Abstract
The association between low exercise capacity and all-cause morbidity and mortality is statistically strong yet mechanistically unresolved. By connecting clinical observation with a theoretical base, we developed a working hypothesis that variation in capacity for oxygen metabolism is the central mechanistic determinant between disease and health (aerobic hypothesis). As an unbiased test, we show that two-way artificial selective breeding of rats for low and high intrinsic endurance exercise capacity also produces rats that differ for numerous disease risks including the metabolic syndrome, cardiovascular complications, premature aging, and reduced longevity. This contrasting animal model system may prove to be translationally superior, relative to more widely-used simplistic models for understanding geriatric biology and medicine.
Clinical observations initiated the Aerobic Hypothesis
Large-scale clinical studies over the past two decades show low exercise capacity to be a stronger predictor of morbidity and mortality relative to other commonly reported risk factors including hypertension, type II diabetes, obesity, and smoking (Kavanagh et al., 2003, Kokkinos et al., 2008, Myers et al., 2002). Dysfunctional aerobic energy metabolism has been implicated in essentially all age-related disease conditions including cardiac arrhythmias and sudden cardiac death (Akar et al., 2005). Moreover, it is well known that regular physical activity reduces the risk of developing a large number of chronic diseases and can be beneficial in the treatment of numerous age-related diseases (Chodzko-Zajko et al., 2009). These clinical association studies led us to formulate the idea that variation in capacity for oxygen metabolism is the central mechanistic determinant of the divide between complex disease and health which we termed the Aerobic Hypothesis (Koch and Britton, 2008). We envisioned that the aerobic hypothesis could be tested prospectively by divergent artificial selection for low and high aerobic treadmill running capacity in rats (Koch and Britton, 2001). That is, if the aerobic hypothesis was true, we expected susceptibility to disease would segregate with low running capacity, and resistance to disease would segregate in rats with high running capacity and simultaneously provide unique contrasting models for study. Still a significant challenge was that we knew of no direct principles that could mechanistically account for the strong link between aerobic function with morbidity and mortality, making the initiation of a large-scale selective breeding paradigm based solely upon clinical association an insufficient path.
Forming a Theoretical Framework
In 2011 Phillip Sharp and Robert Langer wrote: “The next challenge for biomedical research will be to solve problems of highly complex and integrated biological systems within the human body. Predictive models of these systems in either normal or disease states are beyond the capability of current knowledge and technology” (Sharp and Langer, 2011). We foresaw this challenge and aligned three scientific views that articulated a principle-based conceptual framework. In 1978, Ilya Prigogine (Prigogine, 1978)used non-equilibrium thermodynamic arguments to defend that systems tend to organize to a higher complexity when the resulting system can dissipate energy faster than the independent parts. A corollary of this idea states that entropy can temporarily decrease and ordered systems can form (order from disorder). Prigogine declared that “Non-equilibrium thermodynamics leads to general results independent of any specific molecular model.” Contemporaneous with Prigogine’s theory, Hans Krebs (Baldwin and Krebs, 1981) defined the concept that metabolic cycles evolved for greater energy transfer and Peter Mitchell discovered the chemiosmotic mechanism of ATP formation (Mitchell, 1961). These two contributions defined critical features for the molecular specification of energy transfer. We synthesized the ideas of Prigogine, Krebs, and Mitchell to formulate two statements that constitute a theoretical base for the aerobic hypothesis: 1) evolution was underwritten by energy dissipating mechanisms (entropy) and, 2) emergence of complexity was coupled to the highly energetic nature afforded by atmospheric oxygen. Thus, life is apparently one example of a molecular model that resulted from the general operation of non-equilibrium thermodynamics towards increased energy dissipation. Although speculative, these ideas do form an explanation for the association between low oxygen metabolism and low health and can perhaps help guide interpretation and further hypothesis development.
