Abstract
“Catch,” a state where some invertebrate muscles sustain high tension over long periods of time with little energy expenditure (low ATP hydrolysis rate) is similar to the “latch” state of vertebrate smooth muscles. Its induction and release involve Ca2+-dependent phosphatase and cAMP-dependent protein kinase, respectively. Molecular mechanisms for catch remain obscure. Here, we describe a quantitative microscopic in vitro assay reconstituting the catch state with proteins isolated from catch muscles. Thick filaments attached to glass coverslips and pretreated with ≈10−4 M free Ca2+ and soluble muscle proteins bound fluorescently labeled native thin filaments tightly in catch at ≈10−8 M free Ca2+ in the presence of MgATP. At ≈10−4 M free Ca2+, the thin filaments moved at ≈4 μm/s. Addition of cAMP and cAMP-dependent protein kinase at ≈10−8 M free Ca2+ caused their release. Rabbit skeletal muscle F-actin filaments completely reproduced the results obtained with native thin filaments. Binding forces >500 pN/μm between thick and F-actin filaments were measured by glass microneedles, and were sufficient to explain catch tension in vivo. Synthetic filaments of purified myosin and twitchin bound F-actin in catch, showing that other components of native thick filaments such as paramyosin and catchin are not essential. The binding between synthetic thick filaments and F-actin filaments depended on phosphorylation of twitchin but not of myosin. Cosedimentation experiments showed that twitchin did not bind directly to F-actin in catch. These results show that catch is a direct actomyosin interaction regulated by twitchin phosphorylation.
Bivalve molluscs need to keep their shells firmly closed or cling tightly to rocks, etc., and so have evolved their adductor and byssus retractor muscles as catch muscles that can maintain high tension with a very low rate of ATP hydrolysis. A large body of experimental evidence from intact and permeabilized invertebrate catch muscles (1, 2) suggests the following sequence of events controlling catch: the muscle is stimulated by acetylcholine (ACh), which induces Ca2+ release into the sarcoplasm; the Ca2+ directly binds to and activates myosin—because there is no thin-filament Ca2+ regulation (3), the muscle develops and maintains its active tension for the duration of the excitation; a decrease in concentration of ACh reduces intracellular free Ca2+ concentration to the resting level (≈10−7 M), leaving the muscle in the catch state (4); and the addition of 5-hydroxytryptamine (serotonin) increases intracellular cAMP concentration, and this increase in cAMP concentration induces rapid relaxation of the muscle (5, 6). Thus, it is believed that there are at least two control mechanisms necessary in catch muscles to produce the active, catch, and relaxed states.
Although studies with intact or permeabilized muscle fibers and cells have revealed such sequences of events of the catch contraction as described above, it is difficult to clarify the underlying mechanisms of high tension in catch because muscle cells contain a variety of proteins whose roles are not completely understood. Thus, it is essential to reconstitute in vitro the phenomenon of catch by using components isolated from muscle cells to understand better the molecular mechanisms of catch and to determine the minimal number of components required. In this article, we describe reconstruction of the catch state in vitro with thick and thin filaments isolated from catch muscles of the mussel Mytilus galloprovincialis. The catch state is caused by tight binding between thick and thin filaments, which we observed in the light microscope. With this powerful new assay system, we have examined thick and thin-filament components essential for the catch state. These components are actin, myosin, and twitchin—an ≈600-kDa protein supposed to be phosphorylated when the muscle is relaxed from the catch state (7). Other filament components such as tropomyosin, paramyosin, and catchin (8) are not essential. These results and the application of this assay system will help elucidate molecular mechanisms of catch contraction and other prolonged tension states in muscles such as latch (9).
Materials and Methods
Proteins.
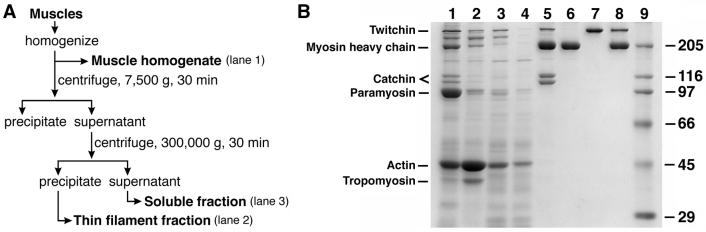
Muscle homogenate, thin-filament fraction, and soluble protein fraction were prepared from byssus retractor muscles of M. galloprovincialis (as shown in Fig. 1A). Protein components of the fractions were analyzed by SDS/PAGE (Fig. 1B). Muscle homogenate (Fig. 1B, lane 1) was obtained by homogenizing the muscles in a standard buffer [80 mM NaCl/5 mM ATP/8 mM MgCl2/2 mM EGTA/10 mM DTT/10 mM Pipes–NaOH (pH 7.0)], and centrifuging at 300 × g for 5 min to remove muscle debris. Na+-based buffer was used to avoid K+-induced contraction of muscle cells during the homogenization. The homogenate was centrifuged at 7,500 × g for 30 min, and the supernatant was centrifuged further at 300,000 × g for 30 min. The precipitate was suspended in standard buffer and used as the thin-filament fraction (Fig. 1B, lane 2). The supernatant was used as the soluble fraction (Fig. 1B, lane 3). To remove most of the twitchin and myosin, the soluble fraction first was dialyzed against 80 mM NaCl/3 mM MgCl2/2 mM EGTA/2 mM DTT/20 mM Pipes–NaOH (pH 7.0) and then applied to an SP Sepharose column (gel vol = 1 ml; Amersham Pharmacia) equilibrated to the same solution; the flow-through fraction then was collected (Fig. 1B, lane 4).
