus mice were stained with a red secondary antibody (B), or with anti-CgA antibody plus red secondary, and analyzed by FACS. The regions R2 and R4 indicate strongly immunopositive cells, and the regions R1 and R3 outline the majority of the Venus-positive cells. D and E, Single-cell upper SI suspensions from GLU-Venus (D) or GIP-Venus (E) mice were stained with antibodies against CgA, and a red secondary, and analyzed by FACS. Venus-positive cells were identified as in Fig 3C, and antibody-positive cells were identified as in Fig 3B. Frequency histograms represent the red fluorescence of Venus-positive cells stained with secondary antibody only (R1, black line), Venus-positive cells stained for CgA (R3, dark shading), or all strongly red fluorescent cells (R4, light shading). Y-axes represent an arbitrary scale.
TF expression profiles
To identify TF that might underlie L- and K-cell development and differentiation, we examined the microarray data for TF that showed more than 10-fold enhanced expression in L- or K-cells compared with controls, or that have been implicated previously in the development of enteroendocrine cells or pancreatic islets (Table 1). Twenty nine TF were detected at more than 10-fold higher intensities in at least one of the enteroendocrine cell populations (upper SI L-cells, colonic L-cells, or upper SI K-cells) relative to their respective nonfluorescent controls. Many of these were not previously known to play a role in K- or L-cells, and most exhibited similar expression profiles across upper SI K- and L-cells and colonic L-cells. Factors differing between upper SI L- and K-cell populations were of particular interest, because they might account for the minor transcriptomic and functional differences between K- and L-cells. None of the 29 identified TF was more than 2-fold elevated in K- vs. L-cells, but Etv1 and Prox1 were more than 10-fold lower in upper SI K- than L-cells (Table 1). These were further assessed by quantitative RT-PCR (Fig. 7), together with Pax4, which has previously been implicated in repression of the GIP promoter (25). Consistent with the microarray findings, Etv1 and Prox1 exhibited enteroendocrine specificity and were found at significantly lower levels in upper SI K- than upper SI and colonic L-cells. Pax4 was not significantly different in K- and L-cells from the upper SI but was lower in K-cells than in colonic L-cells.
Table 1.
Microarray-determined expression of TF intestinal and pancreatic endocrine and control cell populations.
| Gene title | Probe identification | K− | K+ | l- | L+ | LC− | LC+ | α | And β |
|---|---|---|---|---|---|---|---|---|---|
| Ankrd6 | 1437217_at | 19 | 91 | 23 | 269 | 22 | 482 | 221 | 54 |
| Arx | 1450042_at | 23 | 653 | 15 | 1667 | 6 | 837 | 1318 | 10 |
| Atoh1 | 1449822_at | 149 | 216 | 221 | 182 | 937 | 544 | 23 | 31 |
| Bmi1 | 1448733_at | 15 | 182 | 58 | 328 | 98 | 252 | 278 | 717 |
| Dtx1 | 1458643_at | 13 | 122 | 12 | 128 | 16 | 15 | 11 | 25 |
| Egr2 | 1427683_at | 22 | 787 | 33 | 488 | 47 | 50 | 111 | 35 |
| Etv1 | 1450684_at | 19 | 55 | 18 | 2056 | 56 | 2821 | 2480 | 36 |
| Fev | 1425886_at | 52 | 221 | 36 | 763 | 25 | 715 | 215 | 400 |
| Fubp1 | 1433640_at | 23 | 308 | 48 | 323 | 158 | 196 | 459 | 267 |
| Glis3 | 1430353_at | 26 | 49 | 26 | 308 | 106 | 108 | 308 | 667 |
| Grhl1 | 1424030_at | 17 | 313 | 17 | 171 | 47 | 300 | 155 | 134 |