Artificial selection for aerobic capacity
Aerobic capacity, measured as maximal oxygen consumption (VO2max) or an endurance run to exhaustion, is a complicated gene-environment integration between intrinsic factors (inborn, untrained) and those accrued in response to physical training (Bouchard et al., 1992). As an initial path, we explored the role for the perhaps “simpler” intrinsic component of endurance exercise capacity. Maximal endurance capacity on a speed-ramped treadmill running test was adopted as the selection criterion because it provides a strong signal corresponding to whole-body energy transfer and can be measured somewhat objectively in many rats. In 1996 we run-tested a large founder population (n~200) of the genetically heterogeneous N:NIH rat stock (Koch and Britton, 2001) for the initiation of two-way artificial selection. The N:NIH rats were developed by crossbreeding eight inbred strains that represented widely disparate phylogenetic spectrums (Hansen and Spuhler, 1984).
Because aerobic endurance capacity is a highly polygenic trait, it was likely that divergent selection would be successful. By 2011 we had selectively bred for 28 generations (n =11,606 rats phenotyped) and the low and high lines differed in maximal running capacity about 7-fold. The low capacity runners (LCR) exhausted on average after running about 250 meters and the high capacity runners (HCR) exhausted at 2,000 meters. Pedigree-based maximum likelihood methods showed that the proportion of total phenotypic variation explained by the additive effects of genes was about 40% for each line (i.e., narrow sense heritability, h2= 0.40).
The major genetic hypothesis is that functional alleles at multiple interacting loci that affect intrinsic aerobic capacity have been enriched or fixed differentially between the LCR and HCR. It is critical that the models are maintained as genetically heterogeneous lines by using a rotational mating paradigm that minimizes inbreeding (Koch et al., 2011). Compared to inbred strains, in which essentially all loci have been taken to fixation, outbred selected lines maintain genetic complexity, allowing combinations of allelic variants at multiple interacting loci to be enriched by selection pressure (Carlborg et al., 2006). As a result, the LCR-HCR model system is better suited to discover epistatic interactions, modifier genes, and synergistic actions. Importantly, the concurrent breeding of the LCR and HCR at every generation allows the lines to serve as reciprocal controls for environmental influences.
Tests of the Aerobic Hypothesis
At generation 10 of selection, we gathered LCR and HCR rats that differed 4-fold in endurance running capacity to first test if disease features had segregated differentially between the lines. We discovered that adult LCR rats develop cardiovascular risks consistent with the metabolic syndrome including large gain in visceral adiposity, increased blood pressure, dyslipidemia, endothelial dysfunction occurring within carotid arteries, and insulin resistance (Wisloff et al., 2005). Using a more mechanistic approach with LCR and HCR rats from generation 18 (Kivela et al., 2010), we found that gene expression differences related to oxidative phosphorylation and fatty acid metabolism in skeletal muscle correlated significantly with disease risk phenotypes such as physical activity levels, serum high density lipoproteins, and mitochondrial structure. Further extensive study has revealed that LCR, relative to HCR, harbor numerous clinically relevant conditions including increased susceptibility to cardiac ventricular fibrillation (Lujan et al., 2006), hepatic steatosis (Thyfault et al., 2009), disordered sleep (Muncey et al., 2010) , diminished behavioral strategies for coping with stress (Burghardt et al., 2011), increased sensitivity to the deleterious effects of a high fat diet (Noland et al., 2007, Novak et al., 2010), and reduced capacity for oxidation of lipids in skeletal muscle (Lessard et al., 2009), liver (Thyfault et al., 2009), and heart (Wisloff et al., 2005). The segregation of low intrinsic endurance exercise capacity with low health status provided unbiased evidence in support of the aerobic hypothesis. This outcome, no matter how striking, is still associational and does not provide information at the level of proving biologic cause-and-effect.