Figure 1.
(A) A flow-chart showing the preparation of the muscle homogenate, the thin-filament fraction, and the soluble muscle fraction. (B) Protein compositions of preparations used in the present study shown by SDS/PAGE (7.5% polyacrylamide): muscle homogenate (lane 1); the thin-filament fraction (lane 2); and the soluble fraction (lane 3). Most twitchin was removed from the soluble fraction (lane 4) to examine its role in the catch state. To identify thick-filament components essential for the catch state, we prepared paramyosin-free thick filaments (lane 5) and purified myosin filaments (lane 6). We also purified twitchin (lane 7) and added it to the purified myosin filaments (lane 8). Lane 9 shows marker proteins with their molecular masses in kDa shown on the right.
Homogenized muscles were extracted with 0.4 M KCl/4 mM MgCl2/4 mM ATP/4 mM EGTA/2.5 mM DTT/10 mM potassium phosphate buffer (pH 7.0). The 33–50% saturated (NH4)2SO4 precipitate of the extract was dissolved in 0.4 M KCl/2 mM MgCl2/1 mM EGTA/2 mM DTT/10 mM Pipes–KOH (pH 7.0). After centrifugation at 300,000 × g for 30 min, the supernatant was dialyzed against the buffer above containing 0.1 M KCl. The slow decrease in the ionic strength promoted formation of long, thick filaments (10), about 20 μm long and readily observed under a dark-field light microscope. SDS/PAGE showed that they contained myosin, twitchin, and catchin but little paramyosin (Fig. 1B, lane 5).
Myosin (Fig. 1B, lane 6) was purified with a Superose 6 HR 10/30 gel filtration column (Amersham Pharmacia) from the 40–60% saturated (NH4)2SO4 precipitate of the muscle extract dissolved in 0.6 M KCl/2 mM EGTA/1 mM MgCl2/2 mM DTT/10 mM Hepes–KOH (pH 7.5), and filaments were again made by dialysis. To purify twitchin, the 30–40% saturated (NH4)2SO4 precipitate of the muscle extract was dissolved in 0.15 M potassium phosphate (pH 7.5)/1 mM MgCl2/1 mM EGTA/2 mM DTT and loaded onto a Mono Q HR 5/5 column (Amersham Pharmacia). Twitchin (Fig. 1B, lane 7) was eluted stepwise by 0.15 M KCl in the same solution.
Purified twitchin and myosin filaments were putatively dephosphorylated or phosphorylated as follows: soluble fraction containing little myosin or twitchin (see Fig. 1B, lane 4) was mixed with purified twitchin or purified myosin filaments in the presence of ≈10−4 M free Ca2+, and the mixture was left at room temperature for 10 min. The free Ca2+ concentration was lowered to ≈10−7 M by mixing with 4 vol of standard buffer to terminate the Ca2+-dependent dephosphorylation. (Twitchin and myosin filaments treated in this way are designated as “dephosphorylated twitchin” and “dephosphorylated myosin filaments”, respectively.) Then 25 μM cAMP and 5 μg/ml cAMP-dependent protein kinase were added to dephosphorylated twitchin or dephosphorylated myosin filaments and the mixture was incubated for 10 min at room temperature. The phosphorylation reaction was terminated by the addition of 5 μg/ml peptide inhibitor of cAMP-dependent protein kinase (11). (Twitchin and myosin filaments treated in this way are designated as “phosphorylated twitchin” and “phosphorylated myosin filaments”, respectively.)
In Vitro Assays.
Binding of thick and thin filaments in catch was observed in vitro in a flow cell (vol = 15 μl) made of coverslips such as are used often for in vitro motility assays (12). Thick filaments and tetramethylrhodamine (TMR) phalloidin-labeled thin or F-actin filaments were observed under dark-field and Hg-arc fluorescence illumination (U-MWIG filter set; Olympus, Tokyo, Japan), respectively, with an IX-70 light microscope (Olympus). Essentially, standard buffer used for preparation (see the above section) again was used for an assay to simplify the experimental procedures, and Ca2+ concentrations were controlled by adding CaCl2. Concentrations of free Ca2+ were calculated with a computer program, winmaxc (available at http://www.stanford.edu/∼cpatton/maxc.html) (13). The free Ca2+ concentration of the standard buffer was estimated at ≈10−8 M, assuming that total contaminating Ca2+ was 10–100 μM. During fluorescence microscopic observations, 1–5 mg/ml glucose/50 μg/ml glucose oxidase/10 μg/ml catalase and 0.5% (vol/vol) 2-mercaptoethanol were added as oxygen scavengers to the specimen (14), reducing photobleaching and prolonging assay activity.
