Abstract
Background
Inter-observer variability limits the reproducibility of pelvic floor motion measured by magnetic resonance imaging (MRI). Our aim was to develop a semi-automated program measuring pelvic floor motion in a reproducible and refined manner.
Methods
Pelvic floor anatomy and motion during voluntary contraction (squeeze) and rectal evacuation were assessed by MRI in 64 women with fecal incontinence (FI) and 64 age-matched controls. A radiologist measured anorectal angles and anorectal junction motion. A semi-automated program did the same and also dissected anorectal motion into perpendicular vectors representing the puborectalis and other pelvic floor muscles, assessed the pubococcygeal angle, and evaluated pelvic rotation.
Key Results
Manual and semi-automated measurements of anorectal junction motion (r = 0.70; p < 0.0001) during squeeze and evacuation were correlated, as were anorectal angles at rest, squeeze, and evacuation; angle change during squeeze or evacuation were less so. Semi-automated measurements of anorectal and pelvic bony motion were also reproducible within subjects. During squeeze, puborectalis injury was associated (p ≤ 0.01) with smaller puborectalis but not pelvic floor motion vectors, reflecting impaired puborectalis function. The pubococcygeal angle, reflecting posterior pelvic floor motion, was smaller during squeeze and larger during evacuation. However, pubococcygeal angles and pelvic rotation during squeeze and evacuation did not differ significantly between FI and controls.
Conclusion & Inferences
This semi-automated program provides a reproducible, efficient and refined analysis of pelvic floor motion by MRI. Puborectalis injury is independently associated with impaired motion of puborectalis, not other pelvic floor muscles in controls and women with FI.
Keywords: Anorectal, defecography, fecal incontinence, magnetic resonance imaging, pelvic floor, puborectalis
The anal sphincters and pelvic floor muscles preserve fecal continence and participate in defecation (1). By visualizing structural (e.g., rectocele) and functional (e.g., impaired anal relaxation) disturbances, barium defecography and magnetic resonance imaging (MRI) facilitate the diagnosis of defecatory disorders, particularly when anal manometry and a rectal balloon expulsion test are inconclusive (2-6). MRI also reveals puborectalis atrophy and impaired pelvic floor contraction in some women with fecal incontinence (FI) (7-9).
However, the clinical utility of barium defecography and MRI is limited by variable reproducibility of these assessments. For example, in one study, inter-observer agreement was good for identifying enteroceles and rectoceles but suboptimal for identifying intussusception, anismus, or the puborectalis impression by barium defecography (10). Moreover, for some measurements, agreement was much better for experienced observers (10). The American Gastroenterological Association technical review concluded that “there is poor agreement between independent observers in the measurement of the anorectal angle, a parameter thought to be critical to the interpretation of defecography results” (11). This is partly attributable to lack of a standardized definition for the rectal axis, which forms one aspect of the anorectal angle and can be drawn through the anterior, middle or posterior rectal wall. Moreover, the rectum is curvilinear, and the puborectalis indentation on the posterior rectal wall is variable. Hence, even minor differences in the orientation of the rectal axis can profoundly affect the anorectal angle. Lastly, the bony landmarks (e.g., pubic symphysis, coccyx) which are used to measure perineal descent may not be visible during barium defecography but are distinctly visualized on MR images (12). Nonetheless, and despite centralized training, a NIH-sponsored multi-institutional study concluded that “measurement variability adversely affects the utility of many MRI measurements for multicenter pelvic floor disorder research,” although agreement was better for bony than pelvic soft-tissue measurements during dynamic pelvic MRI (13). By contrast, we documented substantial inter-observer agreement for anorectal angles and the anorectal location at rest, during squeeze and evacuation measured by MRI in asymptomatic women and women with defecatory disorders; however, differences between observers were statistically significant (12).
While most attention has focused on anorectal motion and the puborectalis, the coccyx also moves during pelvic floor contraction and rectal evacuation (14). The coccygei, which have a variable proportion of muscle and fibrous tissue, may pull forward and support the coccyx, after it has been pressed backwards, during defecation or parturition (14-16). With the levatores ani and piriforms, they close the posterior part of the pelvic outlet. Previous studies have evaluated the relationship of coccygeal mobility to age, sex, parity, and minor trauma but not to fecal incontinence. It is conceivable that coccygeal weakness may contribute to pelvic weakness in FI.
Hence, the aims of this study were to (i) to develop a user-friendly semi-automated program to measure anorectal motion by defecography; (ii) evaluate the within-subject reproducibility of anorectal motion measured by this semi-automated program; (iii) to compare measurements of anorectal motion by a radiologist and a semi-automated program; and (iv) compare anorectal motion disturbances in women with and without FI. A more robust and refined approach to measure pelvic floor motion by MR defecography may enhance the utility of this technique in clinical practice and research studies.
MATERIALS AND METHODS
Identification of Cases and Controls
This case-control study was conducted in Olmsted County, Minnesota and approved by the Institutional Review Boards at Olmsted Medical Center and Mayo Clinic. As detailed previously (17), a random sample of 5300 Olmsted County women (including 84 nursing home residents), stratified by age (10-year intervals between 20-29 and 80+ years), was drawn from a sampling frame consisting of the unique residents seen at least once during the 10-year period, 1992-2002. A questionnaire-based study on the prevalence and risk factors for FI was conducted among 2800 of 5300 respondents, of whom 507 had FI as defined by accidental leakage of liquid or solid stool unrelated to a short-term, self-limited, diarrheal illness in the past year (18-20). Subsequently, 176 Olmsted County women with and 176 age-matched (± 5 years) women without FI participated in a nested case-control study evaluating the antecedent risk factors for FI (21). Cases did, while controls did not, have FI unrelated to a temporary diarrheal illness over the past year, nor did cases have organic diseases (e.g., neurological disorders, inflammatory bowel disease) known to be associated with FI. From this group, 68 cases and 68 matched controls agreed to have MR imaging to assess anal sphincter and pelvic floor anatomy and pelvic floor motion; the semi-automated analysis described herein was possible in 64 matched pairs.
