Abstract
We measured auditory brainstem responses (ABRs) in eight Rhesus monkeys after implantation of electrodes in the semicircular canals of one ear, using a multi-channel vestibular prosthesis based on cochlear implant technology. In five animals, click-evoked ABR thresholds in the implanted ear were within 10 dB of thresholds in the non-implanted control ear. Threshold differences in the remaining three animals varied from 18 to 69 dB, indicating mild to severe hearing losses. Click- and tone-evoked ABRs measured in a subset of animals before and after implantation revealed a comparable pattern of threshold changes. Thresholds obtained five months or more after implantation – a period in which the prosthesis regularly delivered electrical stimulation to achieve functional activation of the vestibular system – improved in three animals with no or mild initial hearing loss and increased in a fourth with a moderate hearing loss. These results suggest that, although there is a risk of hearing loss with unilateral vestibular implantation to treat balance disorders, the surgery can be performed in a manner that preserves hearing over an extended period of functional stimulation.
1. Introduction
A number of recent animal studies have demonstrated the feasibility of electrical stimulation of the eighth nerve to drive vestibular responses in humans, with the objective of treating balance disorders such as Meniere’s disease or bilateral loss of vestibular end organ function. Merfeld and colleagues, applying rotation-modulated electrical pulse trains to a wire electrode implanted near the ampulla of a single semicircular canal, elicited eye movements in guinea pigs (Gong and Merfeld, 2002) and squirrel monkeys (Lewis et al., 2002; Merfeld et al., 2007) that were consistent with activation of the vestibular-ocular reflex (VOR) pathways. Similar results have been obtained by Della Santina and collaborators using multiple implanted canals in chinchillas (Della Santina et al., 2007; Davidovics et al., 2011) and Rhesus monkeys (Dai et al., 2011). More recently, Phillips and colleagues (Nie et al., 2011; see also Rubinstein et al., in press) described a multi-channel vestibular prosthesis based on commercial cochlear implant technology that was capable of eliciting VOR-like eye movements in Rhesus monkeys.
A major concern of semicircular canal implantation is an adverse effect on hearing function. Given the proximity of the cochlea to the vestibular organs and their shared fluid volumes, complications from surgical implantation can potentially affect cochlear hair cell viability or mobility of the ossicles. Indeed, the reverse has been demonstrated in numerous clinical studies: cochlear implant surgery can, to various degrees, result in reduced vestibular function (Brey et al., 1995; Enticott et al., 2006; Krause et al., 2010). In one recent study in chinchillas, in which the three semicircular canals of one ear were implanted with twisted-pair wire electrodes, four of six animals exhibited an increase in auditory brainstem response (ABR) thresholds of the implanted ear (Tang et al., 2009). A similar proportion of hearing loss was reported for guinea pigs (Tran et al., 2011). In contrast, a recent study in monkeys showed no significant change in ABR threshold in any of the four tested animals (Dai et al., 2011).
In the present study, we assessed ABR thresholds before and after vestibular implantation with a modified cochlear implant in non-human primates. The electrodes were inserted with a technique designed to preserve the normal rotational sensitivity of the semicircular canal and hair cells, an approach that should be beneficial to human patients with only partial or episodic loss of vestibular function. ABR thresholds were also measured several months following implantation, to evaluate the stability of hearing and potential effects of chronic electrical stimulation.
2. Methods
2.1. Animal subjects and experimental overview
Auditory brainstem responses were collected from eight male Rhesus monkeys (Macaca mulatta, 4–7 kg, 2–5 years of age) implanted with the University of Washington/Cochlear vestibular prosthesis. The recordings were made over periods ranging from a few weeks prior to several months after implantation. All procedures were approved by the University of Washington Institutional Animal Care and Use Committee and conducted in accordance to the guidelines established by the National Institutes of Health.
2.2. Surgical implantation of electrode arrays
The University of Washington/Cochlear vestibular prosthesis is a modified Nucleus Freedom cochlear implant array manufactured in collaboration with Cochlear Ltd. (Lane Cove, Australia). The arrays consist of three insulated multi-wire leads, each with three banded electrode contacts separated by 0.2 mm near the lead’s tip. The three leads bifurcate from a common insulated bundle which connects to a Nucleus implant processor and receiver coil. The metal casing of the processor and a separate single-wire lead terminating in a ball electrode serve as current returns.
The implant surgeries were performed in sterile conditions with the animals under inhalant anesthesia. A high-speed drill with cutting burrs was used to perform a mastoidectomy of the right ear, and a diamond burr was used to expose and “blue-line” the semicircular canals. The sterilized body of the implant was positioned under the skin and secured to the mastoid bone and temporalis muscle with suture. Each canal to be implanted was then fenetrated close to the ampullary ending with the diamond burr and pick using techniques similar to that for hybrid cochlear implants. One electrode lead was immediately inserted into each targeted canal parallel to the lumen and pointed toward, but not entering, the ampulla. Considerable effort was made to not penetrate the membranous labyrinth during insertion. For some animals, electrically-evoked compound action potentials were recorded from each canal to ensure a proper insertion depth (Nie et al., 2011). In this manner, the lateral canal and typically one or both of the other canals were implanted. Finally, the leads were secured in place and the subcutaneous tissue and skin were closed with suture.
