Abstract
We describe the use of strain-promoted copper-free click reaction in the post-self-assembly functionalization of organoplatinum(II) metallacycles. The coordination-driven self-assembly of 120°-cyclooctyne tethered dipyridyl donor with 60° and 120°-di-Pt(II) acceptors formed molecular rhomboids and hexagons bearing cyclooctynes. These species undergo post-self-assembly [3 + 2] Huisgen cycloaddition with a variety of azides to give functionalized ensembles under mild conditions.
The functionalization of supramolecular assemblies has been extensively investigated over the past few years with an aim to develop nanoscale ensembles that can find applications in diverse fields such biological systems, host-guest chemistry, cavity directed synthesis, catalysis, photonics, redox activity, magnetic behavior, self-organization, and sensing.1 Although various functionalized nanoscopic systems have been developed through conventional covalent synthesis, the control of functional groups and structural precision, the ability to perform selective encapsulation, and synthetic ease and building-block versatility makes coordination-driven self-assembly a powerful tool to assemble functional supramolecules with relative simplicity. However, potential incompatibilities and interferences by various functional groups with the self-assembly process often limit the library and scope of the functionalized tectons. Thus post-self-assembly modification of supramolecular ensembles through transformation of the organic component of the assemblies may be a way to circumvent the problem. We have recently been able to achieve post-assembly functionalization of metallosupramolecular prisms via covalent modifications to incorporate new functionalities under mild conditions.2 The free amino groups tethered on the edges of the prisms allowed facile reactions with isocyanate or maleic anhydride. Similarly, the free malemide groups tethered on the edges of these prisms underwent Diels-Alders reaction with anthracenyl ferrocenoate. However, the range and scope of using amino group as a handle to introduce various functional groups is limited. The azide-alkyne based “click” reactions are attractive alternatives in this context since they usually involve weakly polarized reactants, minimizing undesired side reactions and thus could be an efficient method for expanding the range of chemical functionalities that can be tethered onto the metallosupramolecules. Post-synthetic modification of metal-organic frameworks3 have been achieved in recent years through copper(I) catalyzed azide-alkyne cycloaddition (CuAAC) reactions.4 However, the application of click chemistry to functionalize discrete assemblies has received much less attention. In a rare example, Zhou et al.5 described the functionalization of porous nanocages bearing free alkyne groups via the CuAAC reaction with azide-terminated PEG to transform the nanocages into water-stable colloids, which showed controlled release of the anticancer drug 5-fluorouracil. However, use of CuAAC reactions in living systems is limited due the cytotoxicity of the Cu(I) catalyst towards living cells. Copper free strain-promoted azide-alkyne cycloaddition (SPAAC) reactions6 recently developed between cyclooctynes and azides have found wide utility in chemical biology, such as labeling of biomolecules - glycans,7 proteins,8 lipids9 and nucleotides,10 modification of oligonucleotides11 and enzymes12, and cell and tissue surface engineering.13 In the field of material science, SPAAC reactions have been used for surface functionalization of dendrimers,14 polymers,15 nano-particles16 and nanowires,17 surface patterning,18 and cross-linking of polymers and hydrogels.19
Herein we report post-self-assembly functionalization of supramolecular ensembles through “copper-free click chemistry”. The 120°-cyclooctyne-tethered dipyridyl donor was synthesized via the amide coupling reaction of 3,5-bis(4-pyridylethynyl)aniline with 1-cyclooctyn-3-glycolic acid20 in dicloromethane leading to the formation of the cyclooctyne-tethered donor 1 (Supporting Information, Scheme S1).