Response to Environments
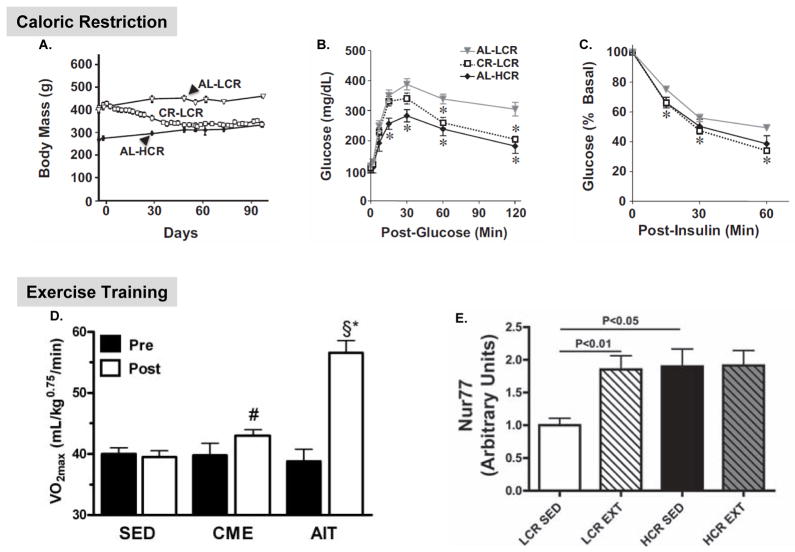
Of major clinical relevance is the response of the LCR/HCR model system to positive and negative health environments. Two environment interventions are shown to retrieve the higher risk features of the LCR. First, caloric restriction (CR) of 30% applied for three months reduced body mass, and reduced both glucose and insulin intolerance (Figure 1A, 1B, and 1C). CR also reduced serum and tissue (liver and muscle) triglyceride levels and reversed inflammation, oxidative stress, and fibrosis in liver (Bowman et al., 2010). Second, exercise training for six weeks was shown to increase maximal oxygen consumption (VO2max) by 38% and was accompanied by significant improvements in morphological and dynamic indices of both diastolic and systolic cardiac function (Hoydal et al., 2007, Wisloff et al., 2005). Of direct clinical relevance, LCR demonstrate a larger increase in VO2max and greater reduction in features of the metabolic syndrome in response to high-intensity aerobic interval training compared to continuous moderate-intensity exercise (Figure 1D) (Haram et al., 2009).
Figure 1.
Positive health environments reversed complex disease risk in low capacity runner (LCR) rats. (A) Caloric restriction (CR) reduced body mass (A) in LCR to reach the equivalent of HCR on ad libitum (AL) feeding and improves glucose (B) and insulin (C) tolerance compared to AL-LCR. (Figure 1A-C is reprinted from Bowman et al: 2010. Caloric restriction reverses hepatic insulin resistance and steatosis in rats with low aerobic capacity. Endocrinology, 151(11), 5157– 64. Copyright 2010, The Endocrine Society). (D) Maximal oxygen uptake (VO2max) increased more with aerobic interval trainig (AIT) relative to continuous moderate exercise (CME) in LCR. Significantly different between AIT and CME (*, P < 0.05). Significantly different from sedentary (SED) (§, P< 0.01; #, P< 0.05). Figure D is reprinted from Haram et al: 2009. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovascular Research, 81(4):723–3 by courtesy from The European Society of Cardiology. (E) Exercise training increased the protein content of several important regulators of carbohydrate and lipid metabolism in skeletal muscle of LCR. The protein content for nuclear orphan receptor Nur77, quantified using Western blot analysis, is shown for LCR compared to high capacity runners (HCR) in the sedentary (SED) and exercise trained (EXT) conditions. Figure E is reprinted from Lessard et al:2011. Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. Am J Physiol by courtesy from The American Physiological Society.