| Hes1 | 1418102_at | 289 | 509 | 418 | 431 | 699 | 467 | 95 | 102 |
| Hopx | 1428662_a_at | 828 | 3388 | 1040 | 6942 | 1162 | 4810 | 554 | 2138 |
| Ikzf4 | 1457342_at | 15 | 194 | 15 | 340 | 18 | 198 | 213 | 268 |
| Insm1 | 1455865_at | 52 | 1395 | 66 | 3424 | 38 | 2890 | 1053 | 2464 |
| Isl1 | 1450723_at | 49 | 3338 | 61 | 4027 | 77 | 2724 | 6055 | 3022 |
| Irx2 | 1426298_at | 23 | 26 | 20 | 31 | 26 | 32 | 563 | 29 |
| Jazf1 | 1433894_at | 13 | 168 | 21 | 322 | 28 | 194 | 555 | 525 |
| Klf12 | 1439847_s_at | 15 | 117 | 12 | 458 | 101 | 310 | 156 | 192 |
| Mafa | 1436092_at | 9 | 11 | 9 | 9 | 10 | 19 | 9 | 5987 |
| Mafb | 1451715_at | 86 | 14 | 51 | 18 | 85 | 21 | 213 | 74 |
| Mlxipl | 1419185_a_at | 1561 | 1675 | 845 | 2286 | 102 | 1028 | 63 | 2025 |
| Myt1 | 1422773_at | 17 | 230 | 18 | 402 | 17 | 232 | 207 | 202 |
| Neurod1 | 1426413_at | 336 | 2017 | 230 | 9579 | 58 | 4369 | 3229 | 4333 |
| Neurog3 | 1432034_at | 95 | 136 | 94 | 77 | 50 | 68 | 94 | 142 |
| Nkx2–2 | 1421112_at | 17 | 376 | 21 | 626 | 10 | 757 | 598 | 845 |
| Nkx6–1 | 1425828_at | 52 | 35 | 33 | 23 | 44 | 34 | 89 | 722 |
| Nkx6–2 | 1427420_at | 14 | 18 | 11 | 19 | 19 | 20 | 15 | 22 |
| Nr4a2 | 1455034_at | 41 | 579 | 65 | 1952 | 96 | 194 | 250 | 125 |
| Pax4 | 1451598_at | 55 | 65 | 35 | 201 | 42 | 339 | 42 | 38 |
| Pax6 | 1419271_at | 16 | 535 | 18 | 1277 | 12 | 872 | 705 | 915 |
| Pdx1 | 1422174_at | 46 | 111 | 55 | 117 | 13 | 16 | 79 | 932 |
| Pou3f4 | 1422165_at | 21 | 21 | 14 | 14 | 15 | 13 | 48 | 24 |
| Prox1 | 1437894_at | 7 | 17 | 7 | 177 | 8 | 79 | 112 | 52 |
| St18 | 1455123_at | 11 | 568 | 19 | 1540 | 26 | 742 | 1984 | 2221 |
| Tbx3 | 1437479_x_at | 46 | 78 | 33 | 331 | 20 | 35 | 8 | 10 |
| Tcf7l2 | 1429427_s_at | 109 | 79 | 58 | 51 | 106 | 100 | 14 | 17 |
| Tle2 | 1436244_a_at | 45 | 332 | 46 | 529 | 84 | 361 | 182 | 145 |
| Zfp179 | 1418360_at | 10 | 11 | 9 | 32 | 13 | 155 | 211 | 95 |
| Zfp644 | 1429623_at | 6 | 15 | 11 | 132 | 21 | 37 | 100 | 41 |
| Zfp667 | 1436122_at | 17 | 102 | 17 | 526 | 83 | 363 | 364 | 613 |
| Zfp90 | 1449126_at | 18 | 204 | 30 | 234 | 81 | 338 | 54 | 107 |
Data represent the mean RMA analyzed intensities for the probes indicated, from mouse Affymetrix 430 2.0 arrays (n = 2–3 per sample).
Fig. 7.
TF expression in enteroendocrine cells. Quantitative RT-PCR analysis of selected TF from Table 1. Expression in upper SI K-cells (K+), upper SI L-cells (L+), colonic L-cells (LC+), and their respective control populations (K−, L−, LC−) is shown relative to that of β-actin, measured in parallel in the same samples. Data represent the mean and se of n = 3 samples. Significant differences between K+, L+, and LC+ cells, identified by one-way ANOVA with post hoc Tukey's test, are indicated; *, P < 0.05; **, P < 0.01.
Discussion
SI enteroendocrine cells are traditionally considered as distinct cell types [e.g. the I-cells (CCK), S-cells (Sct), K-cells (GIP), L-cells (GLP-1/PYY), and D-cells (Sst)] deriving from a common intestinal stem cell precursor (6–8). Our data suggest, however, that these cell populations are less distinct than previously recognized. The extent of the similarities was evident in the global microarray expression profiles of FACS-purified L- and K-cells which showed, surprisingly, that upper SI L-cells were more similar to their neighboring K-cells than to L-cells from the colon, although the latter are classically considered as the same cell type.