Stirring a mixture of 120° cyclooctyne-tethered donor 3 and the 60° organoplatinum(II) acceptor, 3,6-Bis-[trans-Pt(PEt3)2(NO3)2]phenanthrene (4) in a 1:1 ratio in CD2Cl2 for 8 h led to the formation of self-assembled [2 + 2] metallacyclic rhomboid 6. Similarly, self-assembled [3 + 3] hexagon 7 was prepared by mixing the 120° donor ligand 3 with organoplatinum(II) acceptor, 4,4′-[trans-Pt(PEt3)2(NO3)2]diphenylketone (5) in a 1:1 ratio in CD3OD for 8 h. (Scheme 1). Multinuclear NMR (31P and 1H) of the reaction products revealed the formation of discrete, highly symmetric assemblies. These metallacycles contain a pendant cyclooctyne moiety at their vertices. The 31P {1H} NMR spectrum of 6 and 7 displayed sharp singlets at 12.7 and 14.1 ppm with concomitant 195Pt satellites corresponding to a single phosphorus environment (Figure 1). The peaks were shifted upfield from their respective platinum acceptors 4 and 5 by approximately 6.4 and 5.1 ppm. The up-field shift as well as the decrease in coupling constant (ΔJ) for the 195Pt satellites is consistent with back donation from the platinum atoms. In the 1H NMR spectrum of 6, signals due to the α- and β-protons of the pyridine rings showed the expected downfield shifts relative to 3 due to the loss of electron density that occurs upon coordination of the pyridyl-N atom to the Pt(II) metal center. As shown in Figure 2, the α- and β-protons on the pyridine rings are split into two sets of two doublets upon coordination that is consistent with previous observations of similar Pt-based rhomboids.21 The α-pyridyl protons of cyclooctyne-tethered donor 3, which appears as a doublet at 8.62 ppm is split into two doublets at 9.34 and 8.68 ppm. Similarly, β-pyridyl protons (δ = 7.42 ppm) are split into two doublets at 7.96 and 7.81 ppm. The signal at δ = 9.18 ppm was assigned to the amide proton. In the 1H NMR spectrum of 7, sharp signals corresponding to coordinated α- and β-pyridyl protons were identified at 8.89 and 7.87 ppm with downfield shifts relative to 3 (Figure 2).
Scheme 1.
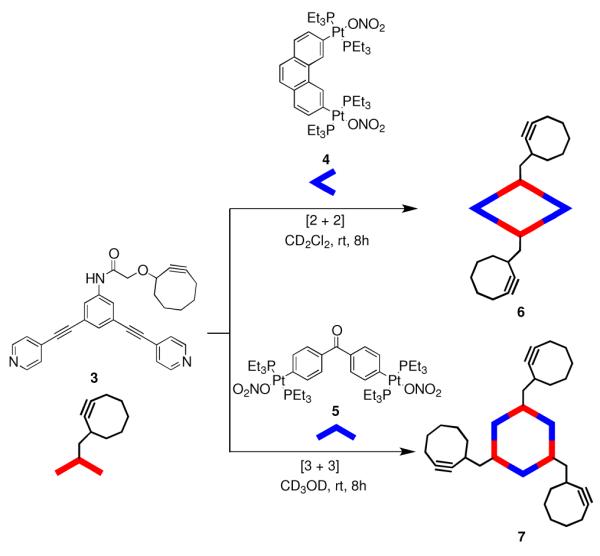
Self-assembly of Discrete Metallacyclic Rhomboids and Hexagons bearing cyclooctyne functionality.
Figure 1.
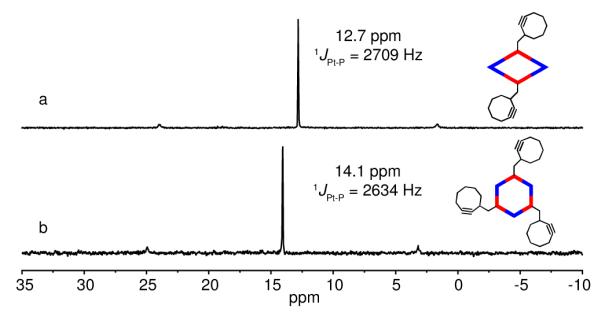
31P {1H} NMR spectra of a) rhomboid 6 and b) hexagon 7.
Figure 2.