Table 1 summarizes the surgical and recording histories of all animals used in this study. Five of the 8 animals underwent at least one revision surgery, as indicated in the second column by values greater than 1. The third column provides a cumulative record of which canals had been implanted at the time of the first post-implantation ABRs (which, for this study, were recorded one week or more after the animal’s last implantation). Pre-implantation ABRs were obtained prior to the first implant surgery in four of the animals, as indicated in the fourth column. Tone ABRs were obtained in the same subset of animals, as indicated in the fifth column. Additionally, in some animals ABR recordings were made at least 5 months after the last surgery, as shown in column 6. The final column indicates animals exhibiting electrically-evoked eye movements (EEM –see Section 2.4) at the time of the first post-implant ABR.
Table 1.
Summary of implanted monkeys. #Surg indicates the number of vestibular implant surgeries performed at the time of the post-implant ABR. Canals signifies semicircular canals implanted at least once over all surgeries; L = lateral, P = posterior, and A = anterior. Pre, >5 mo, and tones indicate the availability of ABR data for pre-implant clicks, longitudinal clicks, and tones, respectively. EEM indicates animals exhibiting a minimum electrically-evoked eye movement (20° /s) for at least one canal at the time of the post-implant ABR.
| Animal | # Surg | Canals | Pre | Tones | >5 mo | EEM |
|---|---|---|---|---|---|---|
| M1 | 2 | L, P | ✓ | |||
| M2 | 3 | L, P, A | ✓ | |||
| M3 | 2 | L, P | ✓ | |||
| M4 | 2 | L, P | ||||
| M5 | 2 | L, P | ✓ | ✓ | ✓ | ✓ |
| M6 | 1 | L, P | ✓ | ✓ | ✓ | ✓ |
| M7 | 1 | L, P | ✓ | ✓ | ✓ | ✓ |
| M8 | 1 | L, P, A | ✓ | ✓ | ✓ | ✓ |
In addition to the semicircular canal implantation described above, the monkeys underwent other procedures that could potentially have affected hearing function. Prior to any vestibular surgery, all animals were implanted with a scleral eye coil and a head-mounted restraint system to facilitate the tracking of eye movements (see Section 2.3). Some animals were also fitted with a microelectrode recording chamber centered over a small craniotomy. Details of the procedures have been given elsewhere (Fuchs et al., 2005). The invasiveness of these surgeries, which required moderate drilling of the skull and occasional application of topical antibiotic, entailed some risk to the peripheral hearing mechanisms (da Cruz et al., 1997; Farzanegan et al., 2010; Soudijn et al., 1976). However, any permanent hearing loss from these factors would likely have been small, and –relevant to the findings of the present study –would not be expected to affect one ear more than the other. Analysis of the ABR data revealed no trend with respect to the presence of a recording chamber at the time of recording.
2.3. Auditory brainstem responses
The monkeys were anesthetized with a mixture of ketamine (7–10 mg/kg) and xylazine (0.6–1.2 mg/kg), with one additional supplemental dose given if needed to maintain sedation. The entire recording procedure generally lasted from 40 to 60 min. Prior to ABR recording, the external ear canals were examined otoscopically and wax or other debris was removed if necessary. Subsequent to the recording session, any animal with elevated thresholds or more than a 20 dB mismatch between ears was checked more thoroughly for signs of middle ear infection and deformities with the use of an operating microscope. None of the animals examined in this manner exhibited indications of conductive hearing loss.
The ABR recordings were made with the anesthetized animal lying on its side or stomach. Scalp potentials were recorded using EEG needle electrodes (Chalgren Enterprises, Gilroy, CA) applied in a 3-electrode configuration: the positive electrode at the midline just above the brow ridge, the negative electrode above the mastoid ipsilateral to the stimulated ear, and the common electrode above the mastoid of the contralateral ear. The recorded signals were amplified with a P55 AC pre-amplifier (Grass Technologies, West Warwick, RI) by a gain of 104 over a passband of 30–3000 Hz. The acoustic stimuli were delivered via an ER-3A insert earphone (Etymotic Research, Elk Grove Village, IL) fitted with an infant-sized ear tip (ER3-14D). Care was taken to insert the ear tip snugly into the external canal of the test ear. A foam earplug was inserted in the opposite ear to reduce ambient noise. Signal collection and stimulus generation were directed through an M-audio Delta 44 multi-channel audio board (Avid Technology, Irwindle, CA) and controlled by custom software running on a Pentium-based personal computer. The software averaged and displayed the digitized signals, which were later exported for analysis in MATLAB (Mathworks, Natick, MA).
The acoustic click and tone stimuli were presented at a rate of 10/s and waveforms were averaged across 500 stimulus repetitions. Clicks were condensation pulses of 100 µs duration. Tones were 10 ms sinusoidal bursts modulated with a 2-ms linear on ramp, a 6-ms plateau, and a 2-ms linear off ramp. Tone frequencies of 500, 2000, and 8000 Hz were used. For each stimulus type, a series of averages was obtained by decreasing the stimulus level in 10 or 20 dB steps until waveform peaks were no longer apparent; the level was then raised or lowered in 5 dB steps to obtain a rough estimate of threshold based on visible inspection. Click series were obtained in both ears for all animals; tone series were obtained only in the right ear for a subset of animals (see Table 1). Stimulus levels in this paper are expressed in decibels as “peak equivalent” sound pressure level (dB pSPL) referred to a 1 kHz standard tone. Calibrations were performed by coupling the earphone to a ¼″ microphone (Larson-Davis, Depew, NY) via a 0.15 cc plastic chamber modeling the external canal of an adult Rhesus macaque (Spezio et al., 2000).