In the upper and lower SI and colon, 75–80% of Venus-positive cells from GLU-Venus mice stained positively for GLP-1. Although the apparent lack of GLP-1 staining in 20–25% of Venus-positive cells could represent Venus fluorescence in a non-L-cell population, detection of GLP-1 by immunofluorescence is inevitably less than 100% efficient because of factors such as antibody affinity and the requirement for cell permeabilization. Indeed, varying affinities between antibodies against different hormones preclude exact comparisons between the percentages of cells stained using different antibodies. We also observed that although Venus fluorescence was found in about 80% of GLP-1-positive cells from GLU-Venus mice, it had a lower penetrance in GIP-Venus mice, being detectable in only approximately 30% of GIP-immunopositive cells. This mirrors the level of Venus fluorescence, which was considerably brighter in enteroendocrine cells from GLU-Venus than GIP-Venus mice, likely due to a higher transgene copy number in the former strain. It is therefore probable that our collected K-cell populations were dominated by cells with high GIP promoter activity, whereas the L-cell populations spanned a broader range of proglucagon promoter activity and might also have included some cells at an earlier stage of differentiation.
The finding that upper SI and, to a lesser extent, lower SI L-cells expressed messages for GIP, CCK, Sct, and Nts, in addition to GLP-1 and PYY, prompted experiments to determine the proportions of L-cells staining positive for different key hormones. Despite the transcriptomic similarities between upper SI K- and L-cells, fewer than 15% of upper SI L-cells contained GIP, or of upper SI K-cells contained GLP-1, as assessed by immunostaining and FACS analysis. Almost all upper SI L-cells, however, were strongly immunoreactive against CCK, accounting for about 50% of the total CCK-positive cell population. This suggests that most upper SI L-cells contain CCK, but that there is also a distinct, similar-sized pool of I-cells that are not GLP-1 positive. Whether the CCK-containing cell populations that are GLP-1 positive or GLP-1 negative differ markedly in their functional responsiveness and physiological role will be interesting topics for future evaluation. Although mRNA analysis indicates that SI L-cell populations express gcg, gip, sct, cck, nts, and pyy, our data do not enable us to distinguish how many different hormones can be produced by any single enteroendocrine cell.
FACS analysis of upper SI K-cells revealed that most are also CCK positive and that there is an overlap of approximately 5% with Sst-producing D-cells. The detection of iapp message in upper SI K-cells is consistent with a previous report that IAPP was identified in cells containing Sst, CCK, or PYY in the rat small intestine (26). Apart from a higher L-cell number and percentage of L-cells coexpressing PYY in the lower SI, FACS analysis revealed only minor differences in the number of L-cells coexpressing GIP and CCK along the length of the SI. Because the lower SI cell suspensions contained many Venus-negative cells that bound secondary antibodies even in the absence of a primary antibody, we were unable to determine whether there are ileal pools of PYY-, GIP-, or CCK-positive cells that do not also express proglucagon. The lower levels of GIP and CCK mRNA expression in L-cell populations from the lower SI suggest, however, that the content of these peptides per L-cell, although detectable by FACS analysis, is less in the distal compared with the proximal SI.
Interestingly, the CgA antibody strongly stained a small population of cells in the upper SI but only weakly labeled the K- and L-cells in this region. The identity of the strongly positive CgA cells was not investigated here, but it was reported previously that the majority of enterochromaffin and gastrin cells are CgA positive, whereas CgA immunostaining was only variably detected in cells containing GIP, Sct, Nts, and PYY (27). Although CgA is widely used as an indicator of enteroendocrine cells, our data therefore confirm that it is not a reliable marker of upper SI K- and L-cells.
We identified a number of TF specific to L- and K-cells, including factors previously implicated in enteroendocrine development, e.g. Fev, Insm1, Isl1, NeuroD1, Nkx2.2, and Pax6 (7, 8, 28–30), and/or pancreatic islet cell formation and function, e.g. Arx, Glis3, Mlxipl, Myt1, Prox1, and Tle2 (31–36). In addition, we found a number of TF not previously documented to play a role in enteroendocrine development, including Ankrd6, Bmi1, Dtx1, Egr2, Etv1, Fubp1, Grhl1, Ikzf4, Jazf1, Klf12, Nr4a2, St18, Tbx3, Zfp90, Zfp179, Zfp644, and Zfp667. The high expression of Mlxipl in colonic L-cells is interesting, because it encodes the carbohydrate response element binding protein, a known glucose-responsive regulator of metabolism (37). This supports the idea that colonic L-cells are glucose sensitive, consistent with their expression of a number of genes involved in glucose metabolism (13).