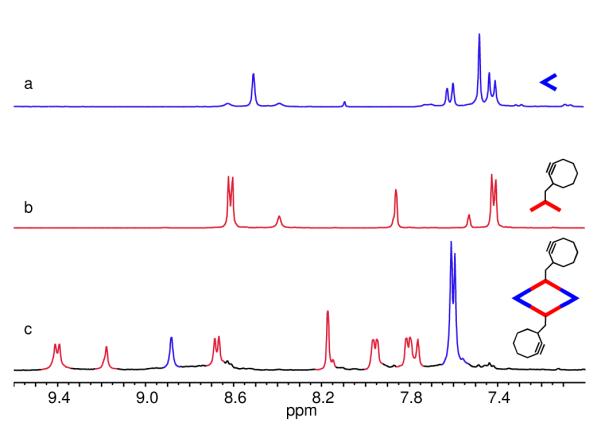
Partial 1H NMR spectra (in CD2Cl2) of a) Pt(II) acceptor 4, b) cyclooctyne tethered donor 3, and c) rhomboid 6.
Electrospray ionization-mass spectroscopic (ESI-MS) studies further confirmed the formation of discrete supramolecular assemblies. The ESI mass spectrum for rhomboid 6 (Supporting Information) showed a peak at m/z = 1560.5, corresponding to [M – 2NO3]2+ and m/z = 1019.02 attributable to [M –3NO3]3+. All the peaks were isotopically resolved, and agree very well with the calculated theoretical distribution. In the ESI-MS of 7, no parent ion peak was observed due to fragmentation. However, unique fragments were observed that support the formation of the hexagonal structure when analyzed in conjunction with the NMR spectra (Supporting information).
In order to gain structural information about the metallacycles, single-point DFT energy minimization were performed using the Gaussian09 package.22 All calculations were performed using the B3LYP hybrid DFT functional,23,24 and a split basis set wherein the 6-31G** basis set25 were used for C, H, N, O and P atoms, while the LANL2DZ basis set26 and pseudo potential were used for Pt. To minimize the computational cost, PEt3 ligands were modeled as PH3. In rhomboid 6, the two cyclooctyne moieties at the vertices lie above and below the plane of the central metallacyclic core. The cyclooctyne groups adopt a more stable boat conformation.27 The alkyne bonds in the cyclooctynes are bent from linearity. Previous studies28 using DFT-based models for the 1,3-dipolar cycloaddition of cyclooctynes with azides have shown that such deviation from linearity distorts the cyclooctynes towards the transition state geometry, thus requiring less distortion energy to reach their preferred transition state geometry relative to a linear alkyne.
The metallacyclic rhomboid 6 was tested for post-assembly functionalization via “copper free click chemistry”. The rhomboid 6 undergoes efficient [3 + 2] Huisgen-type SPAAC reactions with a variety of functionalized azides to give functionalized metallacycles under mild conditions (Scheme 2). Molecular rhomboids 8a-c were obtained upon treatment of 6 with azidomethyl benzene, 1-(azidomethyl)pyrene and 2-(azidoethyl)biotinamide, respectively, in 1:5 ratio in CD2Cl2 for 2 h at room temperature. In all cases, the only product observed were the two regioisomeric 1, 4, 5-trisubstituted-1, 2, 3-triazoles in varying ratios as identified from 1H NMR spectra of 8a-c. The 31P {1H} NMR spectra for all the assemblies remained unchanged with the peak appearing at about the same position relative to their unfunctionalized counterpart. The 31P {1H} NMR spectrum of the functionalized rhomboids 8a-c appear as sharp singlets at 12.7 ppm identical to that of its unfunctionalized rhomboid 7. The 1H NMR spectra of the ensembles showed additional peaks attributable to benzyl, pyrenyl, and biotinyl protons (Supporting information). In the 1H NMR spectrum of 8a, the phenyl protons originating from the benzyl azide appear as a multiplet 7.38 ppm while the benzyl protons appear at 5.60 ppm and are consistent with previous observations.29 The pyrenyl protons in 8b appear as a multiplet at 8.27-8.11 ppm with the benzyl proton appearing at 6.38 ppm.