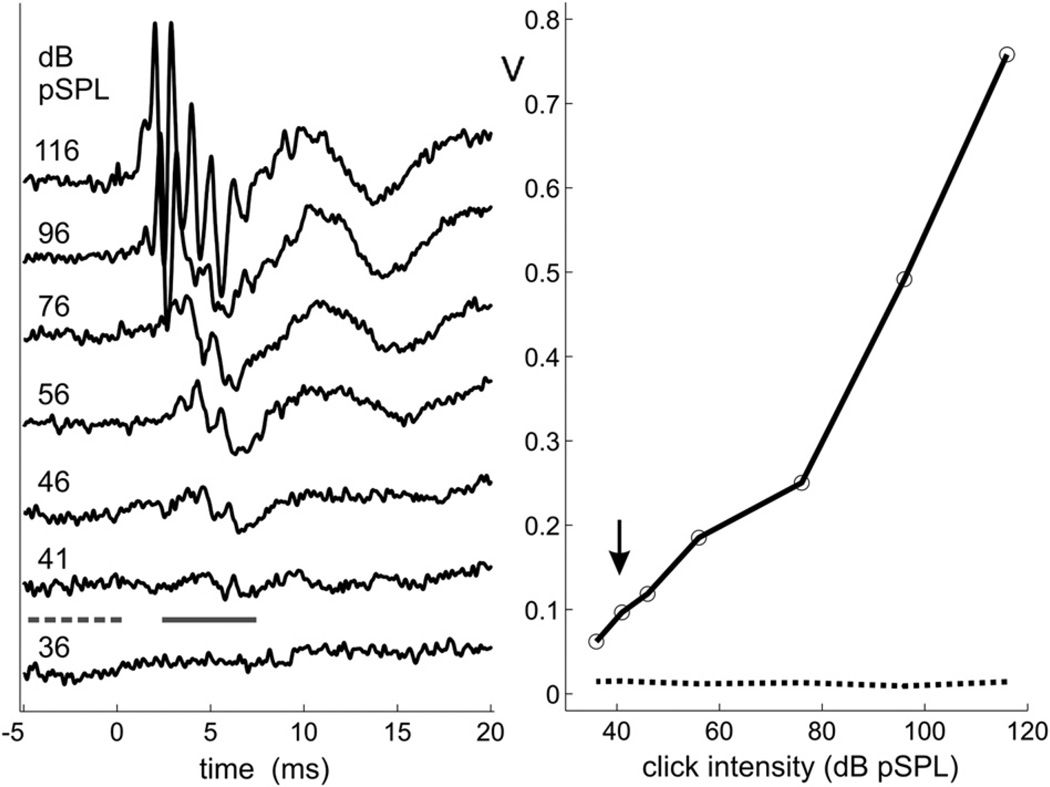
Offline, response threshold was defined objectively as the minimum click intensity that produced an ABR amplitude exceeding a specified criterion level. Fig.1 illustrates the automated procedure we used to estimate thresholds for click stimulation. The left panel displays a typical recording series for the right ear of one animal, 8 days after vestibular implantation. Each trace is the average of 500 click presentations. A baseline noise level was first obtained for each trace by calculating its standard deviation over the 5 ms time segment preceding the click (dashed horizontal bar). Next, the largest positive-to-negative excursion was found within the 2.5–7.5 ms time window following the click (solid horizontal bar). This signal window was intended to encompass waves III–V of the ABR, which were generally the largest components at low stimulus levels; also, the delay between the positive and negative excursions was limited to 2 ms, which effectively provided an estimation of the peak-to-peak amplitude of the largest of these ABR waves. (For the right ear of animal M7, the analysis window was extended to 10 ms to capture an atypical yet salient low-amplitude waveform ABR feature.) Finally, the waveform amplitude versus intensity function was interpolated to 1 dB steps, and the point where the function crossed 6 times the baseline noise level was defined as threshold. The right panel of Fig.1 shows a graph of the interpolated amplitude function (solid line) and noise level (dotted line) for the ABR series given in the left panel. Threshold for this example occurred at 41 dB SPL, as denoted by the arrow.
Fig. 1.
Left panel: ABR recording series for the implanted right ear of animal M5 in response to acoustic clicks. Each trace is the average of 500 stimulus presentations. Solid and dashed lines indicate time windows for analyzing waveform amplitude and noise level, respectively. Right panel: Waveform amplitude (solid line) and noise level (dotted line) in amplified voltage (V) as a function of stimulus level.
The procedure for estimating thresholds to tone stimulation was slightly different than that for clicks. Waveform amplitude was defined as the maximum root-mean-squared energy calculated in any 1 ms time window between 2.5 and 10 ms following tone onset. This detection strategy reflected the more variable shape and frequency-dependent onset latencies of tone-evoked ABR waveforms. The threshold criterion was set to 3 times the baseline noise level.