Anorectal and Pelvic MR Imaging
The anal sphincters were imaged by a disposable endorectal colon coil (MRInnervu®, Medrad, Inc., Indianola, PA, USA) prior to dynamic MR defecography (7, 22). After removing the endoanal coil, dynamic MR defecography was performed by an interactive single-shot, fast spin-echo (SSFSE) imaging technique after placing 120 cc of ultrasound gel into the rectum and a four-element phased-array coil around the pelvis (7, 22, 23). Images were acquired in the supine position with a field of view of 24 to 32 cm, slice thickness of 5 mm, TR (repetition time) of 1400 to 2000 ms, TE (echo time) of 90 ms, and a matrix size of 256 × 160 (NEX 0.5). An oblique sagittal plane bisecting the anorectum was defined by selecting three points from axial images during real-time imaging. Images were acquired every 1.4 to 2 seconds during rest, squeeze and rectal evacuation. Using real-time image reconstruction, we monitored the exam, ensured performance of desired maneuvers, and instructed or encouraged patients.
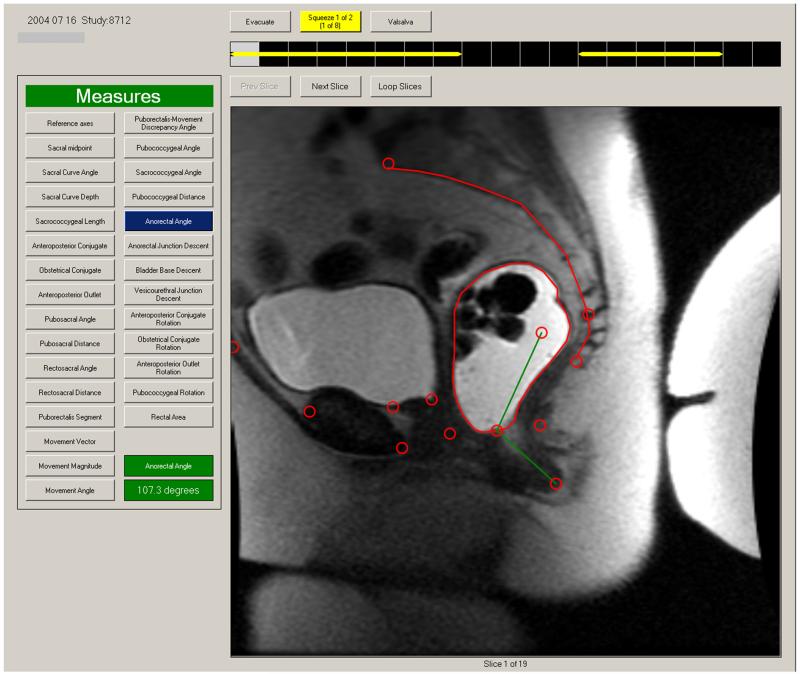
Anorectal motion was measured manually by an experienced radiologist [JF], who measured the perpendicular distance of the anorectal junction from the pubococcygeal line and the anorectal angle at rest, during squeeze, and rectal evacuation. The perpendicular distance of the vesicourethral junction and bladder base from the pubococcygeal line was also measured. Separately, a trained physician (JN) used the custom-made semi-automated program, used for the first time in this study, which runs on a Windows PC, to record these measurements as well as bladder excursion during evacuation. This process comprises 2 steps: First, similar to the process employed by a radiologist, the landmarks necessary to measure anorectal and pelvic floor motion (i.e., anorectal junction and angle, upper and lower border of pubic symphysis, sacral promontory, and sacrococcygeal junction) are identified on images by the user (Figure 1). While images were acquired in real-time (i.e., every 1.2-2 seconds) during rectal evacuation and pelvic floor contraction (squeeze), measurements were only made on images at rest, maximum anorectal excursion from rest during squeeze, and maximum anorectal excursion during evacuation. Thereafter, the program then calculates distances and angles between sets of points over a time sequence of images. Results are tabulated in a spreadsheet.
Figure 1. User interface for semi-automated measurement program.
After a series of DICOM images are loaded, any image from that series can be selected for display in the window. In this example, the landmarks have been demarcated by an observer (circles). Any measurement can be displayed by selecting tabs on the left side of screen. Here, the anorectal angle at rest is shown.
In addition to the measurements specified above, the program also identifies (i) vectors connecting the anorectal junction at rest to the junction during (maximum) squeeze and separately during (maximum) evacuation; (ii) the vector components of these two anorectal motion vectors in a specific coordinate frame (the puborectalis motion vector, along a line which connects the anorectal junction at rest to the pubic symphysis, and the pelvic floor motion vector, which is perpendicular to the puborectalis motion vector. The puborectalis and pelvic floor motion vectors represent the action of the puborectalis muscle and other components of the levator ani muscle, respectively); (iii) pubococcygeal angle (Figure 2), as defined by the lower border of pubic symphysis, sacrococcygeal junction, and tip of coccyx, which measures posterior pelvic floor motion; and (iv) rotation of the bony pelvis during pelvic floor contraction by measuring rotation of the lines connecting the upper border of the pubic symphysis to the sacral promontory (i.e., anteroposterior conjugate) and to the sacrococcygeal junction (i.e., pubococcygeal line). Likewise, rotation of lines connecting the lower border of the pubic symphysis with the sacral promontory (obstetric conjugate) and the sacrococcygeal junction (anteroposterior outlet) was also measured.