Scheme 2.
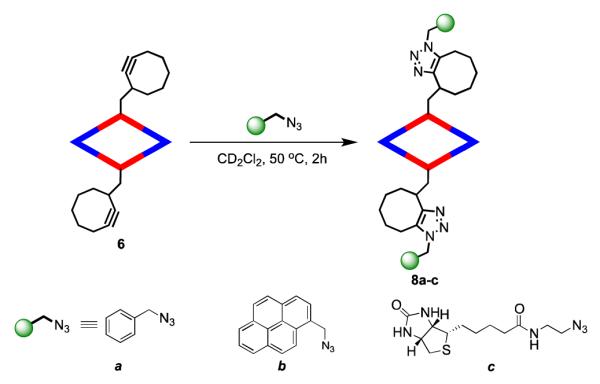
Post-assembly modification of discrete metallacyclic rhomboids with different azides via copper-free click chemistry.
ESI-MS data provided further evidence for the formation of the discrete functionalized species. The ESI mass spectrum for 8a showed isotopically resolved peaks at m/z = 1693.6, corresponding to [M – 2NO3]2+ and m/z = 1108.1 attributable to [M – 3NO3]3+ (Supporting information). Similarly, isotopically resolved peaks due to [M – 2NO3]2+ and [M – 3NO3]3+ were observed at m/z = 1817.6 and 1190.8 in the ESI-MS spectrum of 8b. The ESI-MS spectrum of biotin functionalized species 9c also showed isotopically resolved peak at m/z = 1228.1, corresponding to [M – 3NO3]3+.
In conclusion, we have demonstrated that copper-free strain-promoted azide-alkyne cycloaddition (SPAAC) reactions can be effectively used to functionalize metallacycles having pendant cyclooctyne groups. In contrast to other methodologies for construction of functionalized supramolecules where a pre-functionalized unit is used, this method provides a facile way to incorporate a wide range of chemical functionalities on appropriate supramolecular assemblies with relative synthetic ease. The ready access to a multi-biotin scaffold permits the formation of a biotin multimeric structure for potential enhancement of avidin-biotin assays. Since a multivalent biotin scaffold allows many biotin binding proteins to dock simultaneously and form larger complexes.30 These conjugates may recruit more detection molecules to bind at the site of an analyte that can have a direct effect on the sensitivity of immuno-assays, fluorescent detection, enzyme linked immune-adsorbent assays and western blotting procedures. Functionalization of three-dimensional supramolecular cages via this methodology, having large cavities, with biologically relevant homing devices may lead to better drug delivery devices. Investigation along these lines is currently underway in our laboratory.
Supplementary Material
Figure 3.
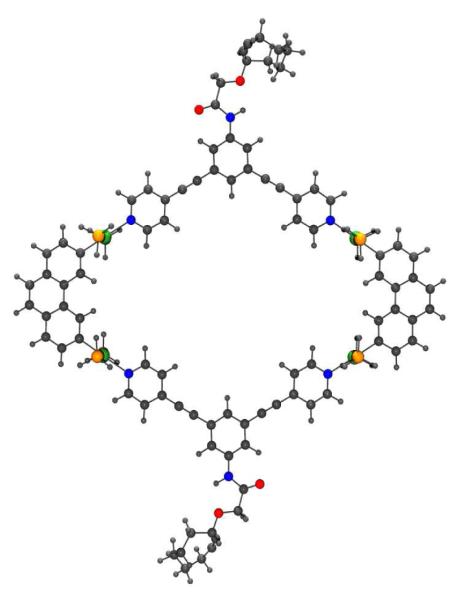
Density Functional Theory (DFT) optimized structure of metallacyclic rhomboid 6. Color code: gray, C; light gray, H; Blue, N; red, O; orange, P; green, Pt.
