Abstract
The serotonin system is implicated in a variety of psychiatric disorders whose clinical presentation and response to treatment differ between males and females, as well as with aging. However, human neurobiological studies are limited. Sex differences in the cerebral metabolic response to an increase in serotonin concentrations were measured, as well as the effect of aging, in men compared to women. Thirty-three normal healthy individuals (14 men/19 women, age range 20–79 years) underwent two resting positron emission tomography (PET) studies with the radiotracer [18F]-2-deoxy-2-fluoro-D-glucose ([18F]-FDG) after placebo and selective serotonin reuptake inhibitor (SSRI, citalopram) infusions on two separate days. Results indicated that women demonstrated widespread areas of increased cortical glucose metabolism with fewer areas of decrease in metabolism in response to citalopram. Men, in contrast, demonstrated several regions of decreased cortical metabolism, but no regions of increased metabolism. Age was associated with greater increases in women and greater decreases in men in most brain regions. These results support prior studies indicating that serotonin function differs in men and women across the lifespan. Future studies aimed at characterizing the influences of age and sex on the serotonin system in patients with psychiatric disorders are needed to elucidate the relationship between sex and age differences in brain chemistry and associated differences in symptom presentation and treatment response.
Keywords: selective serotonin reuptake inhibitors, citalopram, serotonin, positron emission tomography (PET), glucose metabolism, sex differences, aging
INTRODUCTION
The serotonin system plays a critical role in the regulation of many behaviors, including mood, and abnormalities in the functional integrity of this system have been found in numerous psychiatric disorders (as reviewed by Lucki, 1998). Many of these disorders have clinical presentations that differ between men and women, and also change with advancing age. Accordingly, an understanding of the influence of sex and age on the serotonin system will have implications for the pathophysiology of such psychiatric disorders, and inform their intervention and prevention strategies.
Neuroimaging studies have observed sex differences in the serotonin system. The rate of serotonin synthesis in the healthy human brain is reported to be lower in women compared to men (Nishizawa et al., 1997; Frey et al., 2010; Sakai et al., 2006). Studies of receptor binding have yielded less consistent results. In some studies, 5HT2A binding was higher in men compared to women (Biver et al., 1996; Soloff et al., 2010), whereas other studies found no sex difference in binding of the same receptor (Adams et al., 2004; Frøkjaer et al., 2009). Conflicting results may be attributable to the narrower age range of subjects included in the latter compared to the former studies. Conversely, 5HT1A receptor binding has been found to be lower in men compared to women in some studies (Jovanovic et al., 2008; Parsey et al., 2002), although other studies (e.g., Stein et al., 2008) found no sex differences in this receptor, despite studying demographically very similar subjects and using the same methods as studies that did find a sex difference. Small sample sizes and large individual variability may account for the disparate findings.
Although few studies have examined sex differences in serotonin transporter (SERT), higher expression of SERT in the diencephalon and brainstem in healthy women relative to healthy men has been shown (Staley et al., 2001), and higher SERT binding in women compared to men has also been found (Jovanovic et al., 2008).
In addition to sex, normal aging has also been shown to affect some aspects of the serotonin system. For example, Møller and colleagues (2007) found a 10% decline in global mean 5HT1A receptor binding in elderly compared to young adults. Similarly, availability of the 5HT2A receptor appears to decline by approximately 10% per decade (Adams et al., 2004; Meltzer et al., 1998b; Sheline et al., 2002), with an aging effect seen in the anterior cingulate, and orbitofrontal, ventrolateral prefrontal, dorsolateral prefrontal, superior medial frontal, medial inferior temporal, sensorimotor, and parietal cortices, as well as the insula and amygdala/hippocampus (Adams et al., 2004). SERT has been shown to decline with age in both men (Yamamoto et al., 2002) and women (Kuikka et al., 2001)
Unlike serotonin receptors, serotonin synthesis does not appear to change with age (Rosa-Neto et al., 2007). Serotonin function inferred from in the glucose metabolic response to a serotonin-selective reuptake inhibitor (SSRI) challenge has been found to increase with age in some anterior cortical regions and decrease with age in posterior cortical regions (Goldberg et al., 2004). Taken together, these results suggest functional serotonin compensation in normal individuals across the lifespan, in contrast to structural changes in the serotonin system with advancing age.
While both age and sex have been shown to affect various indices of serotonin functioning, few studies have examined the interaction between age and sex. Two investigations found that, in men, increased age is correlated with decreased 5HT1A binding potential (BP; Costes et al., 2005; Moses-Kolko et al., 2011). In women, the opposite effects of aging have been found; 5HT1A receptor BP increases with age (Moses-Kolko et al., 2011).
Thus, results from numerous studies have found sex differences in several aspects of the serotonin system, including serotonin synthesis, but equivocal findings for measures of serotonin receptor binding (5-HT1A, 5-HT2A) have been obtained. Advancing age is associated with a decline in receptor number and region-dependent differences in the glucose metabolic response to an SSRI, but no apparent change in serotonin synthesis. Whether the effect of age on the response of the serotonin system differs between males and females has received little research attention. The aim of this study was to explore the effects of sex and age on the cerebral metabolic response to acute administration of an SSRI, citalopram, in healthy adults.
MATERIALS AND METHODS
Study Design
Thirty-three normal healthy individuals (14 men/19 women) underwent two positron emission tomography (PET) scans on two consecutive days to measure the effects of saline/citalopram administration on cerebral glucose metabolism, as described previously (Smith et al., 2002; Goldberg et al., 2004).