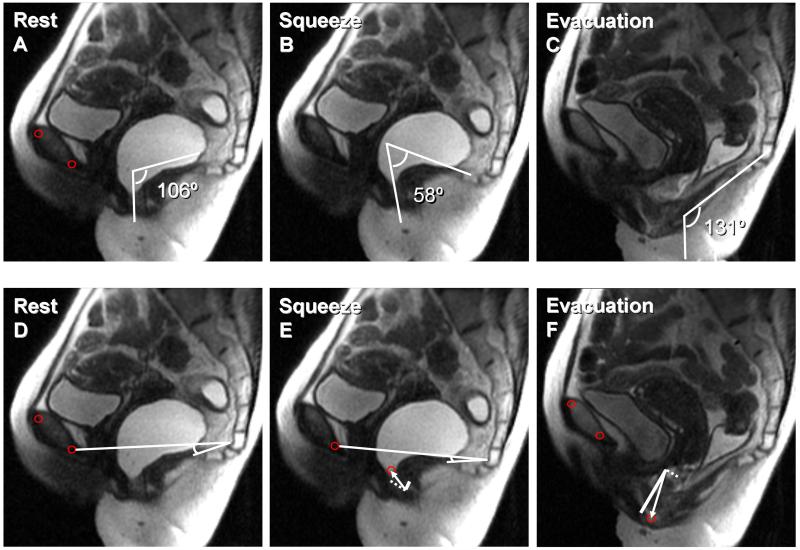
Figure 2. MRI images at rest, during squeeze, and evacuation in a healthy subject.
Anorectal motion was assessed manually (i.e., by measuring anorectal angles, panels A-C) and by the semi-automated program (panels D-F). Panel E depicts the anorectal motion vector, which connects the anorectal junction at rest and squeeze (white arrow). This vector is decomposed into the puborectalis (white dotted line) and pelvic floor (white solid line) components. Panel F depicts the anorectal motion vector from rest to evacuation (white arrow) and its vector components. Also observe that the pubococcygeal angle decreased from 11° at rest to 8° during squeeze (panels D-E).
Eighty six of 127 subjects contracted their pelvic floor muscles twice during dynamic imaging, permitting within-subject reproducibility to be assessed. However, within-subject reproducibility could not be assessed during evacuation because a majority of ultrasound gel was evacuated from the rectum at the first attempt in most patients.
Statistical Analysis
Univariate and multiple variable analyses (i.e., conditional logistic regression models) were used to assess whether structural and functional abnormalities could discriminate between cases and controls. The multiple variable model included age, body mass index (BMI), disease status (i.e., FI or control), and puborectalis injury as defined previously (24). Separate multiple variable models also incorporating age and BMI were used to identify differences in bony pelvic rotation between cases and controls. The concordance between measurements of anorectal and pelvic floor motion during squeeze within subjects was assessed by Lin’s concordance statistic (the concordance correlation coefficient) (25). Within-subject comparisons of anorectal and pelvic floor motion measurements were based on the paired t-test or Wilcoxon rank sum test, as warranted.
RESULTS
Demographic Characteristics and Clinical Features
The demographics, clinical features, findings of MR imaging (endoanal imaging and manual analysis of anorectal motion) have been published previously (24). By design, the age distribution was similar in 64 cases (Mean ± SEM, 57 ± 2 years) and 64 controls (57 ± 2 years), but BMI was higher (p < 0.001) in cases (30.1 ± 0.9 kg/m2) than controls (25.5 ± 0.8 kg/m2). Twenty cases but only 1 control (p < 0.001) had functional diarrhea. Stress urinary incontinence (35 cases, 15 controls) and a cholecystectomy (16 cases, 1 control) were more common (p < 0.001) in cases. Other factors (i.e., obstetric anal sphincter injury, hysterectomy) did not differ significantly between cases and controls.
The duration of FI was 1 to < 5 years in 19 cases (30%), 5 to < 10 years in 17 (27%), 10 to < 15 years in 13 (20%), 15 to < 20 years in 4 (6%), and 20 years or longer in 11 FI patients (17%). Based on the Fecal Incontinence and Constipation Assessment (FICA), (19, 26) the overall severity of FI was mild (17 women [27%]), moderate (44 women [69%]) or severe (3 women [5%]) (21).
Anal Sphincter and Puborectalis Injury
Internal (p = 0.001) and external (p = 0.003) anal sphincter injuries were significantly more common in cases than in controls. Internal sphincter disturbances included mild focal thinning (3 cases, 0 controls), a tear or scar (15 cases, 0 controls), or atrophy (1 case, 3 controls). External sphincter disturbances included a tear or scar (9 cases, 0 controls) or atrophy (6 cases, 3 controls). While puborectalis abnormalities were also more common in cases, differences from controls were not statistically significant. Puborectalis abnormalities included mild asymmetry (2 cases, 0 controls) and unilateral (7 cases, 3 controls) or bilateral atrophy (3 cases, 5 controls).
Comparison of Anorectal and Pelvic Floor Motion by Semi-Automated Method in FI and Controls
The anorectal junction was located 2.8 ± 0.1 cm below the pubococcygeal line in controls and similarly located in cases (Table 1). During squeeze, the anorectal junction moved toward the pubococcygeal line (e.g., by −1.3 ± 0.1 cm in controls). During evacuation, the anorectal junction moved away from this line (e.g., by +3.2 ± 0.2 cm in controls). Figure 2 shows the components (puborectalis and other pelvic floor motion vector) of the anorectal motion vector. Both vectors were greater (p < 0.0001) during evacuation than during squeeze.