Upper SI L-cells were distinguishable from colonic L-cells by expression of several TF, including Dtx1, Egr2, and Tbx3, and from upper SI K-cells by expression of Etv1 and Prox1. Pax4 was higher in colonic L-cells than upper SI K-cells, consistent with the idea that this factor may contribute to repression of the GIP gene (25). Also in agreement with a previous report that Pdx1 expression is required for coexpression of GIP in L-cells (38), we detected Pdx1 in upper small intestinal L- and K-cells but not colonic L-cells. Interestingly, no TF were identified that were both specific to enteroendocrine cells and enriched in upper SI K-cells compared with upper SI or colonic L-cells. Etv1 appeared restricted to cell populations that produced gcg (L- and α-cells), although is not a known regulator of gcg gene expression or of enteroendocrine or pancreatic islet cell development. Jazf1, Glis3, and Prox1, which appeared here as enteroendocrine markers, have been reported as type 2 diabetes susceptibility genes (39–41), so it will be interesting to determine whether human carriers of the susceptible genotypes have reduced incretin responses.
Profiles of TF expression in pancreatic α- and β-cells were consistent with previous observations in mice (42, 43) and largely mirrored a recent transcriptomic analysis of human α- and β-cells (44). Thus, in mice, as in humans, Pdx1, Mafa, and Nkx6.1 were largely β-cell restricted, Mafb, Irx2, and Arx were largely α-cell restricted, and Nkx2.2 was found in both α- and β-cells. We found Mafb to be largely α-cell restricted consistent with previous data from mice, but differing from humans in whom Mafb was detected in both α- and β-cells. Our data also revealed that in mouse islets, Etv1 was strongly expressed in α-cells and Mlxipl was strongly expressed in β-cells. Contrary to the findings in human islets, we did not detect Hdac9 expression in either α- or β-cell populations (data not shown).
Cells coexpressing GLP-1 and GIP have been detected previously by immunostaining (45) and reported to increase in number in subjects with diabetes (46) or in the jejunum of rats that have undergone gastric bypass surgery (47), suggesting that enteroendocrine cell development can be influenced by the metabolic and nutritional environment. GIP expression in the duodenum was also reported to vary according to dietary glucose availability (48). Our data suggest that altered activity of very few TF could shift enteroendocrine cells from one phenotype to another. An interesting question for future research therefore will be to investigate the extent to which diet and metabolic disease influence the spectrum of gut endocrine cells, and whether shifts in the enteroendocrine cell population translate into alterations in feeding behavior or nutrient metabolism. The current interest in developing drugs targeting different intestinal endocrine cell populations, however, underlies the importance of an improved understanding of the interrelationships between these cell types.
Supplementary Material
Acknowledgments
We thank the flow cytometry facility at the Cambridge Institute for Medical Research for assistance with FACS sorting. We also thank G. Dockray (Liverpool, UK) and J.J. Holst (Copenhagen, Denmark) for antibodies against CCK and GIP, respectively. GLUTag cells were kindly provided by D. Drucker (Toronto, Canada).
This work was supported by the Wellcome Trust (Grants WT088357 and WT084210), Medical Research Council [Core funding for the Centre for Obesity and Related Metabolic Diseases (CORD) Cambridge, UK], and the European Union (FP7/2007–2013; grant agreement no. 266408).
Author contributions: F.M.G. and F.R. designed research, analyzed data, and wrote the paper. A.M.H., P.R., and H.E.P. performed research and analyzed data; G.S.H.Y. generated microarray data; L.S.C., C.A.M.B., and T.C.E.M. performed research; G.J.R. developed FACS protocols.
Disclosure Summary: None of the authors have anything to declare.
Footnotes
- aRNA
- Amplified RNA
- CCK
- cholecystokinin
- CgA
- CgB, chromogranin A and B
- FACS
- fluorescence-activated cell sorting
- GIP
- glucose-dependent insulinotropic polypeptide
- GLP-1
- glucagon-like peptide-1
- HBSS
- Hanks' buffered salt solution
- iapp
- islet amyloid polypeptide
- Nts
- neurotensin
- PYY
- PeptideYY
- RMA
- Robust Multi-chip Average
- Sct
- secretin
- SI
- small intestinal/small intestine
- Sst
- somatostatin
- TF
- transcription factor.