Recent work has identified critical features of skeletal muscle metabolism that are diminished in sedentary LCR relative to HCR and can be retrieved by exercise training (Lessard et al., 2009, Lessard et al., 2011). Insulin-stimulated glucose transport, insulin signal transduction, and rates of palmitate oxidation were lower in sedentary LCR versus sedentary HCR. The lower levels of glucose and lipid metabolism in the LCR were associated with decreased protein levels of β2-adrenergic receptor, nuclear orphan receptor Nur77, uncoupling protein-3, and fatty acid transporter (FAT/CD36). Exercise training increased the levels of all four of these proteins and reversed the impairments to glucose and lipid metabolism in the LCR. Nur77 (Figure 1E) is hypothesized to be a critical transcriptional regulator of glucose utilization and lipid metabolism during exercise in humans (Lewis et al., 2010).
Exposure of LCR rats to a high fat diet caused body weight gain, increased fat mass and further elevation in their insulin resistance that is present when on standard rat diet. Surprisingly, these metabolic variables were not changed by a high fat diet in the HCR rats. As a partial explanation, the high fat diet decreased hepatic oxidative capacity in the LCR, but not the HCR rats (Noland et al., 2007). Subsequent work has shown that LCR have a higher economy of fuel use relative to the HCR when fed either a normal or high fats diet (Novak et al., 2010).
Because numerous positive physiological and biochemical features segregated with selection for high aerobic capacity, we hypothesized this would also be true for behavior-related traits (Wikgren et al., 2012) . In discrimination-reversal and T-maze tasks, the HCR rats significantly out-performed the LCR rats, most notably in phases requiring flexible cognition (Wikgren et al., 2012). Further exploration of this initial observation is warranted because behavior is the most complex clinical phenotype to understand. Robust differences between the LCR and HCR for features related to memory and learning would amplify the utility of this animal model system significantly.
Aging
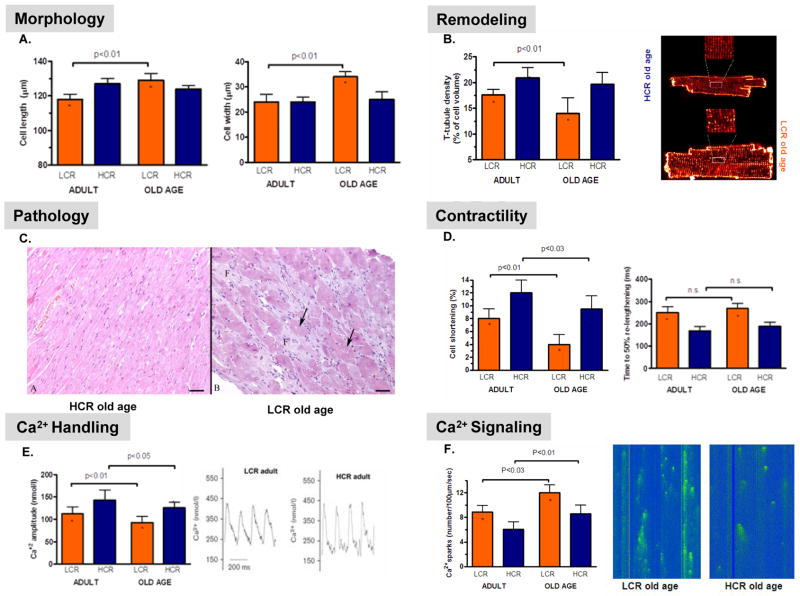
Risk factors for complex diseases together with declines in physical activity and cardiac function occur with aging. Indeed, it has been demonstratedthat a reduction of the usual age-related decline in cardiac function prolongs lifespan (Gonzalez et al., 2008). With the possibility of defining contrasting models, it was of large interest to know if cardiac function declined more rapidly in the LCR relative to the HCR as a function of aging. We evaluated left ventricular cardiomyocyte morphology, contractility, and intracellular Ca2+ handling between LCR and HCR adults (15–20 months) versus old (>25 months). During progression from adult to old age ventricular myocytes from LCR developed pathological morphology (Figure 2A-D) and a substantial decline in contractile function (Figure 2E & F) due to impaired intracellular Ca2+ handling (Figure 2G & H) relative to that observed in HCR. These cellular changes represent an important structural and functional basis for reduced cardiac function in the aged heart (Gonzalez et al., 2008).