Subject Screening and Selection
Subjects were recruited from advertisements in the community. As part of the screening visits, subjects underwent psychiatric evaluation that included a structured clinical interview, (SCID; First et al., 1995), laboratory testing (including CBC and blood chemistry, glucose levels and thyroid function tests), toxicology screening, and magnetic resonance (MR) imaging scans (GE 1.5T Magnetom Vision, General Electric Medical Systems, Milwaukee, Wisconsin). After the screening visits, PET scans were conducted on separate days. A single spoiled gradient-recalled echo (SPGR) MR pulse sequence was used for the volumetric analyses. (TE=5, TR=24, flip angle=20 degrees, NEX=1, 1.5mm slice thickness; Johnson et al., 1993). This sequence yields 124 thin, contiguous images, maximizes contrast among grey matter, white matter and cerebrospinal fluid (CSF) and provides high-resolution delineation of cortical and subcortical structures.
Subjects who had a history of or current neurological disorder, or who met DSM-IV criteria for current or past Axis I psychiatric disorders (including substance abuse) in themselves or their first-degree relatives, or who were not medically stable (including a current diagnosis of insulin-dependent diabetes or poorly controlled hypertension), or who had used prescription (including beta blockers) or over-the-counter medications (e.g., antihistamines, cold medications), or herbal supplements with central nervous system effects within the past two weeks, were excluded. None of the post-menopausal women were taking estrogen replacement preparations.
The mean age for the total sample was 43.4 ± 21.0 years (range 20–79 years). The difference in age between the men (mean age 42.0 ± 21.2 years) and women (mean age 44.5 ± 21.5 years) as a group was not statistically significant (F (1,27)= −0.47, p > 0.5). The mean education level of men (16.0 ± 3.0 years) and women (15.6 ± 2.0 years; t (31) = −0.40, p > .05) did not differ significantly. Twenty-five participants (76% of the sample) were Caucasian; 4 (12%) were African-American, 3 (9%) were Hispanic, and 1 (3%) was Native American. Seventeen of the subjects from the present sample were described in a prior report of the effects of normal aging on brain metabolism in response to citalopram (Goldberg et al., 2004). The demographic characteristics did not differ significantly between subjects previously described and the subjects recruited subsequently. After a complete description of the study to the subjects, written informed consent was obtained according to procedures established by the Institutional Review Board and the Radiation Safety Committee of the North Shore-Long Island Jewish Health System.
Acute Citalopram Intervention
We used glucose metabolism in response to citalopram to interrogate the functional response of the serotonin system because of the sensitivity of glucose metabolism to detecting the effects of antidepressants on brain function (Smith et al., 2011). While measuring changes in receptor availability using a serotonin radiotracer would be a more direct measure of serotonin function, the sensitivity of serotonin receptor radiotracers to a change in endogenous serotonin concentrations is controversial (as reviewed by Paterson et al., 2010). The SSRI citalopram was used because it is the most selective of the SSRI’s, is available for intravenous administration, and is well tolerated across the life span (Alexopoulos et al., 2001).
The subjects were scanned on two consecutive days after an infusion of either placebo (250ml of saline) or citalopram (40mg of the drug diluted in 250ml saline) over 60 minutes. The citalopram dose was chosen and not given on an mg/kg basis to be consistent with the clinical use of citalopram. Accordingly, the 40mg dose was used, as it is the dose that produced a plasma concentration that is comparable to the plasma level obtained after chronic clinical treatment. The radiotracer was injected approximately 30 minutes after the end of the infusion, the time when the maximal effects on cerebral metabolism and neuroendocrine function have been observed (Smith et al., 2002). The study was single blind in that the subjects were told that they would receive either citalopram or placebo (saline) prior to each study, but the investigator knew the identity of the infusion. The order of placebo-drug administration was not randomized because if the drug were administered first, there may be carryover effects to the second scan. As pointed out by Kapitany and colleagues (1999), a time interval of at least three weeks would be necessary between drug and placebo conditions due to the known carry-over effects of serotonergic drugs. As the subjects were studied as a control group for depressed patients, it would not be possible for ethical reasons to maintain the depressed patients un-medicated during such a long time interval. As studies of the test-re-test variability of PET glucose metabolism measures do not show a systematic effect (increase or decrease from the first to second scans), a systematic effect of order (day 1-placebo; day 2-citalopram) on the citalopram effect is not expected (e.g., Bartlett et al., 1988).
On the days of the infusions, serum and plasma samples were obtained at predetermined intervals (end of infusion, and 15, 30, 60, 90, and 120 minutes post-infusion) to measure concentrations of citalopram. The drug levels were analyzed using repeated-measures analysis of variance with condition (2 levels: placebo/citalopram) and time (6 levels: end of infusion, 15, 30, 60, 90, and 120 minutes post-infusion) as within-subject factors. As expected, a significant effect of time was observed (F(5,22)=6.55, p > .05). The main effects of age (F (1,26)=1.99, p > .05), sex (F(1,26)=4.05, p > .05) as well as the interaction (F(1,26)=2.373, p > .05) were not significant. The means and standard deviations for the 30- and 60-minute citalopram levels (ng/ml; during the 18F-FDG uptake) are: 30-minute: young: male 49.1 ± 17.81, female 41.23 ± 17.60; elderly male 41.68 ± 8.81, female 55.23 ± 8.44; 60-minute young: male 40.0 ± 5.47, female 66.17 ± 51.91; elderly: male 41.23 ± 4.93 female 52.87 ± 8.75.