References
- 1. Holst JJ. 2007. The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 2. Baggio LL, Drucker DJ. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 3. Dockray GJ. 2009. Cholecystokinin and gut-brain signalling. Regul Pept 155:6–10 [DOI] [PubMed] [Google Scholar]
- 4. Karra E, Batterham RL. 2010. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol Cell Endocrinol 316:120–128 [DOI] [PubMed] [Google Scholar]
- 5. Ahrén B. 2011. The future of incretin-based therapy: novel avenues–novel targets. Diabetes Obes Metab 13(Suppl 1):158–166 [DOI] [PubMed] [Google Scholar]
- 6. Sjölund K, Sandén G, Håkanson R, Sundler F. 1983. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 85:1120–1130 [PubMed] [Google Scholar]
- 7. Li HJ, Ray SK, Singh NK, Johnston B, Leiter AB. 2011. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab 13( Suppl 1):5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. May CL, Kaestner KH. 2010. Gut endocrine cell development. Mol Cell Endocrinol 323:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. 1997. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 11:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rindi G, Ratineau C, Ronco A, Candusso ME, Tsai M, Leiter AB. 1999. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development 126:4149–4156 [DOI] [PubMed] [Google Scholar]
- 11. Buchan AM, Polak JM, Capella C, Solcia E, Pearse AG. 1978. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry 56:37–44 [DOI] [PubMed] [Google Scholar]
- 12. Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Göke B. 1992. Glucagon-like peptide-1 cells in the gastrointestinal-tract and pancreas of rat, pig and man. Eur J Clin Invest 22:283–291 [DOI] [PubMed] [Google Scholar]
- 13. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. 2008. Glucose sensing in L cells: a primary cell study. Cell Metab 8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. 2009. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parker HE, Reimann F, Gribble FM. 2010. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med 12:e1. [DOI] [PubMed] [Google Scholar]
- 16. Rorsman P. 1997. The pancreatic β-cell as a fuel sensor: an electrophysiologist's viewpoint. Diabetologia 40:487–495 [DOI] [PubMed] [Google Scholar]
- 17. Gribble FM, Williams L, Simpson AK, Reimann F. 2003. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52:1147–1154 [DOI] [PubMed] [Google Scholar]
- 18. Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. 2011. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 152:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers GJ, Tolhurst G, Ramzan A, Habib AM, Parker HE, Gribble FM, Reimann F. 2011. Electrical activity-triggered glucagon-like peptide-1 secretion from primary murine L-cells. J Physiol 589:1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reimann F, Maziarz A, Flock G, Habib AM, Drucker DJ, Gribble FM. 2005. Characterization and functional role of voltage gated cation conductances in the glucagon-like peptide-1 secreting GLUTag cell line. J Physiol 563:161–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedlander RS, Moss CE, Mace J, Parker HE, Tolhurst G, Habib AM, Wachten S, Cooper DM, Gribble FM, Reimann F. 2011. Role of phosphodiesterase and adenylate cyclase isozymes in murine colonic glucagon-like peptide 1 secreting cells. Br J Pharmacol 163:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tung YC, Ma M, Piper S, Coll A, O'Rahilly S, Yeo GS. 2008. Novel leptin-regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus. J Neurosci 28:12419–12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. 1994. Activation of proglucagon gene-transcription by protein kinase-A in a novel mouse enteroendocrine cell-line. Mol Endocrinol 8:1646–1655 [DOI] [PubMed] [Google Scholar]
- 24. Fujita Y, Wideman RD, Asadi A, Yang GK, Baker R, Webber T, Zhang T, Wang R, Ao Z, Warnock GL, Kwok YN, Kieffer TJ. 2010. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet α-cells and promotes insulin secretion. Gastroenterology 138:1966–1975 [DOI] [PubMed] [Google Scholar]
- 25. Musson MC, Jepeal LI, Sharifnia T, Wolfe MM. 2010. Evolutionary conservation of glucose-dependent insulinotropic polypeptide (GIP) gene regulation and the enteroinsular axis. Regul Pept 164:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mulder H, Ekelund M, Ekblad E, Sundler F. 1997. Islet amyloid polypeptide in the gut and pancreas: localization, ontogeny and gut motility effects. Peptides 18:771–783 [DOI] [PubMed] [Google Scholar]