Figure 2.
Cardioventricular myocytes were more compromised in low capacity runner (LCR) compared to high capacity runner (HCR) as a function of aging. Shown here are results for adult rats (15–20 months) compared to rats at old age (25 months). Markers of pathological morphology for the LCR include: (A) increased cell length and cell width, (B) reduced transverse (T)-tubule density quantified from images stained with Di-8-ANEPP, and (C) degeneration/necrosis in light micrographs (hematoxylin and eosin stain) of myocardium in old LCR but not in old HCR; vacuoles are marked with arrowsand interstitial fibroplasia marked by letter F (bar=50 μm). Dynamic functional properties of myocytes were also more diminished in LCR versus HCR as a function of aging and included: (D) reduced isolated fractional shortening and increased time to 50% re-lengthening, (E) lessened amplitude of systolic Ca2+ transients, and (F) reduction in Ca2+spark frequency: images are confocal micrographs from quiescent cells. (Figure 2 is reprinted by courtesy from the American Heart Association. Koch et al.: 2011. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res, 109, 1162–72).
Longevity
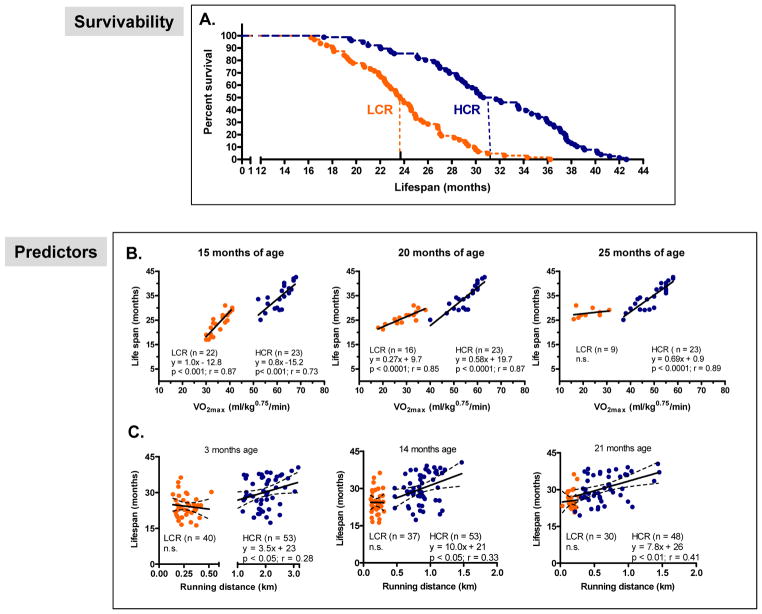
The first tests of survivability (Koch et al., 2011) in these rat models, using generation 14 rats (Norwegian University of Science and Technology, Trondheim, Norway) and repeated at generations 15 and 17 (University of Michigan, Ann Arbor, Michigan), revealed that longevity segregated strongly with capacity between the strains. The median age of death was 23.5 months for LCR and 30.1 months for HCR rats, representing a 28% difference in life expectancy, with no significant difference for maximal lifespan between females and males within lines. The survival outcome for all 3 generations combined is plotted in Figure 3A and a calculated hazard ratio of 5.7 indicates the rate of death in LCR was almost 6 times the rate of death in HCR. Standard necropsy profiles demonstrated, however, that age-related lesions were not different in incidence or severity between the LCR and HCR rats, suggesting that an overt disease condition was not over-represented in rats bred for low intrinsic capacity.
Figure 3.