PET Imaging Procedures
The PET scans were performed with a GE Advance Tomograph in the Center for Neurosciences, Feinstein Institute for Medical Research, as described previously (Smith et al., 2002a,b). Briefly, fifteen to thirty minutes after the end of the infusion of placebo (Day 1)/citalopram (Day 2), 5 mCi of [18F]-2-deoxy-2-fluoro-D-glucose ([18F]-FDG) was injected as an intravenous bolus. A ten-minute transmission scan and a five-minute two-dimensional emission scan were acquired to perform photon attenuation correction, followed by a three-dimensional emission scan 40 minutes after radiotracer injection that lasted for 10 minutes.
Data and Image Analysis
Glucose metabolic rates were calculated (in ml/100g/min) on a pixel-by-pixel basis according to validated methods (Smith et al., 2002a,b). PET data processing was performed on the quantitative glucose metabolism images using the statistical parametric mapping program (Friston et al., 2007). This is a data-driven analytic approach that performs statistical tests on each voxel of each image. The images were smoothed with an isotropic Gaussian kernel (FWHM 8mm for all directions). The glucose metabolic rates were normalized by scaling to a common mean value (50) across all scans, after establishing that the global means did not differ significantly between groups and conditions (p > 0.05). The data were normalized to a global mean because of the greater test-retest variability for absolute compared to relative glucose metabolism observed in numerous studies (e.g., Bartlett et al., 1988). Using SPM5, spatial normalization was applied by aligning the FDG scans to a template in MNI space using affine transformations, and then estimating and writing nonlinear deformations defined by basis functions to warp the FDG scans to MNI space (Ashburner and Friston, 1999). Coordinates in MNI space were transformed to Talairach space using methods described by Lancaster et al. (2007).
The PET and MR data analyses were performed using the flexible factorial option in SPM5. First, the baseline PET data and MR data were analyzed to evaluate sex and age effects. The MR data were analyzed using voxel based morphometry (VBM) as described previously (Smith et al., 2009) to evaluate differences in CSF between groups. For quality control, the images for each subject were visually inspected to check the accuracy of the segmentation prior to the VBM analysis. Due to partial volume effects, increased CSF between groups in cortical and sub-cortical structures may “dilute” the PET and show a reduction in metabolism and, thus, contribute to differences in the PET data (Mazziota et al., 1981).
For the PET data, four separate analyses to evaluate the sex and age effect on the acute glucose metabolic response to citalopram were performed. In summary, to evaluate the acute metabolic response to citalopram in females and males, analyses of variance were performed for each group separately and the contrasts for increase (+1, −1; scan order Post/Pre for all analyses) and decrease (−1, +1) were tested (Table 1). To compare the patterns of increase and decrease in the males to the females, two contrasts were tested (Table 2): Female increase > Male decrease (+1, −1, −1, +1) and Female decrease > Male increase (−1, +1, +1, −1). To evaluate the correlation between age and the acute metabolic response to citalopram, age was included as a covariate and the groups were analyzed separately for positive and negative correlations (Table 3). To compare the patterns of positive and negative correlations with age in the males to the females, both males and females were included in the analysis. Age was included as a covariate. Two contrasts were tested (Table 4): females positive correlation greater than the males negative correlation (+1, −1) and females negative correlation greater than the males positive correlation (−1, +1).
Table 1.
The acute cerebral metabolic response to citalopram in men and women.
| Regions of Decrease in Women | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| Midbrain (substantia nigra) | −5 | −13 | −11 | 3.14 | ||||
| 29 | −71 | 19 | 3.58* | Middle Temporal Gyrus | ||||
| 37 | −86 | 17 | 3.42* | Middle Occipital Gyrus | ||||
| Regions of Increase in Women | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| Superior Frontal Gyrus | −18 | 55 | 15 | 3.67** | ||||
| Anterior Cingulate Gyrus (BA 24) | −4 | 13 | 29 | 2.71 | ||||
| 5 | ;67 | 18 | 2.71 | Posterior Cingulate (BA 31) | −2 | −65 | 14 | 2.98* |
| Uncus (BA 28) | −23 | −12 | −27 | 3.01 | ||||
| Superior Temporal Gyrus | −58 | −39 | 20 | 3.57** | ||||
| 44 | −21 | 12 | 3.12 | Transverse Temporal Gyrus | ||||
| 44 | −59 | 36 | 2.98 | Angular Gyrus | ||||
| 48 | −56 | 45 | 2.89 | Inferior Parietal Lobule (BA 40) | ||||
| 48 | −23 | 52 | 3.36 | Postcentral Gyrus | ||||
| 11 | −44 | 49 | 3.78** | Precuneus (BA 7) | ||||
| Putamen | −21 | 6 | −4 | 3.44* | ||||
| 3 | −17 | 4 | 3.62* | Thalamus | −19 | −34 | 5 | 4.75* |
| Regions of Decrease in Men | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| 0 | −18 | 31 | 2.75** | Anterior Cingulate Gyrus (BA 23) | −6 | −31 | 34 | 4.05* |
| 19 | 40 | −16 | 4.07** | Superior Frontal Gyrus | −8 | 10 | 52 | 3.18 |
| 38 | 28 | 30 | 3.66** | Middle Frontal Gyrus | −30 | 38 | 41 | 3.68 |
| 42 | 8 | 6 | 3.88** | Precentral Gyrus (BA 44) | −36 | 2 | 27 | 3.40 |
| 48 | −55 | 16 | 3.76* | Superior Temporal Gyrus (BA 22) | ||||
| Superior Parietal Lobule (BA 7) | −26 | −58 | 62 | 3.65 | ||||
| 1 | −39 | 22 | 3.12* | Posterior Cingulate | ||||
| 20 | −61 | 52 | 3.21 | Precuneus | ||||
| 61 | −38 | 30 | 3.61* | Inferior Parietal Lobule | ||||
| Regions of Increase in Men: None | ||||||||
Table 2.