Table 1.
Comparison of Anorectal Motion by Semi-Automated Method and by a Radiologist in Fecal Incontinence Cases and Controls
| Semi-Automated Assessmentsa | Cases (N = 64) |
Controls (N = 64) |
|---|---|---|
| Location of anorectal junction at rest (cm)c | 2.7 ± 0.2 | 2.8 ± 0.1 |
| Anorectal junction motion during squeeze (cm)d | −1.3 ± 0.10 | −1.4 ± 0.1 |
| Anorectal junction motion during evacuation (cm)d | 3.2 ± 0.2 | 3.2 ± 0.2 |
| Pelvic floor motion component during squeeze (cm)e | −1.0 ± 0.1 | −1.0 ± 0.1 |
| Pelvic floor motion component during evacuation (cm)e | 2.6 ± 0.1 | 2.8 ± 0.1 |
| Puborectalis motion component during squeeze (cm)f | −0.8 ± 0.1 | −0.9 ± 0.1 |
| Puborectalis motion component during evacuation (cm)f | 1.4 ± 0.1 | 1.6 ± 0.1 |
| Anorectal angle at rest(°) | 108 ± 2 | 108 ± 2 |
| Anorectal angle change during squeeze (°) | −26 ± 2 | −29 ± 2 |
| Anorectal angle change during evacuation (°) | 12 ± 3 | 17 ± 3 |
| Location of bladder base at rest c | −1.1 ± 0.1 | −1.1 ± 0.1 |
| Bladder base motion during squeeze (cm)d | −0.4 ± 0.05 | −0.5 ± 0.06 |
| Bladder base motion during evacuation (cm) d | 3.0 ± 0.2 | 3.0 ± 0.2 |
| Location of vesicourethral junction at rest c | −1.1 ± 0.1 | −0.9 ± 0.1 |
| Vesicourethral motion during squeeze (cm) d | −0.4 ± 0.05 | −0.5 ± 0.06 |
| Vesicourethral motion during evacuation (cm) d | 2.9 ± 0.2 | 2.8 ± 0.2 |
| Pubococcygeal angle at rest c | 38 ± 1.8 | 33 ± 1.5 |
| Pubococcygeal angle change during squeeze (°) | −5.5 ± 1.2 | −5.3 ± 1.2 |
| Pubococcygeal angle change during evacuation (°) | 9.3 ± 1.5 | 10.7 ± 1.4 |
| Assessments by a Radiologist a | ||
| Location of anorectal junction at rest (cm) c | 2.8 ± 0.2 | 2.5 ± 0.1 |
| Anorectal junction motion during squeeze (cm) d | −1.4 ± 0.2 | −1.2 ± 0.1 |
| Anorectal junction motion during evacuation (cm) d | 2.6 ± 0.2 | 3.2 ± 0.2 |
| Anorectal angle at rest (°) | 101 ± 2 | 105 ± 2 |
| Anorectal angle change during evacuation (°) | 14 ± 3.2 | 19.6 ± 2.9 |
| Anorectal angle change during squeeze (°) | −24 ± 2.4 | −32 ± 2.3 |
| Location of bladder base at rest (cm) c | 2.1 ± 0.2 | 1.4 ± 0.1 |
| Location of vesicourethral junction at rest (cm) c | 1.6 ± 0.1 | 1.4 ± 0.1 |
Distances are measured along the perpendicular line connecting the anorectal junction, bladder base or vesicourethral junction (as appropriate) to the line between the lower border of the pubic symphysis and sacrococcygeal junction. Motion and angle changes are assessed as differences (Rest –evacuation) and (Rest – squeeze)
p = 0.01 by paired t test
Positive and negative values reflect location below and above pubococcygeal line respectively
Positive and negative values reflect movement of anorectal junction away from and toward pubococcygeal line respectively
Positive and negative values reflect motion downward and upward perpendicular to the line connecting the anorectal junction and the pubic symphysis respectively
Positive and negative values reflect motion away from and towards pubic bone respectively
During pelvic floor contraction, the pubococcygeal angle was more (p = 0.0001) acute (−5.5 ± 1.2° [FI], −5.3 ± 1.2 [controls]) than at rest. During evacuation, this angle was more (p = 0.0001) obtuse (+9.3 + 1.5 [cases], +10.7 + 1.4° [controls]). None of these measurements was significantly different between cases and controls.
Comparison of Anorectal Motion and Angle Change Measured by Semi-Automated and Manual Analysis
Table 1 also provides corresponding measurements made by a radiologist, which have been presented in detail elsewhere (24). For parameters which were measured by both methods (i.e., by a radiologist and by the semi-automated method), manual and semi-automated values were comparable. Thus, measurements of anorectal junction motion by both techniques were correlated during squeeze (r = 0.80; p < 0.0001) and evacuation (r = 0.70; p < 0.0001); these correlations were also similar in both cases and controls (data not shown). Anorectal junction motion assessed manually was also correlated with the puborectalis motion vector (r = 0.73, p < 0.0001 during squeeze and r = 0.47, p = 0.001 during evacuation) and the pelvic floor motion vector (r = 0.72; p < 0.0001 during squeeze and r = 0.68, p < 0.0001 during evacuation) as computed by semi-automated analysis. Both correlations were again similar in cases and controls (data not shown).
Anorectal angles measured by manual and semi-automated techniques at rest (r = 0.65; p < 0.0001), squeeze (r = 0.52; p < 0.0001), and evacuation (r = 0.33; p = 0.0002) were correlated. However, while the anorectal angle change during evacuation (r = 0.21; p = 0.02) was correlated, the angle change during squeeze was not.