Binding forces between thick and F-actin filaments were measured with glass microneedles to which F-actin filaments were attached by means of N-ethylmaleimide-treated rabbit myosin (15). The stiffness of the needles ranged from 10 to 20 pN/μm. To observe thick filaments in the fluorescence microscope, they were preincubated with TMR-isothiocyanate-labeled myosin rods prepared by proteolysis from rabbit skeletal muscle myosin (16). Muscle homogenate containing native thick filaments in standard buffer on ice was well mixed rapidly with 1/20 vol of 0.4 M KCl solution containing fluorescently labeled myosin rods and left at room temperature for 1 h. These fluorescent rods spontaneously bound to thick filaments. To minimize unexpected effects of rods on the native structure of thick filaments, the quantity of fluorescent rod was minimized so that the thick filaments could be observed under a fluorescence microscope. Therefore, thick and F-actin filaments were simultaneously observed under the same fluorescence illumination but were distinguishable because the fluorescence intensity of the thick filaments was clearly less than that of the F-actin filaments.
Cosedimentation Experiments.
To study the binding between twitchin and F-actin filaments in the catch and relaxed states, cosedimentation experiments were performed. Dephosphorylated or phosphorylated twitchin (4 μg/ml) was mixed with 0.1 mg/ml phalloidin-stabilized rabbit F-actin and centrifuged at 30,000 rpm in a TLA-100.1 rotor (Beckman-Spinco) for 30 min (sufficient to precipitate molecules > 250S) to precipitate F-actin. The experiments were also performed in the presence of 50 μg/ml purified myosin filaments. The protein compositions of the supernatant and the precipitate were analyzed by SDS/PAGE (7.5% acrylamide) and the gel was silver-stained.
Results and Discussion
In Vitro Reconstitution of Catch with Native Filaments.
Muscle homogenate (Fig. 1B, lane 1) was rich in the main components of thick filaments—myosin, paramyosin, twitchin (7), and catchin (8). The thin-filament fraction (Fig. 1B, lane 2) contained mainly actin and tropomyosin. TMR-phalloidin was added to this fraction to make native thin filaments fluorescent. The soluble fraction (Fig. 1B, lane 3) contained a variety of proteins whose roles have not yet been characterized; however, studies using catch muscles imply that soluble fraction contains enzymes, such as Ca2+-dependent phosphatase (17), calmodulin (17), and cAMP-dependent protein kinase (5, 6), required for the regulation of catch.
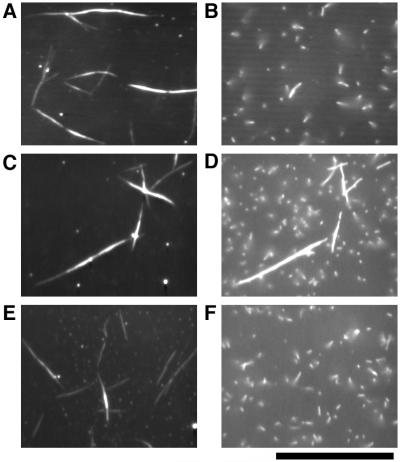
Using these three fractions, we devised an assay based on direct visualization of the binding of thin filaments to thick filaments in vitro under conditions corresponding to the catch state. All of the following experiments were performed in the presence of 5 mM ATP and ≈10−8 M free Ca2+ in standard buffer except where otherwise noted. We first added 25 μM cAMP to the muscle homogenate to induce the relaxed state in the thick filaments; 15 μl of the homogenate was placed on the lower coverslip of the flow cell. Then, the second coverslip was placed on top to complete the flow cell. Thick filaments in this homogenate spontaneously attached to the clean glass surface of the flow cell. Because the thick filaments could not be seen in the fluorescence microscope, their positions were confirmed by dark-field light microscopy as long spindle-shaped images with tapered ends of average length ≈20 μm (Fig. 2A; ref. 18). The flow cell was then perfused with 40 μl of standard buffer containing TMR-phalloidin-labeled thin filaments, and was washed with 80 μl of the buffer to remove any unbound thin filaments. Observation by fluorescence microscopy showed few TMR-labeled thin filaments bound to thick filaments (Fig. 2B), although some short thin filaments bound nonspecifically to the glass surface. The thick filaments attached onto the glass surface then were perfused with 40 μl of soluble fraction (≈0.4 mg/ml proteins) containing 5 μg/ml protein kinase inhibitor (11) in the presence of ≈10−4 M free Ca2+. This treatment (catch treatment) should activate phosphatases in the soluble fraction and initiate the catch state. After a 10-min incubation, this soluble fraction and the protein kinase inhibitor were flushed from the flow cell, and free Ca2+ concentration was reduced to ≈10−8 M with 80 μl of standard buffer. TMR-labeled thin filaments with 40 μl of buffer were added, and unbound filaments were again washed out by perfusion with 80 μl of buffer. In this case, many thin filaments bound so tightly to thick filaments that about 60% of the thick filaments retained bound thin filaments even after the mechanical agitation of buffer flow through the cell (but 40% did not; Fig. 2C). The fact that only 60% did might be because of the heterogeneity of the thick filaments. Although individual cells isolated from Mytilus byssus retractor muscles went into the catch state (4), some cells relaxed after the active contraction much more rapidly than others (N. Ishii, personal communication). Thus, there is the possibility of heterogeneity between cells within catch muscle.
Figure 2.