2.4. Electrically evoked eye movements
The ability of the implanted electrode arrays to functionally activate the vestibular nerve was evaluated by measuring evoked eye movements, as described in an earlier study (Nie et al., 2011). Briefly, horizontal and vertical eye movements were tracked with a scleral search coil system as the animal sat in the dark with its head fixed. Eye positions were recorded and digitized to a personal computer using the Spike 2 data acquisition system (Cambridge Electronic Design, Cambridge, England). Electrical stimulation at individual electrode contacts was controlled with a Cochlear Laura-34 research interface and NIC-2 based custom software.
A week or more after implantation, individual canals were stimulated with the most deeply inserted of the 3 electrode contacts, over a range of stimulus parameters. For the purposes of this study, we define a canal electrode as being functionally active if stimulation reliably produced a nystagmic eye movement for pulse trains of 2-s duration, pulse rates between 100 and 600 pulses per second, pulse widths up to 400 µs, and current levels up to the maximum allowed by the electrode impedance and voltage compliance. As in Nie et al. (2011), the criterion slow-phase velocity of the evoked nystagmus was set conservatively at 20 deg/s.
Animals for which longitudinal ABR data are reported were stimulated at frequent intervals for at least 5 months post-implantation. A typical stimulus was a train of 100 µs/phase pulses, 2–10 s in duration, delivered at a rate of 300 pulses per second and at a current level sufficient to evoke eye movements (usually between 50 and 200 µA). However, due to the nature of the experimental protocols, the electrical stimulation parameters varied considerably from day to day and animal to animal, and included unmodulated as well as amplitude- and frequency-modulated pulse trains. The regularity of stimulation was also variable across animals, but generally pulse trains were delivered 1–2 times per minute over the course of a 1.5 h experimental session, with an average of about 3 sessions per week.
3. Results
3.1. ABR click thresholds
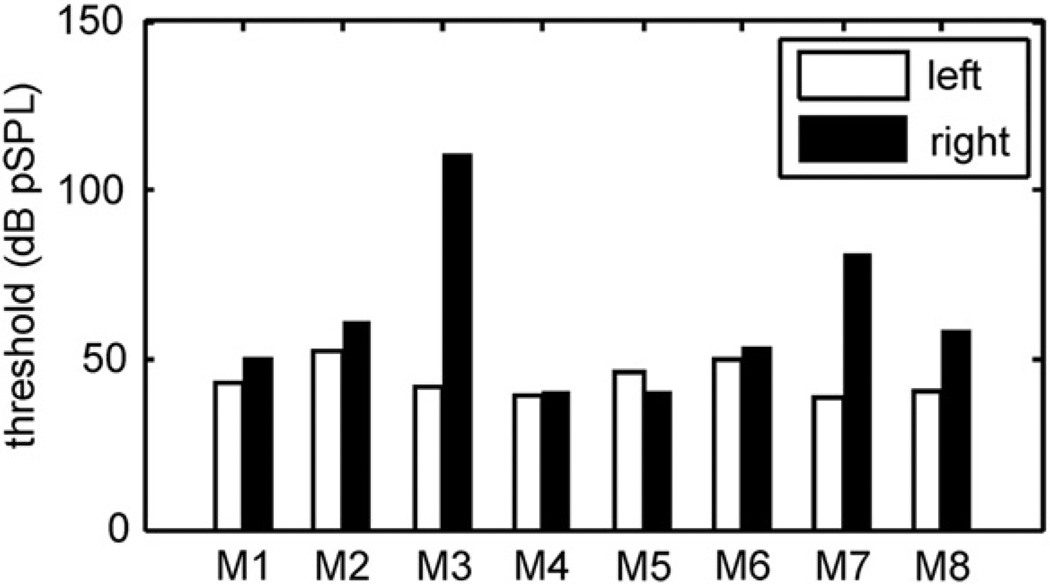
Fig. 2 summarizes the results for acoustic click stimulation for all 8 implanted animals. As noted in Table 1, functional activation of the vestibular system, as assessed with evoked eye movements, was achieved in all but one animal (M4). Post-implantation ABR thresholds for the left (control) and right (implanted) ears are presented as white and black bars in Fig. 2, respectively. For most animals, the thresholds correspond to ABRs recorded within 10 weeks of the last implant surgery; for animals M1 and M3, the recordings were made 12 months and 4.5 months following the last surgery. The non-implanted left ears served as individual controls. The average left ear threshold was 44.3 dB pSPL (±5.0, standard deviation), which is intermediate to average thresholds reported in earlier studies of the Rhesus macaque (Lasky et al., 1999; Torre et al., 2004; Dai et al., 2011). Thresholds for the implanted right ears were much more variable and higher on average (62.4 ± 23.4 dB pSPL). While all but one animal (M5) had a greater right ear threshold compared to control, the higher average threshold was mainly attributable to three of the animals. For M8 the difference between right and left ear thresholds was 18 dB, which could be considered a mild hearing loss. For M7 and M3 the differences were 42 dB and 69 dB, respectively, which are moderate and severe hearing losses. The remaining five animals exhibited threshold differences less than 10 dB (average right-left difference = +3.2 dB).
Fig. 2.
Post-implant ABR thresholds in response to acoustic clicks for all animals (M1–M8). White bars – left (control) ear; black bars – right (implanted) ear.