ACKNOWLEDGMENT
PJS thanks the NIH (Grant GM-057052) for financial support.
Footnotes
Supporting Information. Experimental procedures and characterizations data for all metallacyclic assemblies. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Chakrabarty R, Mukherjee PS, Stang PJ. Chem. Rev. 2011;111:6810. doi: 10.1021/cr200077m. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wang M, Lan W-J, Zheng Y-R, Cook TR, White HS, Stang PJ. J. Am. Chem. Soc. 2011;133:10752. doi: 10.1021/ja204155r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cohen SM. Chem. Rev. 2012;112:970. doi: 10.1021/cr200179u. [DOI] [PubMed] [Google Scholar]; (b) Wang Z, Cohen SM. Chem. Soc. Rev. 2009;38:1315. doi: 10.1039/b802258p. [DOI] [PubMed] [Google Scholar]; (c) Song Y-F, Cronin L. Angew. Chem. Int. Ed. 2008;47:4635. doi: 10.1002/anie.200801631. [DOI] [PubMed] [Google Scholar]
- (4) (a).Goto Y, Sato H, Shinkai S, Sada K. J. Am. Chem. Soc. 2008;130:14354. doi: 10.1021/ja7114053. [DOI] [PubMed] [Google Scholar]; (b) Gadzikwa T, Lu G, Stern CL, Wilson SR, Hupp JT, Nguyen ST. Chem. Commun. 2008:5493. doi: 10.1039/b805101a. [DOI] [PubMed] [Google Scholar]; (c) Gadzikwa T, Farha OK, Malliakas CD, Kanatzidis MG, Hupp JT, Nguyen ST. J. Am. Chem. Soc. 2009;131:13613. doi: 10.1021/ja904189d. [DOI] [PubMed] [Google Scholar]; (d) Savonnet M, Bazer-Bachi D, Bats N, Perez-Pellitero J, Jeanneau E, Lecocq V, Pinel C, Farrusseng D. J. Am. Chem. Soc. 2010;132:4518. doi: 10.1021/ja909613e. [DOI] [PubMed] [Google Scholar]; (e) Kawamichi T, Inokuma Y, Kawano M, Fujita M. Angew. Chem., Int. Ed. 2010;49:2375. doi: 10.1002/anie.201000018. [DOI] [PubMed] [Google Scholar]
- (5).Zhao D, Tan S, Yuan D, Lu W, Rezenom YH, Jiang H, Wang L-Q, Zhou H-C. Adv. Mater. 2011;23:90. doi: 10.1002/adma.201003012. [DOI] [PubMed] [Google Scholar]
- (6) (a).Reviews: Sletten EM, Bertozzi CR. Angew. Chem. Int. Ed. 2009;48:6974. doi: 10.1002/anie.200900942. Best MD. Biochemistry. 2009;48:6571. doi: 10.1021/bi9007726. Becer CR, Hoogenboom R, Schubert U. Angew. Chem. Int. Ed. 2009;48:4900. doi: 10.1002/anie.200900755. Jewett JC, Bertozzi CR. Chem. Soc. Rev. 2010;39:1272. doi: 10.1039/b901970g. Debets MF, van der Doelen CWJ, Rutjes FPJT, van Delft FL. ChemBioChem. 2010;11:1168. doi: 10.1002/cbic.201000064. Sletten EM, Bertozzi CR. Acc. Chem. Res. 2011;44:666. doi: 10.1021/ar200148z. Best MD, Rowland MM, Bostic HE. Acc. Chem. Res. 2011;44:686. doi: 10.1021/ar200060y. Debets MF, van Berkel SS, Dommerholt J, Dirks AJ, Rutjes FPJT, van Delft FL. Acc. Chem. Res. 2011;44:805. doi: 10.1021/ar200059z.