Binding of the TMR-labeled thin filaments to the thick filaments observed with dark-field and fluorescence microscopy. (A) Thick filaments observed in dark-field illumination. (B–D) Thin filaments observed in fluorescence illumination. When the thin filaments were applied onto thick filaments pretreated with cAMP and then washed, they did not remain bound to the thick filaments (B). After the thick filaments were treated with soluble fraction in the presence of ≈10−4 M free Ca2+, thin filaments bound to the thick filaments at ≈10−8 M free Ca2+ (C). Addition of the soluble fraction in the presence of cAMP caused detachment of these thin filaments from the thick filaments (D). All photographs were taken with the same field of view. (Bar = 20 μm.)
Preincubation of thick filaments without either the soluble fraction or Ca2+ did not give rise to such tight binding between thick and thin filaments. Together with studies on muscle (17), this observation suggests that Ca2+-dependent phosphatases in the soluble fraction were activated by ≈10−4 M free Ca2+ and dephosphorylated their target proteins to induce tight binding of thick and thin filaments (“catch complex”) corresponding to the catch state of the muscle. Increasing the free Ca2+ concentration to ≈10−4 M caused the thin filaments, previously bound tightly, to start active sliding along the thick filaments with a velocity of 3.9 ± 0.9 μm/s (n = 30). Thin filaments moved on thick filaments without catch treatment at 3.7 ± 0.8 μm/s (n = 30) under the same conditions. These results indicate (i) that maintenance of the catch complex requires low free Ca2+ concentration, and (ii) that myosin motile activity is not affected directly by the level of target protein phosphorylation. These results also suggest that intracellular Ca2+ simultaneously triggers two independent sequences of events in vivo: the activation of myosin regulated by direct binding of Ca2+ (3), and the induction of the catch state.
To initiate relaxation from catch, we perfused the catch complex of thick and thin filaments described above with soluble fraction containing 25 μM cAMP and ≈10−8 M free Ca2+; we found that almost all of the thin filaments detached from the thick filaments (Fig. 2D). It has been demonstrated that, in muscle fibers, twitchin is phosphorylated on relaxation from the catch state (7). Twitchin, one of the main components of the thick filaments, probably is phosphorylated on addition of cAMP and soluble fraction. It is probable also that soluble fraction contained cAMP-dependent protein kinase. This likelihood was confirmed by the inhibition of detachment of thin filaments with the addition of 5 μg/ml protein kinase inhibitor (11), and by the release of thin filaments from the catch complex with the addition of 5 μg/ml cAMP-dependent protein kinase from bovine heart together with 25 μM cAMP instead of soluble fraction.
Identification of Thin-Filament Components Essential for Catch.
The thin-filament fraction contains several proteins in addition to actin and tropomyosin, the main components of thin filaments (Fig. 1B, lane 2). To identify the minimum number of thin-filament components required for the catch state, purified rabbit skeletal muscle F-actin labeled with TMR-phalloidin was substituted for TMR-labeled native molluscan thin filaments. These purified rabbit F-actin filaments completely reproduced the results obtained with the native thin filaments. This result shows that thin filament-associated proteins such as tropomyosin (see Fig. 1B, lane 2) are not essential, and the catch state can be formed irrespective of the source of actin.
Binding Forces of the Catch Complex.
The binding force between the thick and F-actin filaments in the catch state was measured by using glass microneedles (Fig. 3; ref. 15). A TMR-labeled rabbit skeletal muscle F-actin filament was captured with a microneedle and brought into contact with a thick filament on the glass surface after catch treatment (Fig. 3 A and B). After the F-actin filament bound to the thick filament in the catch complex, the microscope stage was moved at a constant velocity of ≈1 μm/s to apply force to the complex (Fig. 3 C–E). Although the F-actin filament was firmly attached to the microneedle by N-ethylmaleimide-treated myosin molecules (15), this attachment was always broken before the dissociation of the catch complex (Fig. 3 F and G). The force calculated from the deflection of the microneedle just as the F-actin filament detached from the microneedle ranged from 130 to 550 pN/μm of overlap between the F-actin and the thick filament (n = 9). Because the measured force was the lower limit of the binding force, catch complexes can sustain forces greater than 500 pN/μm of F-actin filament. When living muscle fibers develop maximal active tension, the estimated contractile force per thick filament is about 20 nN (19). Because these smooth muscle cells do not have such distinct lattice structures of myofilaments as seen in striated muscles, it is difficult to estimate forces between myofilaments during contraction in living muscles. According to electron microscope studies, however, the average length of the halves of thick filaments is about 10 μm (18), and about 10 thin filaments can interact with 1 thick filament in muscles (20). From these observations we conclude that during active contraction the interaction between 1 thick and 1 thin filament sustains about 200 pN/μm of overlap (or 20 nN per 10 thin filaments per 10 μm). The value obtained in this in vitro study (>500 pN/μm) was sufficient to explain the tension during the catch contraction in vivo (≈200 pN/μm), even if 40% of the thick filaments do not sustain force under the catch state. In the present experiments, we did not take into account the bipolar nature of native thick filaments. Because oppositely oriented myosin heads are not likely to generate so much force (21, 22), properly oriented filaments could sustain more force than that measured in the present study. In addition to this direct measurement of the binding force, our in vitro assay completely followed and reproduced the sequence of events occurring in vivo, and, therefore, we conclude that this reversible tight binding between the thick and thin filaments after catch treatment in vitro corresponds closely to the catch state in living molluscan muscles.
Figure 3.