A. Survival curves for data combined from aerobic rats at generations 14, 15, and 17 (n= 63 LCR and n= 76 HCR). Hazard ratio indicates rate of death in LCR rats was almost 6 times greater than for HCR. B. VO2max measured at 15, 20, and 25 months of age was a strong predictor of survival for rats. C. Distance run to exhaustion estimated at 3, 14, and 21 months of age was a significant, but less strong predictor of survival, relative to VO2max for HCR, but not for LCR rats. Summed, Figure 3 data represent the first demonstration that longevity can segregate with an almost purely intrinsic aerobic endurance phenotype. (Figure 3 is reprinted by courtesy from the American Heart Association. Koch et al.: 2011. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res, 109, 1162–72.)
For humans, each incremental increase in exercise capacity measured in METs (metabolic equivalents, i.e., multiples of resting oxygen uptake) is equivalent to a 13 % increase in survival. That is, those with an exercise capacity of >7 Mets (fit category) have a 50–70% decrease in mortality risk compared with those achieving <5 METS (low-fit category) (Kokkinos et al., 2008). Using our contrasting LCR-HCR model system, we provided first demonstration that serial assessments of intrinsic aerobic capacity measured across lifespan can be significant predictors of age at death (Koch et al., 2011). For example, at mid-life (15 months), each 10 ml/kg0.75/min increase in VO2max associated with 8 and 10 month extensions in lifespan for rats in the HCR and LCR strains, respectively (Figure 3B). VO2max continued as a predictor of lifespan for HCR rats at both 20 and 25 months of age, and at 20 months of age for LCR rats. The more complicated phenotype of distance run to exhaustion served as a significant but less powerful predictor of lifespan for HCR rats, but not LCR. For example, for HCR rats 14 months of age, the capacity to run an additional 0.5 km at exhaustion was associated with living an additional 5 months (Figure 3C).
Concluding Remarks
The broad premise that oxidative energy metabolism is mechanistically connected with longevity is attractive because it has the power to shape the multiplicity of biological networks that influence essentially every phenotype across a lifespan (Kirkwood, 2005). Additionally, endurance capacity fulfills the fundamental criteria for service as a biomarker of aging as suggested by The American Federation for Aging Research. That is, endurance capacity predicts the rate of aging accurately, represents a basic underlying process, can be tested repeatedly without harm, and can be evaluated in animals (Johnson, 2006). Our results, coupled with the view that multicellular complexity was afforded by the steep thermodynamic gradient of an oxygen atmosphere (Falkowski et al., 2005), provide a basic logic for mechanistically connecting complex disease, aging, and longevity with aerobic energy metabolism.
Acknowledgments
This work was supported by Norwegian Council on Cardiovascular Disease, Norwegian Research Council Funding for Outstanding Young Investigators, K.G. Jebsen Foundation, Simon Fougner Hartmanns Family Foundation, and Foundation for Cardiovascular Research at St. Olav’s Hospital, Trondheim, Norway (to U.W.). Funding was also provided by University of Michigan Institute of Gerontology, Nathan Shock Center of Excellence (NIA) grant (P30 AG13283) and Geriatric Center, and Claude D. Pepper Older Americans Independence Center grant (P30 AG024824) Pilot Grant Programs (to L.G.K.) and National Institutes of Health grant (RO1 DK077200) (to S.L.B.). The LCR-HCR rat model system was funded by National Center for Research Resources (NCRR) grant (R24 RR017718) and is currently supported by Office of Research Infrastructure Programs/OD grant (ROD012098A) from the National Institutes of Health (to L.G.K. and S.L.B.). Authors thank Jane Heibel for editorial improvements to this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AKAR FG, AON MA, TOMASELLI GF, O'ROURKE B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–35. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDWIN JE, KREBS H. The evolution of metabolic cycles. Nature. 1981;291:381–2. doi: 10.1038/291381a0. [DOI] [PubMed] [Google Scholar]