The anorectal angle change measured manually was also correlated with pelvic floor (r = 0.34; p = 0.0002) and puborectalis (r = 0. 29; p < 0.002) motion vectors among all subjects. However, these correlations were significant in cases (i.e., pelvic floor motion vector [r = 0.37; p = 0.003] and puborectalis motion vector [r = 0.25; p = 0.046]) but not in controls.
Manual and semi-automated measurements of the location of the bladder base and vesicourethral junction at rest were also correlated (r = 0.55; p < 0.0001) across all subjects.
Relationship between Puborectalis Injury and Anorectal Motion
While anorectal motion during squeeze did not differ between cases and controls, puborectalis injury was associated with reduced anorectal motion during squeeze (Figure 3). During squeeze, puborectalis injury (e.g., asymmetry or atrophy) explained (p ≤ 0.01) 7% of the variation in anorectal motion and 5% of the variation in the magnitude of the puborectalis motion vector among subjects after adjusting for age and BMI. However, puborectalis injury did not explain inter-subject variation in the other pelvic floor motion vector during squeeze or inter-subject variation in any vectors (i.e., anorectal motion, puborectalis, other pelvic floor) during evacuation.
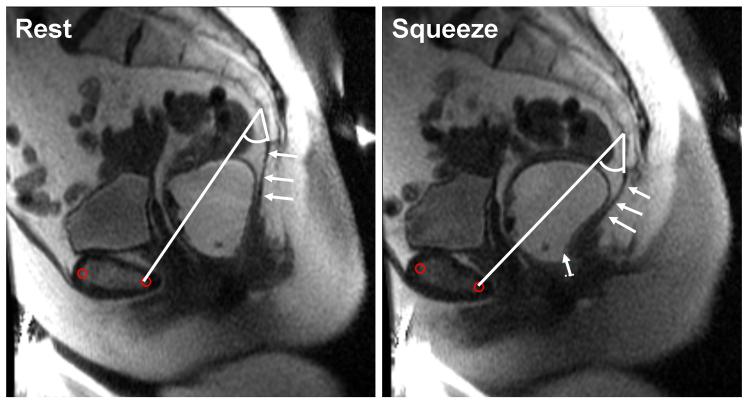
Figure 3. MRI images at rest and during squeeze in a patient with fecal incontinence and puborectalis injury.
The anorectal motion vector from rest to squeeze (white arrow) is decomposed into puborectalis motion vectors (white dotted line), which is very short, and the longer “other pelvic floor muscle” vector. In this example, the “other pelvic floor muscle” vector constitutes the main component of the anorectal motion vector. In contrast to the asymptomatic subject, the pubococcygeal angle increased from 33° at rest to 40° during squeeze. The anococcygeal ligament is indicated by arrows.
Intra-individual Reproducibility of Anorectal and Pelvic Floor Motion during Squeeze
The intra-individual reproducibility of pelvic motion during voluntary contraction (i.e., squeeze) was assessed in 86 subjects who contracted their pelvic floor muscles twice during a maneuver. During contraction, measurements of overall anorectal motion, puborectalis and pelvic floor motion vectors were very reproducible (Table 2). However, the pubococcygeal angle change during voluntary contraction was less reproducible.
Table 2.
Within-Subject Reproducibility of Anorectal and Pelvic Floor Motion During Squeeze Assessed by Semi-Automated Method
| Measurements | Maneuver 1 | Maneuver 2 | CCC (95% CI)* |
|---|---|---|---|
| Movement magnitude, Rest to squeeze | 1.54 ± 0.09 | 1.57 ± 0.09 | 0.71 (0.60-0.82) |
| Puborectalis motion component, Rest to squeeze (cm) |
−0.95 ± 0.09 | −0.93 ± 0.09 | 0.80 (0.73-0.88) |
| Pelvic floor motion component, Rest to squeeze (cm) |
−1.05 ± 0.07 | −1.10 ± 0.06 | 0.60 (0.47-0.74) |
| Anorectal angle at rest (°) | 107.3 ± 1.83 | 103.6 ± 1.76 | 0.80 (0.73-0.88) |
| Anorectal angle change during squeeze (°) | −27.62 ± 1.73 | −26.40 ± 1.70 | 0.79 (0.71-0.87) |
| Anorectal junction at rest (cm) a | 2.73 ± 0.13 | 2.77 ± 0.13 | 0.85 (0.79-0.91) |
| Location of bladder base at rest (cm) a | −1.13 ± 0.07 | −1.12 ± 0.08 | 0.89 (0.85-0.93) |
| Bladder base motion during squeeze (cm) a | −0.45 ± 0.05 | −0.51 ± 0.05 | 0.71 (0.61-0.82) |
| Location of vesicourethral junction at rest (cm) a |
−1.11 ± 0.07 | −1.11 ± 0.08 | 0.89 (0.85-0.94) |
| Vesicourethral motion during squeeze (cm) a |
−0.48 ± 0.05 | −0.51 ± 0.05 | 0.68 (0.58-0.80) |
| Pubococcygeal angle at rest (°) | 34.4 ± 1.4 | 34.9 ± 1.5 | 0.88 (0.84-0.93) |
| Pubococcygeal angle change, Rest to squeeze (°) |
−5.02 ± 1.07 | −6.62 ± 1.10 | 0.57 (0.43-0.71) |
| Rotation of AP conjugate (°) | −4.49 ± 0.88 | −4.97 ±0.93 | 0.94 (0.92-0.96) |
| Rotation of obstetric conjugate (°) | −4.02 ± 0.61 | −3.96 ± 0.65 | 0.90 (0.86-0.94) |
| Rotation of anteroposterior outlet (°) | −1.67 ± 6.3 | −1.88 ± 0.69 | 0.90 (0.86-0.94) |
| Rotation of pubococcygeal outlet (°) | −2.02 ± 0.56 | −2.62 ± 0.62 | 0.90 (0.86-0.94) |
Note – Within-subject reproducibility could only be evaluated during squeeze. During evacuation, ultrasound gel was evacuated between maneuvers. Hence, reproducibility could not be assessed.