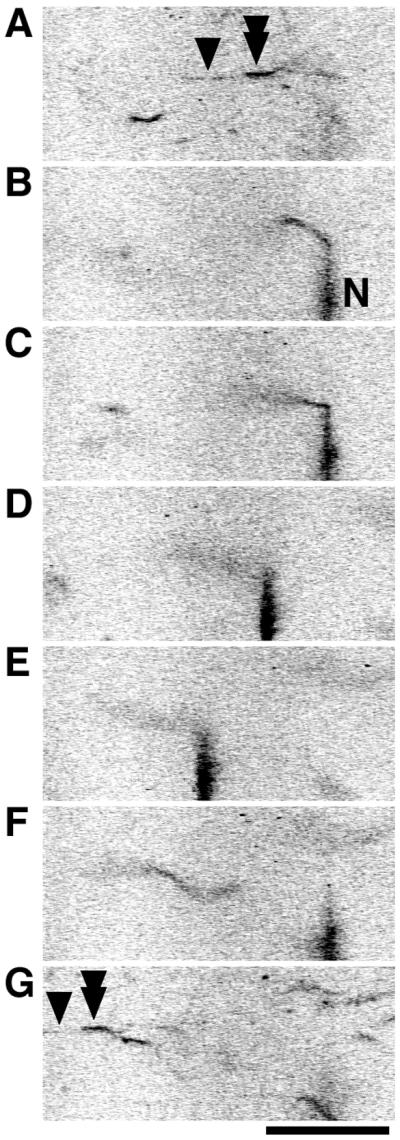
Measurement of binding force of the catch complex. One end of an F-actin filament (tandem arrowhead) was brought into contact with a thick filament (arrowhead) to form the catch complex (A), while the other end was captured with a needle coated with N-ethylmaleimide-treated myosin (indicated by “N”; stiffness of this needle = 18 pN/μm) (B). Then, the microscope stage was moved at a constant velocity of ≈1 μm/s to apply force on the catch complex by the deflection of the needle (C and D). When the deflection of the needle reached a critical extent (21 μm; E), the F-actin filament abruptly detached from the needle. The needle then moved back to the baseline under its own elasticity (F). Even at the break of the bond between the F-actin and the needle, the other end of the F-actin filament (tandem arrowhead) still remained attached to the thick filament (arrowhead) on the glass surface (G). Therefore, the maximal deflection of the needle indicated the lower limit of the binding force sustained by the overlap of the F-actin and thick filaments (1.7 μm). The lower limit of the binding force per overlap was calculated to be 220 pN/μm. (Bar = 20 μm.)
Identification of Thick Filament Components Essential for Catch.
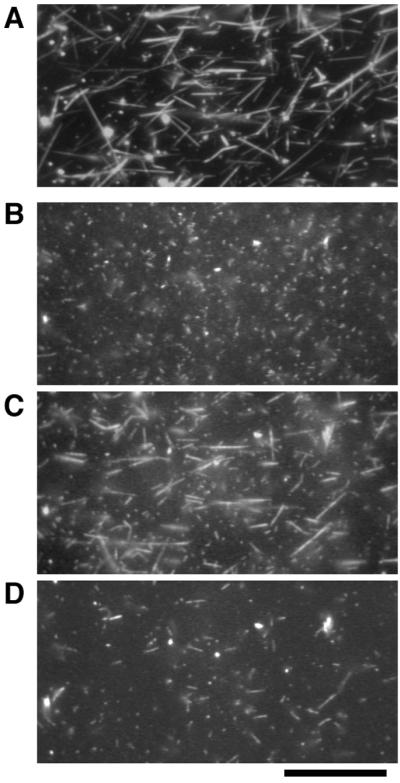
To identify the minimum components of thick filaments essential for the catch state, synthetic filaments were prepared with the purified thick filament proteins myosin, paramyosin, twitchin (7), and catchin (8). First, we synthesized paramyosin-free synthetic thick filaments (Fig. 1B, lane 5) that contained twitchin, myosin, and catchin; they bound F-actin filaments tightly after catch treatment (data not shown). This result shows that paramyosin is not essential for the catch state. Second, three solutions were prepared: soluble fraction containing little twitchin (Fig. 1B, lane 4), purified myosin solution (Fig. 1B, lane 6), and purified twitchin solution (Fig. 1B, lane 7). The synthetic filaments of purified myosin and twitchin were much thinner than the native thick filaments. These synthetic filaments of myosin and twitchin moved F-actin filaments at 2.9 ± 0.3 μm/s (n = 30) at ≈10−4 M free Ca2+, which is comparable to native thick filaments. However, we performed the catch treatment in test tubes to minimize the effects of surfaces on the binding properties of these filaments. The filaments first were left at room temperature with the soluble fraction at ≈10−4 M free Ca2+ in a test tube, and then 2–4 vol of standard buffer was added to reduce the free Ca2+ concentration to ≈10−7 M. TMR-phalloidin-labeled F-actin was then added to the test tube and a sample was observed in the fluorescence microscope. Filaments formed by pure myosin did not bind F-actin filaments after the catch treatment (Fig. 4 A and B). In contrast, when myosin filaments were mixed first with purified twitchin (Fig. 1B, lane 8), F-actin filaments bound to the myosin filaments after the same catch treatment (Fig. 4 C and D). When cAMP and cAMP-dependent protein kinase were added after the catch treatment, F-actin did not bind (Fig. 4 E and F). These observations showed that myosin and twitchin are essential components of the thick filament for induction of the catch state, and that other components such as paramyosin and catchin are not required.