- BOUCHARD C, DIONNE FT, SIMONEAU JA, BOULAY MR. Genetics of aerobic and anaerobic performances. Exerc Sport Sci Rev. 1992;20:27–58. [PubMed] [Google Scholar]
- BOWMAN TA, RAMAKRISHNAN SK, KAW M, LEE SJ, PATEL PR, GOLLA VK, BOUREY RE, HARAM PM, KOCH LG, BRITTON SL, WISLOFF U, LEE AD, NAJJAR SM. Caloric restriction reverses hepatic insulin resistance and steatosis in rats with low aerobic capacity. Endocrinology. 2010;151:5157–64. doi: 10.1210/en.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGHARDT PR, FLAGEL SB, BURGHARDT KJ, BRITTON SL, GERARD-KOCH L, WATSON SJ, AKIL H. Risk-assessment and coping strategies segregate with divergent intrinsic aerobic capacity in rats. Neuropsychopharmacology. 2011;36:390–401. doi: 10.1038/npp.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLBORG O, JACOBSSON L, AHGREN P, SIEGEL P, ANDERSSON L. Epistasis and the release of genetic variation during long-term selection. Nat Genet. 2006;38:418–20. doi: 10.1038/ng1761. [DOI] [PubMed] [Google Scholar]
- CHODZKO-ZAJKO WJ, PROCTOR DN, FIATARONE SINGH MA, MINSON CT, NIGG CR, SALEM GJ, SKINNER JS. Exercise and Physical Activity for Older Adults. Medicine & Science in Sports & Exercise. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- FALKOWSKI PG, KATZ ME, MILLIGAN AJ, FENNEL K, CRAMER BS, AUBRY MP, BERNER RA, NOVACEK MJ, ZAPOL WM. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science. 2005;309:2202–4. doi: 10.1126/science.1116047. [DOI] [PubMed] [Google Scholar]
- GONZALEZ A, ROTA M, NURZYNSKA D, MISAO Y, TILLMANNS J, OJAIMI C, PADIN-IRUEGAS ME, MULLER P, ESPOSITO G, BEARZI C, VITALE S, DAWN B, SANGANALMATH SK, BAKER M, HINTZE TH, BOLLI R, URBANEK K, HOSODA T, ANVERSA P, KAJSTURA J, LERI A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- HANSEN C, SPUHLER K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res. 1984;8:477–9. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- HARAM PM, KEMI OJ, LEE SJ, BENDHEIM MO, AL-SHARE QY, WALDUM HL, GILLIGAN LJ, KOCH LG, BRITTON SL, NAJJAR SM, WISLOFF U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81:723–32. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYDAL MA, WISLOFF U, KEMI OJ, BRITTON SL, KOCH LG, SMITH GL, ELLINGSEN O. Nitric oxide synthase type-1 modulates cardiomyocyte contractility and calcium handling: association with low intrinsic aerobic capacity. Eur J Cardiovasc Prev Rehabil. 2007;14:319–25. doi: 10.1097/hjr.0b013e3280128bef. [DOI] [PubMed] [Google Scholar]
- JOHNSON TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–6. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- KAVANAGH T, MERTENS DJ, HAMM LF, BEYENE J, KENNEDY J, COREY P, SHEPHARD RJ. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003;42:2139–43. doi: 10.1016/j.jacc.2003.07.028. [DOI] [PubMed] [Google Scholar]