Comparison between the acute cerebral metabolic response to citalopram in men compared to women.
| Regions of greater increase in Women than Men | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| 0 | −22 | 33 | 3.54** | Anterior Cingulate | −20 | 41 | −6 | 4.0* |
| Medial Frontal Gyrus | −20 | 52 | −3 | 3.76* | ||||
| 55 | 15 | 7 | 3.44** | Precentral Gyrus | ||||
| 40 | −25 | 14 | 3.14 | Transverse Temporal Gyrus | ||||
| Supramarginal Gyrus | −48 | −44 | 35 | 3.15* | ||||
| Inferior Parietal Lobule | −60 | −36 | 25 | 3.65** | ||||
| Regions of greater decrease in Women than Men | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| 12 | −45 | −31 | 3.33 | Cerebellum (Anterior Lobe) | ||||
| 16 | −63 | −40 | 3.49 | Cerebellum (Posterior Lobe) | ||||
Table 3.
Correlations between the acute cerebral metabolic response to citalopram and age in men and women.
| Women: Regions of Positive Correlations with Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| 60 | −34 | −14 | 3.79 | Middle Temporal Gyrus | ||||
| 3 | −65 | 31 | 3.01 | Cuneus | ||||
| 32 | −71 | −37 | 3.08 | Cerebellum | −29 | −61 | −23 | 3.01 |
| Women: Regions of Negative Correlations with Age: None | ||||||||
| Men: Regions of Positive Correlations with Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| 20 | −87 | 34 | 3.48* | Cuneus | ||||
| Men: Regions of Negative Correlations with Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| 18 | 45 | 44 | 3.12 | Superior Frontal Gyrus | −34 | 49 | 22 | 2.98 |
| 45 | 47 | 6 | 3.07* | Middle Frontal Gyrus | ||||
| 51 | 16 | 10 | 3.12* | Inferior Frontal Gyrus (BA 44) | ||||
| 12 | −8 | 12 | 3.70* | Thalamus | −14 | −19 | 3 | 3.57 |
Table 4.
Comparison of correlations between the acute cerebral metabolic response to citalopram and age in men compared to women.
| Greater Decreases Correlated with Increasing Age in Men compared to Women | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| Superior Frontal Gyrus | −10 | 60 | 21 | 3.26* | ||||
| Middle Frontal Gyrus | −9 | 25 | −18 | 3.09* | ||||
| Caudate | −10 | 12 | 13 | 2.98 | ||||
| Thalamus | −19 | −28 | 0 | 3.35 | ||||
| Greater Increases Correlated with Increasing Age in Women compared to Men | ||||||||
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | Brain Region | Left Hemisphere | ||||||
| Talairach Coordinates | Talairach Coordinates | |||||||
| x (mm) | y (mm) | z (mm) | Z score | x (mm) | y (mm) | z (mm) | Z score | |
| 39 | −80 | 21 | 3.09* | Middle Temporal Gyrus | ||||
| 28 | −87 | 26 | 3.35* | Cuneus | ||||
| 26 | −88 | 19 | 3.66* | Middle Occipital Gyrus | ||||
Significant regions are reported at a t threshold greater than 3.51 (z > 2.98, p < 0.00288; uncorrected for multiple independent comparisons) and a cluster size greater than 50 voxels. The comparisons that were significant at the cluster level (p < 0.05) corrected (**) and uncorrected (*) are indicated.
The comparisons were considered significant at a t threshold greater than 3.51 (z > 2.98, p < 0.00288; uncorrected for multiple independent comparisons) and a cluster size greater than 50 voxels. The comparisons that were significant at the cluster level (p < 0.01) are also indicated in the tables.
RESULTS
Baseline differences in glucose metabolism, cerebrospinal fluid (CSF), and gray matter (GM) concentrations by age and sex (data not shown)
Baseline metabolism was greater in women than in men in the right middle temporal gyrus. In contrast, men had higher baseline metabolism than women in pre-central gyrus (BA 6) and cuneus (BA 19) bilaterally, and right precuneus (BA 31). Regarding the difference in correlation between baseline glucose metabolism and age between men and women, greater baseline metabolism was correlated with increasing age to a greater extent in men compared to women in the left pre-central gyrus (BA 6) and right lingual gyrus (BA 17). For CSF, the positive correlation with age was observed to a greater extent in women compared to men in the medial frontal and middle temporal gyri (bilaterally), right insula, and right cuneus.
The acute cerebral metabolic response to citalopram in men and women (Table 1, Figure 1)
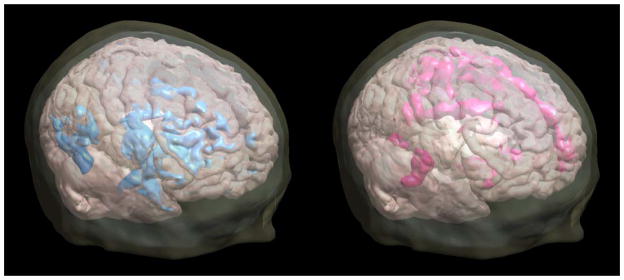
Figure 1.
Changes in cerebral glucose metabolism after acute citalopram administration (40mg, IV). In males (depicted on the left) the predominant change was a decrease in metabolism, and in females (depicted on the right) the predominant change was an increase in metabolism. The “blobs” indicate significant regions of change above the statistical threshold (p < 0.00288). Figures are superimposed on a three- dimensional rendering of a 7T MR scan.