- (7) (a).Prescher JA, Bertozzi CR. Cell. 2006;126:851. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]; (b) Chang PV, Prescher JA, Hangauer MJ, Bertozzi CR. J. Am. Chem. Soc. 2007;129:8400. doi: 10.1021/ja070238o. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ning X, Guo J, Wolfert MA, Boons G-J. Angew. Chem. Int. Ed. 2008;47:2253. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1821. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Baskin JM, Dehnert KW, Laughlin ST, Amacher SL, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10360. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Dehnert KW, Beahm BJ, Huynh TT, Baskin JM, Laughlin ST, Wang W, Wu P, Amacher SL, Bertozzi CR. ACS Chem. Biol. 2011;6:547. doi: 10.1021/cb100284d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8) (a).Link AJ, Vink MKS, Agard NJ, Prescher JA, Bertozzi CR, Tirrell DA. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10180. doi: 10.1073/pnas.0601167103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tanrikulu IC, Schmitt E, Mechulam Y, Goddard WA, III, Tirrell DA. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15285. doi: 10.1073/pnas.0905735106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Uttamapinant C, White KA, Baruah H, Thompson S, Fernández-Suárez M, Puthenveetil S, Ting AY. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10914. doi: 10.1073/pnas.0914067107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Plass T, Milles S, Koehler C, Schultz C, Lemke EA. Angew. Chem. Int. Ed. 2011;50:3878. doi: 10.1002/anie.201008178. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yao JZ, Uttamapinant C, Poloukhtine AA, Baskin JM, Codelli JA, Sletten EM, Bertozzi CR, Popik VV, Ting AY. J. Am. Chem. Soc. 2012;134:3720. doi: 10.1021/ja208090p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9) (a).Fernández-Suárez M, Baruah H, Martínez-Hernández L, Xie KT, Baskin JM, Bertozzi CR, Ting AY. Nat. Biotechnol. 2007;25:1483. doi: 10.1038/nbt1355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Neef AB, Schultz C. Angew. Chem. Int. Ed. 2009;48:1498. doi: 10.1002/anie.200805507. [DOI] [PubMed] [Google Scholar]; (c) Bostic HE, Smith MD, Poloukhtine AA, Popik VV, Best MD. Chem. Commun. 2012;48:1431. doi: 10.1039/c1cc14415d. [DOI] [PubMed] [Google Scholar]
- (10).Marks IS, Kang JS, Jones BT, Landmark KJ, Cleland AJ, Taton TA. Bioconjugate Chem. 2011;22:1259. doi: 10.1021/bc1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11) (a).Gutsmiedl K, Wirges CT, Ehmke V, Carell T. Org. Lett. 2009;11:2405. doi: 10.1021/ol9005322. [DOI] [PubMed] [Google Scholar]; (b) Jayaprakash KN, Peng CG, Butler D, Varghese JP, Maier MA, Rajeev KG, Manoharan M. Org. Lett. 2010;12:5410. doi: 10.1021/ol102205j. [DOI] [PubMed] [Google Scholar]; (c) Sharma AK, Heemstra JM. J. Am. Chem. Soc. 2011;133:12426. doi: 10.1021/ja205518e. [DOI] [PubMed] [Google Scholar]; (d) Singh I, Heaney F. Chem. Commun. 2011;47:2706. doi: 10.1039/c0cc03985c. [DOI] [PubMed] [Google Scholar]; (d) Shelbourne M, Chen X, Brown T, El-Sagheer AH. Chem. Commun. 2011;47:6257. doi: 10.1039/c1cc10743g. [DOI] [PubMed] [Google Scholar]