- KIRKWOOD TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- KIVELA R, SILVENNOINEN M, LEHTI M, RINNANKOSKI-TUIKKA R, PURHONEN T, KETOLA T, PULLINEN K, VUENTO M, MUTANEN N, SARTOR MA, REUNANEN H, KOCH LG, BRITTON SL, KAINULAINEN H. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J. 2010;24:4565–74. doi: 10.1096/fj.10-157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH LG, BRITTON SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- KOCH LG, BRITTON SL. Aerobic metabolism underlies complexity and capacity. J Physiol. 2008;586:83–95. doi: 10.1113/jphysiol.2007.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH LG, KEMI OJ, QI N, LENG SX, BIJMA P, GILLIGAN LJ, WILKINSON JE, WISLOFF H, HOYDAL MA, ROLIM N, ABADIR PM, VAN GREVENHOF EM, SMITH GL, BURANT CF, ELLINGSEN O, BRITTON SL, WISLOFF U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109:1162–72. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKKINOS P, MYERS J, KOKKINOS JP, PITTARAS A, NARAYAN P, MANOLIS A, KARASIK P, GREENBERG M, PAPADEMETRIOU V, SINGH S. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–22. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- LESSARD SJ, RIVAS DA, CHEN ZP, VAN DENDEREN BJ, WATT MJ, KOCH LG, BRITTON SL, KEMP BE, HAWLEY JA. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology. 2009;150:4883–91. doi: 10.1210/en.2009-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESSARD SJ, RIVAS DA, STEPHENSON EJ, YASPELKIS BB, 3RD, KOCH LG, BRITTON SL, HAWLEY JA. Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R175–82. doi: 10.1152/ajpregu.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS GD, FARRELL L, WOOD MJ, MARTINOVIC M, ARANY Z, ROWE GC, SOUZA A, CHENG S, MCCABE EL, YANG E, SHI X, DEO R, ROTH FP, ASNANI A, RHEE EP, SYSTROM DM, SEMIGRAN MJ, VASAN RS, CARR SA, WANG TJ, SABATINE MS, CLISH CB, GERSZTEN RE. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUJAN HL, BRITTON SL, KOCH LG, DICARLO SE. Reduced susceptibility to ventricular tachyarrhythmias in rats selectively bred for high aerobic capacity. Am J Physiol Heart Circ Physiol. 2006;291:H2933–41. doi: 10.1152/ajpheart.00514.2006. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MUNCEY AR, SAULLES AR, KOCH LG, BRITTON SL, BAGHDOYAN HA, LYDIC R. Disrupted sleep and delayed recovery from chronic peripheral neuropathy are distinct phenotypes in a rat model of metabolic syndrome. Anesthesiology. 2010;113:1176–85. doi: 10.1097/ALN.0b013e3181f56248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS J, PRAKASH M, FROELICHER V, DO D, PARTINGTON S, ATWOOD JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- NOLAND RC, THYFAULT JP, HENES ST, WHITFIELD BR, WOODLIEF TL, EVANS JR, LUST JA, BRITTON SL, KOCH LG, DUDEK RW, DOHM GL, CORTRIGHT RN, LUST RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293:E31–41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- NOVAK CM, ESCANDE C, BURGHARDT PR, ZHANG M, BARBOSA MT, CHINI EN, BRITTON SL, KOCH LG, AKIL H, LEVINE JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58:355–67. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIGOGINE I. Time, Structure, and Fluctuations. Science. 1978;201:777–785. doi: 10.1126/science.201.4358.777. [DOI] [PubMed] [Google Scholar]
- SHARP PA, LANGER R. Research agenda. Promoting convergence in biomedical science. Science. 2011;333:527. doi: 10.1126/science.1205008. [DOI] [PubMed] [Google Scholar]
- THYFAULT JP, RECTOR RS, UPTERGROVE GM, BORENGASSER SJ, MORRIS EM, WEI Y, LAYE MJ, BURANT CF, QI NR, RIDENHOUR SE, KOCH LG, BRITTON SL, IBDAH JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–16. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIKGREN J, MERTIKAS GG, RAUSSI P, TIRKKONEN R, AYRAVAINEN L, PELTO-HUIKKO M, KOCH LG, BRITTON SL, KAINULAINEN H. Selective breeding for endurance running capacity affects cognitive but not motor learning in rats. Physiology & Behavior. 2012;106:95–100. doi: 10.1016/j.physbeh.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISLOFF U, NAJJAR SM, ELLINGSEN O, HARAM PM, SWOAP S, AL-SHARE Q, FERNSTROM M, REZAEI K, LEE SJ, KOCH LG, BRITTON SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–20. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]