From the “EEM” column of Table 1, it is evident that all three animals with elevated implanted thresholds (M3, M7, and M8) achieved the criterion velocity of eye motion in response to electrical stimulation. Most of the animals with less than a 10 dB right-left threshold difference achieved the minimum EEM level as well. Thus, the ability of the implant to elicit functional activation of the vestibular system was not an apparent factor in the reduction or preservation of hearing function. The number of surgeries and total canals implanted also did not have a clear relationship to the incidence of hearing loss.
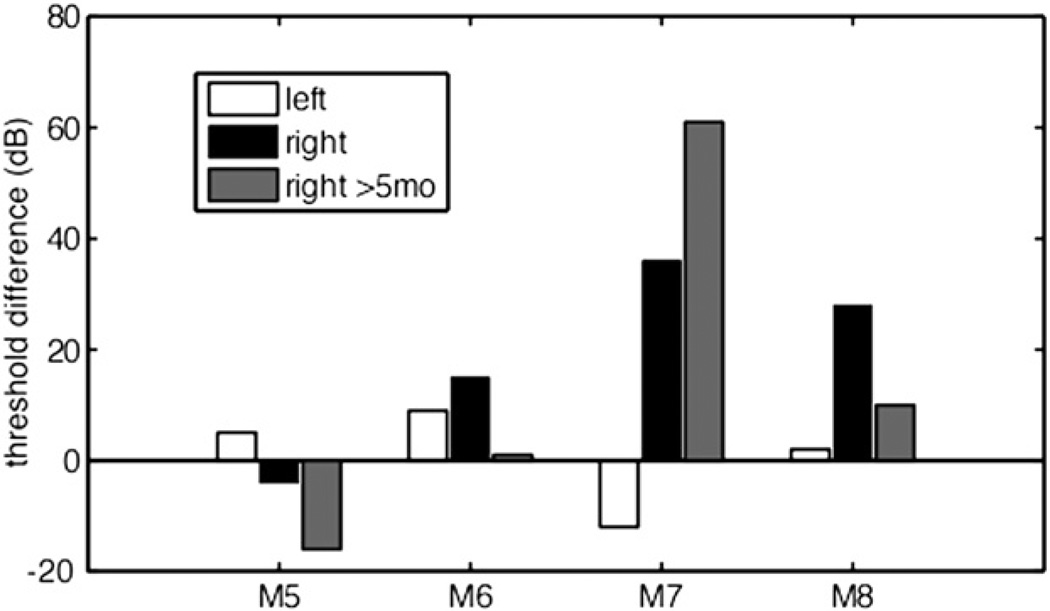
In four animals, thresholds were obtained prior to the first implantation surgery, providing a pre-implantation control for both ears. Fig. 3 presents this data as the change in ABR threshold from the pre-implant recording to the recording made 8 days after each animal’s final implantation, for both left (white bars) and right ears (black bars). For all four animals, thresholds in the non-implanted left ear remained within 12 dB of their pre-surgery values. As in Fig. 2, threshold differences for the right ear were more variable. For animals M5 and M6, the right ear threshold at 8 days post-surgery was within 15 dB of the pre-implant threshold. For M7, the change in threshold was 36 dB, comparable to the left vs. right post-implant difference of 42 dB observed in Fig. 2. For M8 the change was 28 dB, notably larger than the left vs. right threshold difference of 18 dB. Part of this discrepancy was due to an especially low pre-implant threshold measured in the right ear (31 dB pSPL). In general, however, the pre/post threshold comparisons gave qualitatively similar results as the left/right comparisons, with animal M7 showing the largest difference for both analyses.
Fig. 3.
Difference between post- and pre-implant ABR thresholds in response to acoustic clicks for a subset of animals. White and black bars represent, for the left (control) and right (implanted) ears respectively, post-implant ABRs collected on the 8th day following the last vestibular surgery. Gray bars represent longitudinal data for the right ear, obtained at least 5 months after surgery.
For each of the animals shown in Fig. 3, ABRs were also obtained 5 months or more after the last implant surgery (gray bars). Thresholds in the implanted ear of two of the animals, M5 and M6, improved slightly over their 8-day levels by 12 and 14 dB, respectively. Likewise, the threshold for M8, which had a mild hearing loss originally, decreased by 18 dB. In contrast, the right ear threshold of M7, which at 8 days was the most elevated of the four animals, increased by another 25 dB. Together these findings suggest that, when hearing function is severely enough compromised by vestibular implantation, threshold will not improve and may even worsen in time, but that when hearing function is minimally affected by implantation, threshold will remain stable or improve.