Figure 4.
Binding of TMR-labeled F-actin filaments to synthetic myosin filaments without (A and B) and with (C–F) twitchin. Dark-field observation shows myosin filaments (A, C, and E) and fluorescence observation shows F-actin filaments (B, D, and F) in the same fields. Although F-actin filaments did not bind to myosin filaments without twitchin after the catch treatment (B), they bound to myosin filaments with twitchin after the same treatment (D). F-actin filaments did not bind to myosin filaments with twitchin when they were treated with cAMP-dependent protein kinase after the catch treatment (F). (Bar = 20 μm.)
Role of Myosin in Catch.
When dephosphorylated twitchin was mixed with phosphorylated myosin filaments, the resultant filaments bound fluorescently labeled F-actin filaments in a test tube. In contrast, when phosphorylated twitchin was mixed with dephosphorylated myosin filaments, the resultant filaments did not bind. These results indicate that catch is regulated by phosphorylation of twitchin as shown by Siegman et al. (7), although myosin can be phosphorylated (23, 24).
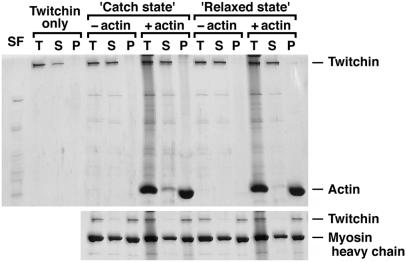
To see whether twitchin directly binds F-actin filaments without myosin in catch, cosedimentation experiments were performed. Neither dephosphorylated twitchin (Fig. 5, “Catch state”) nor phosphorylated twitchin (Fig. 5, “Relaxed state”) precipitated in the absence of F-actin (−actin). In the presence of an excess amount of F-actin (+actin), only a small amount of twitchin precipitated, but there was no significant difference in the amount of precipitated twitchin between dephosphorylated and phosphorylated. In the presence of myosin (Fig. 5 Lower), twitchin precipitated irrespectively of its phosphorylation states. These results indicate that twitchin binds to myosin rather than F-actin filaments in all cases, and it is likely that the catch complex results from the direct binding of myosin to F-actin filaments. It should be noted that a significant amount of myosin remained in the supernatant in these experiments (Fig. 5 Lower). In the presence of MgATP, Mytilus myosin is somewhat soluble even at low ionic strength (24). Indeed, in the absence of ATP, all myosin precipitated under these sedimentation conditions (sufficient to precipitate molecules > 250S; data not shown). Because most twitchin precipitated in all conditions tested (Fig. 5 Lower), twitchin does not bind to soluble myosin but binds to filamentous myosin.
Figure 5.
Cosedimentation of twitchin with and without F-actin either in the absence (Upper) or presence (Lower) of myosin. SF, the soluble fraction present in the experiments with the catch state and the relaxed state; T, total proteins before centrifugation; S and P, the supernatant and precipitate, respectively, after centrifugation sufficient to precipitate 250S molecules. When purified twitchin (4 μg/ml) was centrifuged (Twitchin only), it remained in the supernatant. In the absence of phalloidin-stabilized F-actin (−actin) and of myosin, most of the twitchin remained in the supernatant in both the catch and relaxed states. In the presence of 0.1 mg/ml F-actin (+actin), a little twitchin precipitated in both states with no significant difference in the amounts. In contrast, in the presence of 50 μg/ml myosin (Lower), most of the twitchin precipitated in all conditions. Note that significant amounts of myosin remained in the supernatant in all conditions.
We also examined the effect of vanadate, a known inhibitor of actomyosin interaction (25), and found that as soon as 1 mM vanadate in standard buffer was introduced into the flow cell containing the catch complex of native thick and thin filaments, thin filaments detached from the catch complex. Another inhibitor of actomyosin interaction, 2 mM pyrophosphate (26), also decreased the number of bound thin filaments in the same experiment (data not shown). These results strongly suggest that the basic mechanism for catch is actomyosin interaction regulated by twitchin phosphorylation.
Two principal hypotheses for the mechanism of catch have been proposed (1, 2): the paramyosin hypothesis, in which paramyosin forms cross-bridges between the thick filaments creating the catch state; the actomyosin or “linkage” hypothesis, which assumes that catch tension is caused by interactions between the thick and thin filaments through actomyosin cross-bridges. The paramyosin hypothesis may be excluded because we successfully reproduced the catch state in vitro without paramyosin. Also, this result excludes the possibility that myosin is regulated by the phosphorylation level of the underlying paramyosin in the thick filaments (27). In summary, the state of catch muscles is controlled at three levels. (i) Ca2+ acts directly on myosin to initiate and terminate the active contraction. (ii) Ca2+ independently triggers the dephosphorylation of twitchin, and at low free Ca2+ concentrations, this dephosphorylation results in the catch state by means of the actomyosin interaction. (iii) cAMP triggers the phosphorylation of twitchin which results in the relaxation of catch.
Relation to Other Studies.