- (12).Debets MF, van Berkel SS, Schoffelen S, Rutjes FPJT, van Hest JCM, van Delft FL. Chem. Commun. 2010;46:97. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- (13).Wilson JT, Krishnamurthy VR, Cui W, Qu Z, Chaikof EL. J. Am. Chem. Soc. 2009;131:18228. doi: 10.1021/ja908887v. [DOI] [PubMed] [Google Scholar]
- (14) (a).Ornelas C, Broichhagen J, Weck M. J. Am. Chem. Soc. 2010;132:3923. doi: 10.1021/ja910581d. [DOI] [PubMed] [Google Scholar]; (b) Ledin PA, Friscourt F, Guo J, Boons G-J. Chem. Eur. J. 2011;17:839. doi: 10.1002/chem.201002052. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huang B, Desai A, Zong H, Tang S, Leroueil P, Baker JR., Jr. Tet. Lett. 2011;52:1411. doi: 10.1016/j.tetlet.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Canalle LA, van Berkel SS, de Haan LT, van Hest JCM. Adv. Funct. Mater. 2009;19:3464. [Google Scholar]
- (16) (a).Lallana E, Fernandez-Megia E, Riguera R. J. Am. Chem. Soc. 2009;131:5748. doi: 10.1021/ja8100243. [DOI] [PubMed] [Google Scholar]; (b) Jølck RI, Sun H, Berg RH, Andresen TL. Chem. Eur. J. 2011;17:3326. doi: 10.1002/chem.201003131. [DOI] [PubMed] [Google Scholar]
- (17).Henriksson A, Friedbacher G, Hoffmann H. Langmuir. 2011;27:7345. doi: 10.1021/la200951x. [DOI] [PubMed] [Google Scholar]
- (18) (a).DeForest CA, Polizzotti BD, Anseth KS. Nat. Mater. 2009;8:659. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DeForest CA, Sims EA, Anseth KS. Chem. Mater. 2010;22:4783. doi: 10.1021/cm101391y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Orski SV, Poloukhtine AA, Arumugam S, Mao L, Popik VV, Locklin J. J. Am. Chem. Soc. 2010;132:11024. doi: 10.1021/ja105066t. [DOI] [PubMed] [Google Scholar]
- (19) (a).Johnson JA, Baskin JM, Bertozzi CR, Koberstein JT, Turro NJ. Chem. Commun. 2008:3064. doi: 10.1039/b803043j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu J, Filion TM, Prifti F, Song J. Chem. Asian J. 2011;6:2730. doi: 10.1002/asia.201100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem. Biol. 2006;10:644. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- (21).Pollock JB, Cook TR, Stang PJ. J. Am. Chem. Soc. 2012;134:10607. doi: 10.1021/ja3036515. [DOI] [PubMed] [Google Scholar]
- (22).Frisch MJ, et al. Gaussian09. Revision C.01. Gaussian, Inc.; Wallingford, CT: 2010. See supporting information for full reference. [Google Scholar]
- (23).Becke AD. J. Chem. Phys. 1993;98:5648. [Google Scholar]
- (24).Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- (25).Hehre WJ, Ditchfield R, Pople JA. J. Chem. Phys. 1972;56:2257. [Google Scholar]
- (26).Hay PJ, Wadt WR. J. Chem. Phys. 1985;82:299. [Google Scholar]
- (27).Schoenebeck F, Ess DH, Jones GO, Houk KN. J. Am. Chem. Soc. 2009;131:8121. doi: 10.1021/ja9003624. [DOI] [PubMed] [Google Scholar]
- (28) (a).Gordon CG, Mackey JL, Jewett JC, Sletten EM, Houk KN, Bertozzi CR. J. Am. Chem. Soc. 2012;134:9199. doi: 10.1021/ja3000936. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ess DH, Jones GO, Houk KN. Org. Lett. 2008;10:1633. doi: 10.1021/ol8003657. [DOI] [PubMed] [Google Scholar]
- (29).Campbell-Verduyn L, Elsinga PH, Mirfeizi L, Dierckx RA, Ferringa BL. Org. Biomol. Chem. 2008;6:3461. doi: 10.1039/b812403e. [DOI] [PubMed] [Google Scholar]
- (30).Xu H, Regino CAS, Koyama Y, Hama Y, Gunn AJ, Bernardo M, Kobayashi H, Choyke PL, Brechbiel MW. Bioconjugate Chem. 2007;18:1474. doi: 10.1021/bc0701085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