3.2. ABR tone thresholds
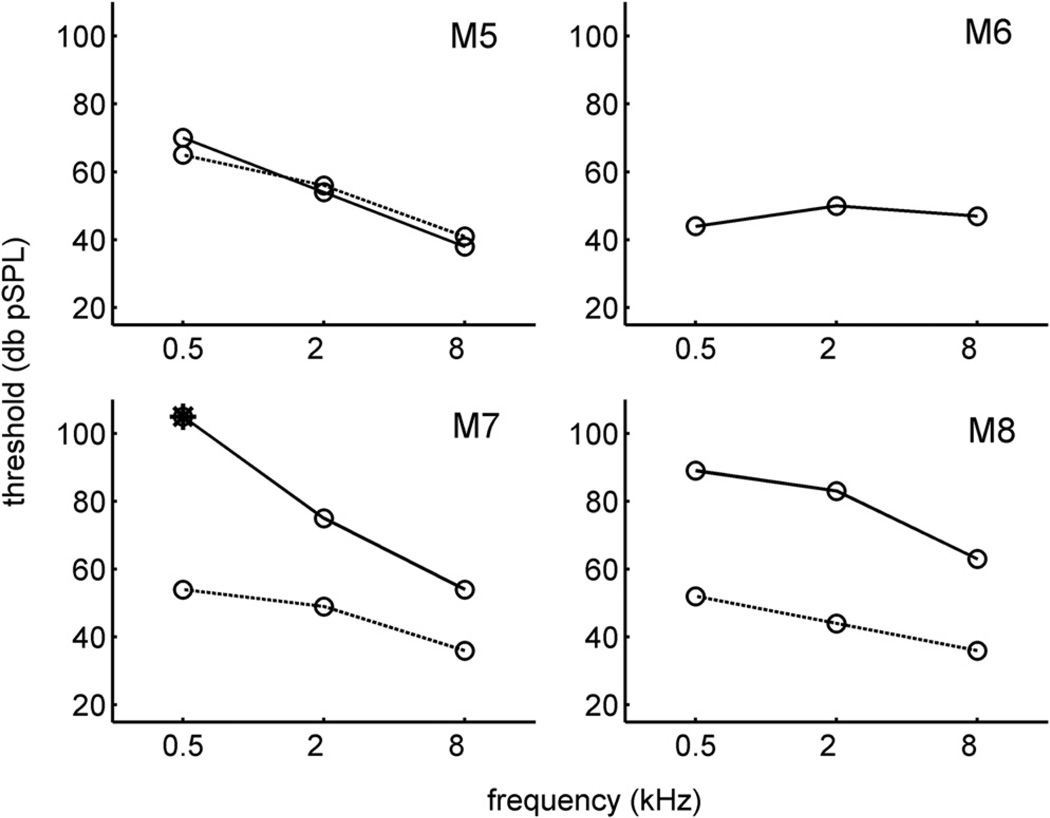
Pre- and post-implant ABR thresholds in response to tone pips are shown in Fig. 4. Prior to implantation, the threshold pattern across the three tested frequencies has a low-pass characteristic, with 500 Hz tones eliciting the highest thresholds. This pattern is similar to that observed previously in the Rhesus monkey (Lasky et al., 1999), for ABR as well as behavioral thresholds. In general, the changes in right ear threshold after implantation mirrored the threshold changes observed for click stimulation in Fig. 3. For animal M5, the tone thresholds at all tested frequencies changed by less than 5 dB, comparable to the result for clicks. Similarly, for M6 (for whom pre-implant tone data was not available), tone thresholds remained relatively low after implantation. For M7 and M8, however, threshold increases of ~20 dB or more occurred at each frequency, comparable to the 36 and 28 dB increases for those animals observed in Fig. 3 for clicks.
Fig. 4.
Pre-implant (dotted lines) and post-implant (solid lines) thresholds for the right ear in response to tone pips at 500, 2000, and 8000 Hz. Post-implant ABRs were obtained 8 days after surgery. There is no pre-implant tone data for M6. Asterisk for M7 denotes a recording for which an ABR waveform was not detected.
4. Discussion
To summarize the present findings, the implanted ears of 5 of 8 animals exhibited ABR click-evoked thresholds that were generally higher but within 10 dB of the thresholds in the non-implanted control ears (Fig. 2). The remaining 3 animals had threshold differences ranging from +18 to +69 dB. ABRs recorded before and after implantation in a subset of these animals indicated that threshold elevations were specific to the side of surgery (Fig. 3). Threshold changes obtained with tonal stimuli over a range of frequencies were largely consistent with the threshold changes observed with clicks (Fig. 4).
The primary result that 5 of 8 animals exhibited little or no hearing loss after vestibular implantation is similar to previous reports in rodents (Tang et al., 2009; Tran et al., 2011), though the proportion of animals with preserved hearing in the present study was greater. However, it contrasts with a recent study using the same non-human primate model (Dai et al., 2011), in which all four implanted animals exhibited ABR threshold changes less than 10 dB. The main methodological difference between the present study and that of Dai et al. (2011) is the method of implantation. In the earlier study, the electrode leads were implanted in a small window made very close to the ampulla of the targeted semicircular canal. The UW/Cochlear electrode leads, in contrast, were designed to be inserted through a fenestra made more distant from the ampulla and course parallel to the lumen of the canal between the bony and membranous labyrinths. This approach was intended to preserve natural vestibular function. Both electrode insertion techniques risk disruption of the membranous labyrinth, a factor that can negatively affect cochlear function (Smouha and Inouye, 1999; Isaacson and Antonelli, 1999). However, the relative risk associated with the two techniques is difficult to assess.
In some respects, the risk of hearing loss with the present insertion procedure may be comparable to that of canal plugging (e.g. Parnes and McClure, 1985; Gentine et al., 2008). It should be noted that in two monkeys, not included in this study, three canals were implanted with single-channel wire electrodes and intentionally plugged. One of these animals had a normal post-implant ABR threshold and the other had a 25 dB threshold elevation compared to the control ear. Given the demonstrated risk to auditory function (and to vestibular function, if plugging occurs) with canal insertion, a surgical approach that does not involve insertion directly into the canal may be a viable alternative. Such an approach has been explored by Guyot and colleagues in a small number of human patients (Wall et al., 2007; Guyot et al., 2011).