Distances are measured along the perpendicular line connecting the anorectal junction, bladder base or vesicourethral junction (as appropriate) to the line between the lower border of the pubic symphysis and sacrococcygeal junction. Motion and angle changes are assessed as differences (Rest –evacuation) and (Rest – squeeze) CCC – concordance correlation coefficient
Relationship between Anorectal Location at Rest and Motion during Evacuation and Squeeze
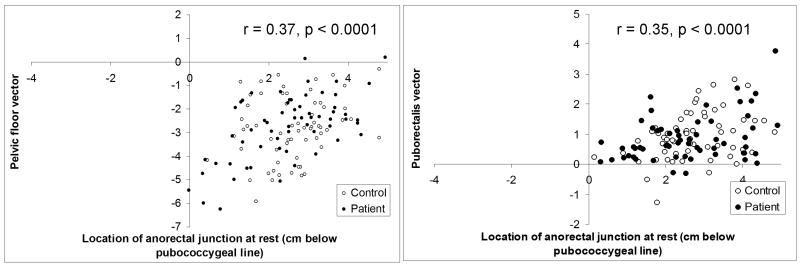
Anorectal motion during evacuation was related to the location of the anorectal junction at rest. When the anorectal junction was higher at rest, evacuation was accompanied by a longer (r = −0.37; p < 0.0001) pelvic floor motion vector (Figure 4), but a shorter (r = 0.33; p = 0.0002) puborectalis motion vector. In contrast, during squeeze, the puborectalis motion vector was greater (r = −0.35; p < 0.0001) when the anorectal junction was lower at rest. Similar correlations were observed separately in cases and controls (data not shown).
Figure 4. Relationship between anorectal location at rest and motion during evacuation (left panel) and squeeze (right panel).
When the anorectal junction was higher at rest (i.e., closer to pubococcygeal line), the pelvic floor motion vector was longer during evacuation. In contrast, during squeeze, the puborectalis motion vector was longer when the anorectal junction was lower (i.e., further from the pubococcygeal line) at rest.
Comparison of Pelvic Bony Motion in Cases and Controls
The pubococcygeal line and anteroposterior outlet were identifiable in all subjects (Figure 5). However, the anteroposterior and obstetric conjugates could only be drawn when the sacral promontory was visible (i.e., in 55 controls and 44 cases). After adjusting for age and BMI, clockwise rotation of the of the bony pelvis as measured by the anteroposterior conjugate (4.1 ± 0.7° [cases], 5.2 ± 1.2° [controls]), obstetric conjugate (4.6 ± 0.7° [cases], 4.0 ± 0.8° [controls]), pubococcygeal line (2.4 ± 0.7° [cases], 2.8 ± 0.7° [controls]), and anteroposterior outlet (2.2 ± 0.8° [cases], 2.4 ± 0.8° [controls]) were not significantly different between cases and controls.
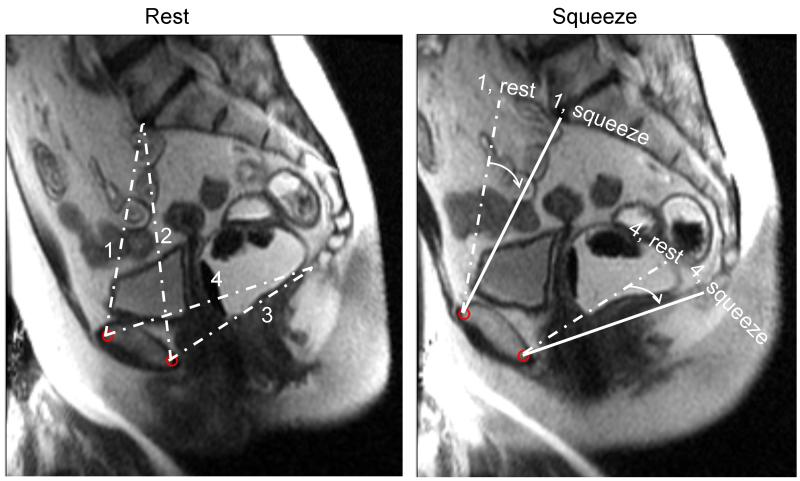
Figure 5. Assessment of pelvic rotation by MRI.
Left panel shows anteroposterior conjugate (1), obstetric conjugate (2), anteroposterior outlet (3), and pubococcygeal line (4) at rest. During squeeze (right panel), observe pelvic rotation as measured by the angle between anteroposterior conjugate and anteroposterior outlet diameters at rest (dashed line) and (solid line) in a control subject.