In this study, the structural components essential for the catch state—actin, myosin, and twitchin—have been clarified. Actin did not need to be from catch muscles, because purified rabbit skeletal muscle F-actin filaments also formed the catch complex. This fact means that the elements specific for catch reside in either myosin or twitchin, or both. Indeed, there are some differences between catch-type and non-catch-type myosin heavy chains from the same species of scallop (28, 29). However, they are made by alternative transcription from the same gene. Their amino acid sequences are more than 90% identical, with the differences located in some limited parts of the sequences. The differences affect the ATPase rate (29). Although myosin essential light chains are identical (30), there are some differences also in the regulatory light chains (30, 31). It is not clear whether the unique parts of catch-type myosin are essential for the catch state. The other component, twitchin, sometimes called minititin, also resides in both types of muscle in scallop (32, 33), but it is not clear whether there are differences in amino acid sequences of twitchin between the two types of muscle. The present assay will be able to clarify which differences are important for catch contraction by the use of hybrid synthetic thick filaments consisting of catch-type myosin and non-catch-type twitchin, and vice versa.
Twitchin is found in many other invertebrates, such as Caenorhabditis elegans (34) and Aplysia (35, 36). Indeed, in Aplysia accessory radula closer muscle, twitchin is phosphorylated by cAMP-dependent protein kinase, and this phosphorylation accelerates the relaxation of the muscle (35). From this point of view, twitchin in both Mytilus and Aplysia seems to play a similar role. The only differences between them are that relaxation is somewhat slower in Mytilus than in Aplysia, and that the extracellular messenger that increases intracellular cAMP concentration in Mytilus is serotonin but in Aplysia it is the small cardioactive peptides and the myomodulins (35). Thus, it is possible that twitchins other than that of bivalve catch muscles strengthen the interaction between actin and myosin filaments at rest depending on their level of phosphorylation. The present assay will be useful for clarifying whether they have an effect similar to that of catch muscle twitchin in other animal species.
Twitchin has a protein kinase domain similar to vertebrate myosin light chain kinase and a calmodulin (CaM)-binding domain near its C terminus (37, 38). A possible target of twitchin kinase is the myosin light chain, but Siegman et al. (7) did not find any incorporation of phosphate into the light chains in the transitions to and from catch. Thus, twitchin kinase itself might not be involved in catch contraction, but it may have another function, indicating that twitchin has at least two different functions. The CaM-binding site might be important for the induction of catch because the present study revealed that Ca2+ is essential for the induction of catch, and the soluble fraction used to induce catch may well have contained CaM.
Future studies should clarify the interaction between twitchin and myosin and the nucleotide state of myosin ATPase in the catch state. During the latch state, in which tension is maintained for a long period after the active contraction in vertebrate smooth muscles, MgADP seems to remain bound to the myosin (39). This reaction intermediate of myosin ATPase could be relevant to the catch state in molluscan smooth muscles (40).
The present in vitro assay permits study of the catch state at the molecular level, because small quantities of rare, mutant, and recombinant proteins can be used; further investigations with this assay combined with single-molecule enzymology (41) should elucidate the molecular mechanisms of catch contraction and other related phenomena in muscle.
Acknowledgments
We thank Drs. Michael Anson, Bernhard Brenner, and David R. Trentham for discussion and comments on the manuscript. This work was partly supported by a Grant-in-Aid from the Ministry of Education, Science and Culture of Japan (to K.O.) and a grant from Japan Science and Technology Corporation (Cooperative System for Supporting Priority Research).
Abbreviations
- ACh
acetylcholine
- TMR
tetramethylrhodamine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rüegg J C. Physiol Rev. 1971;51:201–248. doi: 10.1152/physrev.1971.51.1.201. [DOI] [PubMed] [Google Scholar]
- 2.Watabe S, Hartshorne D J. Comp Biochem Physiol B Biochem Mol Biol. 1990;96:639–646. doi: 10.1016/0305-0491(90)90207-a. [DOI] [PubMed] [Google Scholar]
- 3.Kendrick-Jones J, Lehman W, Szent-Györgyi A G. J Mol Biol. 1970;54:313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- 4.Ishii N, Simpson A W M, Ashley C C. Science. 1989;243:1367–1368. doi: 10.1126/science.2922614. [DOI] [PubMed] [Google Scholar]
- 5.Cornelius F. J Gen Physiol. 1982;79:821–834. doi: 10.1085/jgp.79.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfitzer G, Rüegg J C. J Comp Physiol. 1982;147:137–142. [Google Scholar]