Another methodological difference with the study by Dai et al. (2011) is that the number of animals in the present study was twice as large. Moreover, all but one of the animals in our study underwent multiple implantation surgeries, resulting in a total of 24 attempted canal insertions at the time of post-implant ABR recording. This compares to only 12 insertions (3 canals in each of 4 monkeys) by Dai et al. If each insertion is considered an independent risk to hearing, the chance of incurring a hearing loss would be considerably greater in the present study. From that standpoint, it is worth highlighting that 4 of the 5 animals exhibiting a <10 dB threshold difference in Fig. 2 had at least one revision surgery, and one of these had two revision surgeries.
One animal with a substantial initial hearing loss (M7) exhibited an even higher threshold after 5 months. Two possible explanations for the delayed threshold increase are that 1) adverse effects from the implantation surgery were still progressing at the time of the initial post-implant ABR, at 8 days; or 2) frequent electrical stimulation during the 5-month time period caused some type of cochlear damage. Several lines of evidence, however, suggest electrical stimulation probably has a minimal effect on hearing. First, the animals with a small (M5 and M6) or mild (M8) pre- to post-implant threshold change exhibited slight decreases in threshold after 5 months of implantation. Second, across all tested animals, post-implant thresholds did not depend on the ability of the vestibular implant to elicit VOR-like eye movements. Third, previous studies with cochlear implants suggest that chronic electrical stimulation does not damage spiral ganglion neurons or cochlear hair cells distant from the implant, and may even act as a trophic factor for their preservation (Leake et al., 1999; Coca et al., 2007). Because the semicircular canal electrode leads were not in close proximity to cochlear cells, a similar outcome would be expected for vestibular stimulation. Consistent with the present findings, Tran et al. (2011) demonstrated no additional ABR threshold increases in vestibular implanted guinea pigs after several days of electrical stimulation.
We conclude that, despite a potential risk of hearing loss, unilateral implantation of the semicircular canals can be performed in a manner that preserves hearing over an extended period of functionally effective electrical stimulation. By analogy to cochlear implantation, which provides a benefit to deaf patients that outweighs its risk to vestibular function, vestibular implantation might be worth some loss of hearing if it can help patients suffering from severe balance disorders.
Acknowledgments
The authors thank Brandon Warren for development of the ABR software. This research was supported by a contract from the National Institute on Deafness and Other Communication Disorders, N01-DC-6-005 and an NCRR ITHS ignition award, RR00166. The Anspach Effort, Inc. provided surgical drills.
Footnotes
Disclosure
All authors participated in some aspect of the experiment design and data acquisition, and all have approved the final manuscript. Authors SMB, LL, KN, JTR, CRSK, AF, and JOP have patent applications pending on the vestibular prosthesis.
Contributor Information
Steven M. Bierer, Email: sbierer@uw.edu.
Leo Ling, Email: lling@uw.edu.
Kaibao Nie, Email: niek@uw.edu.
Albert F. Fuchs, Email: fuchs@uw.edu.
Chris R.S. Kaneko, Email: kaneko@uw.edu.
Trey Oxford, Email: treyo@uw.edu.
Amy L. Nowack, Email: aln@uw.edu.
Sarah J. Shepherd, Email: shepsh@uw.edu.
Jay T. Rubinstein, Email: rubinj@uw.edu.
James O. Phillips, Email: jop@uw.edu.
References
- Brey RH, Facer GW, Trine MB, Lynn SG, Peterson AM, Suman VJ. Vestibular effects associated with implantation of a multiple channel cochlear prosthesis. Am. J. Otolaryngol. 1995;16(4):424–430. [PubMed] [Google Scholar]
- Coca A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007;225(1–2):60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz MJ, Fagan P, Atlas M, McNeill C. Drill-induced hearing loss in the nonoperated ear. Otolaryngol. Head Neck Surg. 1997;117:555–558. doi: 10.1016/s0194-5998(97)70030-5. [DOI] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Della Santina CC. Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys. Hear Res. 2011;277(1–2):204–210. doi: 10.1016/j.heares.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovics NS, Fridman GY, Chiang B, Della Santina CC. Effects of biphasic current pulse frequency, amplitude, duration, and interphase gap on eye movement responses to prosthetic electrical stimulation of the vestibular nerve. IEEE Trans. Neural Syst. Rehabil. Eng. 2011;19(1):84–94. doi: 10.1109/TNSRE.2010.2065241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina CC, Migliaccio AA, Patel AH. A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-d vestibular sensation. IEEE Trans. Biomed. Eng. 2007;54(6 Pt 1):1016–1030. doi: 10.1109/TBME.2007.894629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott JC, Tari S, Koh SM, Dowell RC, O’Leary SJ. Cochlear implant and vestibular function. Otol. Neurotol. 2006;27(6):824–830. doi: 10.1097/01.mao.0000227903.47483.a6. [DOI] [PubMed] [Google Scholar]