DISCUSSION
These findings demonstrate that a new software program provides a semi-automated, reproducible, efficient, and refined analysis of pelvic floor motion assessed by MR defecography. In addition to the traditional measurements (i.e., anorectal motion and anorectal angles), this semi-automated program can also analyze puborectalis and pelvic floor motion vectors, the pubococcygeal angle, and pelvic rotation. These measurements were validated by several approaches: First, intra-subject agreement was evaluated by comparing anorectal motion during squeeze in 86 women who contracted their pelvic floor muscles twice during the study. With one exception (i.e., pubococcygeal angle change during squeeze), within-subject concordance correlation coefficients for pelvic floor motion variables during squeeze were approximately 0.6 or higher, indicating substantial agreement. The concordance correlation coefficient assesses both the accuracy and the precision of the agreement between two quantitative measures, whereas the usual (Pearson) correlation assesses just the precision of the agreement. Consistent with a previous study that evaluated inter-observer agreement (13), agreement between 2 pelvic floor contractions was somewhat better for bone pelvimetry than for soft tissue measurements. This is not surprising since subjects were asked to contract their pelvic floor muscles but not move the pelvis during contraction. Hence, it is inevitable that pelvic floor and anorectal motion measurements are more subject to variations in human effort between maneuvers between maneuvers than bony pelvis motion. Also, bone contours are more distinct than soft tissue contours. Concurrent validity was assessed by comparing manual and semi-automated measurements; and this agreement was also substantial for anorectal junction motion during squeeze and evacuation and for the anorectal angle at rest, squeeze and evacuation. However, concurrent validity for the anorectal angle change during squeeze and evacuation was suboptimal, perhaps because it is challenging to reproducibly define the rectal and anal boundaries of this angle during these maneuvers (11). To overcome this limitation, the anorectal motion vector was separated into its vector components, which were both significantly correlated with the anorectal angle. Puborectalis injury was associated with a weaker puborectalis but not with a weaker pelvic floor motion vector during pelvic floor contraction. This supports the construct validity of the puborectalis motion vector. Together, these findings suggest that the semi-automated measurement of anorectal motion and its component vectors may be preferable to measuring the anorectal angle during defecography.
We previously observed that perineal descent during evacuation was more pronounced when the anorectal junction was higher at rest in women with defecatory disorders (12). This study confirms those observations in a different cohort; moreover, the pelvic floor motion vector during evacuation was also greater when the anorectal junction was higher at rest. These findings reinforce the need to consider the location of the anorectal junction and the pelvic floor at rest when interpreting anorectal motion during maneuvers and pelvic organ prolapse. To emphasize this point, an assessment of pelvic organ prolapse that is based only on descent during evacuation or a Valsalva maneuver may underestimate pelvic laxity in patients with a low pelvic floor at rest. Conversely, when the anorectal junction is higher (i.e., located closer to the pubococcygeal line) at rest, the puborectalis motion vector during evacuation and squeeze (i.e., contraction) is shorter. Perhaps this suggests that women with a higher anorectal junction at rest have excellent pelvic floor support with a relatively taut puborectalis muscle that permits less motion during contraction and relaxation.
The pubococcygeus muscle decussates in the midline and is attached to the coccyx by the anococcygeal ligament (27). Consistent with a previous study (14), the pubococcygeal angle was more acute during pelvic floor contraction and more obtuse during evacuation. Narrowing of the pubococcygeal angle is attributable to the coccygeus, which pulls forward and supports the coccyx. Conversely, the muscle relaxes together with other components of the levator ani, contributing to widening of the anorectal angle and thereby enabling evacuation. However, changes in the pubococcygeal angle and rotation of the bony pelvis, which reflect activation of accessory pelvic floor muscles, was not significantly different between women with and without FI.
In contrast to conventional fluoroscopy, dynamic MRI is generally performed in the supine position. Some but not all comparative studies observed more pelvic organ prolapse and perineal descent during fluoroscopy than dynamic MRI (28-30) (31). Among patients with dyschezia, there was good correlation between these techniques for detecting rectoceles, enteroceles, and perineal descent (32). This user friendly PC-based semi-automated program can be installed on standard workstations and facilitate the assessment of motion by pelvic MR imaging particularly in multi-center studies. While this initial study was focused on the anorectum, the software can also be used for bone pelvimetry and should be adaptable to measure rectocele and enterocele size and uterine descent. The current program measures anorectal motion in two dimensions. However, since obstetric trauma can cause asymmetric injury to the levator ani, it may be advantageous to analyze motion in 3 dimensions (33). Because the program relies upon two dimensional coordinates and clear identification of bony landmarks on digitized images, it may be more challenging to analyze barium defecography images.
In summary, these findings suggest that real-time images of anorectal motion by MR defecography can be efficiently and accurately analyzed by a semi-automated technique, which can dissect relative contributions of the puborectalis and other pelvic floor muscles to anorectal motion. This semi-automated program should facilitate the analysis of pelvic floor motion by MR defecography in clinical practice and research studies. While pelvic floor motion overall was not significantly different between women with FI and age-matched asymptomatic women, puborectalis injury was associated with impaired puborectalis motion.
ACKNOWLEDGEMENTS FUNDING
The project described was supported by USPHS NIH Grant R01 DKDK78924, NIH Grant RR018898 and Grant Number 1 UL1 RR024150* from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research, and made possible by the Rochester Epidemiology Project (Grant #R01-AG034676 from the National Institute on Aging).” Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Jessica Noelting, David Lake, and Armando Manduca – data analysis
Adil E. Bharucha - substantial contributions to conception and design, data analysis, drafting article
J. G. Fletcher - data acquisition
Stephen J. Riederer and L. Joseph Melton, III - substantial contributions to conception and design
Alan Zinsmeister - substantial contribution to design and statistical analysis
All - critically revising article and final approval of published version
Footnotes
DISCLOSURES: Competing Interests: the authors have no competing interests.