Decreased metabolism after citalopram administration (metabolism after citalopram administration minus metabolism in the placebo condition) in women was observed in the left midbrain, and right middle temporal, and right middle occipital gyri (magnitude change −1.80% ± 1.86; range −5.52 to 1.24). The areas of increase were more extensive and included the left superior frontal gyrus, left anterior cingulate gyrus, left superior temporal gyrus, left uncus, left putamen, thalamus bilaterally (magnitude change left: 2.75% ± 2.62; range 1.88 to 7.83), and posterior cingulate gyri (bilaterally). Other areas of increase observed in women were in the right transverse temporal gyrus, and other right parietal cortical regions (including angular gyrus, inferior parietal gyrus, post-central gyrus, precuneus; magnitude change 1.96% ± 2.19; range −2.25 to 6.18).
In contrast to the women, the predominant change in metabolism after citalopram administration in the men was that of decreased metabolism in the anterior cingulate (magnitude change left: 2.42% ± 2.28; range −5.85 to 1.73), superior frontal (magnitude change right: −2.17% ±1.83; range 4.27 to 1.20), and pre-central gyri (bilaterally), right superior temporal gyrus, left superior parietal lobule, right posterior cingulate, right precuneus, and right inferior parietal lobule. There were no significant areas of increase in men. Figure 1 depicts the regions for which significant effects (i.e., t-values) were found; the magnitude of the effects are not indicated.
Comparison between the acute cerebral metabolic response to citalopram in men and women (Table 2)
For the comparison of the acute cerebral metabolic response to citalopram, greater decreases in women compared to men were observed in the right cerebellum (magnitude change female: −1.02% ± 3.00; range −6.74 to 3.62; male: 2.50% ± 3.11; range −3.13 to 6.47), whereas greater increases in women compared to men were observed in the anterior cingulate (bilaterally; left magnitude change female: −1.46% ± 2.16; range −3.04 to 4.86; male: −1.47% ± 1.84; range −4.48 to 1.58), left medial frontal, right pre-central, right transverse temporal, left supramarginal, and left inferior parietal gyri.
Correlations between the acute cerebral metabolic response to citalopram and age in men and women (Table 3)
In men, the change in metabolism after citalopram was positively correlated with age in the right cuneus (R = 0.74) and negatively correlated with age in the right and left superior frontal gyri and thalamus (R = −0.73), and in the right middle and inferior frontal gyri. Thus, in men, greater decreases in frontal and thalamic regions and greater increases in the occipital cortex were observed with increasing age. In women, greater increases in the right middle temporal gyrus (R= 0.60) and right cuneus, and in the cerebellum (bilaterally; right, R = 0.59) were associated with increasing age. Thus, for both men and women, the predominant effect (decrease in men and increase in women) is accentuated with age.
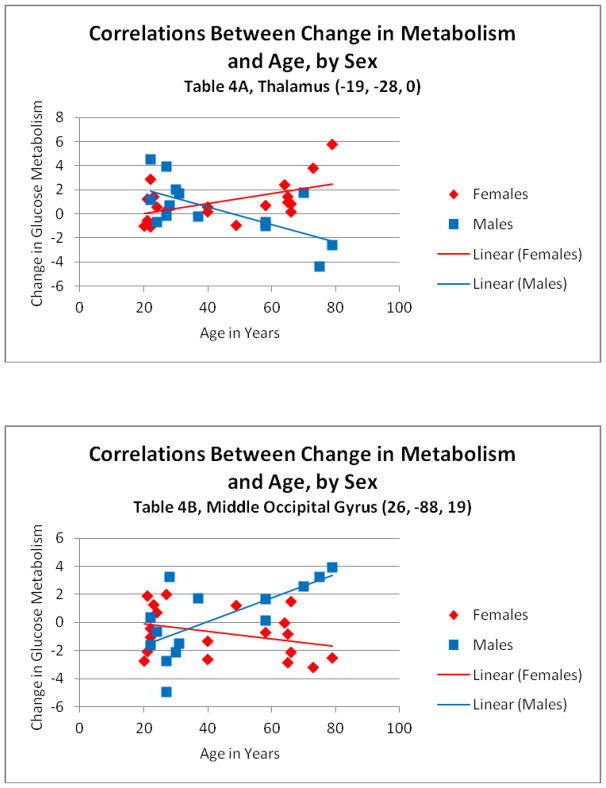
Comparison of correlations between the acute cerebral metabolic response to citalopram and age in men versus women (Table 4; Figure 2)
Figure 2.
Correlations between change in cerebral glucose metabolism in response to citalopram and age in the thalamus (A) and middle occipital gyrus (B), depicted in men and women separately.
With respect to the comparison of correlations with age between the sexes, greater decreases in the left superior frontal (female/male R = 0.52/−.66) and middle frontal gyri, left caudate, and left thalamus (female/male R = 0.59/0.53) were correlated with increasing age to a greater extent in men compared to women. Greater increases in the right middle temporal and middle occipital gyri (female/male R = −0.33/0.68) and right cuneus were associated with increasing age in women compared to men. Thus greater decreases in frontal and subcortical regions are observed with advancing age in men and greater increases in posterior cortical regions in women.