- Farzanegan G, Ghasemi M, Panahi F, Raza M, Alghasi M. Does drill-induced noise have an impact on sensorineural hearing during craniotomy procedure? Br. J. Neurosurg. 2010;24(1):40–45. doi: 10.3109/02688690903374059. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Ling L, Phillips JO. Behavior of the pvp interneurons of the vestibulo-ocular reflex during head-free gaze shifts in the monkey. J. Neurophysiol. 2005;94(6):4481–4490. doi: 10.1152/jn.00101.2005. [DOI] [PubMed] [Google Scholar]
- Gentine A, Martin E, Schultz P, Debry C, Charpiot A. Lateral semicircular canal plugging: a simple and effective surgical treatment against incapacitating Menière’s disease. Rev. Layrngol. Otol. Rhinol. 2008;129(1):11–16. [PubMed] [Google Scholar]
- Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans. Biomed. Eng. 2002;49(2):175–181. doi: 10.1109/10.979358. [DOI] [PubMed] [Google Scholar]
- Guyot JP, Sigrist A, Pelizzone M, Feigl GC, Kos MI. Eye movements in response to electrical stimulation of the lateral and superior ampullary nerves. Ann. Otol. Rhinol. Laryngol. 2011;120(2):81–87. doi: 10.1177/000348941112000202. [DOI] [PubMed] [Google Scholar]
- Isaacson DJ, Antonelli PJ. Labyrinthine fenestration in the guinea pig. Otolaryngol. Head Neck Surg. 1999;120(3):394–399. doi: 10.1016/S0194-5998(99)70282-2. [DOI] [PubMed] [Google Scholar]
- Krause E, Louza JP, Wechtenbruch J, Gürkov R. Influence of cochlear implantation on peripheral vestibular receptor function. Otolaryngol. Head Neck Surg. 2010;142(6):809–813. doi: 10.1016/j.otohns.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Soto AA, Luck ML, Laughlin NK. Otoacoustic emission, evoked potential, and behavioral auditory thresholds in the rhesus monkey (Macaca mulatta) Hear Res. 1999;136:35–43. doi: 10.1016/s0378-5955(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J. Comp. Neurol. 1999;412(4):543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Gong W, Ramsey M, Minor L, Boyle R, Merfeld DM. Vestibular adaptation studied with a prosthetic semicircular canal. J. Vestib. Res. 2002;12(2–3):87–94. [PubMed] [Google Scholar]
- Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans. Biomed. Eng. 2007;54(6 Pt 1):1005–1015. doi: 10.1109/TBME.2007.891943. [DOI] [PubMed] [Google Scholar]
- Nie K, Bierer SM, Ling L, Oxford T, Rubinstein JT, Phillips JO. Characterization of the electrically evoked compound action potential of the vestibular nerve. Otol. Neurotol. 2011;32:88–97. doi: 10.1097/mao.0b013e3181f6ca45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes LS, McClure JA. Effect on brainstem auditory evoked responses of posterior semicircular canal occlusion in guinea pigs. J. Otolaryngol. 1985;14(3):145–150. [PubMed] [Google Scholar]
- Rubinstein JT, Bierer S, Fuchs AF, Kaneko C, Ling L, Nie K, Oxford T, Newlands S, Santos F, Risi F, Abbas PJ, Phillips J. OImplantation of the semicircular canals with preservation of hearing and rotational sensitivity: a vestibular neurostimulator suitable for clinical research. Otol. Neurotol. doi: 10.1097/MAO.0b013e318254ec24. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudijn ER, Bleeker JD, Hoeksema PE, Molenaar I, van Rooyen JP, Ritsma RJ. Scanning electron microscopic study of the organ of Corti in normal and sound-damaged guinea pigs. Ann. Otol Rhinol Laryngol. Suppl. 1976;85(4 Pt2, Suppl. 29):1–58. doi: 10.1177/00034894760850S401. [DOI] [PubMed] [Google Scholar]
- Smouha EE, Inouye M. Partial labyrinthectomy with hearing preservation: frequency-specific data using tone-burst auditory brain stem response. Otolaryngol. Head Neck Surg. 1999;120(2):146–152. doi: 10.1016/s0194-5998(99)70398-0. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Keller CH, Marrocco RT, Takahashi TT. Head-related transfer functions of the Rhesus monkey. Hear Res. 2000;144:73–88. doi: 10.1016/s0378-5955(00)00050-2. [DOI] [PubMed] [Google Scholar]
- Tang S, Melvin TN, Della Santina CC. Effects of semicircular canal electrode implantation on hearing in chinchillas. Acta Otolaryngol. 2009;129:481–486. doi: 10.1080/00016480802252243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre P, Mattison JA, Fowler CG, Lane MA, Roth GS, Ingram DK. Assessment of auditory function in rhesus monkeys (Macaca mulatta): effects of age and caloric restriction. Neurobiol. Aging. 2004;2:945–954. doi: 10.1016/j.neurobiolaging.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Tran H, de Waele C, Beraneck M, Vassias I, Gioanni H, Huy PT, Herman P, Vidal PP, Kania RE. Auditory outcomes after implantation and electrical stimulation of the lateral ampullar nerve in guinea pig. Ear Hear. 2011;33(1):118–123. doi: 10.1097/AUD.0b013e31822f6726. [DOI] [PubMed] [Google Scholar]
- Wall C, 3rd, Kos MI, Guyot JP. Eye movements in response to electric stimulation of the human posterior ampullary nerve. Ann. Otol Rhinol Laryngol. 2007;116(5):369–374. doi: 10.1177/000348940711600509. [DOI] [PubMed] [Google Scholar]