REFERENCES
- 1.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterology & Motility. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 2.Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW. Defecography in normal volunteers: results and implications. Gut. 1989;30:1737–1749. doi: 10.1136/gut.30.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamant NE, Kamm MA, Wald A, Whitehead WE. American Gastroenterological Association Medical Position Statement on Anorectal Testing Techniques. Gastroenterology. 1999;116:732–760. doi: 10.1016/s0016-5085(99)70194-0. [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE. Update of tests of colon and rectal structure and function. Journal of Clinical Gastroenterology. 2006;40:96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 6.Reiner CS, Tutuian R, Solopova AE, Pohl D, Marincek B, Weishaupt D. MR defecography in patients with dyssynergic defecation: spectrum of imaging findings and diagnostic value. British Journal of Radiology. 2011;84:136–144. doi: 10.1259/bjr/28989463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terra MP, Beets-Tan RGH, Vervoorn I, et al. Pelvic floor muscle lesions at endoanal MR imaging in female patients with faecal incontinence. European Radiology. 2008;18:1892–1901. doi: 10.1007/s00330-008-0951-8. [DOI] [PubMed] [Google Scholar]
- 9.Lewicky-Gaupp C, Brincat C, Yousuf A, Patel DA, Delancey JOL, Fenner DE. Fecal incontinence in older women: are levator ani defects a factor? American Journal of Obstetrics & Gynecology. 2010;202:491.e491–496. doi: 10.1016/j.ajog.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobben AC, Wiersma TG, Janssen LWM, et al. Prospective assessment of interobserver agreement for defecography in fecal incontinence.[see comment] American Journal of Roentgenology. 2005;185:1166–1172. doi: 10.2214/AJR.04.1387. AJR. [DOI] [PubMed] [Google Scholar]
- 11.Diamant NE, Kamm MA, Wald A, Whitehead WE. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 12.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic Variation in Functional Disorders of Defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart ME, Fielding JR, Richter HE, et al. Reproducibility of dynamic MR imaging pelvic measurements: a multi-institutional study. Radiology. 2008;249:534–540. doi: 10.1148/radiol.2492072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassi R, Lombardi G, Reginelli A, et al. Coccygeal movement: assessment with dynamic MRI. European Journal of Radiology. 2007;61:473–479. doi: 10.1016/j.ejrad.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Gray H. Gray’s Anatomy. 36 edn Churchill Livingstone; Edinburgh, London, Melbourne and New York: 1980. [Google Scholar]
- 16.Wendell-Smith CPaW PM. Musculature of the Pelvic Floor. 2nd edn Heinemann Medical; London: 1977. [Google Scholar]
- 17.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: A population based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clinical Gastroenterology & Hepatology. 2006;4:1004–1009. doi: 10.1016/j.cgh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Bharucha AE, Seide B, Zinsmeister AR, Melton JL. Relation of bowel habits to fecal incontinence in women. American Journal of Gastroenterology. 2008;103:1470–1475. doi: 10.1111/j.1572-0241.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharucha AE, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559–1566. doi: 10.1053/j.gastro.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. American Journal of Gastroenterology. 2003;98:399–411. doi: 10.1111/j.1572-0241.2003.07235.x. [DOI] [PubMed] [Google Scholar]
- 23.Busse RF, Riederer SJ, Fletcher JG, Bharucha AE, Brandt KR. Interactive fast spin-echo imaging. Magnetic Resonance in Medicine. 2000;44:339–348. doi: 10.1002/1522-2594(200009)44:3<339::aid-mrm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Bharucha AE, Fletcher JG, Melton LJ, 3rd, Zinsmeister AR. Obstetric Trauma, Pelvic Floor Injury And Fecal Incontinence: A Population-Based Case-Control Study. American Journal of Gastroenterology. 2012 doi: 10.1038/ajg.2012.45. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrasco JL, Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics. 2003;59:849–858. doi: 10.1111/j.0006-341x.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 26.Bharucha AE, Locke GR, Seide B, Zinsmeister AR. A New Questionnaire for Constipation and Fecal Incontinence. Alimentary Pharmacology & Therapeutics. 2004;20:355–364. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 27.Shafik A. New concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. II. Anatomy of the levator ani muscle with special reference to puborectalis. Investigative Urology. 1975;13:175–182. [PubMed] [Google Scholar]
- 28.Fielding JR, Griffiths DJ, Versi E, Mulkern RV, Lee ML, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. Ajr. 1998;171:1607–1610. doi: 10.2214/ajr.171.6.9843296. [DOI] [PubMed] [Google Scholar]
- 29.Lienemann A, Anthuber C, Baron A, Kohz P, Reiser M. Dynamic MR colpocystorectography assessing pelvic-floor descent. Eur Radiol. 1997;7:1309–1317. doi: 10.1007/s003300050294. [DOI] [PubMed] [Google Scholar]
- 30.Kelvin FM, Maglinte DD, Hale DS, Benson JT. Female pelvic organ prolapse: a comparison of triphasic dynamic MR imaging and triphasic fluoroscopic cystocolpoproctography. Ajr. 2000;174:81–88. doi: 10.2214/ajr.174.1.1740081. [DOI] [PubMed] [Google Scholar]
- 31.Vanbeckevoort D, Van Hoe L, Oyen R, Ponette E, De Ridder D, Deprest J. Pelvic floor descent in females: comparative study of colpocystodefecography and dynamic fast MR imaging. J Magn Reson Imaging. 1999;9:373–377. doi: 10.1002/(sici)1522-2586(199903)9:3<373::aid-jmri2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Vitton V, Vignally P, Barthet M, et al. Dynamic anal endosonography and MRI defecography in diagnosis of pelvic floor disorders: comparison with conventional defecography. Diseases of the Colon & Rectum. 2011;54:1398–1404. doi: 10.1097/DCR.0b013e31822e89bc. [DOI] [PubMed] [Google Scholar]
- 33.Heilbrun ME, Nygaard IE, Lockhart ME, et al. Correlation between levator ani muscle injuries on magnetic resonance imaging and fecal incontinence, pelvic organ prolapse, and urinary incontinence in primiparous women. American Journal of Obstetrics & Gynecology. 2010;202:488.e481–486. doi: 10.1016/j.ajog.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]