DISCUSSION
The goal of this study was to investigate the influence of sex and age on serotonin function, inferred from the cerebral glucose metabolic response to an acute citalopram pharmacologic intervention. The results demonstrated that women exhibited a different metabolic response to citalopram than did men with respect to the direction of the effect (increase versus decrease) and the brain regions involved. In particular, citalopram infusion in women produced widespread increases in glucose metabolism, with fewer areas of decreased metabolism. In contrast, men demonstrated no areas of increased metabolism, whereas several areas of decreased metabolism were observed. In comparing men and women, we found that relative to men, women had greater decreases in metabolism in the right cerebellum, but greater increases in the frontal (anterior cingulate [bilaterally], left medial frontal, right pre-central gyri), temporal (right transverse temporal gyrus) and parietal (left supramarginal and left inferior parietal gyri) cortices. In this sample, baseline cerebral glucose metabolism is higher in men in some regions (precentral gyrus, cuneus and precuneus) that show greater increases in response to citalopram in females (data not shown). Thus, it is possible that the differences in the direction of the effects between men and women may be explained by a normalization of cerebral metabolism since the comparison of metabolism in the citalopram condition did not differ significantly between men and women (data not shown). With respect to the possible influence of cerebral atrophy in interpreting the glucose metabolism data, there were few regions of increased CSF in comparing males and females and also in the comparing the correlations with age between the sexes. The regions that demonstrated greater CSF did not overlap with regions that showed sex or age related differences in response to citalopram. Thus, cerebral atrophy is not likely to contribute to the findings observed.
In prior studies (e.g., Sakai et al., 2006; Staley et al., 2001; Jovanovic et al., 2008), females show a greater capacity to synthesize serotonin, as well as greater SERT and 5-HT1A receptor densities compared to males, although some studies find no sex differences (e.g., Stein et al., 2008). The sex differences in the metabolic response to citalopram found in the current study may therefore be related to greater serotonin function and innervation in females compared to males and a greater net effect of serotonin transporter reuptake inhibition in females than males. Thus, the consequences of serotonin reuptake inhibition may be different in women compared to men.
While the direction of the effect was different, similar cortical regions were affected by citalopram in women and men. The regions affected are areas implicated in affective and cognitive processing, as well as appetitive behaviors that are serotonergically mediated (Liotti et al., 2000, Fletcher et al., 1995, Tataranni et al., 1999). The regions affected include components of the default mode network (e.g., anterior cingulate gyrus, middle frontal gyrus, precuneus; Buckner et al., 2005), as well as regions affected earliest in Alzheimer’s disease. The sensitivity of these regions to citalopram is of interest as serotonin degeneration is observed early in the course of AD, as well as in amyloid transgenic mouse models (as reviewed by Meltzer, et al., 1998a; Liu et al., 2008).
In normal aging, decreases in SERT, 5-HT1A and 5-HT2A receptors are observed (e.g., Møller et al., 2007; Adams et al., 2004; Yamamoto et al., 2002), in contrast to the lack of an aging effect on serotonin synthesis (Rosa-Neto et al., 2007). In the present study, we found that in normal aging the pattern of sex difference is accentuated, with greater increases in women and greater decreases in men associated with advancing age. As differential decreases with age as a function of sex have not been well studied, the significance of the accentuation of these sex differences in metabolic response with aging should be investigated. Furthermore, in our prior work, we found that the metabolic effects of citalopram in geriatric depressed patients are predominantly that of a decrease or normalization of metabolism relative to cerebral metabolism observed in demographically matched control subjects (Smith et al., 2009). Whether these metabolic effects differ between male and female depressed patients warrants further study.
Sex hormones, particularly estrogen and progesterone, have been shown to increase serotonin production (Sanchez et al., 2005) and possibly prevent serotonin cell death (Bethea et al., 2011; see also Fink et al., 1998). In fact, increased 5-HT2A receptor availability has been observed after estrogen and progesterone treatment in post-menopausal women (Moses et al., 2000). It is also the case that while citalopram has a primary mechanism of action by blocking serotonin reuptake and increasing serotonin concentrations, effects on other neurotransmitter systems (e.g., glutamate, norepinephrine, dopamine, and acetylcholine) have been reported (Golembiowska and Dziubina, 2000; Invernizzi et al., 1997; Lucas et al., 2000; Mateo et al., 2000; Hilgert et al., 2000). Given the pattern of alterations in cortical glucose metabolism and the strong evidence for interactions between serotonin and glutamate in neocortical pyramidal cells (as reviewed by Marek and Aghajanian, 1998), it is possible that secondary effects on glutamate concentrations may contribute to the cortical metabolic changes observed.
The impact of testosterone, which declines steadily in men beginning in middle adulthood, is difficult to evaluate as studies have focused on other neurotransmitters, not the serotonin system (see Zheng, 2009 for a review). Taken together with results from studies on sex hormones and their relation to the serotonin system, the accentuation of the pattern of changes in glucose metabolism by citalopram observed with advancing age may represent a compensatory response to these changes associated with decreased sex hormone levels with advancing age.
Two limitations that may contribute to the results obtained should be acknowledged. First, we did not control for phase of the menstrual cycle in women, which may have affected the results in premenopausal women. However, the evidence for changes in serotonin receptor binding during the menstrual cycle is limited and controversial (Jovanovic et al., 2006, 2009). Second, genotyping of the SERT promoter (5-HHTLPR) was not performed. 5-HTTLPR polymorphisms have been shown to influence the direction and laterality of the cerebral metabolic response to citalopram in earlier studies (Smith et al., 2004). Future studies will control for these potentially important variables.
CONCLUSIONS
The current findings indicate that citalopram-induced changes in cerebral metabolism are positively correlated with age in women, whereas in men the changes are primarily negatively correlated with age. Thus, greater increases in metabolism are observed in women and decreases in men with normal aging. These results are not only in line with prior findings suggesting that serotonin function differs in men and women, but also indicate that the nature of this difference changes with advancing age. Future studies aimed at characterizing the influence of age and sex on the serotonin system in patients with depression will further elucidate the relation between brain chemistry and treatment response in elderly men and women with depression.