- 27. Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. 1997. Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J Histochem Cytochem 45:815–822 [DOI] [PubMed] [Google Scholar]
- 28. Gierl MS, Karoulias N, Wende H, Strehle M, Birchmeier C. 2006. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic β cells and intestinal endocrine cells. Genes Dev 20:2465–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jepeal LI, Boylan MO, Wolfe MM. 2003. Cell-specific expression of the glucose-dependent insulinotropic polypeptide gene functions through a GATA and an ISL-1 motif in a mouse neuroendocrine tumor cell line. Regul Pept 113:139–147 [DOI] [PubMed] [Google Scholar]
- 30. Wang YC, Zuraek MB, Kosaka Y, Ota Y, German MS, Deneris ES, Bergsland EK, Donner DB, Warren RS, Nakakura EK. 2010. The ETS oncogene family transcription factor FEV identifies serotonin-producing cells in normal and neoplastic small intestine. Endocr Relat Cancer 17:283–291 [DOI] [PubMed] [Google Scholar]
- 31. Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. 2003. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 17:2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang HS, Kim YS, ZeRuth G, Beak JY, Gerrish K, Kilic G, Sosa-Pineda B, Jensen J, Pierreux CE, Lemaigre FP, Foley J, Jetten AM. 2009. Transcription factor Glis3, a novel critical player in the regulation of pancreatic β-cell development and insulin gene expression. Mol Cell Biol 29:6366–6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. da Silva Xavier G, Sun G, Qian Q, Rutter GA, Leclerc I. 2010. ChREBP regulates Pdx-1 and other glucose-sensitive genes in pancreatic β-cells. Biochem Biophys Res Commun 402:252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gu G, Wells JM, Dombkowski D, Preffer F, Aronow B, Melton DA. 2004. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131:165–179 [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Kilic G, Aydin M, Burke Z, Oliver G, Sosa-Pineda B. 2005. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev Biol 286:182–194 [DOI] [PubMed] [Google Scholar]
- 36. Hoffman BG, Zavaglia B, Beach M, Helgason CD. 2008. Expression of Groucho/TLE proteins during pancreas development. BMC Dev Biol 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Postic C, Dentin R, Denechaud PD, Girard J. 2007. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr 27:179–192 [DOI] [PubMed] [Google Scholar]
- 38. Fujita Y, Chui JW, King DS, Zhang T, Seufert J, Pownall S, Cheung AT, Kieffer TJ. 2008. Pax6 and Pdx1 are required for production of glucose-dependent insulinotropic polypeptide in proglucagon-expressing L cells. Am J Physiol Endocrinol Metab 295:E648–E657 [DOI] [PubMed] [Google Scholar]
- 39. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, et al. 2010. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rees SD, Hydrie MZ, O'Hare JP, Kumar S, Shera AS, Basit A, Barnett AH, Kelly MA. 2011. Effects of 16 genetic variants on fasting glucose and type 2 diabetes in South Asians: ADCY5 and GLIS3 variants may predispose to type 2 diabetes. PLoS One 6:e24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Boström KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, et al. 2008. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bramswig NC, Kaestner KH. 2011. Transcriptional regulation of α-cell differentiation. Diabetes Obes Metab 13(Suppl 1):13–20 [DOI] [PubMed] [Google Scholar]
- 43. Servitja JM, Ferrer J. 2004. Transcriptional networks controlling pancreatic development and β cell function. Diabetologia 47:597–613 [DOI] [PubMed] [Google Scholar]
- 44. Dorrell C, Schug J, Lin CF, Canaday PS, Fox AJ, Smirnova O, Bonnah R, Streeter PR, Stoeckert CJ, Jr, Kaestner KH, Grompe M. 2011. Transcriptomes of the major human pancreatic cell types. Diabetologia 54:2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mortensen K, Christensen LL, Holst JJ, Orskov C. 2003. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114:189–196 [DOI] [PubMed] [Google Scholar]
- 46. Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. 2006. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290:E550–E559 [DOI] [PubMed] [Google Scholar]
- 47. Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. 2011. Duodenal-jejunal bypass protects GK rats from β-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab 300:E923–E932 [DOI] [PubMed] [Google Scholar]
- 48. Shimada M, Mochizuki K, Goda T. 2008. Dietary resistant starch reduces levels of glucose-dependent insulinotropic polypeptide mRNA along the jejunum-ileum in both normal and type 2 diabetic rats. Biosci Biotechnol Biochem 72:2206–2209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.