- 7.Siegman M J, Funabara D, Kinoshita S, Watabe S, Hartshorne D J, Butler T M. Proc Natl Acad Sci USA. 1998;95:5383–5388. doi: 10.1073/pnas.95.9.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada A, Yoshio M, Oiwa K, Nyitray L. J Mol Biol. 2000;295:169–178. doi: 10.1006/jmbi.1999.3349. [DOI] [PubMed] [Google Scholar]
- 9.Dillon P F, Aksoy M O, Driska S P, Murphy R A. Science. 1981;211:495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- 10.Katsura I, Noda H. J Biochem (Tokyo) 1971;69:219–229. doi: 10.1093/oxfordjournals.jbchem.a129449. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H-C, Kemp B E, Pearson R B, Smith A J, Misconi L, Van Patten S M, Walsh D A. J Biol Chem. 1986;261:989–992. [PubMed] [Google Scholar]
- 12.Kron S J, Spudich J A. Proc Natl Acad Sci USA. 1986;83:6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bers D M, Patton C W, Nuccitelli R. Methods Cell Biol. 1994;40:3–29. doi: 10.1016/s0091-679x(08)61108-5. [DOI] [PubMed] [Google Scholar]
- 14.Harada Y, Sakurada K, Aoki T, Thomas D D, Yanagida T. J Mol Biol. 1990;216:49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- 15.Kishino A, Yanagida T. Nature (London) 1988;334:74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- 16.Margossian S S, Lowey S. Methods Enzymol. 1982;85:55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- 17.Castellani L, Cohen C. FEBS Lett. 1992;309:321–326. doi: 10.1016/0014-5793(92)80798-l. [DOI] [PubMed] [Google Scholar]
- 18.Yamada A, Ishii N, Shimmen T, Takahashi K. J Muscle Res Cell Motil. 1989;10:124–134. doi: 10.1007/BF01739968. [DOI] [PubMed] [Google Scholar]
- 19.Neumann T, Fauver M, Pollack G H. Biophys J. 1998;75:938–947. doi: 10.1016/S0006-3495(98)77582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett P M, Elliott A. J Muscle Res Cell Motil. 1989;10:297–311. doi: 10.1007/BF01758426. [DOI] [PubMed] [Google Scholar]
- 21.Yamada A, Takahashi K. J Biochem (Tokyo) 1992;111:676–680. doi: 10.1093/oxfordjournals.jbchem.a123817. [DOI] [PubMed] [Google Scholar]
- 22.Ishijima A, Kojima H, Higuchi H, Harada Y, Funatsu T, Yanagida T. Biophys J. 1996;70:383–400. doi: 10.1016/S0006-3495(96)79582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellani L, Cohen C. Science. 1987;235:334–337. doi: 10.1126/science.3026049. [DOI] [PubMed] [Google Scholar]
- 24.Castellani L, Cohen C. Proc Natl Acad Sci USA. 1987;84:4058–4062. doi: 10.1073/pnas.84.12.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodno C C, Taylor E W. Proc Natl Acad Sci USA. 1982;79:21–25. doi: 10.1073/pnas.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene L E, Eisenberg E. J Biol Chem. 1980;255:543–548. [PubMed] [Google Scholar]
- 27.Cohen C. Proc Natl Acad Sci USA. 1982;79:3176–3178. doi: 10.1073/pnas.79.10.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyitray L, Jancsó Á, Ochiai Y, Gráf L, Szent-Györgyi A G. Proc Natl Acad Sci USA. 1994;91:12686–12690. doi: 10.1073/pnas.91.26.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perreault-Micale C L, Kalabokis V N, Nyitray L, Szent-Györgyi A G. J Muscle Res Cell Motil. 1996;17:543–553. doi: 10.1007/BF00124354. [DOI] [PubMed] [Google Scholar]
- 30.Perreault-Micale C L, Jancsó Á, Szent-Györgyi A G. J Muscle Res Cell Motil. 1996;17:533–542. doi: 10.1007/BF00124353. [DOI] [PubMed] [Google Scholar]
- 31.Miyanishi T, Maita T, Morita F, Kondo S, Matsuda G. J Biochem (Tokyo) 1985;97:541–551. doi: 10.1093/oxfordjournals.jbchem.a135089. [DOI] [PubMed] [Google Scholar]
- 32.Vibert P, Edelstein S M, Castellani L, Elliott B W., Jr J Muscle Res Cell Motil. 1993;14:598–607. doi: 10.1007/BF00141557. [DOI] [PubMed] [Google Scholar]
- 33.Vibert P, York M L, Castellani L, Edelstein S, Elliott B, Nyitray L. Adv Biophys. 1996;33:199–209. [PubMed] [Google Scholar]
- 34.Benian G M, Kiff J E, Neckelmann N, Moerman D G, Waterston R H. Nature (London) 1989;342:45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
- 35.Probst W C, Cropper E C, Heierhorst J, Hooper S L, Jaffe H, Vilim F, Beushausen S, Kupfermann I, Weiss K R. Proc Natl Acad Sci USA. 1994;91:8487–8491. doi: 10.1073/pnas.91.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heierhorst J, Probst W C, Vilim F S, Buku A, Weiss K R. J Biol Chem. 1994;269:21086–21093. [PubMed] [Google Scholar]
- 37.Olson N J, Pearson R B, Needleman D S, Hurwitz M Y, Kemp B E, Means A R. Proc Natl Acad Sci USA. 1990;87:2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallagher P J, Herring B P, Stull J T. J Muscle Res Cell Motil. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- 39.Khromov A, Somlyo A V, Somlyo A P. Biophys J. 1998;75:1926–1934. doi: 10.1016/S0006-3495(98)77633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi M, Morita F. J Biochem (Tokyo) 1989;106:868–871. doi: 10.1093/oxfordjournals.jbchem.a122944. [DOI] [PubMed] [Google Scholar]
- 41.Oiwa K, Eccleston J F, Anson M, Kikumoto M, Davis C T, Reid G P, Ferenczi M A, Corrie J E T, Yamada A, Nakayama H, Trentham D R. Biophys J. 2000;78:3048–3071. doi: 10.1016/S0006-3495(00)76843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]