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST
The present study was supported in part by National Institute of Health: MH 01621 (GSS), MH 49936 (GSS), MH 57078 (GSS), MH 64823 (GSS), M01 RR 018535 (Chiorazzi). David Bjelke, CNMT and Claude Margouleff, B.S. are gratefully acknowledged for their contribution to the conduct of the PET studies. The Geriatric Psychopharmacology Laboratory, Department of Psychiatry, University of Pittsburgh School of Medicine are gratefully acknowledged for providing the intravenous formulation of citalopram and performing the analyses of the citalopram samples.
Financial Disclosures: Dr. Smith received research funding from the National Institute of Mental Health and the National Alliance for Research in Schizophrenia and Depression. Drs. Kramer, Hermann, Ma, Chaly, Dhawan, Munro, and Eidelberg and Mr. Workman reported no biomedical finance interests or potential conflicts of interest.
References
- Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbøl S, Madsen K, Frøkjaer V, Martiny L, Paulson OB, Knudsen GM. A database of [(18)F]-altanserin binding to 5-HT(2A) receptors in normal volunteers: normative data and relationship to physiological and demographic variables. Neuroimage. 2004;21(3):1105–1113. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G, Katz IR, Reynolds CF, III, Carpenter D, Docherty JP. Expert Consensus Panel for Pharmacotherapy of Depressive Disorders in Older Patients. Postgrad Med Spec Report. 2001:1–86. [PubMed] [Google Scholar]
- Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M. Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cerebr Blood Flow Metab. 1988;8:502–512. doi: 10.1038/jcbfm.1988.91. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Smith AW, Centeno ML, Reddy AP. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience. 2011;192:675–688. doi: 10.1016/j.neuroscience.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, Goldman S. Sex difference in 5HT2 receptor in the living human brain. Neurosci Lett. 1996;204(1–2):25–28. doi: 10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, Ryvlin P, Le Bars D. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J Nucl Med. 2005;46(12):1980–1989. [PubMed] [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25(10):764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis 1 disorders-patient edition (SCID-I/P) New York Psychiatric Institute; New York: 1995. [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye--precuneus activation in memory-related imagery. Neuroimage. 1995;2(3):195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Frey BN, Skelin I, Sakai Y, Nishikawa M, Diksic M. Gender differences in α-[11C]MTrp brain trapping, an index of serotonin synthesis, in medication-free individuals with major depressive disorder: A positron emission tomography study. Psychiatry Res Neuroimaging. 2010;183:157–166. doi: 10.1016/j.pscychresns.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Frøkjaer VG, Erritzoe D, Madsen J, Paulson OB, Knudsen GM. Gender and the use of hormonal contraception in women are not associated with cerebral cortical 5-HT 2A receptor binding. Neuroscience. 2009;163:640–645. doi: 10.1016/j.neuroscience.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; London, UK: 2007. [Google Scholar]
- Goldberg S, Smith GS, Barnes A, Ma Y, Kramer E, Robeson K, Kirschner M, Pollock BG, Eidelberg D. Serotonin modulation of cerebral glucose metabolism in normal aging. Neurobiology of Aging. 2004;25:167–174. doi: 10.1016/s0197-4580(03)00088-5. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Dziubina A. Effect of acute and chronic administration of citalopram on glutamate and aspartate release in the rat prefrontal cortex. Pol J Pharmacol. 2000;52:441–448. [PubMed] [Google Scholar]
- Henderson JA, Bethea CL. Differential effects of ovarian steroids and raloxifene on serotonin 1A and 2C receptor protein expression in macaques. Endocrine. 2008;33(3):285–293. doi: 10.1007/s12020-008-9087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgert M, Buchholzer M, Jeltsch H, Kelche C, Cassel JC, Klein J. Serotonergic modulation of hippocampal acetylcholine release after long-term neuronal grafting. Neuroreport. 2000;11:3063–3065. doi: 10.1097/00001756-200009280-00006. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Velasco C, Bramante M, Longo A, Samanin R. Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology. 1997;36(4–5):467–473. doi: 10.1016/s0028-3908(97)00060-9. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Cerin A, Karlsson P, Lundberg J, Halldin C, Nordström AL. A PET study of 5-HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res. 2006;148(2–3):185–193. doi: 10.1016/j.pscychresns.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Karlsson P, Cerin A, Halldin C, Nordström AL. 5-HT(1A) receptor and 5-HTT binding during the menstrual cycle in healthy women examined with [(11)C] WAY100635 and [(11)C] MADAM PET. Psychiatry Res. 2009;172(1):31–37. doi: 10.1016/j.pscychresns.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordström AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39(3):1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Kapitany T, Schindl M, Schindler SD, Hesselmann B, Füreder T, Barnas C, Sieghart W, Kasper S. The citalopram challenge test in patients with major depression and in healthy controls. Psychiatry Res. 1999;88(2):75–88. doi: 10.1016/s0165-1781(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Kuikka JT, Tammela L, Bergström KA, Karhunen L, Uusitupa M, Tiihonen J. Effects of ageing on serotonin transporters in healthy females. Eur J Nucl Med. 2001;28(7):911–913. doi: 10.1007/s002590100540. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol Psychiatry. 2000;48(1):30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, Mamounas L, Lyons WE Blue ME, Lee MK. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2008;28(51):13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas G, De Deurwaerdere P, Porras G, Spampinato U. Endogenous serotonin enhances the release of dopamine in the striatum only when nigro-striatal dopaminergic transmission is activated. Neuropharmacology. 2000;39:1984–1995. doi: 10.1016/s0028-3908(00)00020-4. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. The electrophysiology of prefrontal serotonin systems: therapeutic implications for mood and psychosis. Biol Psychiatry. 1998;44:1118–1127. doi: 10.1016/s0006-3223(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Ruiz-Ortega JA, Pineda J, Ugedo L, Meana JJ. Inhibition of 5-hydroxytryptamine reuptake by the antidepressant citalopram in the locus coeruleus modulates the rat brain noradrenergic transmission in vivo. Neuropharmacology. 2000;39:2036–2043. doi: 10.1016/s0028-3908(00)00041-1. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Phelps ME, Plummer D, Kuhl DE. Quantitation in positron emission tomography: 5. Physical-anatomical effects. J Comput Assist Tomogr. 1981;5:734–743. doi: 10.1097/00004728-198110000-00029. [DOI] [PubMed] [Google Scholar]
- Meltzer C, Smith G, DeKosky S, Mathis C, Pollock B, Moore R, Kupfer D, Reynolds C. Serotonin in aging, late life depression, and Alzheimer’s disease: The emerging role of functional imaging. Neuropsychopharmacology. 1998a;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, Price JC, Reynolds CF, 3rd, Mathis CA, Greer P, Lopresti B, Mintun MA, Pollock BG, Ben-Eliezer D, Cantwell MN, Kaye W, DeKosky ST. Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: Persistence of effect after partial volume correction. Brain Res. 1998b;813(1):167–171. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- Møller M, Jakobsen S, Gjedde A. Parametric and regional maps of free serotonin 5HT1A receptor sites in human brain as function of age in healthy humans. Neuropsychopharmacology. 2007;32(8):1707–1714. doi: 10.1038/sj.npp.1301310. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Shah N, Berga S, Sereika SM, Fisher PM, Coleman R, Becker C, Mason NS, Loucks T, Meltzer CC. Age, sex, and reproductive hormone effects on brain serotonin-1A and serotonin-2A receptor binding in a healthy population. Neuropsychopharmacology. 2011;36(13):2729–2740. doi: 10.1038/npp.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa SS, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954(2):173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab. 2010;30(10):1682–706. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Neto P, Benkelfat C, Sakai Y, Leyton M, Morais JA, Diksic M. Brain regional alpha-[11C]methyl-L-tryptophan trapping, used as an index of 5-HT synthesis, in healthy adults: absence of an age effect. Eur J Nucl Med Mol Imaging. 2007;34(8):1254–1264. doi: 10.1007/s00259-007-0365-x. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Nishikawa M, Leyton M, Benkelfat C, Young SN, Diksik M. Cortical trapping of α-[11C]methyl-L-tryptophan, an index of serotonin synthesis, is lower in females than males. Neuroimage. 2006;33:815–824. doi: 10.1016/j.neuroimage.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135(1–2):194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mintun MA, Moerlein SM, Snyder AZ. Greater loss of 5-HT(2A) receptors in midlife than in late life. Am J Psychiatry. 2002;159(3):430–435. doi: 10.1176/appi.ajp.159.3.430. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Hermann CR, Goldberg S, Ma Y, Dhawan V, Barnes A, Chaly T, Belakhleff A, Laghrissi-Thode F, Greenwald B, Eidelberg D, Pollock BG. Acute and chronic effects of citalopram on cerebral glucose metabolism in geriatric depression. Am J Geriatric Psychiatry. 2002a;10:715–723. [PubMed] [Google Scholar]
- Smith GS, Kramer E, Hermann C, Ma Y, Dhawan V, Chaly T, Eidelberg D. Serotonin modulation of cerebral glucose metabolism in depressed older adults. Biol Psychiatry. 2009;66(3):259–266. doi: 10.1016/j.biopsych.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Lotrich F, Malhotra A, Lee A, Ma Y, Kramer E, Gregersen P, Eidelberg D, Pollock B. The effect of serotonin transporter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–2234. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- Smith GS, Ma Y, Dhawan V, Gunduz H, Carbon M, Kirshner M, Larson J, Chaly T, Belakhleff A, Kramer E, Greenwald B, Kane JM, Laghrissi-Thode F, Pollock BG, Eidelberg D. Serotonin modulation of cerebral glucose metabolism measured with positron emission tomography (PET) in human subjects. Synapse. 2002b;45(2):105–112. doi: 10.1002/syn.10088. [DOI] [PubMed] [Google Scholar]
- Smith GS, Workman CI, Kramer E, Hermann CR, Ginsberg R, Ma Y, Dhawan V, Chaly T, Eidelberg D. The relationship between the acute cerebral metabolic response to citalopram and chronic citalopram treatment outcome. Am J Geriatr Psychiatry. 2011;19(1):53–63. doi: 10.1097/jgp.0b013e3181eafde4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Price JC, Mason NS, Becker C, Meltzer CC. Gender, personality, and serotonin-2A receptor binding in healthy subjects. Psychiatry Res: Neuroimaging. 2010;181:77–84. doi: 10.1016/j.pscychresns.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fugita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis R. Sex differences in [123I]β-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Stein P, Savli M, Wadsak W, Mitterhauser M, Fink M, Spindelegger C, Mien LK, Moser U, Dudczak R, Kletter K, Kasper S, Lanzenberger R. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C]WAY-100635. Eur J Nucl Med Mol Imaging. 2008;35:2159–2168. doi: 10.1007/s00259-008-0850-x. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Suhara T, Okubo Y, Ichimiya T, Sudo Y, Inoue M, Takano A, Yasuno F, Yoshikawa K, Tanada S. Age-related decline of serotonin transporters in living human brain of healthy males. Life Sci. 2002;71:751–757. doi: 10.1016/s0024-3205(02)01745-9. [DOI] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89(2):134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]